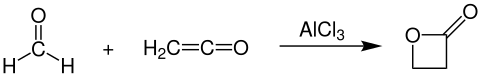Β-Propiolactone (original) (raw)
From Wikipedia, the free encyclopedia
(Redirected from Beta-Propiolactone)
"Propiolactone" redirects here. For α-propiolactone, see Methyloxiranone.
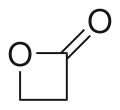
β-Propiolactone
 |
|
|---|---|
 |
|
| Names | |
| Preferred IUPAC name Oxetan-2-one | |
| Other namesPropiolactone2-Oxetanone3-Hydroxypropanoic acid lactone | |
| Identifiers | |
| CAS Number | 57-57-8  Y Y |
| 3D model (JSmol) | Interactive image |
| ChEBI | CHEBI:49073  Y Y |
| ChEMBL | ChEMBL1200627  N N |
| ChemSpider | 2275  Y Y |
| DrugBank | DB09348 |
| ECHA InfoCard | 100.000.309 |
| EC Number | 200-340-1 |
| KEGG | D05630  Y Y |
| PubChem CID | 2365 |
| RTECS number | RQ7350000 |
| UNII | 6RC3ZT4HB0  Y Y |
| UN number | 2810 |
| CompTox Dashboard (EPA) | DTXSID8021197 |
InChI InChI=1S/C3H4O2/c4-3-1-2-5-3/h1-2H2  YKey: VEZXCJBBBCKRPI-UHFFFAOYSA-N YKey: VEZXCJBBBCKRPI-UHFFFAOYSA-N  YInChI=1/C3H4O2/c4-3-1-2-5-3/h1-2H2Key: VEZXCJBBBCKRPI-UHFFFAOYAQ YInChI=1/C3H4O2/c4-3-1-2-5-3/h1-2H2Key: VEZXCJBBBCKRPI-UHFFFAOYAQ |
|
| SMILES O=C1OCC1 | |
| Properties | |
| Chemical formula | C3H4O2 |
| Molar mass | 72.063 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | slightly sweet[1] |
| Density | 1.1460 g/cm3 |
| Melting point | −33.4 °C (−28.1 °F; 239.8 K) |
| Boiling point | 162 °C (324 °F; 435 K) (decomposes) |
| Solubility in water | 37 g/100 mL |
| Solubility in organic solvents | Miscible |
| Vapor pressure | 3 mmHg (25°C)[1] |
| Refractive index (_n_D) | 1.4131 |
| Hazards | |
| GHS labelling: | |
| Pictograms |    |
| Signal word | Danger |
| Hazard statements | H315, H319, H330, H350 |
| Precautionary statements | P201, P202, P260, P264, P271, P280, P281, P284, P302+P352, P304+P340, P305+P351+P338, P308+P313, P310, P320, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 |
| Flash point | 74 °C; 165 °F; 347 K[1] |
| Explosive limits | 2.9%-?[1] |
| NIOSH (US health exposure limits): | |
| PEL (Permissible) | OSHA-Regulated carcinogen[1] |
| REL (Recommended) | Ca[1] |
| IDLH (Immediate danger) | Ca [N.D.][1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).  N verify (what is N verify (what is  Y Y N ?) Infobox references N ?) Infobox references |
Chemical compound
β-Propiolactone, often simply called propiolactone, is an organic compound with the formula CH2CH2CO2. It is a lactone family, with a four-membered ring. It is a colorless liquid with a slightly sweet odor, highly soluble in water and organic solvents.[2][3] The carcinogenicity of this compound has limited its commercial applications.[4]
β-Propiolactone is prepared industrially by the reaction of formaldehyde and ethenone in the presence of aluminium- or zinc chloride as catalyst:[5]
In the research laboratory, propiolactones have been produced by the carbonylation of epoxides.[6]
Reactions and applications
[edit]
It reacts with many nucleophiles in a ring-opening reactions. With water hydrolysis occurs to produce 3-hydroxypropionic acid (hydracryclic acid). Ammonia gives the β-alanine, which is a commercial process.[5]
Propiolactone was once widely produced as an intermediate in the production of acrylic acid and its esters. That application has been largely displaced in favor of safer and less expensive alternatives. β-Propiolactone is an excellent sterilizing and sporicidal agent, but its carcinogenicity precludes that use.[2] It is used to inactivate a wide variety of viruses,[7] for instance the influenza virus, as a step in vaccine production.[8] The principal use of propiolactone is an intermediate in the synthesis of other chemical compounds.[5]
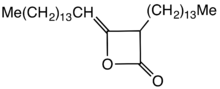
Structure of an alkyl ketene dimer (AKD), a propiolactone derivative that is used in papermaking.
β-Propiolactone is "reasonably anticipated to be a human carcinogen" (IARC, 1999).[2] It is one of 13 "OSHA-regulated carcinogens," chemicals regarded occupational carcinogens by the U.S. Occupational Safety and Health Administration, despite not having an established permissible exposure limit.[9]
- 3-Oxetanone, an isomer of β-propiolactone
- Malonic anhydride (2,4-oxetanone)
- α-Propiolactone
- ^ a b c d e f g NIOSH Pocket Guide to Chemical Hazards. "#0528". National Institute for Occupational Safety and Health (NIOSH).
- ^ a b c "β-Propiolactone CAS No. 57-57-8" - US Department of Health and Human Services, Report on Carcinogens, National Toxicology Program, Thirteenth Edition, 2 October 2014. Accessed on 2015-01-03.
- ^ Merck Index, 12th Edition, entry 8005.
- ^ Miller, Raimund; Abaecherli, Claudio; Said, Adel; Jackson, Barry (2001). "Ketenes". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a15_063. ISBN 978-3-527-30385-4.
- ^ a b c Miltenberger, Karlheinz (2000). "Hydroxycarboxylic Acids, Aliphatic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a13_507. ISBN 978-3527306732.
- ^ John W. Kramer; Daniel S. Treitler; Geoffrey W. Coates (2009). "Low Pressure Carbonylation of Epoxides to β-Lactones". Org. Synth. 86: 287. doi:10.15227/orgsyn.086.0287.
- ^ Logrippo, Gerald A. (1960). "Investigations of the Use of Beta-Propiolactone in Virus Inactivation". Annals of the New York Academy of Sciences. 83 (4): 578–594. Bibcode:1960NYASA..83..578L. doi:10.1111/j.1749-6632.1960.tb40931.x. PMID 14417982. S2CID 6025589.
- ^ Qiang Gao; Linlin Bao; Haiyan Mao; Lin Wang; Kangwei Xu; Minnan Yang; Yajing Li; Ling Zhu; Nan Wang; Zhe Lv; Hong Gao; Xiaoqin Ge; Biao Kan; Yaling Hu; Jiangning Liu; Fang Cai; Deyu Jiang; Yanhui Yin; Chengfeng Qin; Jing Li; Xuejie Gong; Xiuyu Lou; Wen Shi; Dongdong Wu; Hengming Zhang; Lang Zhu; Wei Deng; Yurong Li; Jinxing Lu; Changgui Li; Xiangxi Wang; Weidong Yin; Yanjun Zhang; Chuan Qin (2020-07-03). "Development of an inactivated vaccine candidate for SARS-CoV-2". Science. 369 (6499): 77–81. Bibcode:2020Sci...369...77G. doi:10.1126/science.abc1932. PMC 7202686. PMID 32376603.
- ^ "Appendix B - Thirteen OSHA-Regulated Carcinogens" - Centers for Disease Control and Prevention. Accessed on 2013-11-06.