Adenovirus: Background, Pathophysiology, Epidemiology (original) (raw)
Background
Adenovirus, a DNA virus, was first isolated in the 1950s in adenoid tissue–derived cell cultures, hence the name. These primary cell cultures were often noted to spontaneously degenerate over time, and adenoviruses are now known to be a common cause of asymptomatic respiratory tract infection that produces in vitro cytolysis in these tissues. [1, 2, 3]
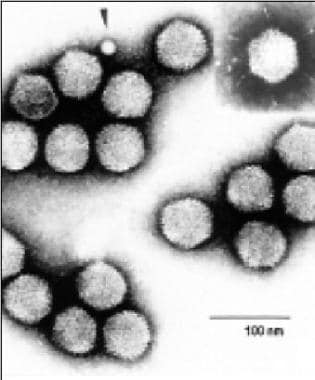
A virus image from the International Committee on Taxonomy of Viruses, in The Big Picture Book of Viruses, available at https://www.virology.net/Big\_Virology/BVDNAadeno.html.
An extremely hardy virus, adenovirus is ubiquitous in human and animal populations, survives long periods outside a host, and is endemic throughout the year. Possessing over60 serotypes, adenovirus is recognized as the etiologic agent of various diverse syndromes. It is transmitted via direct inoculation to the conjunctiva, a fecal-oral route, [1, 2] aerosolized droplets, or exposure to infected tissue or blood.
The virus is capable of infecting multiple organ systems; however, most infections are asymptomatic. Adenovirus often is cultured from the pharynx and stool of asymptomatic children, and most adults have measurable titers of anti-adenovirus antibodies, implying prior infection. Adenovirus is known to be oncogenic in rodents but not in humans.
Adenovirus has been associated with both sporadic and epidemic disease and, with regard to infections among military recruits, who were routinely immunized against types 4 and 7 from 1971 until the cessation of vaccine production in 1996. Adenovirus became a significant cause of economic cost and morbidity in this setting. A live oral vaccine against adenovirus types 4 and 7 was approved for use in this population by the US Food and Drug Administration (FDA) in 2011, and subsequent incidence of acute respiratory disease declined.
Of interest is the role of adenoviruses as viral vectors in vaccination and in gene therapy. For example, in viral vector vaccines against SARS-CoV2, the vector virus is used to deliver RNA encoding SARS-CoV2 spike protein into target cells. [4, 5, 6] Adenoviruses can infect various cells, both proliferating and quiescent, and thus hold the promise of targeting many different tissues and diseased cell lines.
The genome of adenovirus is well known and can be modified with relative ease to induce lysis or cytotoxicity of a specified cell line without affecting others.
The virus itself can be engineered to remove its replicative capacity by removing essential genes. Additionally, specific genes can be inserted into the virus that then can repair defective metabolic, enzymatic, or synthetic pathways in the host. Suicide gene systems that convert nontoxic systemically delivered prodrugs to active chemotherapeutic agents have been delivered via adenoviral vectors directly into cancer cells. However, the greatest challenge in viral gene therapy, as might be expected, is the immune response to the viral vector itself.
The complex mechanisms by which viral vectors may be incorporated into gene therapy and the rapid growth in this field put further discussion beyond the scope of this text.

Pathophysiology
Adenoviruses are a family of >60 serotypes, divided into seven subgroups or species (A through G). Measuring 70-90 nm, its double-stranded DNA genome is surrounded by a non-enveloped icosahedron capsid with fiber-like projections from each of its 12 vertices. Certain serotypes are associated with distinct clinical manifestations, reflecting preferential infection of the respiratory, gastrointestinal, and urinary tracts and conjunctiva. Serotype-specific clinical manifestations may be partially determined by differences in cell tropism. Adenoviruses are immunogenic and elicit strong innate and adaptive immune responses. Recovery from adenovirus infection is associated with the development of serotype-specific neutralizing antibodies.
Upon infection with adenovirus, one of three different interactions with the cells may occur.
The first is lytic infection, which occurs when an adenovirus enters human epithelial cells and continues through an entire replication cycle, which results in cytolysis, cytokine production, and induction of host inflammatory response.
The second is chronic or latent infection, the exact mechanism of which is unknown, which frequently involves asymptomatic infection of lymphoid tissue.
Lastly, oncogenic transformation has been observed in rats. During oncogenesis, the replication cycle is truncated, and adenoviral DNA is then integrated into the host cell’s DNA. Thereafter, adenovirus produces potent E1A proteins that immortalize primary rodent cells by altering cellular transcription, ultimately leading to deregulation of apoptosis and malignant transformation.

Epidemiology
Frequency
United States
Adenoviruses are isolated most commonly in infants and children and cause 5-10% of all febrile illnesses in this population. Adenovirus infections are prevalent in daycare centers and in households with young children. An increased incidence of infection was found in military recruits until the introduction of an effective vaccine against serotype 4 (Ad4) and serotype 7 (Ad7) in 1971. The economy-driven cessation of vaccine production by its sole producer in 1996 resulted in re-emergence of outbreaks, with Ad4 predominating in 98% of cases. The reservoirs exist within the crowded training environment itself, and Ad4 has been detected on lockers, rifles, and bedding. Ad4 seropositivity of new recruits has been demonstrated to rise from 30% to almost 100%. Prolonged pharyngeal shedding and communal quarters contribute to outbreaks, with illness most commonly arising in weeks 3 to 5.
Lost productivity and interrupted military training prompted reinvestigation of vaccine production. Live oral adenovirus types 4 and 7 vaccine was approved by the FDA in 2011, significantly decreasing and the incidence of febrile respiratory illness. Notably, co-infection with non-vaccine strains (B1 and E) have developed following vaccination, [7] and surveillance for emerging non-vaccine strains is still needed.
In 2007, media attention following adenovirus outbreaks in the United States focused on serotype 14. The CDC's Morbidity and Mortality Weekly Review published an article entitled "Acute Respiratory Disease Associated with Adenovirus Serotype 14—Four States, 2006-2007."
Mortality/Morbidity
Severe morbidity and mortality associated with adenovirus infections are rare in immunocompetent hosts. Uncommon complications that increase the risk of mortality include meningoencephalitis and pneumonitis.
Severe adenovirus infections have been reported in immunocompromised patients, such as transplant patients and those with inherited and acquired immunodeficiency states. Mortality rates associated with adenovirus infections among pediatric and adult transplant recipients have varied from 6-70%. [8]
Morbidity and deaths due to pronounced host inflammatory responses have occurred in past gene vector trials.
As with polio vaccines, live adenovirus vaccines in the 1950s became contaminated with simian virus 40 (SV40), with resulting concern that this virus caused various cancers. After subsequent long-term follow-up, some studies have found a moderate association between SV40 and human cancers as a transforming virus, whereas some other studies have reported no such findings. [9, 10]
Race
No racial predilection has been described.
Sex
Adenovirus urinary tract infections are more common in males. The prevalence of other syndromes does not appear to be affected by the sex of the individual.
Age
Adenovirus infection typically affects children from infancy to school age, but children of any age may be affected, including neonates. Young adults in any setting of close quarters and stress may be affected, as in the case of military trainees.

Prognosis
The prognosis of adenovirus infection is generally good in immunocompetent hosts, but mortality rates may be as high as 70% in immunocompromised individuals.

Patient Education
Frequent hand hygiene with soap and water or sanitizers that specify coverage of adenoviruses and avoidance of towel and pillow sharing among household contacts of patients with conjunctivitis is helpful.
Children should be taught to perform frequent hand hygiene, especially once entering group care and educational settings. The effectiveness of education should not be underestimated. Studies have shown that provision of soap and promotional materials about hand hygiene reduces illness by 40-50% in developing nations impacted by childhood diarrhea. [11, 12]
Patients should be advised of the contagiousness and possible long-term ocular sequelae of ophthalmologic disease.

- Usman N, Suarez M. Adenoviruses. StatPearls. Treasure Island, FL: StatPearls Publishing; 2024 Jan. [Full Text].
- Febbo J, Revels J, Ketai L. Viral Pneumonias. Infect Dis Clin North Am. 2024 Mar. 38 (1):163-182. [QxMD MEDLINE Link].
- Zilberbeg MD, Khan I, Shorr AF. Respiratory Viruses in Nosocomial Pneumonia: An Evolving Paradigm. Viruses. 2023 Jul 31. 15 (8):[QxMD MEDLINE Link].
- Kopecky-Bromberg SA, Palese P. Recombinant vectors as influenza vaccines. Curr Top Microbiol Immunol. 2009. 333:243-67. [QxMD MEDLINE Link].
- Harvey AR, Hellström M, Rodger J. Gene therapy and transplantation in the retinofugal pathway. Prog Brain Res. 2009. 175:151-61. [QxMD MEDLINE Link].
- Limbach KJ, Richie TL. Viral vectors in malaria vaccine development. Parasite Immunol. 2009 Sep. 31(9):501-19. [QxMD MEDLINE Link].
- Vora GJ, Lin B, Gratwick K et al. Co-infections of adenovirus species in previously vaccinated patients. Emerg Infect Dis. June 2006. 12:921-30. [QxMD MEDLINE Link].
- Echavarría M. Adenoviruses in immunocompromised hosts. Clin Microbiol Rev. 2008 Oct. 21(4):704-15. [QxMD MEDLINE Link]. [Full Text].
- Shah KV. SV40 and human cancer: a review of recent data. Int J Cancer. 2007 Jan 15. 120(2):215-23. [QxMD MEDLINE Link].
- Martini F, Corallini A, Balatti V, Sabbioni S, Pancaldi C, Tognon M. Simian virus 40 in humans. Infect Agent Cancer. 2007. 2:13. [QxMD MEDLINE Link].
- Solomon ET, Gari SR, Kloos H, Alemu BM. Handwashing effect on diarrheal incidence in children under 5 years old in rural eastern Ethiopia: a cluster randomized controlled trial. Trop Med Health. 2021 Mar 23. 49(1):26. [QxMD MEDLINE Link]. [Full Text].
- Luby SP, Agboatwalla M, Painter J, Altaf A, Billhimer W, Keswick B, et al. Combining drinking water treatment and hand washing for diarrhoea prevention, a cluster randomised controlled trial. Trop Med Int Health. 2006 Apr. 11(4):479-89. [QxMD MEDLINE Link]. [Full Text].
- Killerby ME, Rozwadowski F, Lu X, Caulcrick-Grimes M, McHugh L, Haldeman AM, et al. Respiratory Illness Associated With Emergent Human Adenovirus Genome Type 7d, New Jersey, 2016-2017. Open Forum Infect Dis. 2019 Jan 11. ofz017. [QxMD MEDLINE Link]. [Full Text].
- Kujawski SA, Lu X, Schneider E, Blythe D, Boktor S, Farrehi J, et al. Outbreaks of adenovirus-associated respiratory illness on five college campuses in the United States. Clin Infect Dis. 2020 Apr 23. ciaa465. [QxMD MEDLINE Link]. [Full Text].
- Tabain I, Ljubin-Sternak S, Cepin-Bogovic J, Markovinovic L, Knezovic I, Mlinaric-Galinovic G. Adenovirus Respiratory Infections in Hospitalized Children: Clinical Findings in Relation to Species and Serotypes. Pediatr Infect Dis J. 2012 Apr 18. [QxMD MEDLINE Link].
- Faden H, Wynn RJ, Campagna L, Ryan RM. Outbreak of adenovirus type 30 in a neonatal intensive care unit. J Pediatr. 2005 Apr. 146(4):523-7. [QxMD MEDLINE Link].
- Kajon AE, Moseley JM, Metzgar D, Huong HS, Wadleigh A, Ryan MA. Molecular epidemiology of adenovirus type 4 infections in US military recruits in the postvaccination era (1997-2003). J Infect Dis. 2007 Jul 1. 196(1):67-75. [QxMD MEDLINE Link].
- Russell KL, Hawksworth AW, Ryan MA et al. Vaccine-preventable adenoviral respiratory illness in US military recruits,1999-2004. Vaccine. April 2006. 15:2835-42. [QxMD MEDLINE Link].
- Wirsing von König CH, Rott H, Bogaerts H, Schmitt HJ. A serologic study of organisms possibly associated with pertussis-like coughing. Pediatr Infect Dis J. 1998 Jul. 17(7):645-9. [QxMD MEDLINE Link].
- Versteegh FG, Mooi-Kokenberg EA, Schellekens JF, Roord JJ. Bordetella pertussis and mixed infections. Minerva Pediatr. 2006 Apr. 58(2):131-7. [QxMD MEDLINE Link].
- Saiki-Macedo S, Valverde-Ezeta J, Cornejo-Tapia A, Castillo ME, Petrozzi-Helasvuo V, Aguilar-Luis MA, et al. Identfication of viral and bacterial etiologic agents of the pertussis-like syndrome in children under 5 years old hospitalized. BMC Infect Dis. 2019 Jan 21. 19(1):75. [QxMD MEDLINE Link]. [Full Text].
- Dart JK, El-Amir AN, Maddison T, Desai P, Verma S, Hughes A, et al. Identification and control of nosocomial adenovirus keratoconjunctivitis in an ophthalmic department. Br J Ophthalmol. 2009 Jan. 93(1):18-20. [QxMD MEDLINE Link].
- Bruno B, Zager RA, Boeckh MJ, et al. Adenovirus nephritis in hematopoietic stem-cell transplantation. Transplantation. 2004 Apr 15. 77(7):1049-57. [QxMD MEDLINE Link].
- Nakamura Y, Ohashi K, Nakano N, Nemoto T, Funada N, Ando M. [Acute necrotizing tubulointerstitial nephritis due to adenoviral infection following hematopoietic stem cell transplantation: clinical findings obtained from 4 autopsy cases]. Nippon Jinzo Gakkai Shi. 2008. 50(8):1036-43. [QxMD MEDLINE Link].
- Uhnoo I, Wadell G, Svensson L, Johansson ME. Importance of enteric adenoviruses 40 and 41 in acute gastroenteritis in infants and young children. J Clin Microbiol. 1984 Sep. 20(3):365-72. [QxMD MEDLINE Link]. [Full Text].
- Jalal H, Bibby DF, Tang JW, Bennett J, Kyriakou C, Peggs K. First reported outbreak of diarrhea due to adenovirus infection in a hematology unit for adults. J Clin Microbiol. 2005 Jun. 43(6):2575-80. [QxMD MEDLINE Link].
- Munoz FM, Piedra PA, Demmler GJ. Disseminated adenovirus disease in immunocompromised and immunocompetent children. Clin Infect Dis. 1998 Nov. 27(5):1194-1200. [QxMD MEDLINE Link]. [Full Text].
- Ison MG. Adenovirus infections in transplant recipients. Clin Infect Dis. 2006 Aug 1. 43(3):331-9. [QxMD MEDLINE Link].
- Hedderwick SA, Greenson JK, McGaughy VR, Clark NM. Adenovirus cholecystitis in a patient with AIDS. Clin Infect Dis. 1998 Apr. 26(4):997-9. [QxMD MEDLINE Link].
- Liu M, Worley S, Arrigain S, Aurora P, Ballmann M, Boyer D, et al. Respiratory viral infections within one year after pediatric lung transplant. Transpl Infect Dis. 2009 Aug. 11(4):304-12. [QxMD MEDLINE Link].
- Echavarria M, Sanchez JL, Kolavic-Gray SA, Polyak CS, Mitchell-Raymundo F, Innis BL, et al. Rapid detection of adenovirus in throat swab specimens by PCR during respiratory disease outbreaks among military recruits. J Clin Microbiol. 2003 Feb. 41(2):810-2. [QxMD MEDLINE Link].
- Lu X, Trujillo-Lopez E, Lott L, Erdman DD. Quantitative real-time PCR assay panel for detection and type-specific identification of epidemic respiratory human adenoviruses. J Clin Microbiol. 2013 Apr. 51(4):1089-93. [QxMD MEDLINE Link]. [Full Text].
- Lion T, Baumgartinger R, Watzinger F, Matthes-Martin S, Suda M, Preuner S, et al. Molecular monitoring of adenovirus in peripheral blood after allogeneic bone marrow transplantation permits early diagnosis of disseminated disease. Blood. 2003 Aug 1. 102(3):1114-20. [QxMD MEDLINE Link]. [Full Text].
- Marchiori E, Escuissato DL, Gasparetto TD, Considera DP, Franquet T. "Crazy-paving" patterns on high-resolution CT scans in patients with pulmonary complications after hematopoietic stem cell transplantation. Korean J Radiol. 2009 Jan-Feb. 10(1):21-4. [QxMD MEDLINE Link]. [Full Text].
- Lion T. Adenovirus infections in immunocompetent and immunocompromised patients. Clin Microbiol Rev. 2014. 27(3):441-462. [QxMD MEDLINE Link]. [Full Text].
- Matthes-Martin S, Feuchtinger T, Shaw PJ, Engelhard D, Hirsch HH, Cordonnier C, et al. Fourth European Conference on Infections in Leukemia. European guidelines for diagnosis and treatment of adenovirus infection in leukemia and stem cell transplantation: summary of ECIL-4 (2011). Transpl Infect Dis. 2012 Dec. 14(6):Epub. [QxMD MEDLINE Link]. [Full Text].
- Humar A, Kumar D, Mazzulli T, Razonable RR, Moussa G, Paya CV, et al. A surveillance study of adenovirus infection in adult solid organ transplant recipients. Am J Transplant. 2005 Oct. 5 (10):2555-9. [QxMD MEDLINE Link].
- Faiyumi MA, Betensley A. Successful Treatment of Severe Adenovirus Pneumonia With Cidofovir in a Lung Transplant Recipient. Chest. 2013. 144:172A-172B.
- Feuchtinger T, Lücke J, Hamprecht K, Richard C, Handgretinger R, Schumm M. Detection of adenovirus-specific T cells in children with adenovirus infection after allogeneic stem cell transplantation. Br J Haematol. 2005 Feb. 128(4):503-9. [QxMD MEDLINE Link].
- Wy Ip W, Qasim W. Management of adenovirus in children after allogeneic hematopoietic stem cell transplantation. Adv Hematol. 2013. 2013:176418. [QxMD MEDLINE Link]. [Full Text].
- Creidy R, Moshous D, Touzot F, et al. Specific T cells for the treatment of cytomegalovirus and/or adenovirus in the context of hematopoietic stem cell transplantation. J Allergy Clin Immunol. 2016 Sep. 138 (3):920-924.e3. [QxMD MEDLINE Link].
- Horlock C, Skulte A, Mitra A, Stansfield A, Bhandari S, Ip W, et al. Manufacture of GMP-compliant functional adenovirus-specific T-cell therapy for treatment of post-transplant infectious complications. Cytotherapy. 2016 Sep. 18 (9):1209-18. [QxMD MEDLINE Link].
- Barrett AJ, Prockop S, Bollard CM. Virus-Specific T Cells: Broadening Applicability. Biol Blood Marrow Transplant. 2018 Jan. 24 (1):13-18. [QxMD MEDLINE Link].
- Carter BA, Karpen SJ, Quiros-Tejeira RE, et al. Intravenous Cidofovir therapy for disseminated adenovirus in a pediatric liver transplant recipient. Transplantation. 2002 Oct 15. 74(7):1050-2. [QxMD MEDLINE Link].
- Hayashi M, Lee C, de Magalhaes-Silverman M. Adenovirus infections in BMT patients successfully treated with cidofovir. Blood. 2000. 96 (suppl 1):189a.
- Muller WJ, Levin MJ, Shin YK, Robinson C, Quinones R, Malcolm J. Clinical and in vitro evaluation of cidofovir for treatment of adenovirus infection in pediatric hematopoietic stem cell transplant recipients. Clin Infect Dis. 2005 Dec 15. 41(12):1812-6. [QxMD MEDLINE Link].
- Neofytos D, Ojha A, Mookerjee B, Wagner J, Filicko J, Ferber A, et al. Treatment of adenovirus disease in stem cell transplant recipients with cidofovir. Biol Blood Marrow Transplant. 2007 Jan. 13(1):74-81. [QxMD MEDLINE Link].
- Yusuf U, Hale GA, Carr J, Gu Z, Benaim E, Woodard P. Cidofovir for the treatment of adenoviral infection in pediatric hematopoietic stem cell transplant patients. Transplantation. 2006 May 27. 81(10):1398-404. [QxMD MEDLINE Link].
- Saquib R, Melton LB, Chandrakantan A, Rice KM, Spak CW, Saad RD, et al. Disseminated adenovirus infection in renal transplant recipients: the role of cidofovir and intravenous immunoglobulin. Transpl Infect Dis. 2009 Sep 15. [QxMD MEDLINE Link].
- Sofer A, Arger N, Vest M. Successful Treatment of Adenovirus-Induced ARDS With Cidofovir and IVIG. Chest. 2013. 144:229A-229B.
- Prasad VK, Papanicolaou GA, Maron GM et al. Treatment of Adenovirus (AdV) Infection in Allogeneic Hematopoietic Cell Transplant (allo HCT) Patients (pts) with Brincidofovir: Final 36 Week Results from the AdVise Tria. Biology of Blood and Marrow Transplantation. 2017. 23:S57–S58.
- Brundage TM, Vainorius E, Chittick G et al. Brincidofovir Decreases Adenovirus Viral Burden, Which is Associated with Improved Mortality in Pediatric Allogeneic Hematopoietic Cell Transplant Recipients. Biology of Blood and Marrow Transplantation. 2018. 24:S372.
- Grimley MS, Chemaly RF, Englund JA, Kurtzberg J, Chittick G, Brundage TM, et al. Brincidofovir for Asymptomatic Adenovirus Viremia in Pediatric and Adult Allogeneic Hematopoietic Cell Transplant Recipients: A Randomized Placebo-Controlled Phase II Trial. Biol Blood Marrow Transplant. 2017 Mar. 23 (3):512-521. [QxMD MEDLINE Link].
- Chimerix, Inc. Expanded Access. Chimerix.com. Available at https://www.chimerix.com/patient-resources/expanded-access/#. Accessed: 13 April 2021.
- Romanowski EG, Hussein ITM, Cardinale SC, Butler MM, Morin LR, Bowlin TL, et al. Filociclovir Is an Active Antiviral Agent against Ocular Adenovirus Isolates In Vitro and in the Ad5/NZW Rabbit Ocular Model. Pharmaceuticals (Basel). 2021 Mar 26. 14(4):294. [QxMD MEDLINE Link]. [Full Text].
- Barraza EM, Ludwig SL, Gaydos JC et al. Reemergence of adenovirus type 4 acute respiratory disease in military trainees:report of an outbreak during a lapse in vaccination. J Infect Dis. June 1999. 179:1531-3. [QxMD MEDLINE Link].
- Ryan MA, Gray GC, Smith B, et al. Large epidemic of respiratory illness due to adenovirus types 7 and 3 in healthy young adults. Clin Infect Dis. 2002 Mar 1. 34(5):577-82. [QxMD MEDLINE Link].
- Vora GJ, Lin B, Gratwick K et al. o-infections of adenovirus species in previously vaccinated patients. Emerg Infect Dis. June 2006. 12:921-30. [QxMD MEDLINE Link].
- Rutala WA, Peacock JE, Gergen MF, Sobsey MD, Weber DJ. Efficacy of hospital germicides against adenovirus 8, a common cause of epidemic keratoconjunctivitis in health care facilities. Antimicrob Agents Chemother. 2006 Apr. 50 (4):1419-24. [QxMD MEDLINE Link].
- Fanourgiakis P, Georgala A, Vekemans M, Triffet A, De Bruyn JM, Duchateau V. Intravesical instillation of cidofovir in the treatment of hemorrhagic cystitis caused by adenovirus type 11 in a bone marrow transplant recipient. Clin Infect Dis. 2005 Jan 1. 40(1):199-201. [QxMD MEDLINE Link].
Author
Sandra G Gompf, MD, FACP, FIDSA Professor of Infectious Disease and International Medicine, University of South Florida Morsani College of Medicine; Chief, Infectious Diseases Section, Director, Occupational Health and Infection Control Programs, James A Haley Veterans Hospital
Sandra G Gompf, MD, FACP, FIDSA is a member of the following medical societies: American College of Physicians, Infectious Diseases Society of America
Disclosure: Nothing to disclose.
Coauthor(s)
Tuhina Cornelius, MD, MBBS Fellow in Infectious Disease, Florida Department of Health, University of South Florida Morsani College of Medicine
Tuhina Cornelius, MD, MBBS is a member of the following medical societies: American College of Physicians
Disclosure: Nothing to disclose.
Specialty Editor Board
Francisco Talavera, PharmD, PhD Adjunct Assistant Professor, University of Nebraska Medical Center College of Pharmacy; Editor-in-Chief, Medscape Drug Reference
Disclosure: Received salary from Medscape for employment. for: Medscape.
Richard B Brown, MD, FACP Chief, Division of Infectious Diseases, Baystate Medical Center; Professor, Department of Internal Medicine, Tufts University School of Medicine
Richard B Brown, MD, FACP is a member of the following medical societies: Alpha Omega Alpha, American College of Chest Physicians, American College of Physicians, American Medical Association, American Society for Microbiology, Infectious Diseases Society of America, Massachusetts Medical Society
Disclosure: Nothing to disclose.
Chief Editor
Michael Stuart Bronze, MD David Ross Boyd Professor and Chairman, Department of Medicine, Stewart G Wolf Endowed Chair in Internal Medicine, Department of Medicine, University of Oklahoma Health Science Center; Master of the American College of Physicians; Fellow, Infectious Diseases Society of America; Fellow of the Royal College of Physicians, London
Michael Stuart Bronze, MD is a member of the following medical societies: Alpha Omega Alpha, American College of Physicians, American Medical Association, Association of Professors of Medicine, Infectious Diseases Society of America, Oklahoma State Medical Association, Southern Society for Clinical Investigation
Disclosure: Nothing to disclose.
Additional Contributors
David Hall Shepp, MD Program Director, Fellowship in Infectious Diseases, Department of Medicine, North Shore University Hospital; Associate Professor, New York University School of Medicine
David Hall Shepp, MD is a member of the following medical societies: Infectious Diseases Society of America
Disclosure: Received salary from Gilead Sciences for management position.
Richard Oehler, MD Associate Professor, Department of Internal Medicine, Division of Infectious Diseases and International Medicine, University of South Florida College of Medicine; Director of Clinical Education, Division of Infectious Diseases, Tampa Veterans Affairs Medical Center
Richard Oehler, MD is a member of the following medical societies: American College of Physicians, American Medical Association, Infectious Diseases Society of America, Society for Healthcare Epidemiology of America
Disclosure: Nothing to disclose.