Droxicam (original) (raw)
From Wikipedia, the free encyclopedia
NSAID analgesic drug
Pharmaceutical compound
Droxicam
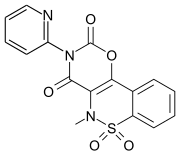 |
|
|---|---|
 |
|
| Clinical data | |
| ATC code | M01AC04 (WHO) |
| Identifiers | |
| IUPAC name 2_H_,5_H_-1,3-Oxazino(5,6-c)(1,2)benzothiazine-2,4(3_H_)-dione, 5-methyl-3-(2-pyridinyl)-, 6,6-dioxide | |
| CAS Number | 90101-16-9 |
| PubChem CID | 65679 |
| ChemSpider | 59108  Y Y |
| UNII | F24ADO1E2D |
| KEGG | D07267  Y Y |
| ChEBI | CHEBI:76133  Y Y |
| ChEMBL | ChEMBL1213420  Y Y |
| CompTox Dashboard (EPA) | DTXSID70238035 |
| Chemical and physical data | |
| Formula | C16H11N3O5S |
| Molar mass | 357.34 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| SMILES CN1c2c(oc(=O)n(c2=O)c3ccccn3)-c4ccccc4S1(=O)=O | |
InChI InChI=1S/C16H11N3O5S/c1-18-13-14(10-6-2-3-7-11(10)25(18,22)23)24-16(21)19(15(13)20)12-8-4-5-9-17-12/h2-9H,1H3  YKey:OEHFRZLKGRKFAS-UHFFFAOYSA-N YKey:OEHFRZLKGRKFAS-UHFFFAOYSA-N  Y Y |
Droxicam is a non-steroidal anti-inflammatory drug of the oxicam class. A prodrug of piroxicam, it is used for the relief of pain and inflammation in musculoskeletal disorders such as rheumatoid arthritis and osteoarthritis.[1]
When heated, phenyl pyridin-2-ylcarbamate (1) decomposes to 2-isocyanatopyridine (2) which reacts with the heterocyclic compound (3) to give droxicam.[2][3][4]
- ^ Jané F, Rodríguez de la Serna A (1991). "Droxicam: a pharmacological and clinical review of a new NSAID". European Journal of Rheumatology and Inflammation. 11 (4): 3–9. PMID 1365488.
- ^ US patent 4563452, Jose M. Ribalta-Baro and Jordi F. Rigola-Constansa, "Benzothiazine derivatives and their applications as medicinal products or as synthesis intermediates for medicinal products", issued 1992-07-21, assigned to Laboratorios del Dr Esteve SA
- ^ "Droxicam". Thieme. Retrieved 2024-07-04.
- ^ "Droxicam". chemdrug.com. Retrieved 2024-07-04.
