Malvidin (original) (raw)
From Wikipedia, the free encyclopedia
Malvidin
 |
|
|---|---|
| Names | |
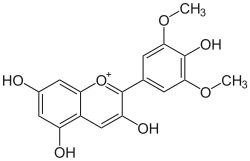
| IUPAC name 3,4′,5,7-Tetrahydroxy-3′,5′-dimethoxyflavylium | |
| Systematic IUPAC name 3,5,7-Trihydroxy-2-(4-hydroxy-3,5-dimethoxy)-1λ4-benzopyran-1-ylium | |
| Identifiers | |
| CAS Number | 643-84-5  Y Y |
| 3D model (JSmol) | Interactive image |
| ChEBI | CHEBI:6674  N N |
| ChEMBL | ChEMBL255753  YChEMBL592005 YChEMBL592005  Y Y |
| ChemSpider | 140095  Y Y |
| KEGG | C08716  Y Y |
| PubChem CID | 159287 |
| UNII | GL5KGZ4D8U  Y Y |
| CompTox Dashboard (EPA) | DTXSID30146622 |
InChI InChI=1S/C17H14O7/c1-22-14-3-8(4-15(23-2)16(14)21)17-12(20)7-10-11(19)5-9(18)6-13(10)24-17/h3-7H,1-2H3,(H3-,18,19,20,21)/p+1  YKey: KZMACGJDUUWFCH-UHFFFAOYSA-O YKey: KZMACGJDUUWFCH-UHFFFAOYSA-O  YInChI=1/C17H14O7/c1-22-14-3-8(4-15(23-2)16(14)21)17-12(20)7-10-11(19)5-9(18)6-13(10)24-17/h3-7H,1-2H3,(H3-,18,19,20,21)/p+1Key: KZMACGJDUUWFCH-IKLDFBCSAG YInChI=1/C17H14O7/c1-22-14-3-8(4-15(23-2)16(14)21)17-12(20)7-10-11(19)5-9(18)6-13(10)24-17/h3-7H,1-2H3,(H3-,18,19,20,21)/p+1Key: KZMACGJDUUWFCH-IKLDFBCSAG |
|
| SMILES Oc1cc2c(O)cc(O)cc2[o+]c1c3cc(OC)c(O)c(OC)c3 | |
| Properties | |
| Chemical formula | C17H15O7+ |
| Molar mass | 331.2968 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).  N verify (what is N verify (what is  Y Y N ?) Infobox references N ?) Infobox references |
Chemical compound
Malvidin is an O-methylated anthocyanidin, the 3',5'-methoxy derivative of delphinidin. As a primary plant pigment, its glycosides are highly abundant in nature.
Natural occurrences
[edit]
Malvidin is responsible for the blue color found in petals of the Primula plants of the polyanthus group. Blue flowers of the blue pimpernel (Anagallis monelli) have also a higher concentration of malvidin.
It is responsible primarily for the color of red wine, Vitis vinifera being one of its sources.[1] It is also present in other berries, such as blueberries (Vaccinium corymbosum) or the saskatoon berries (Amelanchier alnifolia).[2][3]
Slightly acidic and neutral solutions of malvidin are characteristically of a red color, while basic solutions of malvidin yield a blue color.
The breakdown of malvidin releases syringic acid.
Use as a marker in archaeology
[edit]
The breakdown of malvidin releases syringic acid as revealed in the examination of jars containing shedeh, a drink of Ancient Egypt. Malvidin is also present in the site of the Areni-1 winery, a 6,100-year-old winery discovered in 2007 in the Areni-1 cave complex in the village of Areni in the Vayots Dzor province of Armenia.
- Malvin is a malvidin diglucoside.
- Oenin is the malvidin-3-glucoside.
- Primulin is the 3-O-galactoside of malvidin.
- Malvidin 3-rutinoside is a pigment responsible for bract color in Curcuma alismatifolia (the Siam tulip).[4] Acylated malvidin 3-rutinosides are responsible for the violet color of Petunia integrifolia subsp. inflata.[5]
- Malvidin-3-O-glucoside-5-O-(6-acetylglucoside) is a pigment responsible for the blue color in 'Johnson's Blue' and other 'blue' geraniums[6]
- List of phytochemicals in food
- List of antioxidants in food
- ^ "Phytochemicals: Malvidin". Top Cultures. Archived from the original on April 1, 2010. Retrieved 2009-05-20.
- ^ Mazza G (2005). "Compositional and functional properties of saskatoon berry and blueberry". International Journal of Fruit Science. 5 (3): 99–118. doi:10.1300/J492v05n03_10.
- ^ Bakowska-barczak; Marianchuk, M; Kolodziejczyk, P (2007). "Survey of bioactive components in Western Canadian berries". Canadian Journal of Physiology and Pharmacology. 85 (11): 1139–52. doi:10.1139/y07-102. PMID 18066116.
- ^ Nakayama, M; Roh, MS; Uchida, K; Yamaguchi, Y; Takano, K; Koshioka, M (2000). "Malvidin 3-rutinoside as the pigment responsible for bract color in Curcuma alismatifolia". Bioscience, Biotechnology, and Biochemistry. 64 (5): 1093–5. doi:10.1271/bbb.64.1093. PMID 10879491.
- ^ Tatsuzawa, F (1999). "Acylated malvidin 3-rutinosides in dusky violet flowers of Petunia integrifolia subsp. Inflata". Phytochemistry. 52 (2): 351–355. Bibcode:1999PChem..52..351T. doi:10.1016/S0031-9422(99)00095-3.
- ^ Markham, Kenneth R.; Mitchell, Kevin A.; Boase, Murray R. (1997). "Malvidin-3-O-glucoside-5-O-(6-acetylglucoside) and its colour manifestation in 'Johnson's Blue' and other 'Blue' geraniums". Phytochemistry. 45 (2): 417–423. Bibcode:1997PChem..45..417M. doi:10.1016/S0031-9422(96)00831-X.