Copper in PDB 9bnh: X-Ray Crystal Structure of Cu-TZ4H Tryptophan Zipper Metallo-Peptide (original) (raw)
Protein crystallography data
The structure of X-Ray Crystal Structure of Cu-TZ4H Tryptophan Zipper Metallo-Peptide, PDB code: 9bnhwas solved by V.T.Dang, A.Nguyen, with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 16.48 / 1.12 |
|---|---|
| Space group | P 1 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 23.198, 25.261, 24.149, 90, 110.32, 90 |
| R / Rfree (%) | 12 / 14.5 |
Other elements in 9bnh:
The structure of X-Ray Crystal Structure of Cu-TZ4H Tryptophan Zipper Metallo-Peptide also contains other interesting chemical elements:
Copper Binding Sites:
The binding sites of Copper atom in the X-Ray Crystal Structure of Cu-TZ4H Tryptophan Zipper Metallo-Peptide (pdb code 9bnh). This binding sites where shown within 5.0 Angstroms radius around Copper atom.
In total 2 binding sites of Copper where determined in the X-Ray Crystal Structure of Cu-TZ4H Tryptophan Zipper Metallo-Peptide, PDB code: 9bnh:
Jump to Copper binding site number: 1; 2;
Copper binding site 1 out of 2 in 9bnh
Go back to  Copper Binding Sites List in 9bnh
Copper Binding Sites List in 9bnh 
Copper binding site 1 out of 2 in the X-Ray Crystal Structure of Cu-TZ4H Tryptophan Zipper Metallo-Peptide
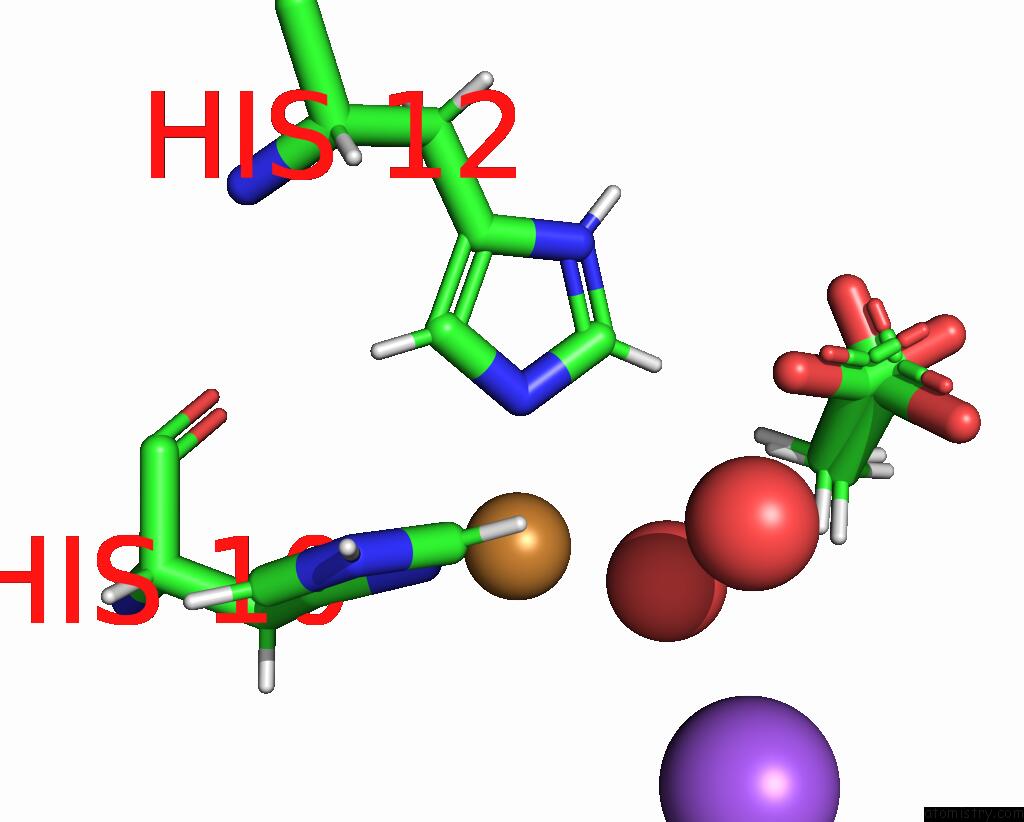
Mono view
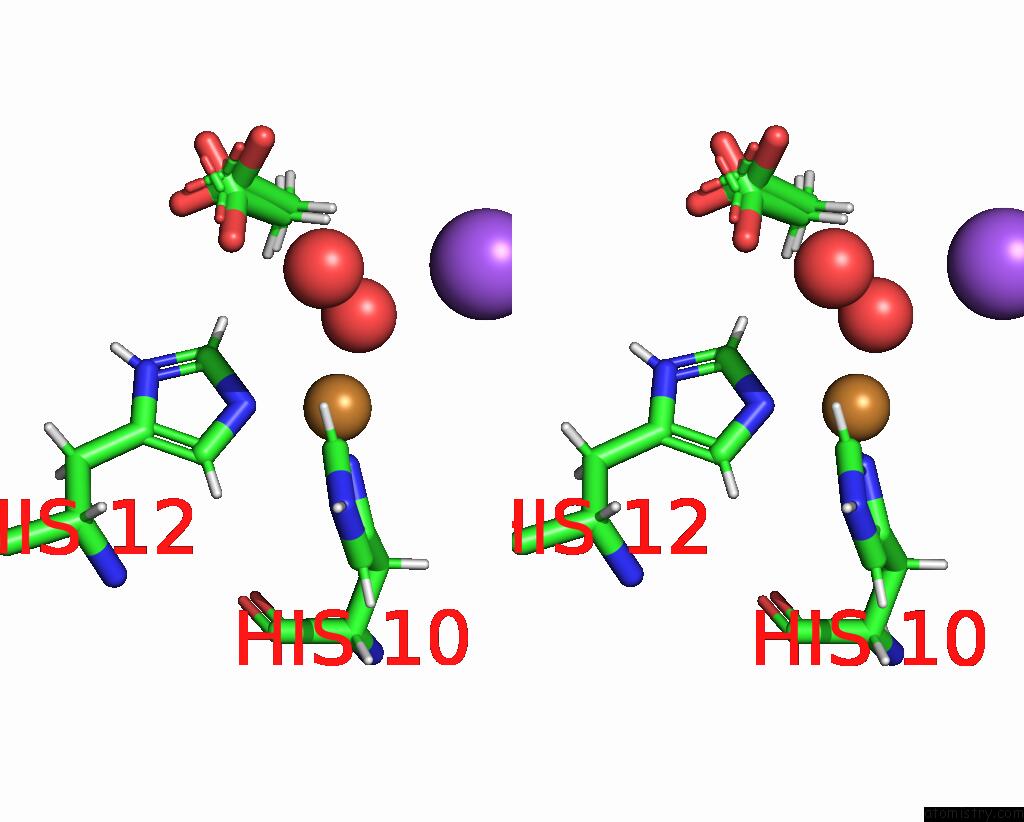
Stereo pair view
| | A full contact list of Copper with other atoms in the Cu binding site number 1 of X-Ray Crystal Structure of Cu-TZ4H Tryptophan Zipper Metallo-Peptide within 5.0Å range: probe atom residue distance (Å) B Occ A:Cu103 b:9.1 occ:0.97 NE2 A:HIS12 2.0 9.4 1.0 ND1 A:HIS10 2.0 10.3 1.0 O A:HOH215 2.2 13.4 1.0 HB3 A:HIS10 2.8 11.3 1.0 CE1 A:HIS12 2.9 10.5 1.0 CE1 A:HIS10 3.0 10.7 1.0 CD2 A:HIS12 3.0 9.0 1.0 CG A:HIS10 3.0 9.8 1.0 HE1 A:HIS12 3.1 12.6 1.0 HE1 A:HIS10 3.2 12.8 1.0 HD2 A:HIS12 3.3 10.8 1.0 CB A:HIS10 3.4 9.4 1.0 H3 A:ACY102 3.6 14.5 0.7 H1 A:ACY102 3.6 14.5 0.3 HB2 A:HIS10 3.7 11.3 1.0 H1 A:ACY102 4.0 14.5 0.7 ND1 A:HIS12 4.1 10.2 1.0 NE2 A:HIS10 4.1 11.7 1.0 CG A:HIS12 4.2 9.6 1.0 CD2 A:HIS10 4.2 10.8 1.0 H2 A:ACY102 4.2 14.5 0.3 CH3 A:ACY102 4.2 12.1 0.7 NA A:NA105 4.3 41.3 1.0 CH3 A:ACY102 4.4 12.1 0.3 O A:HIS10 4.5 9.3 1.0 O A:ACY102 4.7 13.8 0.7 CA A:HIS10 4.7 9.0 1.0 HD1 A:HIS12 4.8 12.3 1.0 C A:HIS10 4.8 9.2 1.0 HE2 A:HIS10 4.9 14.0 1.0 H2 A:ACY102 5.0 14.5 0.7 O A:HOH201 5.0 25.0 1.0 | | ---------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------- |
Copper binding site 2 out of 2 in 9bnh
Go back to  Copper Binding Sites List in 9bnh
Copper Binding Sites List in 9bnh 
Copper binding site 2 out of 2 in the X-Ray Crystal Structure of Cu-TZ4H Tryptophan Zipper Metallo-Peptide
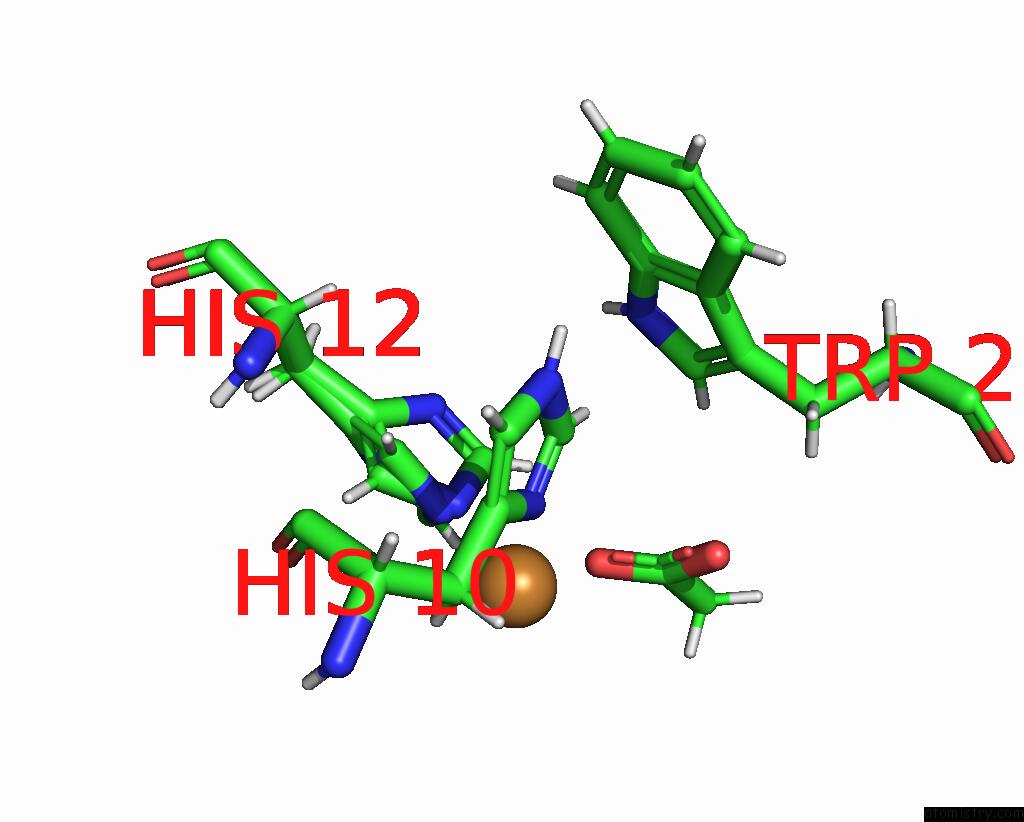
Mono view
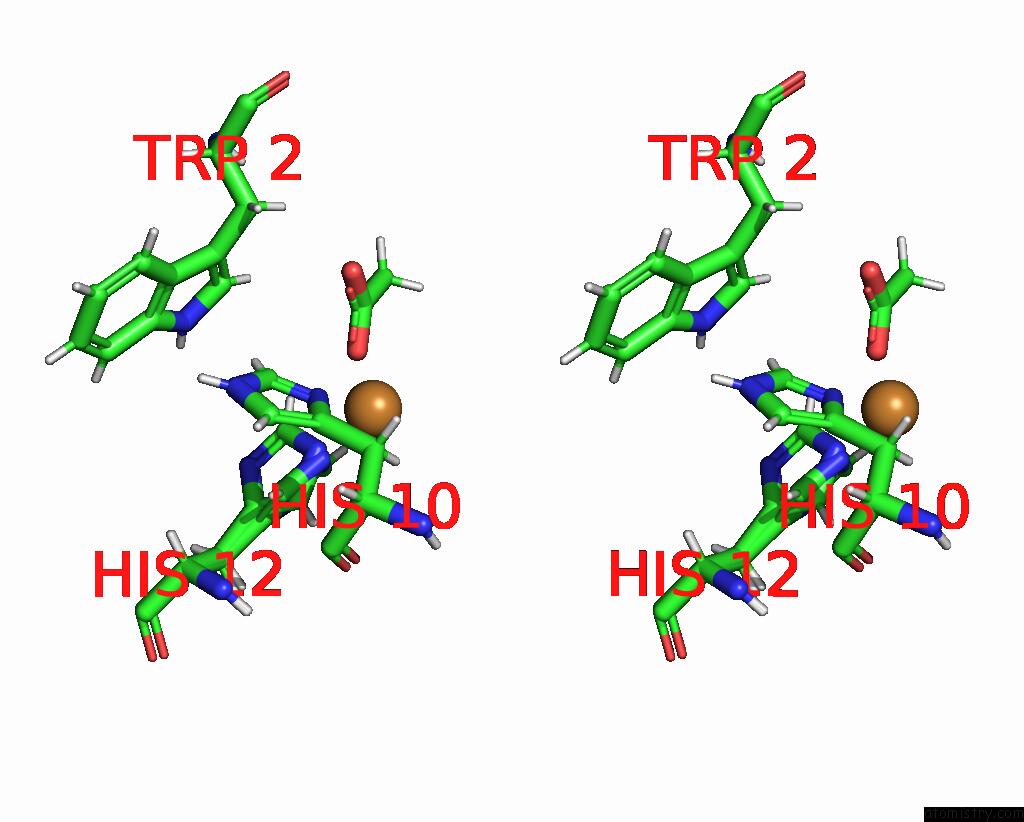
Stereo pair view
| | A full contact list of Copper with other atoms in the Cu binding site number 2 of X-Ray Crystal Structure of Cu-TZ4H Tryptophan Zipper Metallo-Peptide within 5.0Å range: probe atom residue distance (Å) B Occ B:Cu108 b:8.3 occ:1.00 ND1 B:HIS10 2.0 9.3 1.0 NE2 B:HIS12 2.0 8.6 0.5 NE2 B:HIS12 2.0 7.9 0.5 O B:ACY104 2.3 13.9 1.0 HB3 B:HIS10 2.8 11.0 1.0 CE1 B:HIS12 3.0 7.9 0.5 CE1 B:HIS10 3.0 9.9 1.0 CE1 B:HIS12 3.0 8.8 0.5 CD2 B:HIS12 3.0 8.8 0.5 CG B:HIS10 3.0 9.2 1.0 HE1 B:HIS12 3.1 9.4 0.5 CD2 B:HIS12 3.1 8.0 0.5 C B:ACY104 3.1 14.8 1.0 HE1 B:HIS10 3.2 11.9 1.0 HE1 B:HIS12 3.2 10.5 0.5 HD2 B:HIS12 3.2 10.6 0.5 HD2 B:HIS12 3.3 9.6 0.5 CB B:HIS10 3.4 9.2 1.0 OXT B:ACY104 3.5 17.5 1.0 HB2 B:HIS10 3.7 11.0 1.0 NE2 B:HIS10 4.1 10.9 1.0 ND1 B:HIS12 4.1 8.9 0.5 ND1 B:HIS12 4.1 7.4 0.5 CD2 B:HIS10 4.1 10.0 1.0 CG B:HIS12 4.1 8.8 0.5 CG B:HIS12 4.2 8.2 0.5 CH3 B:ACY104 4.4 12.8 1.0 O B:HIS10 4.4 8.7 1.0 H2 B:ACY104 4.4 15.3 1.0 CA B:HIS10 4.7 8.7 1.0 H1 B:ACY104 4.7 15.3 1.0 C B:HIS10 4.8 8.2 1.0 HE2 B:HIS10 4.9 13.0 1.0 HD1 A:TRP2 4.9 13.2 1.0 HD2 B:HIS10 5.0 12.0 1.0 | | ----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------- |
Reference:
V.T.Dang, A.Engineer, D.Mcelheny, A.Drena, J.Telser, K.Tomczak, A.I.Nguyen. Crystallography Reveals Metal-Triggered Restructuring of Beta-Hairpins. Chemistry 02101 2024.
ISSN: ISSN 0947-6539
PubMed: 39152095
DOI: 10.1002/CHEM.202402101
Page generated: Sat Sep 28 19:56:01 2024