Home page | Telomerase Database (original) (raw)
The Telomerase Database is a Web-based tool for the study of structure, function, and evolution of the telomerase ribonucleoprotein.
The objective of this database is to serve the research community by providing a comprehensive compilation of information known about the telomerase enzyme and its substrate, telomeres. The collection of information includes sequences, alignments, secondary and tertiary structures, mutations known to cause human telomerase-deficiency diseases, and current telomerase researchers.

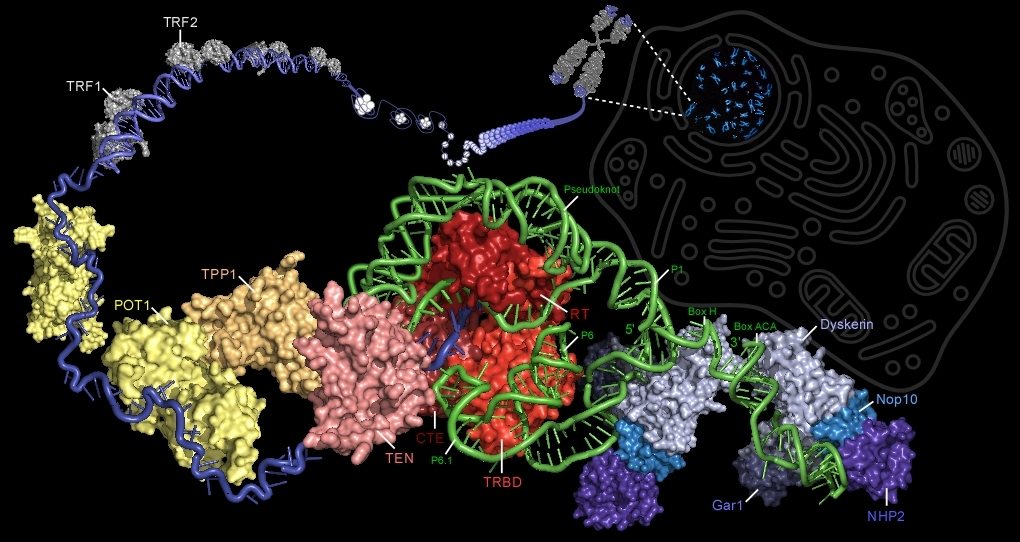
Illustration of the vertebrate telomerase ribonucleoprotein complexed with its substrate, telomeric DNA (blue). Telomerase consists of a reverse transcriptase (TERT, red), RNA component (TR, green), and the dyskerin protein complex (blue). The TERT protein contains the essential telomerase N-terminal domain (TEN, pink), telomerase RNA-binding domain (TRBD, light red), the reverse transcriptase domain (RT, red), and the C-terminal extension (CTE, dark red). TR contains base-pairing region P1b that defines the template boundary, the template that encodes the telomeric DNA repeat sequence, the pseudoknot, conserved regions 4 and 5 (CR4/5) that include P6 and P6.1, and the 3’-proximal H/ACA stem-loops each bound by a dyskerin complex. The dyskerin complex is composed of dyskerin (light blue), Nop10 (sky blue), NHP2 (violet), and Gar1 (dark blue). The pseudoknot is believed to be bound by TEN while P6/P6.1 is bound by TRBD . Six proteins associate with telomeric DNA and form the shelterin complex. TRF1 (grey), TRF2 (grey), and POT1 (yellow) directly bind telomeric DNA. TIN2 (not shown), TPP1 (orange), and Rap1 (not shown) associated with these telomeric DNA binding proteins. TRF1 and TRF2 bind double-stranded telomeric DNA, while POT1 binds to the single-stranded 3'-overhang.
Please cite: Podlevsky, J.D., Bley, C.J., Omana, R.V., Qi, X., Chen, J. (2007) The Telomerase Database. Nucleic Acids Res. 36 D339-D343.