Calcium iodate (original) (raw)
From Wikipedia, the free encyclopedia
Calcium iodate
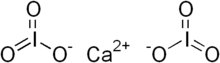 |
|
|---|---|
| Names | |
| IUPAC name Calcium diiodate | |
| Other namesLautarite | |
| Identifiers | |
| CAS Number | 7789-80-2 (anhydrous)  Y10031-33-1 (hexahydrate) Y10031-33-1 (hexahydrate)  N N |
| 3D model (JSmol) | Interactive image |
| ChemSpider | 23021  Y Y |
| ECHA InfoCard | 100.029.265 |
| EC Number | 232-191-3 |
| E number | E916 (glazing agents, ...) |
| PubChem CID | 24619 |
| UNII | L8MN4Y57BR (anhydrous)  Y Y |
| CompTox Dashboard (EPA) | DTXSID40960972 |
InChI InChI=1S/Ca.2HIO3/c;2*2-1(3)4/h;2*(H,2,3,4)/q+2;;/p-2  YKey: UHWJJLGTKIWIJO-UHFFFAOYSA-L YKey: UHWJJLGTKIWIJO-UHFFFAOYSA-L  YInChI=1/Ca.2HIO3/c;2*2-1(3)4/h;2*(H,2,3,4)/q+2;;/p-2Key: UHWJJLGTKIWIJO-NUQVWONBAT YInChI=1/Ca.2HIO3/c;2*2-1(3)4/h;2*(H,2,3,4)/q+2;;/p-2Key: UHWJJLGTKIWIJO-NUQVWONBAT |
|
| SMILES [Ca+2].[O-]I(=O)=O.[O-]I(=O)=O | |
| Properties | |
| Chemical formula | Ca(IO3)2 |
| Molar mass | 389.88 g/mol (anhydrous)407.90 g/mol (monohydrate) |
| Appearance | white solid |
| Density | 4.519 g/cm3 (monohydrate) |
| Melting point | 540 °C (1,004 °F; 813 K) (monohydrate) |
| Boiling point | decomposes |
| Solubility in water | 0.09 g/100 mL (0 °C) 0.24 g/100 mL (20 °C) 0.67 g/100 mL (90 °C) |
| Solubility product (_K_sp) | 6.47×10−6 |
| Solubility | soluble in nitric acid insoluble in alcohol |
| Magnetic susceptibility (χ) | -101.4·10−6 cm3/mol |
| Structure | |
| Crystal structure | monoclinic (anhydrous) cubic (monohydrate) orthorhombic (hexahydrate) |
| Hazards | |
| Flash point | non-flammable |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).  N verify (what is N verify (what is  Y Y N ?) Infobox references N ?) Infobox references |
Chemical compound
Calcium iodate is any of two inorganic compounds with the formula Ca(IO3)2(H2O)x, where x = 0 or 1. Both are colourless salts that occur as the minerals lautarite and bruggenite, respectively. A third mineral form of calcium iodate is dietzeite, a salt containing chromate with the formula Ca2(IO3)2CrO4.[1] These minerals are the most common compounds containing iodate.
Production and uses
[edit]
Lautarite, described as the most important mineral source of iodine, is mined in the Atacama Desert.[1] Processing of the ore entails reduction of its aqueous extracts with sodium bisulfite to give sodium iodide. This comproportionation reaction is a major source of the sodium iodide.[1]
Calcium iodate can be produced by the anodic oxidation of calcium iodide or by passing chlorine into a hot solution of lime in which iodine has been dissolved.
Calcium iodate is used as an iodine supplement in chicken feed.[1] Ethylenediamine dihydroiodide (EDDI) is a more typical source of nutritional iodine.
- ^ a b c d Lyday, Phyllis A.; Kaiho, Tatsuo (2015). "Iodine and Iodine Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. pp. 1–13. doi:10.1002/14356007.a14_381.pub2. ISBN 978-3527306732.