Congenital Malformations of the Neck: Embryology, Branchial Malformations, Thyroglossal Duct Cysts/Ectopic Thyroid (original) (raw)
Embryology
Branchial apparatus
During the third week of development, the flat trilaminar embryo undergoes a series of complex folds that result in the formation of a cylindrical embryo. During this time, the laterally placed clefts, known as branchial clefts, appear. These clefts are due to flexion folds of the fetus within the amniotic cavity. The basic tissues of development within the head and neck (ectoderm, endoderm, mesoderm, neuroepithelium) become organized into the pharyngeal apparatus, also known as the branchial apparatus, which is the forerunner of the head and neck structures.
Development of the branchial apparatus begins during the second week of gestation and is complete by week 6-7. The apparatus consists of 5 mesodermal (branchial) arches appearing in the lateral wall of the foregut and separated from each other externally by ectoderm-lined brachial clefts and internally by endoderm-lined pharyngeal pouches. The 5 arches, which are prominent in the lateral profile, are numbered from cranium to cauda as 1, 2, 3, 4, and 6. The fifth arch, which is buried, is called the sixth arch by convention.
The endoderm of the primitive pharynx transiently contacts the ectoderm to form thin, double-layered, branchial membranes at the caudal aspect of each cleft. The mesoderm then separates the ectodermal and endodermal layers of the membranes.
An image depicting congenital malformations of the neck appears below.
Three-week-old patient with congenital midline cervical cleft. Note the nipple-like protrusion of the superior aspect of the cleft.
During the fifth week of development, the first arch and cranial portion of the second arch thicken and enlarge caudally. The caudal portion of the second arch, as well as the third and fourth arches, becomes submerged in the cervical sinus of His, a shallow ectodermal pit. The epipericardial ridge developing from the mesoderm lateral to the sixth arch enlarges the depth of the pit. From this tissue arise the infrahyoid muscles, the sternocleidomastoid-trapezius muscle complex, the muscles of the floor of the mouth, and the muscles of the tongue. Also contained in this ridge are the spinal divisions of the accessory nerve and the hypoglossal nerve. During further development, the sinus of His becomes obliterated and, in the adult, represents the angle between the dorsal surface of the strap muscles and the anterior margin of the sternocleidomastoid.
The human embryo has 5 pairs of pharyngeal pouches. The fifth of these is often considered an appendage of the fourth pouch. During development, the pouches extend away from the pharyngeal wall and communicate with the pharynx via the elongated pharyngobranchial duct.
The primordia of the thymus arise from ventral saccules of the lining epithelium of the third pharyngeal pouch, with a small portion arising from the fourth pouch. During the sixth week of development, the saccules begin to elongate caudally, connecting to the pharynx via the thymopharyngeal ducts. As development continues, the connections of the thymic primordia migrate caudally and medially, and the saccules become obliterated by proliferating epithelium. By 8 weeks' gestation, the thymic primordia of each side unite, attach to the pericardium, and descend into the anterior mediastinum. At this point, the thymopharyngeal ducts degenerate, and the thymus attains its final position in the chest.
Table. Derivatives of pharyngeal arches and pouches* (Open Table in a new window)
| Arch # | Skeletal Derivatives | Muscles | Nerve | Artery | Pouch # and Derivatives |
|---|---|---|---|---|---|
| I. Mandibular | Meckel cartilage, malleus, incus, anterior ligament of malleus, sphenomandibular ligament | Muscles of mastication, mylohyoid, anterior digastric, tensor palatini, tensor tympani | Trigeminal | Maxillary | 1. Eustachian tube, middle ear |
| II. Hyoid | Reichert cartilage1. Stapes2. Styloid process3. Stylohyoid ligament4. Lesser cornu/upper portion of body of hyoid | 1. Stapedius2. Posterior digastric3. Stylohyoid4. Muscles of facial expression | Facial | Stapedial | 2. Palatine tonsil |
| III. Glossopharyngeal | Greater cornu/ lower part of body of hyoid | Stylopharyngeus | Glossopharyngeal | Common carotid, portion of internal carotid | 3. Thymus, inferior parathyroid |
| IV. Plus | Thyroid cartilage, laryngeal cartilages | Pharyngeal constrictors, laryngeal muscles | Vagus | Aorta | 4. Superior parathyroid |
| *Modified from Daniels E, Embryology of the Neck. In: Tewfik TL, Der Kaloustian VK, eds. Congenital Anomalies of the Ear, Nose and Throat. New York: Oxford University Press; 1997. (with permission) |
Aortic arches
Each branchial arch contains an associated aortic arch connecting the paired dorsal and ventral aortas. All 6 pairs of arches are not present at the same time, with the first 2 involuting prior to the appearance of the sixth.
The first arch gives rise to the maxillary artery. The dorsal segment of the second arch forms the stapedial artery. The third arch forms the common carotid artery, as well as the proximal portion of the internal carotid artery. The right fourth arch forms the proximal portion of the right subclavian artery, while the left fourth arch contributes to the aortic arch proper. The fifth arch arteries involute. The sixth pair forms the pulmonary arteries.
Thyroid gland
The thyroid gland begins developing during the first and second week of intrauterine life and is completed by week 11. It arises simultaneously from 3 bodies, the median anlage and 2 lateral anlagen.
The median anlage arises as endodermal thickening from the midline of the ventral pharyngeal wall, between the first and second branchial arches, at the junction of the developing anterior and posterior tongue. This is the site of the foramen cecum.
The median thyroid anlage comes in contact with the aortic sac of the heart. As the sac descends, it pulls the median thyroid caudally, causing the thyroid to accumulate more endodermal cells from the surrounding tissue. As the median thyroid anlage descends, its pharyngeal connection elongates as a stalk, namely, the thyroglossal duct, which normally disappears by the fifth to sixth week. At the end of 7 weeks, the median thyroid reaches its final position over the trachea and consists of a small isthmus and 2 lateral lobes.
The contribution of the lateral thyroid anlagen remains controversial. They are thought to receive contributions from the fourth and sixth branchial pouches. As the lateral anlagen migrate anteriorly, they detach from the pharynx and fuse with the median anlage, contributing up to one third of the volume of the gland.
The parafollicular cells, which produce calcitonin, are derived from neural crest cells. They become incorporated in the lateral thyroid primordia, forming the ultimobranchial bodies.
Lymphatic system
Several theories exist pertaining to the development of the lymphatic system.
The first theory, known as the venous origin with centrifugal spread theory, centers on the development of "sprouts" off of large central veins. These then enlarge, coalesce, and form new sprouts that eventually form the lymphatic system. The direction of spread is toward the periphery.
The second theory, known as the mesenchymal origin with centripetal spread theory, describes the independent development of the lymphatic system from the confluence of mesenchyme spaces. The spaces move centripetally by interacting with other mesenchymal spaces.
The final theory, known as the combined venous-mesenchymal origin theory, states that the lymphatic system originates from the confluence of small venules with spaces in the mesenchyme. In this theory, development progresses through distinct phases.
The first phase begins at approximately 7 weeks of intrauterine life with the development of endothelial buds, known as the lymphatic primordia, sprouting from veins and uniting to form plexuses. These give rise to paired axillary and jugular sacs.
The second phase displays enlargement of these sacs and the development of single communications with the ipsilateral internal jugular veins, contralateral internal jugular veins, and axillary sacs.
The third stage shows enlargement and widening of these sacs and the appearance of other lymphatic primordia (eg, internal thoracic primordia, paratracheal primordia).
Further enlargement continues in the fourth stage, with coalescence of the primordial sprouts.
During the fifth and final stage, all lymphatic primordia fuse, and one continuous system is formed, with permeation of the lymphatic channels into the tissues. The formal thoracic duct and cisterna chyli take shape. Development is complete by 10 weeks.

Branchial Malformations
Classification of branchial anomalies
Congenital anomalies of the neck arise as a consequence of disturbances in the complex development of the branchial apparatus of the fetus. They are classified according to their branchial cleft or pouch of origin as well as their anatomic relationships. They may take the form of a fistula, sinus, or cyst, based on the degree of completion of development of the anomalous structure. [1]
Fistulae represent persistence of both the cleft and the corresponding pouch, thereby forming a communication (ie, fistula) that is epithelial lined. The fistula lies caudal to the structures derived from that particular arch and connects the skin to the foregut. Sinuses may be considered partial fistula, usually opening externally, with no internal opening. They are epithelial lined. Fistulae and sinuses may be lined by stratified squamous, columnar, or ciliated epithelium, and they may contain lymphoid tissue as well.
Cysts have neither an internal nor an external opening and are most often lined by stratified squamous epithelium (ectoderm derived); however, they can be lined by columnar epithelium (endoderm derived from pouches).
First branchial anomalies
First branchial anomalies represent 1% of all branchial anomalies. Work reports the following 2 types [2] :
- A type I branchial anomaly is ectodermally derived and is a duplication of the external auditory canal (EAC). Type I branchial anomalies may manifest posteriorly, adjacent to the pinna and concha. They may terminate near a bony plate at the level of the mesotympanum.
- Type II branchial anomalies contain both ectoderm and mesoderm. They are more common and can originate along the EAC, middle ear cleft, or nasopharynx. Type II branchial anomalies often manifest with a fistula emanating from the concha, EAC, or neck, with a tract extending medially and inferiorly to the EAC. They may pass medial or lateral to the facial nerve and terminate at the level of the anterior border of the sternocleidomastoid muscle. Often an opening is found in the EAC as well as below the jaw.
First branchial anomalies do not usually involve the middle ear or tympanic membrane.
Patients with first branchial anomalies may present with unilateral facial paralysis. First branchial anomalies may be associated with hemifacial microsomia.
A study by Shinn et al of pediatric patients with branchial anomalies indicated that those with first branchial anomalies were more likely than those with second arch anomalies to have otologic complications. Of the 27 children with first anomalies, 22% had otorrhea, 25.9% had otitis media, and 14.8% had cholesteatoma, while 3.8% of the 80 children with second anomalies had otitis. Children with first anomalies were also more likely to require primary incision and drainage than those with second anomalies (59.3% vs 2.5%, respectively) and to experience persistent disease following primary excision (25.9% vs 2.5%, respectively). [3]
Second arch anomalies
Second arch anomalies are the most common and represent 90-95% of branchial anomalies.
They are classified into 4 categories (I-IV) based on anatomic location. Types I-III are the most frequently occurring second arch anomalies, with type II being the most common. Bilaterality of second arch anomalies is uncommon.
- Type I anomalies are located along the anterior margin of sternocleidomastoid muscle at the junction of the middle and lower thirds, deep to the platysma and cervical fascia.
- Type II anomalies lie in contact with the great vessels.
- Type III anomalies pass medially between the internal and external carotid arteries, extending toward the lateral pharyngeal wall and lying above the glossopharyngeal and hypoglossal nerves and below the stylohyoid ligament.
- Type IV anomalies are very rare and are located next to the pharyngeal wall, medial to the great vessels at the level of the tonsillar fossa.
Cystic lesions are more common than fistulae. They tend to manifest as smooth, soft masses in the lateral neck and are located anterior and deep to the sternocleidomastoid muscle. Fistulae tend to manifest as recurrent neck infections, often following an upper respiratory tract infection, below the level of the digastric muscle. The most common time for presentation of second branchial anomalies is during the second decade of life.
Branchiootorenal syndrome (BOR syndrome), or Melnick-Fraser syndrome, is an association of auricular malformations, branchial fistulae, deafness, and renal anomalies. An estimated 2% of profoundly deaf children have BOR syndrome. The estimated prevalence is 1 per 40,000 people. External ear malformations may include preauricular pits, tags, lop-ears, and/or microtia. Anomalies of the ossicles, facial nerve, and fallopian canal have also been described.
Third branchial anomalies
Third branchial anomalies are rarely encountered. Many authors agree that differentiating between third and fourth branchial anomalies on clinical grounds is difficult.
A complete fistula has a cutaneous opening along the anterior border of the sternocleidomastoid muscle. The tract courses posterior to the common and/or internal carotid artery, superior to the hypoglossal nerve, and inferior to the glossopharyngeal nerve; then it medially pierces the posterolateral aspect of the thyrohyoid membrane to open into the pyriform sinus.
In 2010, Nicoucar et al reviewed 202 published cases of congenital third branchial arch anomalies. They found that 88% occur on the left side. They manifest as neck abscess in 39% of cases or acute thyroiditis in one third of patients. [4]
Third branchial anomalies can manifest with upper airway compromise in the neonate and may also manifest with hypoglossal nerve palsy.
Fourth branchial anomalies
Fourth branchial anomalies are extremely rare and, unlike second branchial anomalies, typically manifest in childhood. [5]
Fourth branchial anomalies originate at the apex of the pyriform sinus, traveling anteriorly and inferiorly to the cricothyroid muscle and thyroid cartilage. A lateral cervical cyst with an internal fistula in the pyriform sinus is a common occurrence. The course of the tract then runs superiorly over the hypoglossal nerve and descends in the neck posterior to the common carotid artery.
Fourth branchial anomalies follow a different course on each side of the neck. On the right side, the tract passes underneath the subclavian artery and courses superior to the recurrent laryngeal nerve and inferior to the superior laryngeal nerve. On the left side, the tract passes anteriorly underneath the aorta and courses superiorly in the neck, posterior to the common carotid artery.
Fourth branchial anomalies often terminate in the perithyroid space, thyroid gland, or cervical esophagus, and they may manifest clinically as an abscess in these areas. The cutaneous opening is along the anterior border of the sternocleidomastoid muscle. Nathan et al (2012) described a case of a neonate presenting with stridor secondary to a fourth branchial pouch sinus. [6] Magnetic resonance imaging confirmed the presence of a swelling in the posterolateral pharyngeal wall and a sinus opening in the pyriform fossa on the left side. The swelling was aspirated and the fourth arch pouch was treated without any complications and recovered well.
Diagnosis and management of branchial anomalies
Diagnosis is based on physical examination, including a thorough otologic examination. This should include the use of a Lempert or Vienna bivalve ear speculum to examine the cartilaginous EAC. Furthermore, flexible laryngoscopy may reveal a sinus tract in the pyriform sinus. For patients with suspected pyriform sinus fistulae, perform a barium esophagram.
Radiologic confirmation is best achieved using computed tomography. Typically, the cysts are of low attenuation with smooth walls.
Aspiration of the cystic mass may aid in confirming the diagnosis, thus excluding a neoplastic or inflammatory lesion.
Management of branchial cleft anomalies is surgical excision. Surgery should ideally be performed on the uninfected neck. Institute a course of antibiotics prior to the surgical treatment if infection is suspected.
Special considerations
Each of the branchial cleft types has special considerations.
For first branchial anomalies, the potential for facial nerve involvement must be considered. Exploration of the nerve is mandatory before excision of the tract.
For second branchial anomalies, the tract usually bisects the external and internal carotid arteries just above the bifurcation, traveling laterally and superiorly to the hypoglossal and glossopharyngeal nerves and terminating in the tonsillar fossa. These are typically addressed via an incision along the anterior border of the sternocleidomastoid muscle, although an intraoral approach is possible for isolated pharyngeal cysts.
For third branchial anomalies, the tract travels posteriorly to the internal carotid artery, superiorly to the hypoglossal nerve, and inferiorly to the glossopharyngeal nerve. External approaches along the sternocleidomastoid muscle are preferred.
Approach fourth branchial anomalies through a traditional thyroid incision. Pay special attention to the course of the recurrent laryngeal nerve.
The most common complication of surgical resection is recurrence, which occurs in approximately 3% of cases.

Thyroglossal Duct Cysts/Ectopic Thyroid
Thyroglossal duct cysts
Thyroglossal duct cysts (TGDCs) represent the most common congenital anomaly of the neck, accounting for 2-4% of all neck masses. They are most commonly present in the first decade of life but may also be seen in adults.
TGDCs are caused by a persistent epithelial tract, the thyroglossal duct, during the descent of the thyroid from the foramen cecum to its final position in the anterior neck. They may also give rise to sinuses, fistulae, or cysts.
TGDCs manifest in 6 different varieties. The infrahyoid type accounts for 65% of TGDCs and is mostly found in the paramedian position, while the suprahyoid type accounts for nearly 20% and is positioned in the midline. Juxtahyoid cysts make up 15% of TGDCs. Intralingual location occurs in approximately 2% of TGDCs, and the suprasternal variety occurs in approximately 10% of cases. Intralaryngeal TGDC is very rare. This variety has recently been described in a 42-year-old man and should be differentiated from intralaryngeal lesions.
TGDCs are usually nontender and mobile. Infected TGDCs may manifest as tender masses with associated dysphagia, dysphonia, draining sinus, fever, or increasing neck mass. They often manifest after an upper respiratory tract infection. Airway obstruction is possible, especially with intralingual cysts. Ultrasonography and computed tomography (CT) scanning are the radiologic tools of choice. Ultrasonography can distinguish between solid and cystic components.
A study by Choi et al determined which ultrasonographic characteristics could be used to distinguish between TGDCs and dermoid cysts, finding that TGDCs more often had an irregular shape, a poorly defined margin, hyoid bone attachment, and heterogeneous internal echogenicity. They were also more likely to be multilocular, to be located intramuscularly, and to extend longitudinally into the tongue base. [7]
CT scanning may reveal a well-circumscribed cystic lesion, 2-4 cm in diameter with capsular enhancement. A fistulogram may reveal the course of the tract. Thyroid scanning is suggested to rule out the possibility of the cyst containing the only functioning thyroid tissue, albeit in an ectopic site.
The surgical treatment of choice for thyroglossal cysts is the Sistrunk operation, in which an en block resection of the sinus tract and above (including the midportion of the hyoid bone) is performed. Recurrence is approximately 3-5% and is increased by incomplete excision and a history of recurrent infections. Simon and Magit published a report regarding how incision and drainage of infected TGDCs impact recurrence following the Sistrunk procedure. [8] Their series included 120 patients. Forty-nine of the patients (40.8%) had a history of infection and 6 of these required incision and drainage. The overall recurrence rate was 10.8%. Ten of the patients with preoperative infection (20%) had a recurrence, compared with 3 (4%) of the 71 patients without preoperative infection.
A single-center, retrospective study by Malka Josef et al indicated that in patients with TGDCs, a relationship exists between age and surgical failure rate following the Sistrunk procedure, with such failure occurring least often adults. The investigators reported a failure rate of 3.6% in patients aged 18 years or older, compared with 38.9% in children below age 3 years, and 16.7% in patients aged between 3 and 18 years. An association between postoperative complications and surgical failure was found in the two children’s groups but not in adults. [9]
According to Geller et al, simple excision or "cystectomy" has no place in the treatment of TGDC. The Sistrunk procedure is the procedure of choice in both primary and revision cases. An intimate knowledge of the anatomy in and around the larynx and experience reduce the incidence of recurrence. [10]
Endoscope-assisted thyroglossal duct cyst excision and subtotal thyroidectomy were performed by Cai et al via a small submental incision in 6 patients with TGDCs and benign nodular thyroid lesions. The TGDCs and thyroid lesions were completely excised. No complications were observed, and no recurrent lesions were detected during follow-up. They concluded that patients with TGDCs and benign thyroid lesions can be treated by endoscopic excision. [11]
Similarly, a prospective cohort study by Woo et al reported successful outcomes with the use of endoscope-assisted transoral TGDC resection. The study involved 30 patients, with one experiencing transient morbidity. [12]
Ectopic thyroid
Ectopic thyroid tissue can arise from either the median anlage or lateral anlage (far less common). The tissue may or may not be functioning. Prevalence is higher in females than in males, with a female-to-male ratio of 7:1.
The most common site of ectopic tissue is within the tongue (90%). This is most often located in the midline dorsum, which is attributed to the descent of the thyroid through the foramen cecum, as discussed above.
The ectopic tissue is typically found incidentally, although these masses can be symptomatic and can cause dysphagia, dysphonia, stridor, dyspnea, hemorrhage, or hoarseness. An association has been noted between lingual thyroid tissue and cretinism.
CT scans often reveal a homogenous hyperdense mass within the lingual musculature. Management of the mass is surgical; however, prior to the decision to operate, perform thyroid scanning to assess if the mass is functioning and to determine if this tissue is the only viable thyroid material.

Hemangiomas
Hemangiomas represent the most common of all congenital anomalies, with an incidence of 0.3-2% at birth and 10% at age 1 year.
Hemangiomas result from the inappropriate development of vascular endothelium and channels and associated nervous components.
Classification of hemangiomas is as follows:
- Type I - Forms of neonatal staining, including stork bite and nevus flammeus
- Type II - Intradermal capillary hemangiomas, including salmon patches, port wine stain, and spider angiomas
- Type III - Juvenile capillary hemangiomas, including strawberry marks, strawberry hemangiomas, and capillary cavernous hemangiomas
- Type IV - Arteriovenous fistulas
- Type V - Arteriovenous malformations are present. Most hemangiomas present within the first year of life and continue to grow until approximately age 2 years, after which involution occurs.
The new classification according to Mulliken differentiates between hemangiomas and vascular malformations. They differ primarily in that hemangiomas are proliferative lesions while vascular malformations are vessel malformations, which enlarge only by dilation of the involved vessels.
Hemangiomas are classified by location as either superficial (above the dermis), deep (below the dermis), or mixed. Although a large number of hemangiomas will involute, many (as high as 50%) may require treatment. Primary treatment is high-dose steroids during the proliferative phase (usually in the first year of life), followed by surgical management later. Surgical management may involve a combination of both laser treatments and surgical resection.
Vascular malformations are classified according to the type of blood vessels involved. In contrast to hemangiomas, they grow by distentions of the vessels and not by proliferation. Types of vascular malformations are as follows:
Venular malformations involve small venules (capillaries) in the dermis (formerly classified as capillary malformations). The most common type is the port wine stain. Midline venular lesions (also known as stork-bites) tend to involute by age 1 year.
Venous malformations are incorrectly classified as cavernous hemangiomas. These consist of dilated veins. Their growth later in life is due to dilation of the veins.
Lymphatic malformations are also known as lymphangiomas or cystic hygromas. These lesions form from dilated lymph vessels and may suddenly enlarge following infection or trauma. Lymphatic malformations are subclassified into macrocystic lesions, microcystic lesions, and mixed lesions.
Arteriovenous malformations are abnormal connections of arteries (high-pressure) and veins (low-pressure) that progressively dilate because of high pressures transmitted to the venous side.
Mixed lesions, including different types of vessels (eg, venolymphatic), can also occur. Management of vascular malformations depends on the type of vessel involved. The most common sites of manifestation include the face, neck, and scalp.
Physical examination reveals a soft, painless, and compressible mass, which may be located within the skin or mucosa or within muscle, bone, or salivary gland tissues. A pale telangiectatic lesion often precedes hemangiomas, and an audible bruit may be present.
Some of the distinctive features include the following:
- Salmon patch - Light pink or rust colored, most common on the back of the neck, forehead, and eyelids
- Spider angiomas - Small central arteriole with radiating capillaries
- Strawberry mark - Pale halo, surrounded by telangiectasia
- Capillary hemangioma - Red or purple, well-defined borders, blanches with pressure
- Cavernous hemangioma - Red or purple, "bag of worms" texture, blanches with pressure, increases in size on Valsalva
- Port wine stain - Pink or red, purple in adulthood, do not blanch with pressure, underlying soft tissue hypertrophy
- Syndrome associated hemangiomas - Occur in Von Hippel disease, Maffucci syndrome, Sturge-Weber syndrome, and Kasabach-Merritt syndrome
The diagnosis of hemangiomas is made on clinical grounds. Radiologic confirmation can be achieved with Doppler ultrasonography, which also determines the flow rate within the lesion. CT scanning is used to map the extent of the lesion. Angiography helps to distinguish between hemangiomas and vascular malformations.
Treatment of patients with these lesions can pose considerable difficulty in light of the potentially irreversible cosmetic defects. Spontaneous involution occurs in as many as 90% of cases, with the majority involuting by age 5 years. As such, observation is initially recommended.
Other treatment modalities could be used for the approximately 3-5% of patients with nonresolving lesions. These methods have different success rates. They include electrodesiccation, compression, embolization, cryotherapy, sclerotherapy, radiation, chemotherapy, and carbon dioxide and neodymium: yttrium-aluminum-garnet (ND: YAG) lasers.

Thymic Anomalies
Thymic anomalies arise from the ventral saccules of the epithelium of the third pharyngeal pouch. Anomalies can result from (1) incomplete descent of the thymus into the chest, (2) sequestration of thymic tissue foci along the descent path, or (3) failure of the thymopharyngeal duct to involute.
This may result in an aberrant cervical thymus or thymic cysts. Sequestered cysts can occur along the path of the thymopharyngeal ducts from the level of the mandible to the chest. Occasionally, trapped thyroid or parathyroid remnants may be within the cysts.
Typically, these cysts are asymptomatic masses that are not found at birth but discovered later in life, with two thirds manifesting during the first decade of life. Males are affected more frequently than females are.
Patients most often present with an asymptomatic neck mass, which may be slowly increasing in size. The mass may grow rapidly, secondary to infection, hemorrhage, cystic degeneration, or venous engorgement. Approximately 10% of patients present with symptoms of mass effect and compression, including dysphagia, dyspnea, pain, or hoarseness. Thymic cysts can manifest in association with numerous other conditions, including thyrotoxicosis, aplastic anemia, Down syndrome, neurofibromatosis, and Hodgkin lymphoma, although this is quite rare. Patients may also present with myasthenia gravis, which is associated with neoplasms of the thymus. Transudative pleural effusion due to rupture of a thymic cyst into the pleural cavity is very rare. This was recently documented in a patient who reported chest pain in the right hemithorax and dyspnea on exertion.

Sternocleidomastoid Tumor of Infancy
Sternocleidomastoid tumor of infancy (SCTI) is part of the spectrum of conditions known as congenital muscular torticollis (CMT). It represents the most common neck mass of the immediate perinatal period, within the first 2 months of life. The term tumor is a misnomer because most commonly it is congenital fibrosis within the muscle. The etiology remains unclear, with the most likely cause being peripartum injury. SCTI has a slight male prevalence and occurs more often on the right and with breech presentations. SCTIs are typically a firm, painless mass with fusiform shape, approximately 1-3 cm in length. They are most often located in the inferior to the middle third of the sternocleidomastoid muscle, affecting the sternal or clavicular heads equally.
Diagnosis is based on CT scanning and ultrasonographic confirmation. Open biopsy is reserved for cases that do not resolve. The natural course of the disease is a peak in growth for approximately 8 weeks, with complete resolution by 6 months. Physiotherapy is recommended to achieve full range of motion. Some patients have small areas of residual fibrosis. Surgery is reserved for patients in whom torticollis is present for more than one year, those in whom craniofacial asymmetry develops, and those for whom physiotherapy fails. Long-term follow-up care is essential because of the possibility of recurrence or reappearance of the disease during periods of heightened growth.

Cervical Teratoma/Dermoid Cyst and Midline Cervical Cleft
Cervical teratomas
Cervical teratomas are extremely uncommon lesions of the head and neck. The prevalence of cervical teratomas is 1 in 16,000 individuals, with most of them occurring in full-term, preterm, or stillborn infants.
In the neck, they most often occur on the anterolateral surface, extending midline from the thyroid gland. They may manifest clinically as severe respiratory distress and dysphagia due to compression in the proximity of the trachea and esophagus.
These lesions are often large, extending as far as 12 cm in their longest axis. Histologically, they may contain any combination of tissues from the 3 germ layers.
They are often categorized in relation to their proximity to the thyroid gland. The categories include (1) teratomas of the thyroid gland, deriving their blood supply from the thyroid arteries, (2) teratomas adjacent to the thyroid gland, which displace the gland but in which a definitive blood supply cannot be identified, and (3) teratomas of the neck. This categorization is of little clinical use. An extremely rare case of fetal cervical teratoma presenting at 24 weeks of gestation was recently published. In this case, a submaxillary mass and agenesis of corpus callosum were revealed with ultrasonography and also associated with a subarachnoid cyst.
Cervical teratomas are managed with early surgical excision. This is particularly important in light of the possible malignant degeneration of these lesions. The prognosis in nonneoplastic cases is excellent and approaches 100%. The ex-utero intrapartum treatment (EXIT) procedure is a technique designed to allow partial fetal delivery via caesarean section with establishment of a safe fetal airway. The most common indication for the EXIT procedure is the presence of fetal airway obstruction, which is usually caused by a prenatal diagnosed neck mass. [13]
In a literature review of pediatric patients with congenital head and neck teratomas, Patel et al found that emergent perinatal airway management is required for the majority of these individuals. The investigators reported that at delivery, 80 (58%) of the study’s 137 patients suffered from respiratory distress, with intervention needed; this involved endotracheal intubation in 63 cases (46%). Respiratory distress most commonly occurred in patients with oropharyngeal or anterior cervical tumors (66%). [14]
Dermoid cysts
Dermoid cysts are considered the most common form of teratoma and are characterized by a predominance of ectodermal content. Most dermoids in the head and neck occur in the region of the floor of mouth, with approximately one fourth of dermoids involving the lateral neck or midline regions.
They are believed to be caused by epithelial rests trapped during embryologic development.
Clinically, they manifest as slow-growing lesions that produce symptoms when their cystic lumens become filled with keratin debris. Sebaceous material may also be found in the cyst, alluding to its ectodermal origin.
The management of these cysts is surgical excision. However, a retrospective study by Foo et al indicated that head and neck dermoids in pediatric patients can safely and effectively be treated with ultrasonographically guided cyst puncture and drainage, with subsequent radiofrequency coblation of the cyst wall. The investigators reported that complete drainage can be promoted by emulsifying the cyst contents with 3% sodium tetradecyl sulfate. Of 22 dermoids, 21 (95%) were successfully treated, although in four intraosseous dermoids, CT-scan guidance was used in place of or in addition to ultrasonography. [15]
Midline cervical cleft
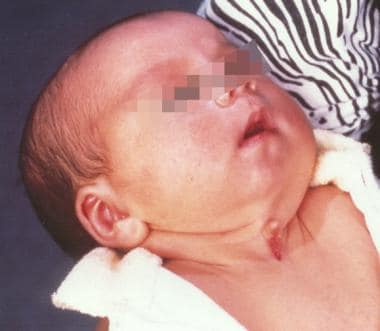
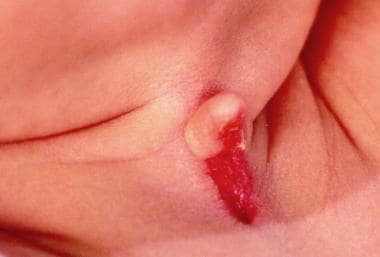
Midline cervical cleft (MCC) is a rare congenital anomaly. Just over 200 cases have been published, the first being described by Bailey in 1924. [16] It is not considered a true cleft because no skin gap exists (see the images below).
Three-week-old patient with congenital midline cervical cleft. Note the nipple-like protrusion of the superior aspect of the cleft.
Close-up view of 3-week-old patient with congenital midline cervical cleft.
The etiology is unclear and is believed to be abnormal fusion of the second and third arches or nonfusion at the level of the ectoderm.
MCC typically manifests at birth with a cleft extending from the inferior aspect of the mentum to the level of the suprasternal notch. Often the area has serous drainage, and the superior aspect of the cleft takes on the appearance of a pseudonipple.
A retrospective study by Bahakim et al of 21 patients with congenital midline cervical cleft found the lesions to be between 0.5 and 5 cm long, with the size of nipple-like protrusion being 0.2 to 1.5 cm. [16]
Physical examination and CT scanning are used to make the diagnosis. Distinguishing this entity from other midline neck masses (eg, thyroglossal duct cysts, bronchogenic cysts) is important. In general, midline cervical clefts are located caudally, while thyroglossal duct cysts are located cranially. Bronchogenic cysts are located in the suprasternal notch, with possible extension into the mediastinum.
Associated defects may be a median cleft of the mandible, tongue, and lower lip. A delay in mandibular development and hypoplasia or absence of neck structures, such as the hyoid bone may be associated. Associated thyroglossal and bronchogenic cysts may occur, as well as defects in other parts of the body such as a sternal cleft. [17] Management involves complete excision of the cleft with surgical repair of the soft tissue defect.

Lymphangioma/Cystic Hygroma
Lymphangiomas are benign multiloculated cystic masses that are soft, compressive, and painless. They usually manifest at or shortly after birth, with 50-60% manifesting by age 1 year and 90% by age 2 years. They are uncommon lesions, accounting for fewer than 5% of all congenital neck masses. The term cystic hygroma is synonymous with lymphangioma.
Approximately 50-75% of all lymphangiomas occur in the neck, most commonly in the anterior and posterior triangles. They can occur in the submental triangle, with extension into the floor of mouth. These masses tend to manifest as slow growing, painless masses with a doughy consistency. They may acutely enlarge following infectious processes (eg, upper respiratory tract infections). As a result of acute enlargement or chronic growth, the hygromas may cause respiratory and swallowing difficulties.
Radiologic diagnosis is often made using ultrasonographic imaging. CT scanning often reveals a multicystic lesion with well-defined boundaries. Rim identification may be difficult in previously uninfected cysts because the lesions do not follow planes of embryologic fusion. MRI may offer better soft tissue delineation than CT scanning. Repeated infection or hemorrhage causes increased signal attenuation. Lymphangioma is classified into 4 histologic subtypes: cavernous lymphangioma, capillary lymphangioma, cystic hygroma, and hemangiolymphangioma.
The management of choice is surgical excision. Other techniques (eg, cryotherapy, sclerotherapy) have met with only marginal success. Even with surgical excision, recurrence rates for lymphangioma are high, reported at 6-50%.