Injector pen (original) (raw)
From Wikipedia, the free encyclopedia
Drug storage and delivery device
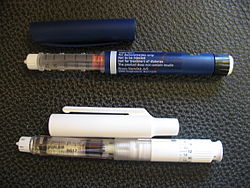
Two disposable injector pens. On the top pen there is a disclaimer that it contains no insulin and is not to be used for treatment of diabetes.
An injector pen (also called a medication pen) is a device used for injecting medication under the skin. First introduced in the 1980s, injector pens are designed to make injectable medication easier and more convenient to use, thus increasing patient adherence. The primary difference between injector pens and traditional vial and syringe administration is the easier use of an injector pen by people with low dexterity, poor vision, or who need portability to administer medicine on time. Injector pens also decrease the fear or adversity towards self-injection of medications, which increases the likelihood that a person takes the medication.
Injector pens are commonly used for medications that are injected repeatedly by a person over a relatively short period of time, especially insulin and insulin analogs used in the treatment of diabetes (called insulin pens). Many other medications are also available as injector pens, including other injectable medicines for diabetes, high cholesterol, migraine prevention, and other monoclonal antibodies. Studies have shown injector pens to be at least as effective as vial and syringe administration, and surveys have shown that a vast majority of people would prefer an injector pen over vial and syringe administration if one was available. After a slow uptake in the United States, injector pens have surpassed vial and syringe administration of insulin in type 2 diabetes.
The primary goal of injector pens is to increase patient adherence by making it easier and more convenient for people to use injectable therapy. This is especially problematic with injectable medications given the extra work associated with an injection, as well as the potential aversion to self-injecting medication.[1][2]
Injector pens increase patient adherence by increasing the ease of self-administering injectable medication, as well as the portability of injection medication.[2] Furthermore, injector pens are easier to handle and use than vials and syringes, making them useful in people with low dexterity, cognitive or visual impairment, or those who worry about being able to properly use a vial and syringe.[2] For medications which do not follow standard dosage in all people, injector pens may be designed to enable easier and more accurate administration of an exact dose, whereas a vial and syringe requires the person to prepare the correct dose themselves.[2] Injector pens may also remove stigma or fear around the use of injection medication in public environments, such as insulin before a meal at a restaurant.[2]
Combination injector pens which include multiple medications used to treat a disease are designed to reduce the number of injections a person must use to administer their medications.[3] The reduction in number of injections required may decrease the risk of non-adherence due to forgetfulness or unwillingness to self-inject medication.[3]
An insulin pen with labels as follows: A) tip; B) medication chamber; C) plunger; D) dose window; E) dose selection dial; F) plunger button
A reusable insulin pen assembled.
The same insulin pen disassembled, showing replaceable cartridge and reusable housing pieces.
An injector pen consists of a chamber or cartridge of medication, a tip to attach a needle, and a piston or plunger to inject the dose.[4] Some pens, including most insulin pens, include dials to adjust the dose of the injection before each administration.[2] Dials enable more accurate dose measuring than traditional vial and syringe administration, especially for low doses of insulin.[2] Injector pens which have dials to adjust dosages may also include a clicking sound or other method to confirm the dose adjustment.[2]
Some pens may include a cartridge filled with medication which can be replaced when empty to enable reuse of the pen itself, whereas other pens are designed to be disposed of after their prefilled chamber is depleted.[2] Injector pens designed for single use may also be autoinjectors, which do not require the user to press a plunger to inject the dose.[4]
Comparison of 4mm and 12.7mm pen needle sizes
All injector pens other than those designed for single use require the use of single-use replaceable pen needles for each injection. These pen needles come in various lengths to accommodate varying depths of subcutaneous tissue under the top of the skin.[5] Pen needles are designed for single use subcutaneous injection of medication and are not designed to be reused for more than one administration.[6] The needles are generally manufactured with an outer protective plastic shell, which is used by a person to attach the needle to the pen, and an inner plastic shell protecting the needle itself. Instruction on how to properly attach and use needles is the responsibility of the doctor or pharmacist to ensure proper use.[2][7]
Today, pen needles are manufactured at shorter needle lengths than required for typical vial and syringe administration, which decreases the pain associated with injection.[2] They are available in multiple lengths and gauge of needle, including 3.5mm, 4mm, 5mm, and 8mm lengths, and 31 through 34 gauge.[8] Over time, needles have also had bevels designed which decrease the force required to penetrate the skin, which decreases the pain associated with injection and may increase the acceptability of self-injection.[8] Furthermore, pen needles are designed for insertion at a 90-degree angle to the skin, as opposed to normal syringes which are designed to be injected at an angle. Pen needles generally do not require pinching of the skin for proper administration, unlike historically used syringes.[9] Pen needles should be disposed of properly after each use, preferably in a purpose-made sharps container, to prevent injury from accidental contact after use.[9]
Comparison to syringe
[edit]
A standard insulin syringe with needle is smaller than an injector pen.
Injector pens are an alternative to the manufacture of medication for injection in vials containing either liquid or a powder to which a diluent such as sterile water is added. When a vial is used as a means of storage, the end-user must use a syringe to "draw up" or remove the medication from the vial to prepare it for administration. The end user must then perform a series of actions to insert the needle of the syringe under the skin, and depress the plunger on the syringe to inject the dose. This requires dexterity which may make it difficult to accurately or completely administer the appropriate doses of medications.[2] Injector pens remove some of the complications of syringes by allowing the pen to be "pushed" against the skin at a 90-degree angle (removing the need to inject at a proper angle as is the case with syringes), as well as by replacing a long, thin plunger of a syringe with a simple button which is depressed and held to inject the dose.[2]
Many insulin analogs and GLP-1 agonists for diabetes treatment are available as injector pens.[2] As with insulin vials, some insulin pens are made with higher concentrations including U-200, U-300, and U-500. Different concentration insulin products may not have the same pharmacokinetic properties as other strengths.[10] The higher concentrations are used to lessen the volume of the injection, and allow the same dose of insulin to be injected with less force.[10] In some cases, these medications may be combined into one pen to be administered daily, for example insulin degludec with liraglutide[6] and insulin glargine with lixisenatide.[11] Combination products are available in fixed-dose ratios and are generally dosed by units of insulin, which will administer a proportional amount of the GLP-1 agonist as well.[12]
Another class of medication commonly available as an injector pen is monoclonal antibodies. Due to the molecular size of monoclonal antibodies, they must be administered via injection. Examples of monoclonal antibodies available or studied as injector pens include adalimumab,[13] secukinumab,[14] and alirocumab.[15] CGRP antagonists which are monoclonal antibodies, used for the prevention of migraines, are also available as injector pens.[16] Other monoclonal antibodies designed for home use may also be manufactured as injector pens.[17][18]
Some medications are formulated as injector pens to quicken the onset of action of the medication. This includes epinephrine, which when used to treat anaphylaxis must work as soon as possible.[19] Contrary to most other injector pens, epinephrine injector pens are designed to administer the medication via intramuscular injection.[19] Another medication formulated as an injector pen to ensure quick onset of action is glucagon for hypoglycemia.[20] Other medications normally administered orally are also available or have been studied as injector pens, either due to different pharmacokinetic properties when administered via injection, or for those who cannot take oral medications. This includes methotrexate for juvenile idiopathic arthritis[21] and sumatriptan for treatment of migraines.[22]
Most injector pens are designed for subcutaneous injection just under the skin, but some are designed for injection into muscle. The desired injection site and the skin profile at the injection site will determine what needle length is appropriate for a person to use.[23] For products with included needles, such as epinephrine pens, different brands may have different included needle lengths, which must be taken into account.[19]
Multiple studies have shown that many people prefer the use of injector pens over other forms of injectable medication, such as vial and syringe.[2] Injector pens in general have also been shown to be at least as effective therapeutically as other injection methods.[2] One study of the use of injector pens for insulin administration found that the chance a person initiated on insulin continued therapy for at least 12 months was higher with insulin pens than with vial and syringe administration.[2] The same study found that the increase in adherence to therapy resulted in increased short-term pharmacy costs (i.e. for the pens/needles) but resulted in an overall decrease in healthcare costs related to diabetes.[2] Insulin pens have also been shown to provide a higher quality of life than traditional injection methods.[2] A 2011 systematic review which examined preference of insulin pens over vial and syringe administration found that in almost all studies and surveys a majority of people preferred insulin pens.[24]
The effectiveness of an injector pen can also depend on the technique used to inject. After fully pressing the plunger button to activate the pen, the button must continue to be held for about 10 seconds to ensure the dose is administered before removing the pen needle from the skin and finally releasing the button.[23] Failure to use the pen as instructed may result in medication leakage and administration of a lower dose than was intended.[23] Another administration problem which may impact effectiveness of an injector pen is lipohypertrophy of the subcutaneous tissue near the injection site. For this reason, it is recommended to rotate the injection site every administration.[23]
The first injector pen was introduced in 1985, by Novo Nordisk to administer insulin products.[25] After their introduction, insulin pens had a slow adoption in the United States, with only 2% of insulin being injected via pen in 1999. A major barrier to adoption in the United States was the increased up-front cost of insulin pens compared to traditional injections.[26] Pen adoption in the United States accelerated after studies showed that the higher up-front cost of insulin pens was offset by the increase in compliance, which decreased overall healthcare costs.[27] Historically, pen needles were manufactured in lengths up to 12.7mm. Over time, pen needles designed for insulin pens have become shorter, and a 4mm long needle is considered sufficient for most people to administer subcutaneously correctly.[23]
In 1989, an injector pen form of human growth hormone was licensed in New Zealand.[28] In the US, a pen form of octreotide was approved by the FDA in 2020, under the brand name Bynfezia.[29]
- ^ Guerci, Bruno; Chanan, Neha; Kaur, Simarjeet; Jasso-Mosqueda, Juan Guillermo; Lew, Elisheva (April 2019). "Lack of Treatment Persistence and Treatment Nonadherence as Barriers to Glycaemic Control in Patients with Type 2 Diabetes". Diabetes Therapy. 10 (2): 437–449. doi:10.1007/s13300-019-0590-x. PMC 6437240. PMID 30850934.
- ^ a b c d e f g h i j k l m n o p q r s Cuddihy, Robert M.; Borgman, Sarah K. (2013). "Considerations for Diabetes: Treatment With Insulin Pen Devices". American Journal of Therapeutics. 20 (6): 694–702. doi:10.1097/MJT.0b013e318217a5e3. PMID 21768872. S2CID 34997991.
- ^ a b Nuffer, Wesley; Guesnier, Ashley; Trujillo, Jennifer M. (March 2018). "A review of the new GLP-1 receptor agonist/basal insulin fixed-ratio combination products". Therapeutic Advances in Endocrinology and Metabolism. 9 (3): 69–79. doi:10.1177/2042018817752315. PMC 5813858. PMID 29492243.
- ^ a b Misra, Ambikanandan, ed. (2010). "11". Challenges in delivery of therapeutic genomics and proteomics. Oxford: Elsevier. pp. 586–587. doi:10.1016/B978-0-12-384964-9.00011-6. ISBN 978-0-12-384964-9.
- ^ Leonardi, Luca; Viganò, Mara; Nicolucci, Antonio (August 2019). "Penetration force and cannula sliding profiles of different pen needles: the PICASSO study". Medical Devices: Evidence and Research. 12: 311–317. doi:10.2147/MDER.S218983. PMC 6717876. PMID 31695523.
- ^ a b Baker, Danial E. (May 2017). "Insulin Degludec/Liraglutide". Hospital Pharmacy. 52 (5): 374–380. doi:10.1177/0018578717715383. PMC 5551637. PMID 28804155.
- ^ Wei, Erin T.; Koh, Eileen; Kelly, Mary S.; Wright, Lorena A.; Tylee, Tracy S. (March 2020). "Patient errors in use of injectable antidiabetic medications: A need for improved clinic-based education". Journal of the American Pharmacists Association. 60 (5): e76–e80. doi:10.1016/j.japh.2020.02.030. PMID 32229089. S2CID 214749788.
- ^ a b Leonardi, Luca; Viganò, Mara; Nicolucci, Antonio (28 August 2019). "Penetration force and cannula sliding profiles of different pen needles: the PICASSO study". Medical Devices: Evidence and Research. 12: 311–317. doi:10.2147/MDER.S218983. PMC 6717876. PMID 31695523.
- ^ a b Bahendeka, Silver; Kaushik, Ramaiya; Swai, Andrew Babu; Otieno, Fredrick; Bajaj, Sarita; Kalra, Sanjay; Bavuma, Charlotte M.; Karigire, Claudine (April 2019). "EADSG Guidelines: Insulin Storage and Optimisation of Injection Technique in Diabetes Management". Diabetes Therapy. 10 (2): 341–366. doi:10.1007/s13300-019-0574-x. PMC 6437255. PMID 30815830.
- ^ a b Reid, Timothy S.; Schafer, Fryn; Brusko, Cynthia (4 July 2017). "Higher concentration insulins: an overview of clinical considerations". Postgraduate Medicine. 129 (5): 554–562. doi:10.1080/00325481.2017.1325311. PMID 28475455.
- ^ "Sanofi Receives FDA Approval of Soliqua 100/33, for the Treatment of Adults with Type 2 Diabetes". Sanofi. November 21, 2016. Archived from the original on July 18, 2017. Retrieved August 20, 2020.
- ^ Aroda, Vanita R; González-Galvez, Guillermo; Grøn, Randi; Halladin, Natalie; Haluzík, Martin; Jermendy, György; Kok, Adri; Őrsy, Petra; Sabbah, Mohamed; Sesti, Giorgio; Silver, Robert (August 2019). "Durability of insulin degludec plus liraglutide versus insulin glargine U100 as initial injectable therapy in type 2 diabetes (DUAL VIII): a multicentre, open-label, phase 3b, randomised controlled trial". The Lancet Diabetes & Endocrinology. 7 (8): 596–605. doi:10.1016/S2213-8587(19)30184-6. PMID 31189519. S2CID 189815494.
- ^ Little, Robert D; Chu, Isabel E; van der Zanden, Esmerij P; Flanagan, Emma; Bell, Sally J; Gibson, Peter R; Sparrow, Miles P; Shelton, Edward; Connor, Susan J; Roblin, Xavier; Ward, Mark G (10 December 2019). "Comparison of Adalimumab Serum Drug Levels When Delivered by Pen Versus Syringe in Patients With Inflammatory Bowel Disease. An International, Multicentre Cohort Analysis". Journal of Crohn's and Colitis. 13 (12): 1527–1536. doi:10.1093/ecco-jcc/jjz103. PMID 31094417.
- ^ Paul, C.; Lacour, J.-P.; Tedremets, L.; Kreutzer, K.; Jazayeri, S.; Adams, S.; Guindon, C.; You, R.; Papavassilis, C. (June 2015). "Efficacy, safety and usability of secukinumab administration by autoinjector/pen in psoriasis: a randomized, controlled trial (JUNCTURE)". Journal of the European Academy of Dermatology and Venereology. 29 (6): 1082–1090. doi:10.1111/jdv.12751. PMID 25243910. S2CID 25330244.
- ^ Farnier, Michel; Jones, Peter; Severance, Randall; Averna, Maurizio; Steinhagen-Thiessen, Elisabeth; Colhoun, Helen M.; Du, Yunling; Hanotin, Corinne; Donahue, Stephen (January 2016). "Efficacy and safety of adding alirocumab to rosuvastatin versus adding ezetimibe or doubling the rosuvastatin dose in high cardiovascular-risk patients: The ODYSSEY OPTIONS II randomized trial". Atherosclerosis. 244: 138–146. doi:10.1016/j.atherosclerosis.2015.11.010. hdl:10447/183606. PMID 26638010.
- ^ Dodick, David W.; Silberstein, Stephen D.; Bigal, Marcelo E.; Yeung, Paul P.; Goadsby, Peter J.; Blankenbiller, Tricia; Grozinski-Wolff, Melissa; Yang, Ronghua; Ma, Yuju; Aycardi, Ernesto (15 May 2018). "Effect of Fremanezumab Compared With Placebo for Prevention of Episodic Migraine: A Randomized Clinical Trial". JAMA. 319 (19): 1999–2008. doi:10.1001/jama.2018.4853. PMC 6583237. PMID 29800211.
- ^ Inman, Taylor R.; Plyushko, Erika; Austin, Nicholas P.; Johnson, Jeremy L. (May 2018). "The role of basal insulin and GLP-1 receptor agonist combination products in the management of type 2 diabetes". Therapeutic Advances in Endocrinology and Metabolism. 9 (5): 151–155. doi:10.1177/2042018818763698. PMC 5958427. PMID 29796245.
- ^ van den Bemt, Bart J. F.; Gettings, Lynda; Domańska, Barbara; Bruggraber, Richard; Mountain, Irina; Kristensen, Lars E. (1 January 2019). "A portfolio of biologic self-injection devices in rheumatology: how patient involvement in device design can improve treatment experience". Drug Delivery. 26 (1): 384–392. doi:10.1080/10717544.2019.1587043. PMC 6442222. PMID 30905213.
- ^ a b c Dreborg, Sten; Tsai, Gina; Kim, Harold (July 2019). "Implications of variation of epinephrine auto-injector needle length". Annals of Allergy, Asthma & Immunology. 123 (1): 89–94. doi:10.1016/j.anai.2019.04.027. PMID 31071440. S2CID 149445920.
- ^ "Drugs@FDA". www.accessdata.fda.gov. FDA. Retrieved 20 August 2020.
- ^ Roszkiewicz, Justyna; Swacha, Zbigniew; Smolewska, Elżbieta (December 2020). "Prefilled pen versus prefilled syringe: a pilot study evaluating two different methods of methotrexate subcutaneous injection in patients with JIA". Pediatric Rheumatology. 18 (1): 64. doi:10.1186/s12969-020-00455-4. PMC 7425569. PMID 32787934.
- ^ Andre, Anthony D.; Brand-Schieber, Elimor; Ramirez, Margarita; Munjal, Sagar; Kumar, Rajesh (19 January 2017). "Subcutaneous sumatriptan delivery devices: comparative ease of use and preference among migraineurs". Patient Preference and Adherence. 11: 121–129. doi:10.2147/PPA.S125137. PMC 5261843. PMID 28176899.
- ^ a b c d e Frid, Anders H.; Kreugel, Gillian; Grassi, Giorgio; Halimi, Serge; Hicks, Debbie; Hirsch, Laurence J.; Smith, Mike J.; Wellhoener, Regine; Bode, Bruce W.; Hirsch, Irl B.; Kalra, Sanjay; Ji, Linong; Strauss, Kenneth W. (September 2016). "New Insulin Delivery Recommendations". Mayo Clinic Proceedings. 91 (9): 1231–1255. doi:10.1016/j.mayocp.2016.06.010. PMID 27594187.
- ^ Anderson, Barbara J.; Redondo, Maria J. (November 2011). "What Can We Learn from Patient-Reported Outcomes of Insulin Pen Devices?". Journal of Diabetes Science and Technology. 5 (6): 1563–1571. doi:10.1177/193229681100500633. PMC 3262728. PMID 22226279.
- ^ Rex, Jørn; Jensen, Klaus H; Lawton, Simon A (2006). "A Review of 20??Years??? Experience with the Novopen?? Family of Insulin Injection Devices". Clinical Drug Investigation. 26 (7): 367–401. doi:10.2165/00044011-200626070-00001. PMID 17163272. S2CID 20771626.
- ^ Bohannon, Nancy J. V. (January 1999). "Insulin delivery using pen devices: Simple-to-use tools may help young and old alike". Postgraduate Medicine. 106 (5): 57–68. doi:10.3810/pgm.1999.10.15.751. PMID 10560468.
- ^ Alemayehu, B; Speiser, J; Bloudek, L; Sarnes, E (July 2018). "Costs associated with long-acting insulin analogues in patients with diabetes". The American Journal of Managed Care. 24 (8 Spec No): SP265–SP272. PMID 30020738.
- ^ Gluckman, P D; Cutfield, W S (1 June 1991). "Evaluation of a pen injector system for growth hormone treatment". Archives of Disease in Childhood. 66 (6): 686–688. doi:10.1136/adc.66.6.686. PMC 1793174. PMID 2053787.
- ^ "Drugs@FDA". www.accessdata.fda.gov. Food and Drug Administration. Retrieved 20 August 2020.
- "Insulin pen packaging and dispensing". U.S. Food and Drug Administration (FDA). 13 October 2020.