Zomebazam (original) (raw)
From Wikipedia, the free encyclopedia
Chemical compound
Zomebazam
 |
|
|---|---|
| Identifiers | |
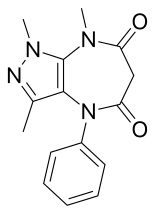
| IUPAC name 1,3,8-trimethyl-4-phenylpyrazolo[3,4-_b_][1,4]diazepine-5,7-dione | |
| CAS Number | 78466-70-3  Y Y |
| PubChem CID | 132677 |
| ChemSpider | 117132  N N |
| UNII | G563Y6G60K |
| CompTox Dashboard (EPA) | DTXSID60868481 |
| Chemical and physical data | |
| Formula | C15H16N4O2 |
| Molar mass | 284.319 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| SMILES O=C1N(c2c(N(C(=O)C1)C)n(nc2C)C)c3ccccc3 | |
InChI InChI=1S/C15H16N4O2/c1-10-14-15(18(3)16-10)17(2)12(20)9-13(21)19(14)11-7-5-4-6-8-11/h4-8H,9H2,1-3H3  NKey:BFWACMHTIVUWJS-UHFFFAOYSA-N NKey:BFWACMHTIVUWJS-UHFFFAOYSA-N  N N |
|
 N N Y (what is this?) (verify) Y (what is this?) (verify) |
Zomebazam[1] produced by Hoechst is a pyrazolodiazepinone derivative drug with anxiolytic properties. It is structurally related to razobazam and zometapine.[2]
The catalytic hydrogenation of N,2,5-trimethyl-4-phenyldiazenylpyrazol-3-amine[a] (1) over Raney nickel gives 4-amino-1,3-dimethyl-5-methylaminopyrazole[b] (2). Treatment with methyl malonyl chloride[c] (3) gives 4-α-ethoxycarbonylacetylamino-1,3-dimethyl-5-methylaminopyrazole[d] (4). Base-catalyzed lactamization gives (5). The Goldberg reaction completes the synthesis of zomebazam (6).[3][4]
Synthesis of zomebazam
^ US 3558605, "4-Aryl-5,6,7,8-tetrahydropyrazolo(3,4-B)-(1,5)diazepine-1H,4H-5,7-diones and medicaments containing same"
^ "Zomebazam". psychotropics.dk. 2003. Retrieved 7 December 2009.
^ Renger B (1985). "Direkte N-Arylierung von Amiden: Eine Verbesserung der Goldberg-Reaktion". Synthesis. 1985 (9): 856–560. doi:10.1055/s-1985-31364. S2CID 93397774.
^ US 4302468, Rackur G, Hoffmann I, issued 1981, assigned to Hoechst Aktiengesellschaft
^ CAS# [37517-81-0]
