Ethynol (original) (raw)
From Wikipedia, the free encyclopedia
Ethynol
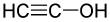 Structural formula of ethynol Structural formula of ethynol  Spacefill model of ethynol Spacefill model of ethynol |
|
|---|---|
| Names | |
| Systematic IUPAC name Ethynol[1] | |
| Other namesYnol, ethynyl alcohol, hydroxyacetylene | |
| Identifiers | |
| CAS Number | 32038-79-2  Y Y |
| 3D model (JSmol) | Interactive imageInteractive image |
| ChemSpider | 110037  N N |
| PubChem CID | 123441 |
| CompTox Dashboard (EPA) | DTXSID90185855 |
InChI InChI=1S/C2H2O/c1-2-3/h1,3H  NKey: QFXZANXYUCUTQH-UHFFFAOYSA-N NKey: QFXZANXYUCUTQH-UHFFFAOYSA-N  N N |
|
| SMILES OC#CC#CO | |
| Properties | |
| Chemical formula | C2H2O |
| Molar mass | 42.037 g·mol−1 |
| Density | 0.981 g·cm-3 |
| Boiling point | 77.1 °C (170.8 °F; 350.2 K) @ 760mmHg |
| Hazards | |
| Flash point | 14.7 °C (58.5 °F; 287.8 K) |
| Thermochemistry | |
| Std enthalpy of formation (Δf_H_⦵298) | 41.6 kJ mol−1 |
| Related compounds | |
| Related compounds | Propargyl alcohol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).  N verify (what is N verify (what is  Y Y N ?) Infobox references N ?) Infobox references |
Chemical compound
Ethynol (or hydroxyacetylene, ethynyl alcohol) is an alkyne–alcohol (ynol) with the formula C2H2O. It is the much-less-stable tautomer of ethenone.
At low temperature in a solid argon matrix it is possible to tautomerise ethenone to form ethynol.[2][3]
- Ethanol (ethyl alcohol)
- Ethenol (vinyl alcohol)
- Acetylenediol
- ^ "Ethynol". The PubChem Project. USA: National Center for Biotechnology Information.
- ^ Hochstrasser, Remo; Wirz, Jakob (1990). "Reversible Photoisomerisierung von Keten zu Ethinol". Angewandte Chemie. 102 (4): 454. Bibcode:1990AngCh.102..454H. doi:10.1002/ange.19901020438.
- ^ Hochstrasser, Remo; Wirz, Jakob (1989). "Ethinol: Photochemische Erzeugung in einer Argonmatrix, IR-Spektrum und Photoisomerisierung zu Keten". Angewandte Chemie. 101 (2): 183. Bibcode:1989AngCh.101..183H. doi:10.1002/ange.19891010209.