Subclavian Steal Syndrome (Subclavian-Vertebral Artery Steal Syndrome) Imaging: Practice Essentials, Computed Tomography, Magnetic Resonance Imaging (original) (raw)
Practice Essentials
Subclavian steal syndrome (SSS), also known as subclavian-vertebral artery steal syndrome, is a type of peripheral artery disease in which reduction or absence of flow in the proximal subclavian artery reverses the normal direction of blood flow in the vertebral artery. Blood is drawn from the contralateral vertebral, basilar, or carotid artery to provide flow in the affected subclavian artery distal to the stenosis. [1] Subclavian steal phenomena (SSP) refers to retrograde flow in the vertebral artery only. The term SSS is reserved for retrograde vertebral artery flow associated with transient neurologic symptoms related to cerebral ischemia. [2, 3]
Coronary subclavian steal syndrome (CSSS) is defined as retrograde flow in the internal mammary artery graft, after coronary artery bypass surgery (CABG), resulting in anterior wall myocardial ischemia. [4, 5, 6, 7]
The subclavian steal, in the absence of other anatomic anomalies, is usually asymptomatic and often an incidental finding. Rarely, however, some patients may provoke the syndrome with exercise and present with transient ipsilateral arm claudication, ataxia, and/or angina.
The most common risk factor is atherosclerosis. SSS has been associated with vasculitis, thoracic outlet syndrome, congenital abnormalities, and prior radiation treatment. [8]
Imaging modalities
First diagnosed angiographically in the early 1960s, SSS is now most commonly diagnosed during Doppler ultrasound (US) examination of the neck arteries. [9, 10, 11, 12, 13, 14] Color Doppler US is the preferred examination for subclavian steal syndrome, but it is operator dependent. In addition, direct examination of the proximal subclavian artery is compromised by the overlying clavicle, ribs, and sternum.
CT and MR angiography are used to confirm any suspected subclavian steal cases found on ultrasound exams, because not all retrograde vertebral flow seen on ultrasound examinations constitute true subclavian steal. Some have been shown to stem from proximal vertebral artery stenosis or occlusion. These techniques can directly show the anatomic features of subclavian stenosis or occlusion, the presence of retrograde vertebral flow, and any associated extracranial and intracranial stenoses. [15, 16]
(See the images below of subclavian steal syndrome.)
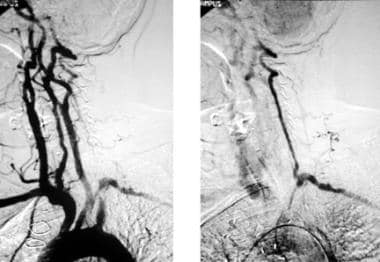
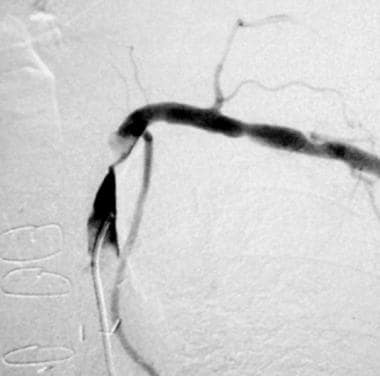
Arch aortogram initially shows apparent absence of left vertebral artery opacification. With delayed imaging on the same patient (right image), the left vertebral artery fills retrogradely to supply the left subclavian artery, confirming left subclavian steal phenomenon secondary to a severe stenosis of the proximal left subclavian artery.
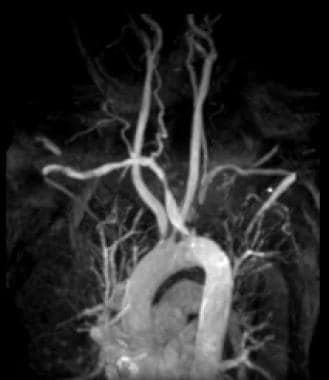
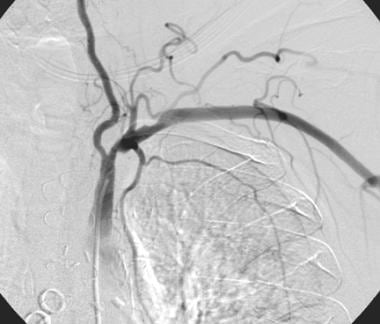
Gadolinium-enhanced magnetic resonance angiography maximum intensity projection image shows left subclavian artery occlusion in a patient with left subclavian steal phenomenon and aberrant right subclavian artery. The patient had no neurologic or arm symptoms, but the subclavian lesion eliminated the left internal mammary artery as a coronary bypass graft option.
Intervention
Most commonly, subclavian artery steno-occlusive disease is not associated with symptoms and therefore can be managed conservatively with attention to diabetes and hyperlipidemia management, cessation of smoking, and antiplatelet therapy. When symptoms are present, coincident significant carotid system stenoses should be excluded. Endovascular treatment is considered the first-line intervention in medically refractory patients with symptomatic subclavian steal syndrome. Surgical options, such as carotid-subclavian bypass, are generally reserved for patients in whom endovascular treatment has failed. [1]
A study comparing early and long-term outcomes of endovascular repair with those of open surgical repair in patients with subclavian artery atherosclerotic occlusive disease found the 2 approaches to be comparably safe, effective, and durable. [17]

Computed Tomography
Contrast-enhanced CT multidetector scans (with 3-D reconstruction techniques) are replacing conventional catheter angiography in the diagnosis of subclavian steal steno-occlusive disease. [18] With contrast enhancement, particularly using test bolus or bolus tracking techniques, the degree of subclavian steno-occlusion, mural thrombus, ulceration, and arterial wall calcification can be evaluated. [16, 19, 20]
Calcified plaque (which may have a density similar to contrast) has the potential to compromise estimates of the degree of stenosis and occlusion. Atheroma-related calcification projected over the proximal subclavian artery may be apparent. The absence of calcification does not rule out subclavian steno-occlusive disease, nor does the presence of calcification confirm it. [19, 20]
(See the image below.)
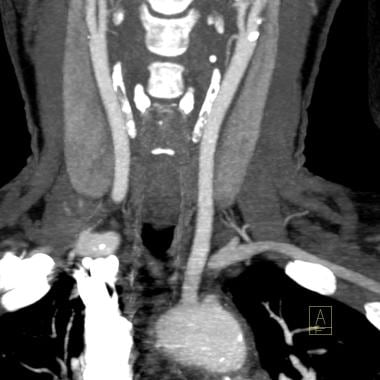
A 12-mm thick maximum intensity projection reconstruction from CT angiogram shows a complete short segment occlusion of the proximal left subclavian artery with filling of the more distal subclavian artery via the left vertebral artery. Note minor atherosclerotic change at both internal carotid artery origins without significant stenosis. This patient was asymptomatic and was managed without revascularization.

Magnetic Resonance Imaging
Contrast-enhanced 3-D MRA after localizing 2-dimensional time-of-flight can confirm subclavian steal phenomenon (SSP). [21] Phase-contrast MRA measures vertebral artery flow direction and velocity. MRA techniques do not require iodinated contrast (and its accompanying nephrotoxic and allergic side effects), but they can be time consuming. Lack of widespread availability, claustrophobia, and contraindications to MR (eg, pacemakers) limit MRA use (see the images below).
Gadolinium-enhanced magnetic resonance angiography maximum intensity projection image shows left subclavian artery occlusion in a patient with left subclavian steal phenomenon and aberrant right subclavian artery. The patient had no neurologic or arm symptoms, but the subclavian lesion eliminated the left internal mammary artery as a coronary bypass graft option.
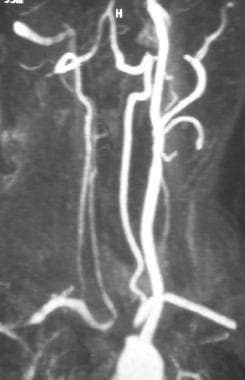
Gadolinium-enhanced magnetic resonance angiogram (MRA) of right carotid-subclavian subclavian steal phenomenon. This tangential aortic arch maximum intensity projection view shows innominate artery occlusion and severe left proximal subclavian artery steno-occlusive lesion. Two-dimensional time-of-flight MRA confirmed retrograde flow in the right vertebral but not in the left vertebral artery. MRA tends to exaggerate the severity of steno-occlusive disease. While this MRA suggests a short occlusion of the left proximal subclavian, a severe stenosis rather than occlusion was documented on conventional catheter arteriography.
With head and neck coils and high-gradient MRI technology, gadolinium-enhanced MRA enables accurate depiction of proximal subclavian artery steno-occlusive disease.
Gadolinium-based contrast agents have been linked to the development of nephrogenic systemic fibrosis (NSF) or nephrogenic fibrosing dermopathy (NFD). The disease has occurred in patients with moderate to end-stage renal disease after being given a gadolinium-based contrast agent to enhance MRI or MRA scans. NSF/NFD is a debilitating and sometimes fatal disease. Characteristics include red or dark patches on the skin; burning, itching, swelling, hardening, and tightening of the skin; yellow spots on the whites of the eyes; joint stiffness with trouble moving or straightening the arms, hands, legs, or feet; pain deep in the hip bones or ribs; and muscle weakness.

Ultrasonography
Subclavian steal phenomenon (SSP) most commonly is diagnosed incidentally during carotid and vertebral artery color Doppler US.
Transcranial color Doppler US enables examination of the basilar artery and arteries of the circle of Willis, characterizing collateral pathways. [11, 12] Flow reversal in the basilar artery is more likely when subclavian steal is symptomatic, particularly when carotid disease is present and is a potential alternative source of symptoms (see the images below).
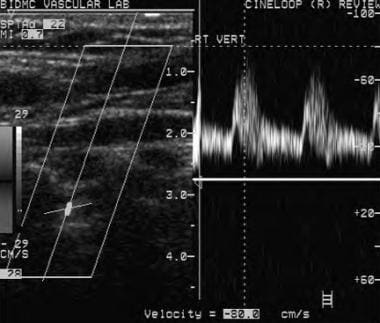
Normal antegrade right vertebral artery flow on Doppler ultrasound. With cephalad direction of insonation, the negative Doppler scale reading is consistent with flow going away from the direction of insonation in a normal cephalad antegrade fashion.
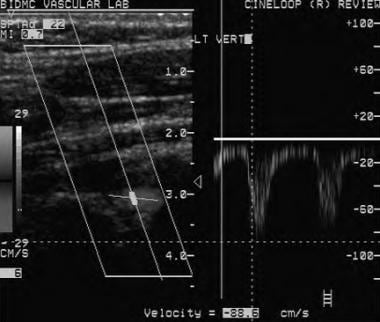
Doppler ultrasound shows reversed flow in the left vertebral artery consistent with left subclavian steal phenomenon. Doppler scale reading shows a negative deflection again; however, this time the direction of insonation is caudad, thus a negative scale Doppler reading indicates flow in a caudad direction and flow reversal in the left vertebral artery.
The earliest waveform change associated with subclavian artery stenosis is a transient decrease in ipsilateral vertebral artery midsystolic velocity. Demonstration of ipsilateral vertebral artery flow reversal with a parvus tardus waveform in the ipsilateral subclavian artery confirms the diagnosis of SSP. A parvus tardus waveform (prolonged systolic acceleration time with decreased peak systolic velocity) in the distal subclavian artery and brachial artery is expected with severe subclavian stenosis or occlusion; a monophasic waveform also replaces the usual triphasic Doppler signal in these vessels. US examination can document other arterial disease. Investigate further if symptoms are atypical, suggesting a different intracranial or cardiac etiology.
Although total vertebral artery reversal of flow correlates with severe subclavian artery stenosis or occlusion, it does not indicate the presence of related neurologic symptoms.
With progressive stenosis of the subclavian artery, midsystolic velocity slows further, then reverses direction until both systolic and diastolic flow are reversed. Reactive hyperemia provoked by blood pressure cuff maneuver and arm exercise exaggerates this waveform change (subclavian steal test).
A rare finding is vertebral artery flow reversal related to proximal vertebral artery occlusion (spinal artery steal) but without subclavian disease. In this syndrome, posterior fossa symptoms can occur with a normal subclavian artery, normal upper extremity blood pressures, and normal triphasic subclavian and brachial Doppler waveforms.
Lack of vertebral artery flow reversal can occur with severe left steno-occlusive subclavian disease when the left vertebral artery originates directly from the aortic arch, a variant that occurs in 6% of individuals and accounts for 14% of all arch vessel anomalies.

Angiography
Conventional film-screen or digital subtraction angiography is the traditional test used for subclavian steal diagnosis but is now reserved for the time of endovascular intervention. [22]
Arch aortography shows subclavian stenosis with antegrade vertebral flow in more minor subclavian stenosis.
In progressive subclavian steno-occlusion, the filling of the vertebral artery becomes less obvious until, eventually, it fills in a retrograde fashion on more delayed imaging (see the images below).
Arch aortogram initially shows apparent absence of left vertebral artery opacification. With delayed imaging on the same patient (right image), the left vertebral artery fills retrogradely to supply the left subclavian artery, confirming left subclavian steal phenomenon secondary to a severe stenosis of the proximal left subclavian artery.
Selective arteriogram (same patient as shown in previous image) shows a severe proximal subclavian stenosis proximal to the left internal mammary artery. The patient presented with angina and had a left internal mammary artery-left anterior descending coronary graft 12 months previously. Note nonopacification of the left vertebral artery and the filling defect consistent with in situ thrombus.
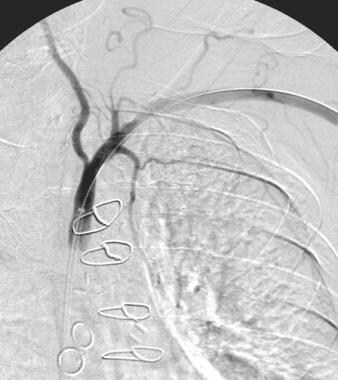
Evaluation should detail the presence of concomitant disease in carotid, cerebral, and vertebrobasilar systems (see the images below).
Patient presented with cardiac ischemia after a left internal mammary artery-left anterior descending coronary graft and multiple coronary artery vein grafts. Subclavian steal cannot occur in this patient, as the left subclavian stenosis is distal to the left vertebral artery origin. Note antegrade flow in the left vertebral artery.
Postangioplasty with a 9-mm balloon. The pressure gradient across the mid subclavian stenosis was reduced from 60 to 0 mm Hg.
Misregistration and motion artifacts can compromise digital subtraction arteriography; if postprocessing does not overcome these problems, repeat contrast injection.
Spinal artery steal can cause vertebral artery flow reversal without subclavian steno-occlusive disease. Subclavian steno-occlusive disease distal to the vertebral artery origin or left subclavian steno-occlusive disease associated with left vertebral origin directly from the aorta cannot be associated with vertebral artery flow reversal.

- Satti SR, Golwala SN, Vance AZ, Tuerff SN. Subclavian steal: Endovascular treatment of total occlusions of the subclavian artery using a retrograde transradial subintimal approach. Interv Neuroradiol. 2016 Jun. 22 (3):340-8. [QxMD MEDLINE Link]. [Full Text].
- Shankar Kikkeri N, Nagalli S. Subclavian Steal Syndrome. 2023 Jan. [QxMD MEDLINE Link]. [Full Text].
- Rafailidis V, Li X, Chryssogonidis I, et al. Multimodality Imaging and Endovascular Treatment Options of Subclavian Steal Syndrome. Can Assoc Radiol J. 2018 Nov. 69 (4):493-507. [QxMD MEDLINE Link].
- Moccetti F, Brinkert M, Wolfrum M, Toggweiler S. Coronary subclavian steal syndrome. Eur Heart J. 2020 Apr 1. 41 (13):1345. [QxMD MEDLINE Link].
- De Roeck F, Tijskens M, Segers VFM. Coronary-subclavian steal syndrome, an easily overlooked entity in interventional cardiology. Catheter Cardiovasc Interv. 2020 Sep 1. 96 (3):614-619. [QxMD MEDLINE Link].
- Cua B, Mamdani N, Halpin D, Jhamnani S, Jayasuriya S, Mena-Hurtado C. Review of coronary subclavian steal syndrome. J Cardiol. 2017 Nov. 70 (5):432-437. [QxMD MEDLINE Link].
- Jones-Ince I, Todd G. Unexpected Stealing From the Heart. Am J Case Rep. 2016 Mar 21. 17:182-5. [QxMD MEDLINE Link]. [Full Text].
- Woo JD, Markowitz JE. A Great Heist: Subclavian Steal Syndrome Causing Posterior Transient Ischemic Attack and Stroke. Perm J. 2022 Dec 19. 26 (4):106-109. [QxMD MEDLINE Link]. [Full Text].
- Uematsu H, Noguchi Y, Osugi Y. Point-of-care ultrasonography in the diagnosis of subclavian steal syndrome. BMJ Case Rep. 2023 Apr 7. 16 (4):[QxMD MEDLINE Link].
- Shemesh E, Karkabi B, Zissman K. Multimodality imaging in subclavian steal syndrome. Oxf Med Case Reports. 2021 Jul. 2021 (7):omab048. [QxMD MEDLINE Link].
- Harper C, Cardullo PA, Weyman AK, Patterson RB. Transcranial Doppler ultrasonography of the basilar artery in patients with retrograde vertebral artery flow. J Vasc Surg. 2008 Oct. 48(4):859-64. [QxMD MEDLINE Link].
- Moghazy KM. Value of color Doppler sonography in the assessment of hemodialysis access dysfunction. Saudi J Kidney Dis Transpl. 2009 Jan. 20(1):35-43. [QxMD MEDLINE Link].
- Kliewer MA, Hertzberg BS, Kim DH, et al. Vertebral artery Doppler waveform changes indicating subclavian steal physiology [published erratum appears in AJR Am J Roentgenol 2000 May;174(5):1464]. AJR Am J Roentgenol. 2000 Mar. 174(3):815-9. [QxMD MEDLINE Link].
- Takach TJ, Reul GJ, Cooley DA, Duncan JM, Livesay JJ, Ott DA, et al. Myocardial thievery: the coronary-subclavian steal syndrome. Ann Thorac Surg. 2006 Jan. 81(1):386-92. [QxMD MEDLINE Link].
- Tanaka T, Fukushima K, Goto H, Momozaki N. Brain Magnetic Resonance Angiography of Subclavian Steal Syndrome. JMA J. 2022 Oct 17. 5 (4):551-552. [QxMD MEDLINE Link].
- Toprak C, Yesin M, Mustafa Tabakci M, Demirel M, Avci A. Coronary Subclavian Steal Syndrome Evaluated with Multimodality Imaging. Intern Med. 2015. 54 (21):2717-20. [QxMD MEDLINE Link].
- Benhammamia M, Mazzaccaro D, Ben Mrad M, Denguir R, Nano G. Endovascular and Surgical Management of Subclavian Artery Occlusive Disease: Early and Long-Term Outcomes. Ann Vasc Surg. 2020 Jul. 66:462-469. [QxMD MEDLINE Link].
- Alegret RE, Blandon RJ, Kirsch J. Poor left internal mammary artery opacification on coronary CT angiography: an indirect sign of subclavian steal. J Vasc Interv Radiol. 2008 Dec. 19(12):1791-2. [QxMD MEDLINE Link].
- Rezende PC, da Costa LM, Scudeler TL, Nakamura D, Giorgi MC, Hueb W. Recurrent angina caused by coronary subclavian steal syndrome confirmed by positron emission tomography. Ann Thorac Surg. 2015. 99 (5):e111-4. [QxMD MEDLINE Link].
- Barfett JJ, Velauthapillai N, Fierstra J, Crawley A, Coolens C, Crean A, et al. Intra-vascular blood velocity and volumetric flow rate calculated from dynamic 4D CT angiography using a time of flight technique. Int J Cardiovasc Imaging. 2014 Oct. 30 (7):1383-92. [QxMD MEDLINE Link].
- Bauer AM, Amin-Hanjani S, Alaraj A, Charbel FT. Quantitative Magnetic Resonance Angiography in the Evaluation of the Subclavian Steal Syndrome: Report of 5 Patients. J Neuroimaging. 2008 Sep 20. [QxMD MEDLINE Link].
- Govedarski V, Genadiev S, Nedevska M, Zakhariev T, Nachev G. [Endovascular recanalisation of subclavian artery stenotic-occlusive lesions]. Khirurgiia (Sofiia). 2008. 30-3. [QxMD MEDLINE Link].
- Arch aortogram initially shows apparent absence of left vertebral artery opacification. With delayed imaging on the same patient (right image), the left vertebral artery fills retrogradely to supply the left subclavian artery, confirming left subclavian steal phenomenon secondary to a severe stenosis of the proximal left subclavian artery.
- Selective arteriogram (same patient as shown in previous image) shows a severe proximal subclavian stenosis proximal to the left internal mammary artery. The patient presented with angina and had a left internal mammary artery-left anterior descending coronary graft 12 months previously. Note nonopacification of the left vertebral artery and the filling defect consistent with in situ thrombus.
- A 12-mm thick maximum intensity projection reconstruction from CT angiogram shows a complete short segment occlusion of the proximal left subclavian artery with filling of the more distal subclavian artery via the left vertebral artery. Note minor atherosclerotic change at both internal carotid artery origins without significant stenosis. This patient was asymptomatic and was managed without revascularization.
- Thrombolysis was considered before angioplasty. As no antegrade left vertebral flow was demonstrated on the original digital subtraction arteriogram, thrombolysis was not performed. This line of treatment was determined in the belief that if any clot embolized to the coronary or vertebral circulations after percutaneous transluminal angioplasty (also a risk of thrombolysis alone), thrombolysis could then be performed with selective catheterization. After administration of vasodilators and anticoagulants, angioplasty with a 7-mm balloon restored antegrade flow to the left vertebral artery. The patient has had sustained relief from angina without embolic complication to either vertebral or coronary circulations. Both radial and ulnar pulses improved post angioplasty.
- Gadolinium-enhanced magnetic resonance angiography maximum intensity projection image shows left subclavian artery occlusion in a patient with left subclavian steal phenomenon and aberrant right subclavian artery. The patient had no neurologic or arm symptoms, but the subclavian lesion eliminated the left internal mammary artery as a coronary bypass graft option.
- Gadolinium-enhanced magnetic resonance angiogram (MRA) of right carotid-subclavian subclavian steal phenomenon. This tangential aortic arch maximum intensity projection view shows innominate artery occlusion and severe left proximal subclavian artery steno-occlusive lesion. Two-dimensional time-of-flight MRA confirmed retrograde flow in the right vertebral but not in the left vertebral artery. MRA tends to exaggerate the severity of steno-occlusive disease. While this MRA suggests a short occlusion of the left proximal subclavian, a severe stenosis rather than occlusion was documented on conventional catheter arteriography.
- Conventional arteriogram in a 70-year-old man who previously had a carotid-subclavian bypass for dizziness related to subclavian steal syndrome. The bypass is occluded and a severe stenosis of the proximal subclavian artery is apparent. Note poor filling of right vertebral artery caused by retrograde flow documented on Doppler ultrasound; note also the bovine arch with common origin of right brachiocephalic trunk and left common carotid artery.
- After stent placement, antegrade flow is restored in the right vertebral artery. Primary stenting was chosen because of theoretical risk of embolism to the left vertebral artery territory; however, there appears to be a time lag before restoration of antegrade vertebral artery flow after proximal subclavian revascularization that protects against this embolism risk. Other techniques to decrease the risk of vertebral artery embolism include decreasing peripheral resistance in the ipsilateral upper extremity (eg, with intra-arterial vasodilators or temporary blood pressure cuff inflation above systolic pressure) to promote vertebral artery retrograde flow during angioplasty or stenting.
- Patient presented with cardiac ischemia after a left internal mammary artery-left anterior descending coronary graft and multiple coronary artery vein grafts. Subclavian steal cannot occur in this patient, as the left subclavian stenosis is distal to the left vertebral artery origin. Note antegrade flow in the left vertebral artery.
- Postangioplasty with a 9-mm balloon. The pressure gradient across the mid subclavian stenosis was reduced from 60 to 0 mm Hg.
- Normal antegrade right vertebral artery flow on Doppler ultrasound. With cephalad direction of insonation, the negative Doppler scale reading is consistent with flow going away from the direction of insonation in a normal cephalad antegrade fashion.
- Doppler ultrasound shows reversed flow in the left vertebral artery consistent with left subclavian steal phenomenon. Doppler scale reading shows a negative deflection again; however, this time the direction of insonation is caudad, thus a negative scale Doppler reading indicates flow in a caudad direction and flow reversal in the left vertebral artery.


Author
Specialty Editor Board
Bernard D Coombs, MB, ChB, PhD Consulting Staff, Department of Specialist Rehabilitation Services, Hutt Valley District Health Board, New Zealand
Disclosure: Nothing to disclose.
Douglas M Coldwell, MD, PhD Professor of Radiology, Director, Division of Vascular and Interventional Radiology, University of Louisville School of Medicine
Douglas M Coldwell, MD, PhD is a member of the following medical societies: American Association for Cancer Research, American College of Radiology, American Heart Association, American Physical Society, American Roentgen Ray Society, Society of Interventional Radiology, Special Operations Medical Association
Disclosure: Nothing to disclose.
Chief Editor
Kyung J Cho, MD, FACR, FSIR William Martel Emeritus Professor of Radiology (Interventional Radiology), Frankel Cardiovascular Center, University of Michigan Health System
Kyung J Cho, MD, FACR, FSIR is a member of the following medical societies: American College of Radiology, American Heart Association, American Medical Association, American Roentgen Ray Society, Association of University Radiologists, Radiological Society of North America
Disclosure: Nothing to disclose.
Additional Contributors
Robert A Koenigsberg, DO, MSc, FAOCR Professor, Director of Neuroradiology, Program Director, Diagnostic Radiology and Neuroradiology Training Programs, Department of Radiology, Hahnemann University Hospital, Drexel University College of Medicine
Robert A Koenigsberg, DO, MSc, FAOCR is a member of the following medical societies: American Osteopathic Association, American Society of Neuroradiology, Radiological Society of North America, Society of NeuroInterventional Surgery
Disclosure: Nothing to disclose.