Bixin (original) (raw)
From Wikipedia, the free encyclopedia
Bixin[1]
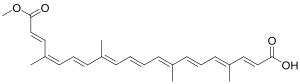 |
|
|---|---|
| Names | |
| IUPAC name (2_E_,4_E_,6_E_,8_E_,10_E_,12_E_,14_E_,16_Z_,18_E_)-20-Methoxy-4,8,13,17-tetramethyl-20-oxoicosa-2,4,6,8,10,12,14,16,18-nonaenoic acid | |
| Other names_cis_-Bixin; α-Bixin; 9-_cis_-6,6'-Diapo-ψ,ψ-carotenedioic acid, 6-methyl ester | |
| Identifiers | |
| CAS Number | 6983-79-5  Y39937-23-0 (trans) Y39937-23-0 (trans)  Y Y |
| 3D model (JSmol) | Interactive image |
| ChEBI | CHEBI:3136 |
| ChEMBL | ChEMBL1172615  N N |
| ChemSpider | 4444638  N N |
| ECHA InfoCard | 100.027.499 |
| PubChem CID | 5281226 |
| UNII | 9L7T4VB66G  Y6JH6LEZ7HY (trans) Y6JH6LEZ7HY (trans)  Y Y |
| CompTox Dashboard (EPA) | DTXSID1024629 |
InChI InChI=1S/C25H30O4/c1-20(12-8-14-22(3)16-18-24(26)27)10-6-7-11-21(2)13-9-15-23(4)17-19-25(28)29-5/h6-19H,1-5H3,(H,26,27)/b7-6+,12-8+,13-9+,18-16+,19-17+,20-10+,21-11+,22-14+,23-15+  YKey: RAFGELQLHMBRHD-IFNPSABLSA-N YKey: RAFGELQLHMBRHD-IFNPSABLSA-N  YInChI=1/C25H30O4/c1-20(12-8-14-22(3)16-18-24(26)27)10-6-7-11-21(2)13-9-15-23(4)17-19-25(28)29-5/h6-19H,1-5H3,(H,26,27)/b7-6+,12-8+,13-9+,18-16+,19-17+,20-10+,21-11+,22-14+,23-15-Key: RAFGELQLHMBRHD-SLEZCNMEBU YInChI=1/C25H30O4/c1-20(12-8-14-22(3)16-18-24(26)27)10-6-7-11-21(2)13-9-15-23(4)17-19-25(28)29-5/h6-19H,1-5H3,(H,26,27)/b7-6+,12-8+,13-9+,18-16+,19-17+,20-10+,21-11+,22-14+,23-15-Key: RAFGELQLHMBRHD-SLEZCNMEBU |
|
| SMILES O=C(O)\C=C\C(=C\C=C\C(=C\C=C\C=C(\C=C\C=C(/C=C/C(=O)OC)C)C)C)C | |
| Properties | |
| Chemical formula | C25H30O4 |
| Molar mass | 394.511 g·mol−1 |
| Appearance | Orange crystals |
| Melting point | 198 °C (cis-isomer) 217 °C (trans-isomer) |
| Solubility in water | Insoluble |
| Hazards | |
| NFPA 704 (fire diamond) |  1 1 0 1 1 0 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).  N verify (what is N verify (what is  Y Y N ?) Infobox references N ?) Infobox references |
Chemical compound
Bixin is an apocarotenoid found in the seeds of the achiote tree (Bixa orellana)[2] from which it derives its name. It is commonly extracted from the seeds to form annatto, a natural food coloring, containing about 5% pigments, of which 70–80% are bixin.[3]
Red seeds of the achiote tree
Bixin is one of the colorants used in the snack Cheetos.
Several thousand tons are harvested annually.[4]
Chemical properties
[edit]
Bixin is unstable. It isomerizes into _trans_-bixin (β-bixin), the double-bond isomer.[1]
Chemical structure of _trans_-bixin
Bixin is soluble in fats and alcohols but insoluble in water. Upon exposure to alkali, the methyl ester is hydrolyzed to produce the dicarboxylic acid norbixin, a water-soluble derivative.
Chemical structure of norbixin
- ^ a b Merck Index, 11th Edition, 1320
- ^ Bouvier, Florence; Dogbo, Odette; Camara, Bilal (2003). "Biosynthesis of the Food and Cosmetic Plant Pigment Bixin (Annatto)". Science. 300 (5628): 2089–2091. Bibcode:2003Sci...300.2089B. doi:10.1126/science.1085162. ISSN 0036-8075. JSTOR 3834418. PMID 12829782. S2CID 560600.
- ^ Executive Summary Bixin Archived July 21, 2011, at the Wayback Machine, National Toxicology Program
- ^ Stringheta, Paulo C.; Silva, Pollyanna I.; Costa, André G.V. (2018). "Annatto/Urucum— Bixa orellana". Exotic Fruits. pp. 23–30. doi:10.1016/B978-0-12-803138-4.00006-X. ISBN 9780128031384.



