Sodium (original) (raw)
Data Zone | Discovery | Facts | Appearance & Characteristics | Uses | Abundance & Isotopes | References
The chemical element sodium is classed as an alkali metal. It was discovered in 1807 by Sir Humphry Davy.

Data Zone
| Classification: | Sodium is an alkali metal |
|---|---|
| Color: | silvery-white |
| Atomic weight: | 22.98977 |
| State: | solid |
| Melting point: | 97.72 oC, 370.87 K |
| Boiling point: | 883 oC, 1156 K |
| Electrons: | 11 |
| Protons: | 11 |
| Neutrons in most abundant isotope: | 12 |
| Electron shells: | 2,8,1 |
| Electron configuration: | 1s2 2s2 2p6 3s1 |
| Density @ 20oC: | 0.971 g/cm3 |
Show more, including: Heats, Energies, Oxidation,
Reactions, Compounds, Radii, Conductivities
| Atomic volume: | 23.7 cm3/mol |
|---|---|
| Structure: | bcc: body-centered cubic |
| Hardness: | 0.5 mohs |
| Specific heat capacity | 1.23 J g-1 K-1 |
| Heat of fusion | 2.598 kJ mol-1 |
| Heat of atomization | 107 kJ mol-1 |
| Heat of vaporization | 96.960 kJ mol-1 |
| 1st ionization energy | 495.8 kJ mol-1 |
| 2nd ionization energy | 4562.4 kJ mol-1 |
| 3rd ionization energy | 6912.2 kJ mol-1 |
| Electron affinity | 52.868 kJ mol-1 |
| Minimum oxidation number | -1 |
| Min. common oxidation no. | 0 |
| Maximum oxidation number | 1 |
| Max. common oxidation no. | 1 |
| Electronegativity (Pauling Scale) | 0.93 |
| Polarizability volume | 23.6 Å3 |
| Reaction with air | vigorous, ⇒ Na2O2 |
| Reaction with 15 M HNO3 | vigorous, ⇒ NaNO3, NOx |
| Reaction with 6 M HCl | vigorous, ⇒ H2, NaCl |
| Reaction with 6 M NaOH | vigorous, ⇒ H2, NaOH |
| Oxide(s) | Na2O |
| Hydride(s) | NaH |
| Chloride(s) | NaCl |
| Atomic radius | 186 pm |
| Ionic radius (1+ ion) | 116 pm |
| Ionic radius (2+ ion) | – |
| Ionic radius (3+ ion) | – |
| Ionic radius (1- ion) | – |
| Ionic radius (2- ion) | – |
| Ionic radius (3- ion) | – |
| Thermal conductivity | 142 W m-1 K-1 |
| Electrical conductivity | 20.1 x 106 S m-1 |
| Freezing/Melting point: | 97.72 oC, 370.87 K |

Untarnished sodium stored under oil.

Sodium metal with oxide layer on uncut surfaces.

A sodium salt held in a hot, blue flame burns with a characteristic yellow flame. Image by Herge.
Discovery of Sodium
In 1806 Sir Humphry Davy discovered that chemical bonding was electrical in nature and that he could use electricity to split substances into their basic building blocks – the chemical elements.
In 1807, at the Royal Institution, London, a few days after isolating potassium for the first time, he isolated sodium for the first time by electrolysis of dried sodium hydroxide, which had been very slightly moistened.
The electrolysis was powered by the combined output of three large batteries he had built.
Davy noted that the metal which formed at the wire electrode he placed in the sodium hydroxide was a liquid, but became solid on cooling and “appeared to have the lustre of silver.” (1)
“It is exceedingly malleable and is much softer than any of the common metallic substances… this property does not diminish when it is cooled to 32 oF (0 oC).”
Davy also noted that, when added to water, sodium decomposed the water, releasing hydrogen.
He asked whether the new substance should be classed as a metal and noted that most other scientists thought it should, despite the fact that its density was much lower than the other metals then known:
“…for amongst the metals themselves there are remarkable differences in this respect, platina [_we now call it platinum_] being nearly four times as heavy as tellurium.”
He named the new metal sodium, because he had used caustic soda or, more simply, soda, as his source of the element. (2),(3)
In Germany caustic soda was known as natronlauge and L. W. Gilbert suggested the new element should be called natronium.
Jacob Berzelius preferred the shorter natrium, from which we get the chemical symbol for sodium, Na. (4)
Interesting Facts about Sodium
- It’s possible this page could have been titled ‘Sodagen.’ This is the name Sir Humphry Davy gave the new metallic element in his laboratory notebook, before deciding he preferred ‘Sodium.’ (5)
- Sodium and its close periodic table neighbor potassium are solids at room temperature. Their alloys however are not. NaK alloys containing 10 to 60 percent of sodium by weight are liquids at room temperature. The commercially available 78% K, 22% Na alloy stays liquid at temperatures as low as -12.6 oC (9.3 oF).
- Humans and other animals need sodium to maintain the correct fluid balance in their cells. An immediate effect of low sodium can be seen in heat cramping, when athletes’ muscles seize up after exertion. Heat cramping is caused by the loss of sodium ions when salt is removed from the body in sweat.
- Sodium is produced in heavy stars, mainly when atoms of neon gain a proton. (The neon atoms were themselves produced by carbon atoms coming together in nuclear fusion reactions.)
- If all the sodium chloride (table salt) in the oceans could be extracted and dried, it would cover the entire surface of the USA to a depth of almost a mile and a half (about 2.3 km). The same salt could cover all the land on Earth to a depth of almost 500 feet (150 m).

This laser beam is tuned to energize sodium atoms left behind by the passage of meteorites in Earth’s mesosphere. The energized sodium atoms glow, forming a small, bright spot. Anyone looking from ground level sees a new, artificial star. The twinkles of this ‘little star’ are fed into an adaptive optics system to restore the image of this ‘star’ to a sharp point. The atmosphere’s distortions are therefore removed, allowing very clear images to be taken of real stars. Image by ESO/G. Hüdepohl (www.atacamaphoto.com).
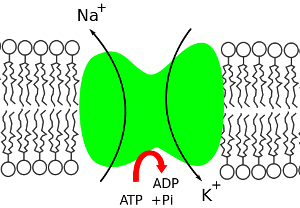
The importance of the sodium-potassium pump to our lives is shown by the fact that it uses one-third of our resting energy. The pump maintains our cells’ electrolyte balance, with excess potassium ions inside cells and excess sodium ions outside cells. This concentration gradient creates a voltage across the cell wall, which allows electrical signals to be transmitted in neurons and in muscles. It also provides the energy for processes in cell-membranes. Image by Phi-Gastrein.

A sphere containing 13 tons of rotating liquid sodium will be used in experiments to learn more about Earth’s magnetic field.
What happens when 20,000 lb of sodium meets a lake?
Harmful effects:
Sodium is considered to be non-toxic. Contact with the skin may, however, cause irritation and burns.
Characteristics:
Sodium is a soft, silvery-white metal. It is soft enough to cut with the edge of a coin.
Freshly cut surfaces oxidize rapidly in air to form a dull, oxide coating.
Sodium burns in air with a brilliant yellow flame.
Sodium floats on water, because its density is lower than water’s. It also reacts vigorously with water – violently if more than a small amount of sodium meets water (see video on left) – to produce sodium hydroxide and hydrogen gas. Sodium reacts with water more vigorously than lithium and less vigorously than potassium. Explosions occur when the heat generated by the sodium-water reaction ignites the resulting hydrogen gas.
Uses of Sodium
Metallic sodium is used in the manufacture of sodamide and esters, and in the preparation of organic compounds. The metal also may be used to modify alloys such as aluminum–silicon by improving their mechanical properties and fluidity. Sodium is used to descale (smooth the surface of) metals and to purify molten metals.
Sodium vapor lamps are highly efficient in producing light from electricity and are often used for street lighting in cities.
Sodium is used as a heat transfer agent; for example, liquid sodium is used to cool nuclear reactors.
Sodium chloride (table salt, NaCl) is vital for good nutrition. Sodium ions facilitate transmission of electrical signals in the nervous system and regulate the water balance between body cells and body fluids.
Abundance and Isotopes
Abundance earth’s crust: 2.4 % by weight, 2.1 % by moles
Abundance solar system: 40 parts per million by weight, 2 parts per million by moles
Cost, pure: $25 per 100g
Cost, bulk: $ per 100g
Source: Due to its high reactivity, sodium is found in nature only as a compound and never as the free element. Sodium is our planet’s sixth most abundant element and it is the most abundant alkali metal. Sodium is obtained commercially by electrolysis of molten sodium chloride.
Isotopes: Sodium has 16 isotopes whose half-lives are known, with mass numbers 20 to 35. Naturally occurring sodium consists of its one stable isotope, 23Na.

References
- Humphry Davy, The Bakerian Lecture, on some new phenomena of chemical changes produced by electricity, particularly the decomposition of the fixed alkalies, and the exhibition of the new substances… etc., Philosophical Transactions of the Royal Society (1808) p1.
- Elementymology & Elements: Sodium
- Saul S. Hauben, J. Chem. Educ., 1933, 10 (4), p 227.
- Martin R. Feldman, J. Chem. Educ., 1980, 57 (12), p 877.
- John M. Thomas, Peter P. Edwards, Vladimir L. Kuznetsov, Sir Humphry Davy: Boundless Chemist, Physicist, Poet and Man of Action., ChemPhysChem., 2008, 9 , p 59.
Cite this Page
For online linking, please copy and paste one of the following:
or
To cite this page in an academic document, please use the following MLA compliant citation:
"Sodium." Chemicool Periodic Table. Chemicool.com. 18 Oct. 2012. Web.
https://www.chemicool.com/elements/sodium.html.