Pourbaix diagram (original) (raw)
Plot of thermodynamically stable phases of an aqueous electrochemical system
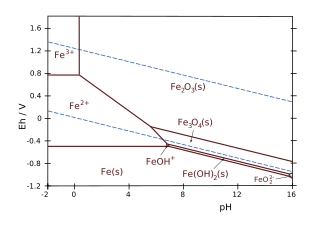
Pourbaix diagram of iron.[1] The Y axis corresponds to voltage potential.
In electrochemistry, and more generally in solution chemistry, a Pourbaix diagram, also known as a potential/pH diagram, EH–pH diagram or a pE/pH diagram, is a plot of possible thermodynamically stable phases (i.e., at chemical equilibrium) of an aqueous electrochemical system. Boundaries (50 %/50 %) between the predominant chemical species (aqueous ions in solution, or solid phases) are represented by lines. As such a Pourbaix diagram can be read much like a standard phase diagram with a different set of axes. Similarly to phase diagrams, they do not allow for reaction rate or kinetic effects. Beside potential and pH, the equilibrium concentrations are also dependent upon, e.g., temperature, pressure, and concentration. Pourbaix diagrams are commonly given at room temperature, atmospheric pressure, and molar concentrations of 10−6 and changing any of these parameters will yield a different diagram.
The diagrams are named after Marcel Pourbaix (1904–1998), the Russian-born Belgian chemist who invented them.
The Pourbaix diagram for uranium in a non-complexing aqueous medium (e.g. perchloric acid / sodium hydroxide)[2]
Pourbaix diagrams are also known as _E_H-pH diagrams due to the labeling of the two axes.
The vertical axis is labeled _E_H for the voltage potential with respect to the standard hydrogen electrode (SHE) as calculated by the Nernst equation. The "H" stands for hydrogen, although other standards may be used, and they are for room temperature only.
For a reversible redox reaction described by the following chemical equilibrium:
a A + b B ⇌ c C + d D
With the corresponding equilibrium constant K:
K = [ C ] c [ D ] d [ A ] a [ B ] b , {\displaystyle K={\frac {[C]^{c}[D]^{d}}{[A]^{a}[B]^{b}}},}
The Nernst equation is:
E H = E 0 − R T z F ln K , {\displaystyle E_{\text{H}}=E^{0}-{\frac {RT}{zF}}\ln {K},}
E H = E 0 − R T z F ln [ C ] c [ D ] d [ A ] a [ B ] b , {\displaystyle E_{\text{H}}=E^{0}-{\frac {RT}{zF}}\ln {\frac {[C]^{c}[D]^{d}}{[A]^{a}[B]^{b}}},}
sometimes formulated as:
E H = E 0 − V T λ z log [ C ] c [ D ] d [ A ] a [ B ] b , {\displaystyle E_{\text{H}}=E^{0}-{\frac {V_{T}\lambda }{z}}\log {\frac {[C]^{c}[D]^{d}}{[A]^{a}[B]^{b}}},}
or, more simply directly expressed numerically as:
E H = E 0 − 0.05916 z log [ C ] c [ D ] d [ A ] a [ B ] b , {\displaystyle E_{\text{H}}=E^{0}-{\frac {0.05916}{z}}\log {\frac {[C]^{c}[D]^{d}}{[A]^{a}[B]^{b}}},}
where:
- V T = R T / F ≈ 0.02569 {\displaystyle V_{T}=RT/F\approx 0.02569}
volt is the thermal voltage or the "Nernst slope" at standard temperature
- λ = ln(10) ≈ 2.30, so that V T λ ≈ 0.05916 {\displaystyle V_{T}\lambda \approx 0.05916}
volt.
The horizontal axis is labeled pH for the −log function of the H+ ion activity.
pH = − log 10 ( a H + ) = log 10 ( 1 a H + ) . {\displaystyle {\text{pH}}=-\log _{10}(a_{{\ce {H+}}})=\log _{10}\left({\frac {1}{a_{{\ce {H+}}}}}\right).}
The lines in the Pourbaix diagram show the equilibrium conditions, that is, where the activities are equal, for the species on each side of that line. On either side of the line, one form of the species will instead be said to be predominant.[3]
In order to draw the position of the lines with the Nernst equation, the activity of the chemical species at equilibrium must be defined. Usually, the activity of a species is approximated as equal to the concentration (for soluble species) or partial pressure (for gases). The same values should be used for all species present in the system.[3]
For soluble species, the lines are often drawn for concentrations of 1 M or 10−6 M. Sometimes additional lines are drawn for other concentrations.
If the diagram involves the equilibrium between a dissolved species and a gas, the pressure is usually set to _P_0 = 1 atm = 101325 Pa, the minimum pressure required for gas evolution from an aqueous solution at standard conditions.[3]
In addition, changes in temperature and concentration of solvated ions in solution will shift the equilibrium lines in accordance with the Nernst equation.
The diagrams also do not take kinetic effects into account, meaning that species shown as unstable might not react to any significant degree in practice.
A simplified Pourbaix diagram indicates regions of "immunity", "corrosion" and "passivity", instead of the stable species. They thus give a guide to the stability of a particular metal in a specific environment. Immunity means that the metal is not attacked, while corrosion shows that general attack will occur. Passivation occurs when the metal forms a stable coating of an oxide or other salt on its surface, the best example being the relative stability of aluminium because of the alumina layer formed on its surface when exposed to air.
Applicable chemical systems
[edit]
The Pourbaix diagram for uranium in carbonate solution. The dashed green lines show the stability limits of water in the system.[2]
While such diagrams can be drawn for any chemical system, it is important to note that the addition of a metal binding agent (ligand) will often modify the diagram. For instance, carbonate (CO2−3) has a great effect upon the diagram for uranium. (See diagrams at right). The presence of trace amounts of certain species such as chloride ions can also greatly affect the stability of certain species by destroying passivating layers.
Even though Pourbaix diagrams are useful for a metal corrosion potential estimation they have, however, some important limitations:[4]: 111
- Equilibrium is always assumed, though in practice it may differ.
- The diagram does not provide information on actual corrosion rates.
- Does not apply to alloys.[a]
- Does not indicate whether passivation (in the form of oxides or hydroxides) is protective or not. Diffusion of oxygen ions through thin oxide layers are possible.
- Excludes corrosion by chloride ions (Cl−, Cl3+ etc.).[b]
- Usually applicable only to temperature of 25 °C (77 °F), which is assumed by default. The Pourbaix diagrams for higher temperatures exist.
Expression of the Nernst equation as a function of pH
[edit]
The E h {\displaystyle E_{h}} 



a A + b B + h H + + z e − ↽ − − ⇀ c C + d D {\displaystyle a\,A+b\,B+h\,{\ce {H+}}+z\,e^{-}\quad {\ce {<=>}}\quad c\,C+d\,D}
The equilibrium constant K of this reduction reaction is:
K = { C } c { D } d { A } a { B } b { H + } h = ( γ c ) c [ C ] c ( γ d ) d [ D ] d ( γ a ) a [ A ] a ( γ b ) b [ B ] b ( γ h + ) h [ H + ] h = ( γ c ) c ( γ d ) d ( γ a ) a ( γ b ) b ( γ h ) h × [ C ] c [ D ] d [ A ] a [ B ] b [ H + ] h {\displaystyle K={\frac {\{C\}^{c}\{D\}^{d}}{\{A\}^{a}\{B\}^{b}\{{\ce {H+}}\}^{h}}}={\frac {(\gamma _{c})^{c}\left[C\right]^{c}\ (\gamma _{d})^{d}\left[D\right]^{d}}{(\gamma _{a})^{a}\left[A\right]^{a}\ (\gamma _{b})^{b}\left[B\right]^{b}\ (\gamma _{h+})^{h}\left[{\ce {H+}}\right]^{h}}}={\frac {(\gamma _{c})^{c}(\gamma _{d})^{d}}{(\gamma _{a})^{a}(\gamma _{b})^{b}(\gamma _{h})^{h}}}{\text{×}}{\frac {\left[C\right]^{c}\left[D\right]^{d}}{\left[A\right]^{a}\left[B\right]^{b}\left[{\ce {H+}}\right]^{h}}}}
where curly braces { } indicate activities (a), rectangle braces [ ] denote molar or molal concentrations (C), γ {\displaystyle \gamma } 
Activities correspond to thermodynamic concentrations and take into account the electrostatic interactions between ions present in solution. When the concentrations are not too high, the activity ( a i {\displaystyle a_{i}} 


a i = γ i C i {\displaystyle a_{i}=\gamma _{i}\,C_{i}}
The half-cell standard reduction potential E red ⊖ {\displaystyle E_{\text{red}}^{\ominus }} 
E red ⊖ ( volt ) = − Δ G ⊖ z F {\displaystyle E_{\text{red}}^{\ominus }({\text{volt}})=-{\frac {\Delta G^{\ominus }}{zF}}}
where Δ G ⊖ {\displaystyle \Delta G^{\ominus }} 

E h = E red = E red ⊖ − R T z F log ( { C } c { D } d { A } a { B } b ) − R T h z F pH {\displaystyle E_{h}=E_{\text{red}}=E_{\text{red}}^{\ominus }-{\frac {RT}{zF}}\log \left({\frac {\{C\}^{c}\{D\}^{d}}{\{A\}^{a}\{B\}^{b}}}\right)-{\frac {RT\,h}{zF}}{\text{pH}}}
In the following, the Nernst slope (or thermal voltage) V T = R T / F {\displaystyle V_{T}=RT/F} 
E h = E red = E red ⊖ − 0.05916 z log ( { C } c { D } d { A } a { B } b ) − 0.05916 h z pH {\displaystyle E_{h}=E_{\text{red}}=E_{\text{red}}^{\ominus }-{\frac {0.05916}{z}}\log \left({\frac {\{C\}^{c}\{D\}^{d}}{\{A\}^{a}\{B\}^{b}}}\right)-{\frac {0.05916\,h}{z}}{\text{pH}}}
This equation is the equation of a straight line for E red {\displaystyle E_{\text{red}}} 

This equation predicts lower E red {\displaystyle E_{\text{red}}} 



Calculation of a Pourbaix diagram
[edit]
When the activities ( a i {\displaystyle a_{i}} 



E h = E red = E red ⊖ − 0.05916 z log ( [ C ] c [ D ] d [ A ] a [ B ] b ) − 0.05916 h z pH {\displaystyle E_{h}=E_{\text{red}}=E_{\text{red}}^{\ominus }-{\frac {0.05916}{z}}\log \left({\frac {\left[C\right]^{c}\left[D\right]^{d}}{\left[A\right]^{a}\left[B\right]^{b}}}\right)-{\frac {0.05916\,h}{z}}{\text{pH}}}
There are three types of line boundaries in a Pourbaix diagram: Vertical, horizontal, and sloped.[5][6]
Vertical boundary line
[edit]
When no electrons are exchanged (z = 0), the equilibrium between A, B, C, and D only depends on [H+] and is not affected by the electrode potential. In this case, the reaction is a classical acid-base reaction involving only protonation/deprotonation of dissolved species. The boundary line will be a vertical line at a particular value of pH. The reaction equation may be written:
a A + b B + h H + ↽ − − ⇀ c C + d D {\displaystyle a\,A+b\,B+h\,{\ce {H+}}\quad {\ce {<=>}}\quad c\,C+d\,D}
and the energy balance is written as Δ G ∘ = − R T ln K {\displaystyle \Delta G^{\circ }=-RT\ln K} 
K = [ C ] c [ D ] d [ A ] a [ B ] b [ H + ] h {\displaystyle K={\frac {\left[C\right]^{c}\left[D\right]^{d}}{\left[A\right]^{a}\left[B\right]^{b}\left[{\ce {H+}}\right]^{h}}}}
Thus:
Δ G ∘ = − R T ln ( [ C ] c [ D ] d [ A ] a [ B ] b [ H + ] h ) {\displaystyle \Delta G^{\circ }=-RT\ln \left({\frac {\left[C\right]^{c}\left[D\right]^{d}}{\left[A\right]^{a}\left[B\right]^{b}\left[{\ce {H+}}\right]^{h}}}\right)}
or, in base-10 logarithms,
Δ G ∘ = − R T λ ( log ( [ C ] c [ D ] d [ A ] a [ B ] b ) + h pH ) {\displaystyle \Delta G^{\circ }=-RT\lambda \,\left(\log \left({\frac {\left[C\right]^{c}\left[D\right]^{d}}{\left[A\right]^{a}\left[B\right]^{b}}}\right)+h\,{\ce {pH}}\right)}
which may be solved for the particular value of pH.
For example[5] consider the iron and water system, and the equilibrium line between the ferric ion Fe3+ ion and hematite Fe2O3. The reaction equation is:
2 Fe 3 + ( aq ) + 3 H 2 O ( l ) ↽ − − ⇀ Fe 2 O 3 ( s ) + 6 H + ( aq ) {\displaystyle {\ce {2 Fe^{3+}(aq) + 3 H_2 O (l) <=> Fe_2 O_3 (s) + 6 H^+ (aq)}}}
which has Δ G ∘ = − 8242.5 J / m o l {\displaystyle \Delta G^{\circ }=-8242.5\,\mathrm {J/mol} } 
pH = − 1 6 ( Δ G ∘ R T λ + log ( [ Fe 2 O 3 ] [ Fe 3 + ] 2 [ H 2 O ] 3 ) ) {\displaystyle {\ce {pH}}=-{\frac {1}{6}}\left({\frac {\Delta G^{\circ }}{RT\lambda }}+\log \left({\frac {{\ce {[Fe2O3]}}}{{\ce {[Fe^{3+}]^2[H2O]^3}}}}\right)\right)}
Because the activities (or the concentrations) of the solid phases and water are equal to unity:
[Fe2O3] = [H2O] = 1, the pH only depends on the concentration in dissolved Fe3+
:
pH = − 1 6 ( Δ G ∘ R T λ + log ( 1 [ Fe 3 + ] 2 ) ) {\displaystyle {\ce {pH}}=-{\frac {1}{6}}\left({\frac {\Delta G^{\circ }}{RT\lambda }}+\log \left({\frac {1}{[{\ce {Fe^{3+}}}]^{2}}}\right)\right)}
At STP, for [Fe3+] = 10−6, this yields pH = 1.76.
Horizontal boundary line
[edit]
When H+ and OH− ions are not involved in the reaction, the boundary line is horizontal and independent of pH.
The reaction equation is thus written:
a A + b B + z e − ↽ − − ⇀ c C + d D ( z > 0 , but without H + ) {\displaystyle a\,A+b\,B+z\,e^{-}\quad {\ce {<=>}}\quad c\,C+d\,D\qquad (z>0{\text{, but without}}\ {\ce {H+}})}
As, the standard Gibbs free energy Δ G ∘ = − R T ln K {\displaystyle \Delta G^{\circ }=-RT\ln K} 
Δ G ∘ = − R T ln ( [ C ] c [ D ] d [ A ] a [ B ] b ) {\displaystyle \Delta G^{\circ }=-RT\ln \left({\frac {\left[C\right]^{c}\left[D\right]^{d}}{\left[A\right]^{a}\left[B\right]^{b}}}\right)}
Using the definition of the electrode potential ∆G = -zFE, where F is the Faraday constant, this may be rewritten as a Nernst equation:
E h = E ∘ − V T z ln ( [ C ] c [ D ] d [ A ] a [ B ] b ) {\displaystyle E_{h}={E^{\circ }}-{\frac {V_{T}}{z}}\ln \left({\frac {\left[C\right]^{c}\left[D\right]^{d}}{\left[A\right]^{a}\left[B\right]^{b}}}\right)}
or, using base-10 logarithms:
E h = E ∘ − V T λ z log ( [ C ] c [ D ] d [ A ] a [ B ] b ) {\displaystyle E_{h}={E^{\circ }}-{\frac {V_{T}\lambda }{z}}\log \left({\frac {\left[C\right]^{c}\left[D\right]^{d}}{\left[A\right]^{a}\left[B\right]^{b}}}\right)}
For the equilibrium Fe2+
/Fe3+
, taken as example here, considering the boundary line between Fe2+ and Fe3+, the half-reaction equation is:
Fe 3 + ( aq ) + e − ↽ − − ⇀ Fe 2 + ( aq ) {\displaystyle {\ce {Fe^3+ (aq) + e^- <=> Fe^2+ (aq)}}}
Since H+ ions are not involved in this redox reaction, it is independent of pH. Eo = 0.771 V with only one electron involved in the redox reaction.[7]The potential Eh is a function of temperature via the thermal voltage V T {\displaystyle V_{T}} 
and Fe3+
ions:
E h = E ∘ − V T λ log ( [ Fe 2 + ] [ Fe 3 + ] ) {\displaystyle E_{h}={E^{\circ }}-V_{T}\lambda \log \left({\frac {{\ce {[Fe^{2+}]}}}{{\ce {[Fe^{3+}]}}}}\right)}
For both ionic species at the same concentration (e.g., 10 − 6 M {\displaystyle 10^{-6}\mathrm {M} } 

Sloped boundary line
[edit]
In this case, both electrons and H+ ions are involved and the electrode potential is a function of pH. The reaction equation may be written:
a A + b B + h H + + z e − ↽ − − ⇀ c C + d D {\displaystyle a\,A+b\,B+h\,{\ce {H+}}+z\,e^{-}\quad {\ce {<=>}}\quad c\,C+d\,D}
Using the expressions for the free energy in terms of potentials, the energy balance is given by a Nernst equation:
E h = E ∘ − V T λ z ( log ( [ C ] c [ D ] d [ A ] a [ B ] b ) + h pH ) {\displaystyle E_{h}={E^{\circ }}-{\frac {V_{T}\lambda }{z}}\left(\log \left({\frac {\left[C\right]^{c}\left[D\right]^{d}}{\left[A\right]^{a}\left[B\right]^{b}}}\right)+h\,{\ce {pH}}\right)}
For the iron and water example, considering the boundary line between the ferrous ion Fe2+ and hematite Fe2O3, the reaction equation is:
Fe 2 O 3 ( s ) + 6 H + ( aq ) + 2 e − ↽ − − ⇀ 2 Fe 2 + ( aq ) + 3 H 2 O ( l ) {\displaystyle {\ce {Fe2O3(s) + 6 H+(aq) + 2 e^- <=> 2 Fe^{2+}(aq) + 3 H2O(l)}}}
with E ∘ = 0.728 V {\displaystyle E^{\circ }=0.728\mathrm {V} } 
The equation of the boundary line, expressed in base-10 logarithms is:
E h = E ∘ − V T λ 2 ( log ( [ Fe 2 + ] 2 [ H 2 O ] 3 [ Fe 2 O 3 ] ) + 6 pH ) {\displaystyle E_{h}={E^{\circ }}-{\frac {V_{T}\lambda }{2}}\left(\log \left({\frac {{\ce {[Fe^{2+}]^2[H2O]^3}}}{{\ce {[Fe2O3]}}}}\right)+6\ {\ce {pH}}\right)}
As, the activities, or the concentrations, of the solid phases and water are always taken equal to unity by convention in the definition of the equilibrium constant K: [Fe2O3] = [H2O] = 1.
The Nernst equation thus limited to the dissolved species Fe2+
and H+ is written as:
E h = E ∘ − V T λ 2 ( log [ Fe 2 + ] 2 + 6 pH ) {\displaystyle E_{h}={E^{\circ }}-{\frac {V_{T}\lambda }{2}}\left(\log \ {\ce {[Fe^{2+}]^2}}+6\ {\ce {pH}}\right)}
For, [Fe2+] = 10−6 M, this yields:
E h = 1.0826 − 0.1775 p H ( in volts ) {\displaystyle E_{h}={1.0826}-{0.1775}\ {pH}\quad ({\text{in volts}})}
Note the negative slope (-0.1775) of this line in a Eh–pH diagram.
The stability region of water
[edit]
Pourbaix diagram for water, including stability regions for water, oxygen and hydrogen at standard temperature and pressure (STP). The vertical scale (ordinate) is the electrode potential (of a hydrogen or non-interacting electrode) relative to a SHE electrode, the horizontal scale (abscissa) is the pH of the electrolyte (otherwise non-interacting). Assuming no overpotential, above the top line the out of equilibrium condition involves oxygen gas production, and oxygen will bubble off of the electrode until equilibrium is reached, or water totally consumed. Likewise, below the bottom line, the out of equilibrium condition involves hydrogen gas production, and hydrogen will bubble off of the electrode until equilibrium is reached, or water totally consumed.
In many cases, the possible conditions in a system are limited by the stability region of water. In the Pourbaix diagram for uranium presented here above, the limits of stability of water are marked by the two dashed green lines, and the stability region for water falls between these two lines. It is also depicted here beside by the two dashed red lines in the simplified Pourbaix diagram restricted to the water stability region only.
Under highly reducing conditions (low _E_H), water is reduced to hydrogen according to:[3]
2 H + + 2 e − ⟶ H 2 ( g ) {\displaystyle {\ce {2 H+ + 2e^- -> H2(g)}}} 
and,
2 H 2 O + 2 e − ⟶ H 2 ( g ) + 2 OH − {\displaystyle {\ce {2 H2O + 2e^- -> H2(g) + 2 OH^-}}} 
Using the Nernst equation, setting _E_0 = 0 V as defined by convention for the standard hydrogen electrode (SHE, serving as reference in the reduction potentials series) and the hydrogen gas fugacity (corresponding to chemical activity for a gas) at 1, the equation for the lower stability line of water in the Pourbaix diagram at standard temperature and pressure is:
E H = − V T λ pH {\displaystyle E_{{\ce {H}}}=-V_{T}\lambda \,{\ce {pH}}}
E H = − 0.05916 pH {\displaystyle E_{{\ce {H}}}=-0.05916\,{\ce {pH}}}
Below this line, water is reduced to hydrogen, and it will usually not be possible to pass beyond this line as long as there is still water present in the system to be reduced.
Correspondingly, under highly oxidizing conditions (high _E_H) water is oxidized into oxygen gas according to:[3]
2 H 2 O ⟶ 4 H + + O 2 ( g ) + 4 e − {\displaystyle {\ce {2 H2O -> 4 H+ + O2(g) + 4e^-}}} 
and,
4 OH − ⟶ O 2 ( g ) + 2 H 2 O + 4 e − {\displaystyle {\ce {4 OH^- -> O2(g) + 2 H_2O + 4e^-}}} 
Using the Nernst equation as above, but with E0 = −ΔG0H2O/2_F_ = 1.229 V for water oxidation, gives an upper stability limit of water as a function of the pH value:
E H = E 0 − V T λ pH {\displaystyle E_{{\ce {H}}}=E^{0}-V_{T}\lambda \,{\ce {pH}}}
E H = 1.229 V − 0.05916 pH {\displaystyle E_{{\ce {H}}}=1.229V-0.05916\,{\ce {pH}}}
at standard temperature and pressure. Above this line, water is oxidized to form oxygen gas, and it will usually not be possible to pass beyond this line as long as there is still water present in the system to be oxidized.
The two upper and lower stability lines having the same negative slope (−59 mV/pH unit), they are parallel in a Pourbaix diagram and the reduction potential decreases with pH.
Pourbaix diagrams have many applications in different fields dealing with e.g., corrosion problems, geochemistry, and environmental sciences. Using the Pourbaix diagram correctly will help shedding light not only on the nature of the species present in aqueous solution, or in the solid phases, but may also help to understand the reaction mechanism.[8]
Concept of pe in environmental chemistry
[edit]
Pourbaix diagrams are widely used to describe the behaviour of chemical species in the hydrosphere. In this context, reduction potential pe is often used instead of EH.[3] The main advantage is to directly work with a logarithm scale. pe is a dimensionless number and can easily be related to EH by the equation:
p e = E H V T λ = E H 0.05916 = 16.903 × E H {\displaystyle pe={\frac {E_{H}}{V_{T}\lambda }}={\frac {E_{H}}{0.05916}}=16.903\,{\text{×}}\,E_{H}}
Where, V T = R T F {\displaystyle V_{T}={\frac {RT}{F}}} 
Moreover,
p e = − log [ e − ] {\displaystyle pe=-\log[e^{-}]} ![{\displaystyle pe=-\log[e^{-}]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/e26e524de25ff6405613eec07ac3d67bcfdf0dbc)
pe values in environmental chemistry ranges from −12 to +25, since at low or high potentials water will be respectively reduced or oxidized. In environmental applications, the concentration of dissolved species is usually set to a value between 10−2 M and 10−5 M for the determination of the equilibrium lines.





- Nernst equation
- Dependency of reduction potential on pH
- Ellingham diagram
- Latimer diagram
- Frost diagram
- Ionic partition diagram
- Bjerrum plot
^ a b . Ignasi Puigdomenech, Hydra/Medusa Chemical Equilibrium Database and Plotting Software (2004) KTH Royal Institute of Technology, freely downloadable software at "KTH - Chemistry / Chemical Equilibrium Software". Archived from the original on 2007-09-29. Retrieved 2007-09-29.
^ a b c d e f van Loon, Gary; Duffy, Stephen (2011). Environmental Chemistry – a global perspective (3rd ed.). Oxford University Press. pp. 235–248. ISBN 978-0-19-922886-7.
^ a b c McCafferty, E. (2010). Introduction to corrosion science. New York: Springer. ISBN 978-1-4419-0454-6. OCLC 663096583.
^ a b c d Verink, E.D. Jr. (2000). "Simplified Procedure for Constructing Pourbaix Diagrams" (PDF). In Revie, R. Winston (ed.). Uhlig 's Corrosion Handbook. John Wiley & Sons, Inc. ISBN 0471157775. Archived from the original (PDF) on 6 August 2020. Retrieved 12 February 2021.
^ Kopeliovich, Dimitri. "Pourbaix Diagrams". SubsTech. Retrieved 2017-03-29.
^ "Chemistry of Iron in Natural Water" (PDF). Geological Survey Water-Supply Paper 1459. 1962. Retrieved 2017-03-29.
^ Noyhouzer, Tomer; Bellemare-Alford, Daphnée; Payne, Nicholas A.; Martineau, Eric; Mauzeroll, Janine (July 2018). "Unfolding the Hidden Reactions in Galvanic Cells". Electrocatalysis. 9 (4): 531–538. doi:10.1007/s12678-018-0459-1. S2CID 104177121.
Brookins, D.G. (1988). Eh-pH Diagrams for Geochemistry. Springer-Verlag. ISBN 0-387-18485-6.
Jones, Denny A. (1996). Principles and Prevention of Corrosion (2nd ed.). Prentice Hall. pp. 50–52. ISBN 0-13-359993-0.
Pourbaix, M. (1974). Atlas of electrochemical equilibria in aqueous solutions (2nd ed.). National Association of Corrosion Engineers. ISBN 9780915567980.
Takeno, Naoto (May 2005). Atlas of Eh-pH diagrams (Intercomparison of thermodynamic databases) (PDF) (Report). Tsukuba, Ibaraki, Japan: National Institute of Advanced Industrial Science and Technology: Research Center for Deep Geological Environments. Retrieved 2017-05-16.
Marcel Pourbaix — Corrosion Doctors
DoITPoMS Teaching and Learning Package- "The Nernst Equation and Pourbaix Diagrams"
ChemEQL Free software for calculation of chemical equilibria from Eawag.
FactSage Commercial thermodynamic databank software, also available in a free web application.
The Geochemist's Workbench Commercial geochemical modeling software from Aqueous Solutions LLC.
GWB Community Edition Free download of the popular geochemical modeling software package.
HYDRA/MEDUSA Free software for creating chemical equilibrium diagrams from the KTH Department of Chemistry.
HSC Chemistry Commercial thermochemical calculation software from Outotec Oy.
PhreePlot Free program for making geochemical plots using the USGS code PHREEQC.
Thermo-Calc Windows Commercial software for thermodynamic calculations from Thermo-Calc Software.
Materials Project Public website that can generate Pourbaix diagrams from a large database of computed material properties, hosted at NERSC.


![{\displaystyle K={\frac {[C]^{c}[D]^{d}}{[A]^{a}[B]^{b}}},}](https://wikimedia.org/api/rest_v1/media/math/render/svg/0efa08e603e9d1b3ee7e0f3ac56b0503b331008e)

![{\displaystyle E_{\text{H}}=E^{0}-{\frac {RT}{zF}}\ln {\frac {[C]^{c}[D]^{d}}{[A]^{a}[B]^{b}}},}](https://wikimedia.org/api/rest_v1/media/math/render/svg/db2f7f69f79b51a2875472cbe0c1ddbc65bcbcfe)
![{\displaystyle E_{\text{H}}=E^{0}-{\frac {V_{T}\lambda }{z}}\log {\frac {[C]^{c}[D]^{d}}{[A]^{a}[B]^{b}}},}](https://wikimedia.org/api/rest_v1/media/math/render/svg/e7fd7742bf3507ba72bb30ce42293f8ad4261866)
![{\displaystyle E_{\text{H}}=E^{0}-{\frac {0.05916}{z}}\log {\frac {[C]^{c}[D]^{d}}{[A]^{a}[B]^{b}}},}](https://wikimedia.org/api/rest_v1/media/math/render/svg/7269645d0f868a2c8bbd5b73ada796073447c4a1)



![{\displaystyle K={\frac {\{C\}^{c}\{D\}^{d}}{\{A\}^{a}\{B\}^{b}\{{\ce {H+}}\}^{h}}}={\frac {(\gamma _{c})^{c}\left[C\right]^{c}\ (\gamma _{d})^{d}\left[D\right]^{d}}{(\gamma _{a})^{a}\left[A\right]^{a}\ (\gamma _{b})^{b}\left[B\right]^{b}\ (\gamma _{h+})^{h}\left[{\ce {H+}}\right]^{h}}}={\frac {(\gamma _{c})^{c}(\gamma _{d})^{d}}{(\gamma _{a})^{a}(\gamma _{b})^{b}(\gamma _{h})^{h}}}{\text{×}}{\frac {\left[C\right]^{c}\left[D\right]^{d}}{\left[A\right]^{a}\left[B\right]^{b}\left[{\ce {H+}}\right]^{h}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/db8c47798e7d074071a615df5e3af3961044cccb)




![{\displaystyle E_{h}=E_{\text{red}}=E_{\text{red}}^{\ominus }-{\frac {0.05916}{z}}\log \left({\frac {\left[C\right]^{c}\left[D\right]^{d}}{\left[A\right]^{a}\left[B\right]^{b}}}\right)-{\frac {0.05916\,h}{z}}{\text{pH}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/283a9fe881fa441d01c337d10f7a6e85bf3dd651)

![{\displaystyle K={\frac {\left[C\right]^{c}\left[D\right]^{d}}{\left[A\right]^{a}\left[B\right]^{b}\left[{\ce {H+}}\right]^{h}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/77a14dc085fb0d8ed3b442aa618fd6167506845d)
![{\displaystyle \Delta G^{\circ }=-RT\ln \left({\frac {\left[C\right]^{c}\left[D\right]^{d}}{\left[A\right]^{a}\left[B\right]^{b}\left[{\ce {H+}}\right]^{h}}}\right)}](https://wikimedia.org/api/rest_v1/media/math/render/svg/f700e3ac9c2fc68f8ad9ee7a5674555b77e5c2a2)
![{\displaystyle \Delta G^{\circ }=-RT\lambda \,\left(\log \left({\frac {\left[C\right]^{c}\left[D\right]^{d}}{\left[A\right]^{a}\left[B\right]^{b}}}\right)+h\,{\ce {pH}}\right)}](https://wikimedia.org/api/rest_v1/media/math/render/svg/6f320dae44421362b42c122bb5fefd396468a3ad)

![{\displaystyle {\ce {pH}}=-{\frac {1}{6}}\left({\frac {\Delta G^{\circ }}{RT\lambda }}+\log \left({\frac {{\ce {[Fe2O3]}}}{{\ce {[Fe^{3+}]^2[H2O]^3}}}}\right)\right)}](https://wikimedia.org/api/rest_v1/media/math/render/svg/1e9b6b1f5d8a86be8e29cb048ef827940878a4a4)
![{\displaystyle {\ce {pH}}=-{\frac {1}{6}}\left({\frac {\Delta G^{\circ }}{RT\lambda }}+\log \left({\frac {1}{[{\ce {Fe^{3+}}}]^{2}}}\right)\right)}](https://wikimedia.org/api/rest_v1/media/math/render/svg/d81d7344121f12ae407118dc20b8fc850372b257)

![{\displaystyle \Delta G^{\circ }=-RT\ln \left({\frac {\left[C\right]^{c}\left[D\right]^{d}}{\left[A\right]^{a}\left[B\right]^{b}}}\right)}](https://wikimedia.org/api/rest_v1/media/math/render/svg/77872903c344ee8522e5eaaefffdc3e85963fa4d)
![{\displaystyle E_{h}={E^{\circ }}-{\frac {V_{T}}{z}}\ln \left({\frac {\left[C\right]^{c}\left[D\right]^{d}}{\left[A\right]^{a}\left[B\right]^{b}}}\right)}](https://wikimedia.org/api/rest_v1/media/math/render/svg/e6977406e65dcda045cf0cbaad4782e045b907a3)
![{\displaystyle E_{h}={E^{\circ }}-{\frac {V_{T}\lambda }{z}}\log \left({\frac {\left[C\right]^{c}\left[D\right]^{d}}{\left[A\right]^{a}\left[B\right]^{b}}}\right)}](https://wikimedia.org/api/rest_v1/media/math/render/svg/44a332f15c0d7512469a1a8b0ed0f69f6d0a00fb)

![{\displaystyle E_{h}={E^{\circ }}-V_{T}\lambda \log \left({\frac {{\ce {[Fe^{2+}]}}}{{\ce {[Fe^{3+}]}}}}\right)}](https://wikimedia.org/api/rest_v1/media/math/render/svg/5755676607ee2373af241565bfac30a00e7838cd)
![{\displaystyle E_{h}={E^{\circ }}-{\frac {V_{T}\lambda }{z}}\left(\log \left({\frac {\left[C\right]^{c}\left[D\right]^{d}}{\left[A\right]^{a}\left[B\right]^{b}}}\right)+h\,{\ce {pH}}\right)}](https://wikimedia.org/api/rest_v1/media/math/render/svg/a1e181f6aec9bd252b50fe095dd9edaf05157913)

![{\displaystyle E_{h}={E^{\circ }}-{\frac {V_{T}\lambda }{2}}\left(\log \left({\frac {{\ce {[Fe^{2+}]^2[H2O]^3}}}{{\ce {[Fe2O3]}}}}\right)+6\ {\ce {pH}}\right)}](https://wikimedia.org/api/rest_v1/media/math/render/svg/902998434ac96ba653b671a30d926988cb46b122)
![{\displaystyle E_{h}={E^{\circ }}-{\frac {V_{T}\lambda }{2}}\left(\log \ {\ce {[Fe^{2+}]^2}}+6\ {\ce {pH}}\right)}](https://wikimedia.org/api/rest_v1/media/math/render/svg/520856bc2bd34b111f02dc552b4a3fdaf923790c)






