Eukaryotes (original) (raw)
This tree is derived from a consensus of several different studies that are cited in the Discussion of Phylogenetic Relationships and the individual pages for each lineage shown.
Discussion of Phylogenetic Relationships
Our understanding of eukaryotic relationships has been transformed by the use of molecular data to reconstruct phylogenies (Sogin et al., 1986). Prior to that, the diversity of microbial eukaryotes was vastly underestimated, and the relationships between them and multicellular eukaryotes were difficult to resolve (Taylor, 1978). Early molecular phylogenies based on small subunit ribosomal RNA (SSU rRNA) gene sequences suggested a ladder of basal lineages topped by a ‘crown’ composed of multicellular groups (animals, plants, and fungi) together with a subset of the purely microbial lineages (Sogin, 1989). A great number of the relationships revealed by SSU rRNA phylogeny have stood the test of time, but subsequent analyses based on protein coding genes and more recently very large datasets composed of hundreds of protein coding genes have led to a revision of the overall structure of the tree. The current view of eukaryotic phylogeny is of a small number of large ‘supergroups’, each comprising a spectacular diversity of structures, nutritional modes, and behaviours (Adl et al., 2005; Keeling, 2004; Keeling et al., 2005; Simpson and Roger, 2002). Some of these supergroup hypotheses are well supported, while others remain the subject of vigorous debate (see (Keeling et al., 2005) for a discussion of evidence). Furthermore the relationships between supergroups are poorly understood. Below we summarise the main members of each supergroup, the evidence for its monophyly, and emerging hypotheses for inter-supergroup relationships.
Archaeplastida (Plantae)
The Archaeplastida, or Plantae, comprises glaucophytes, red algae, green algae and plants. They are united by the possession of a plastid derived from primary endosymbiosis (see Symbiosis section). There has long been strong support for the monophyly of plastids in Archaeplastida based on molecular phylogeny and also plastid genome structure (Turner, 1997; Turner et al., 1999), and molecular phylogenies based on large numbers of protein coding genes have more recently demonstrated the monophyly of the nuclear/cytosolic lineage as well (Burki et al., 2008; Moreira et al., 2000; Reyes-Prieto et al., 2007).

Click on an image to view larger version & data in a new window
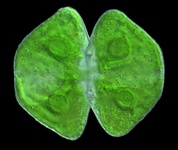
Fig. 7. Some examples of Archaeplastida. From left to right, the red alga Chondracanthus (© Patrick Keeling), the green alga Cosmarium (© Patrick Keeling) and the land plants Typha latifolia (© Patrick Keeling) and Calocedrus decurrens (© 2008 Fort Photo).
Excavata
Excavata is a large and diverse grouping that has been proposed based on a synthesis of morphological and molecular data. Many excavates share a similar feeding groove structure (from which the name is derived) (Simpson and Patterson, 2001; Simpson and Patterson, 1999). Many others lack this structure, but are demonstrably related to lineages that possess it in molecular phylogenies (Simpson, 2003; Simpson et al., 2006; Simpson et al., 2002). Putting this evidence together led to the suggestion of shared ancestry, and some recent multi-gene phylogenies in fact provide tentative support for the monophyly of the whole group (Burki et al., 2008; Rodriguez-Ezpeleta et al., 2007). Many excavates are anaerobes/microaerophiles and contain mitosomes or hydrogenosomes (e.g. diplomonads and parabasalids). Some are important parasites of animals (e.g. trypanosomes, Giardia). One lineage, the euglenids, includes photosynthetic species that have plastids derived from a green alga by secondary endosymbiosis (Breglia et al., 2007; Leander et al., 2007).

Click on an image to view larger version & data in a new window
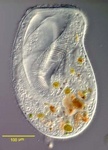
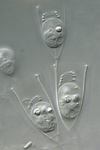
Fig. 8. Some examples of Excavata. From left to right: an SEM of the oxymonad Saccinobaculus minor (© Kevin Carpenter and Patrick Keeling), an SEM of the photosynthetic euglenid Lepocinclis spirogyra (© 2003 Brian S. Leander), a DIC light micrograph of the heterolobosean Stephanopogon minuta (© Naoji Yubuki and Brian S. Leander) and a fluorescence micrograph of the parabasalid Holomastigotes elongatum (© Guy Brugerolle).
Chromalveolata
Chromalveolates comprises six major groups of primarily single celled eukaryotes: apicomplexans, dinoflagellates and ciliates are members of the alveolates, they are hypothesised to be related to stramenopiles, cryptomonads, and haptophytes (Cavalier-Smith, 2004; Keeling, 2009). The basis for this hypothesis is the widespread presence of plastids in these groups that are all derived from secondary endosymbiosis with a red alga. It was therefore proposed that all chromalveolates share a common ancestor where this endosymbiosis took place (Cavalier-Smith, 1999). The monophyly of the plastids has been demonstrated with limited sampling (Hagopian et al., 2004; Rogers et al., 2007; Yoon et al., 2002), and some phylogenies inferred from many different nuclear genes show that the Chromalveolata are monophyletic with the Rhizaria nested within (see below) (Hackett et al., 2007). Additional support comes from two genes with unusual evolutionary histories involving lateral gene transfer and/or re-targeting to the plastid that are most consistent with a common origin of chromalveolate plastids (Fast et al., 2001; Harper and Keeling, 2003; Patron et al., 2004).

Click on an image to view larger version & data in a new window
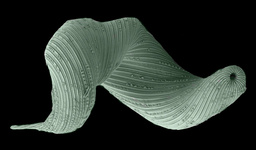
Fig. 9. Some examples of chromalveolates. From left to right: the multiculluar brown alga Macrocystis (© Tom Gruber), the diatom Stephanodiscus (© David G. Mann; this image is derived from the Professor Frank Round Image Archive at the Royal Botanic Garden Edinburgh), the ciliate Bursaria truncatella (© William Bourland, Freshwater and terrestrial microbes of Idaho), the filamentous dinoflagellate Gymnodinium catenatum (© Bob Andersen and D. J. Patterson; this image is of material from Provasoli-Guillard National Center for Culture of Marine Phytoplankton), and the bicosoecid Bicosoeca petiolata (© William Bourland, Freshwater and terrestrial microbes of Idaho).
Rhizaria
Rhizaria comprises several very large and diverse groups of amoebae, flagellates and amoeboflagellates (Cavalier-Smith and Chao, 2003). Many of these will not be familiar to many readers, but they are ubiquitous in nature and important predators in many environments. Major lineages include Cercozoa, Foraminifera, and Radiolaria. Rhizaria is the most recently recognized supergroup, having been identified exclusively from molecular phylogenetic reconstruction (Cavalier-Smith, 2002; Cavalier-Smith, 2003; Nikolaev et al., 2004). Prior to this, there was little reason to anticipate this grouping, because there is no major structural character that unites them. (Although the amoeboid members of the group tend to produce fine pseudopodia, rather than the broad pseudopodia seen in many Amoebozoa – see below.) However, analyses of molecular phylogenies based on nearly all genes examined, as well as rare molecular markers such as insertions and deletions, initially identified the Cercozoa as a group that has then expanded to include the Foraminifera and eventually the Radiolaria (Archibald et al., 2002; Bass et al., 2005; Burki et al., 2007; Burki et al., 2008; Keeling, 2001; Longet et al., 2003; Moreira et al., 2007; Nikolaev et al., 2004; Polet et al., 2004). Analyses of multiple protein coding genes have further supported the monophyly of Rhizaria, and suggested a relationship to chromalveolates (see below).

Click on an image to view larger version & data in a new window
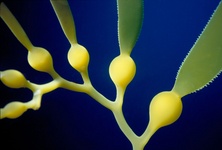
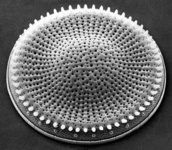
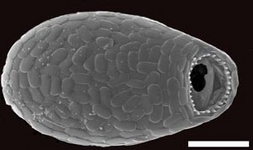
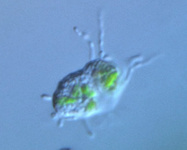
Fig. 10. Some examples of Rhizaria. From left to right: the euglyphid amoeba Corythion dubium (© Edward Mitchell), the chlorarachniophyte Gymnochlora (© Patrick Keeling), the foraminiferans Allogromia (© Jan Pawlowski and José Fahrni) and Calcarina spengleri (© 2008 Michael).
Opisthokonta
Opisthokonta is a grouping consisting of Animals (Metazoa), the true Fungi and their close protistan relatives. The closest relatives of animals include choanoflagellates, which are free-living unicellular or colonial flagellates, and the parasitic Ichthyosporea (also known as Mesomycetozoea). Fungi are most closely related to a group of amoebae called nucleariids. Opisthokonts share two conspicuous features that are uncommon in other eukaryotes: Almost all cells in this group have flat mitochondrial cristae, while flagellated cells typically have a single emergent flagellum that inserts at the posterior end of the cell (Cavalier-Smith, 1987). The monophyly of this group has been shown convincingly by molecular phylogenies (Baldauf and Palmer, 1993; Lang et al., 1999; Ragan et al., 1996; Ruiz-Trillo et al., 2006; Steenkamp et al., 2006; Wainright et al., 1993), and also by a large, conserved insertion within the protein Elongation Factor 1-alpha (Baldauf and Palmer, 1993; Steenkamp et al., 2006). Recently a possible shared lateral gene transfer has been reported (Huang et al., 2005).

Click on an image to view larger version & data in a new window
Fig. 11. Some examples of opisthokonts. From left to right: the choanoflagellate Salpingoeca (© William Bourland), the animals Saimiri sciureus (squirrel monkey, © Luc Viatour) and Cyanea capillata (lion's mane sea jelly, © Patrick Keeling), the fungus Morchella (© Patrick Keeling), and the microsporidian Fibrillanosema crangonycis (© Leon White).
Amoebozoa
The Amoebozoa are a diverse collection of protozoan eukaryotes, almost all of which are amoebae (i.e. cells that produce pseudopodia, but lack flagella) for some or all of their life cycle. Many produce lobose or fan-shaped pseudopodia (in contrast to the elongate, fine pseudopodia typical of Rhizaria), although short, fine sub-pseudopodia are also common. Amoebozoa includes lineages of ‘lobose amoebae’ (e.g the well known Amoeba and Chaos), the lobose testate amoebae (with the cell enclosed in a shell), most of the lineages of ‘slime molds’, the pelobionts and Entamoebae, which lack classical mitochondria, and a few mitochondriate flagellates. Amoebozoa were only recently united as group. Detailed microscopy studies had shown that amoebae as a whole were polyphyletic, and thus when early molecular phylogenetic studies based especially on ribosomal RNA sequences placed slime molds, lobose amoebae, pelobionts and entamoebae as multiple independent lineages (Hinkle et al., 1994; Sogin, 1989), this result seemed plausible. In the last few years, increasingly sophisticated molecular phylogenies incorporating many more taxa and/or genes have tended to unite these previously disparate groups (Bapteste et al., 2002; Fahrni et al., 2003), though not always with strong statistical support. A recent study suggests that the pseudopodia-producing flagellate Breviata represents the deepest branch within a monophyletic amoebozoa clade (Minge et al., 2008).

Click on an image to view larger version & data in a new window
Fig. 12. Some examples of amoebozoans. From left to right: the slime mold Leocarpus fragilis (© 2008 Mavi Rodriguez Garcia), the slime mold Diachea leucopodia (© 2006 Randy Darrah, The Eumycetozoan Project, University of Arkansas), and a lobosean testate amoeba, likely Nebela tubulosa (© 2009 Antonio Guillén, identified by Ralf Meisterfeld).
'Unikonts': A Clade Consisting of Opisthokonts & Amoebozoans
There is now considerable evidence from molecular phylogenies that the opisthokonts and amoebozoans are closely related (Baldauf et al., 2000; Bapteste et al., 2002), and they also share a handful of other molecular characteristics in common (Richards and Cavalier-Smith, 2005). They have been proposed to be a clade called ‘unikonts’ because many of these organisms have a single flagellum (Cavalier-Smith, 2002), but biflagellated lineages are also known in this group. The root of the tree of eukaryotes has been proposed to be somewhere near this lineage, so it is possible the ‘unikonts’ are paraphyletic (Stechmann and Cavalier-Smith, 2002; Stechmann and Cavalier-Smith, 2003).
Do rhizarians branch within the chromalveolates?
There has long been very strong evidence from several kinds of data for the monophyly of alveolates. Multi-gene trees have also consistently and strongly supported a relationship between alveolates and stramenopiles (Burki et al., 2007; Burki et al., 2008; Hackett et al., 2007; Patron et al., 2007; Rodriguez-Ezpeleta et al., 2005; Rodriguez-Ezpeleta et al., 2007; Simpson et al., 2006). There is now also very strong evidence from molecular phylogenies and a shared lateral gene transfer for the monophyly of cryptomonads, haptophytes, and their relatives (Burki et al., 2008; Hackett et al., 2007; Patron et al., 2007; Rice and Palmer, 2006). In addition there is evidence from the plastid genome and plastid targeted proteins for the monophyly of chromalveolates and their plastids (Fast et al., 2001; Hagopian et al., 2004; Harper and Keeling, 2003; Patron et al., 2004; Rogers et al., 2007; Yoon et al., 2002). However, multi-gene trees also consistently show that the entire rhizarian supergroup is closely related to alveolates and stramenopiles (Burki et al., 2007; Burki et al., 2008; Hackett et al., 2007; Rodriguez-Ezpeleta et al., 2007), and some support the monophyly of chromalveolates as a whole with the Rhizaria nested within the group. These relationships will doubtless be refined with further data, but for now we follow the consensus of the available evidence and place the Rhizaria within the Chromalveolata.
References
For additional references about eukaryote phylogeny and evolution please see Eukaryotes - Comprehensive List of References.
Adl, S.M., Simpson, A.G., Farmer, M.A., Andersen, R.A., Anderson, O.R., Barta, J.R., Bowser, S.S., Brugerolle, G., Fensome, R.A., Fredericq, S., James, T.Y., Karpov, S., Kugrens, P., Krug, J., Lane, C.E., Lewis, L.A., Lodge, J., Lynn, D.H., Mann, D.G., McCourt, R.M., Mendoza, L., Moestrup, O., Mozley-Standridge, S.E., Nerad, T.A., Shearer, C.A., Smirnov, A.V., Spiegel, F.W. and Taylor, M.F. (2005) The new higher level classification of eukaryotes with emphasis on the taxonomy of protists. J. Eukaryot. Microbiol., 52, 399-451.
Andersson, S.G. and Kurland, C.G. (1999) Origins of mitochondria and hydrogenosomes. Curr. Opin. Microbiol., 2, 535-541.
Archibald, J.M. (2005) Jumping genes and shrinking genomes--probing the evolution of eukaryotic photosynthesis with genomics. IUBMB Life, 57, 539-547.
Archibald, J.M., Longet, D., Pawlowski, J. and Keeling, P.J. (2002) A novel polyubiquitin structure in Cercozoa and Foraminifera: evidence for a new eukaryotic supergroup. Mol. Biol. Evol., 20, 62-66.
Baldauf, S.L. and Palmer, J.D. (1993) Animals and fungi are each other's closest relatives: congruent evidence from multiple proteins. Proc. Natl. Acad. Sci. USA, 90, 11558-11562.
Baldauf, S.L., Roger, A.J., Wenk-Siefert, I. and Doolittle, W.F. (2000) A kingdom-level phylogeny of eukaryotes based on combined protein data. Science, 290, 972-977.
Bapteste, E., Brinkmann, H., Lee, J., Moore, D., Sensen, C., Gordon, P., Durufle, L., Gaasterland, T., Lopez, P., Muller, M. and Philippe, H. (2002) The analysis of 100 genes supports the grouping of three highly divergent amoebae: Dictyostellium, Entamoeba, and Mastigamoeba. Proc. Natl. Acad. Sci. U S A, 99, 1414-1419.
Bass, D., Moreira, D., Lopez-Garcia, P., Polet, S., Chao, E.E., von der Heyden, S., Pawlowski, J. and Cavalier-Smith, T. (2005) Polyubiquitin insertions and the phylogeny of Cercozoa and Rhizaria. Protist, 156, 149-161.
Breglia, S.A., Slamovits, C.H. and Leander, B.S. (2007) Phylogeny of phagotrophic euglenids (Euglenozoa) as inferred from hsp90 gene sequences. J. Eukaryot. Microbiol., 54, 86-92.
Burki, F., Shalchian-Tabrizi, K., Minge, M., Skjaeveland, A., Nikolaev, S.I., Jakobsen, K.S. and Pawlowski, J. (2007) Phylogenomics reshuffles the eukaryotic supergroups. PLoS ONE, 2, e790.
Burki, F., Shalchian-Tabrizi, K. and Pawlowski, J. (2008) Phylogenomics reveals a new 'megagroup' including most photosynthetic eukaryotes. Biol. Lett., 4(4), 366-369.
Cavalier-Smith, T. (1987) The origin of fungi and pseudofungi. In Rayner, A.D.M., Brasier, C.M. and Moore, D. (eds.), Evolutionary biology of the fungi. Cambridge University Press, Cambridge, pp. 339-353.
Cavalier-Smith, T. (1998) A revised six-kingdom system of life. Biol. Rev. Camb. Philos. Soc., 73, 203-266.
Cavalier-Smith, T. (1999) Principles of protein and lipid targeting in secondary symbiogenesis: euglenoid, dinoflagellate, and sporozoan plastid origins and the eukaryote family tree. J. Eukaryot. Microbiol., 46, 347-366.
Cavalier-Smith, T. (2002) The phagotrophic origin of eukaryotes and phylogenetic classification of Protozoa. Int. J. Sys. Evol. Microbiol., 52, 297-354.
Cavalier-Smith, T. (2003) Protist phylogeny and the high-level classification of Protozoa. Eur. J. Protistol., 39, 338-348.
Cavalier-Smith, T. (2004) Chromalveolate diversity and cell megaevolution: interplay of membranes, genomes and cytoskeleton. In Hirt, R.P. and Horner, D. (eds.), Organelles, Genomes and Eukaryotic Evolution. Taylor and Francis, London, pp. 71-103.
Cavalier-Smith, T. and Chao, E.E. (2003) Phylogeny and classification of phylum Cercozoa (Protozoa). Protist, 154, 341-358.
Douglas, S., Zauner, S., Fraunholz, M., Beaton, M., Penny, S., Deng, L.T., Wu, X., Reith, M., Cavalier-Smith, T. and Maier, U.G. (2001) The highly reduced genome of an enslaved algal nucleus. Nature, 410, 1091-1016.
Embley, T.M. (2006) Multiple secondary origins of the anaerobic lifestyle in eukaryotes. Philos. Trans. R. Soc. Lond. B Biol. Sci., 361, 1055-1067.
Fahrni, J.F., Bolivar, I., Berney, C., Nassonova, E., Smirnov, A. and Pawlowski, J. (2003) Phylogeny of lobose amoebae based on actin and small-subunit ribosomal RNA genes. Mol. Biol. Evol., 20, 1881-1886.
Fast, N.M., Kissinger, J.C., Roos, D.S. and Keeling, P.J. (2001) Nuclear-encoded, plastid-targeted genes suggest a single common origin for apicomplexan and dinoflagellate plastids. Mol. Biol. Evol., 18, 418-426.
Gilson, P.R., Su, V., Slamovits, C.H., Reith, M.E., Keeling, P.J. and McFadden, G.I. (2006) Complete nucleotide sequence of the chlorarachniophyte nucleomorph: nature's smallest nucleus. Proc. Natl. Acad. Sci. USA, 103, 9566-9571.
Gogarten, J.P., Kiblak, H., Dittrich, P., Taiz, L., Bowman, E.J., Bowman, B.J., Manolson, N.F., Poole, R.J., Date, T., Oshima, T., Konishi, J., Denda, K. and Yoshida, M. (1989) Evolution of the vacuolar H+-ATPase: inplications for the origin of eukaryotes. Proc. Natl. Acad. Sci. USA, 86, 6661-6665.
Gould, S.B., Waller, R.F. and McFadden, G.I. (2008) Plastid evolution. Annu. Rev. Plant. Biol., 59, 491-517.
Gray, M.W., Burger, G. and Lang, B.F. (1999) Mitochondrial evolution. Science, 283, 1476-1481.
Gray, M.W. and Doolittle, W.F. (1982) Has the endosymbiont hypothesis been proven? Microbiol Rev., 46, 1-42.
Gray, M.W., Lang, B.F. and Burger, G. (2004) Mitochondria of protists. Annu. Rev. Genet., 38, 477-524.
Hackett, J.D., Yoon, H.S., Li, S., Reyes-Prieto, A., Rummele, S.E. and Bhattacharya, D. (2007) Phylogenomic analysis supports the monophyly of cryptophytes and haptophytes and the association of rhizaria with chromalveolates. Mol. Biol. Evol., 24, 1702-1713.
Hagopian, J.C., Reis, M., Kitajima, J.P., Bhattacharya, D. and de Oliveira, M.C. (2004) Comparative analysis of the complete plastid genome sequence of the red alga Gracilaria tenuistipitata var. liui provides insights into the evolution of rhodoplasts and their relationship to other plastids. J. Mol. Evol., 59, 464-477.
Harper, J.T. and Keeling, P.J. (2003) Nucleus-encoded, plastid-targeted glyceraldehyde-3-phosphate dehydrogenase (GAPDH) indicates a single origin for chromalveolate plastids. Mol. Biol. Evol., 20, 1730-1735.
Harwood, J.L. (1996) Recent advances in the biosynthesis of plant fatty acids. Biochim. Biophys. Acta, 1301, 7-56.
Herrmann, K.M. and Weaver, L.M. (1999) The Shikimate Pathway. Annu. Rev. Plant Physiol. Plant Mol. Biol., 50, 473-503.
Hinkle, G., Leipe, D.D., Nerad, T.A. and Sogin, M.L. (1994) The unusually long small subunit ribosomal RNA of Phreatamoeba balamuthi. Nucleic Acids Res., 22, 465-469.
Huang, J., Xu, Y. and Gogarten, J.P. (2005) The presence of a haloarchaeal type tyrosyl-tRNA synthetase marks the opisthokonts as monophyletic. Mol. Biol. Evol., 22, 2142-2146.
Iwabe, N., Kuma, K.-I., Hasegawa, M., Osawa, S. and Miyata, T. (1989) Evolutionary relationship of archaebacteria, eubacteria, and eukaryotes inferred from phylogenetic trees of duplicated genes. Proc. Natl. Acad. Sci. USA, 86, 9355-9359.
Johnson, M.D., Oldach, D., Delwiche, C.F. and Stoecker, D.K. (2007) Retention of transcriptionally active cryptophyte nuclei by the ciliate Myrionecta rubra. Nature, 445, 426-428.
Keeling, P.J. (2001) Foraminifera and Cercozoa are related in actin phylogeny: two orphans find a home? Mol. Biol. Evol., 18, 1551-1557.
Keeling, P.J. (2004) The diversity and evolutionary history of plastids and their hosts. Am. J. Bot., 91, 1481-1493.
Keeling, P.J. (2009) Chromalveolates and the evolution of plastids by secondary endosymbiosis. J. Eukaryot Microbiol., in press.
Keeling, P.J., Burger, G., Durnford, D.G., Lang, B.F., Lee, R.W., Pearlman, R.E., Roger, A.J. and Gray, M.W. (2005) The tree of eukaryotes. Trends Ecol. Evol., 20, 670-676.
Lang, B.F., Gray, M.W. and Burger, G. (1999) Mitochondrial genome evolution and the origin of eukaryotes. Annu. Rev. Genet., 33, 351-397.
Leander, B.S., Esson, H.J. and Breglia, S.A. (2007) Macroevolution of complex cytoskeletal systems in euglenids. Bioessays, 29, 987-1000.
Lill, R., Diekert, K., Kaut, A., Lange, H., Pelzer, W., Prohl, C. and Kispal, G. (1999) The essential role of mitochondria in the biogenesis of cellular iron-sulfur proteins. Biol. Chem., 380, 1157-1166.
Lill, R. and Kispal, G. (2000) Maturation of cellular Fe-S proteins: an essential function of mitochondria. Trends Biochem. Sci., 25, 352-356.
Longet, D., Archibald, J.M., Keeling, P.J. and Pawlowski, J. (2003) Foraminifera and Cercozoa share a common origin according to RNA polymerase II phylogenies. Int. J. Syst. Evol. Microbiol., 53, 1735 - 1739.
Margulis, L. (1981) Symbiosis in cell evolution. W. H. Freeman and Co., San Francisco.
McFadden, G.I. (1999) Endosymbiosis and evolution of the plant cell. Curr. Opin. Plant. Biol., 2, 513-519.
McFadden, G.I., Gilson, P.R., Douglas, S.E., Cavalier-Smith, T., Hofmann, C.J. and Maier, U.G. (1997) Bonsai genomics: sequencing the smallest eukaryotic genomes. Trends Genet., 13, 46-49.
Minge, M.A., Silberman, J.D., Orr, R.J., Cavalier-Smith, T., Shalchian-Tabrizi, K., Burki, F., Skjaeveland, A. and Jakobsen, K.S. (2008) Evolutionary position of breviate amoebae and the primary eukaryote divergence. Proc. Biol. Sci., 276, 597-604.
Moreira, D., Le Guyader, H. and Phillippe, H. (2000) The origin of red algae and the evolution of chloroplasts. Nature, 405, 69-72.
Moreira, D., von der Heyden, S., Bass, D., Lopez-Garcia, P., Chao, E. and Cavalier-Smith, T. (2007) Global eukaryote phylogeny: Combined small- and large-subunit ribosomal DNA trees support monophyly of Rhizaria, Retaria and Excavata. Mol. Phylogenet. Evol., 44, 255-266.
M�ller, M. (1993) The hydrogenosome. J. Gen. Microbiol., 139, 2879-2889.
Nikolaev, S.I., Berney, C., Fahrni, J.F., Bolivar, I., Polet, S., Mylnikov, A.P., Aleshin, V.V., Petrov, N.B. and Pawlowski, J. (2004) The twilight of Heliozoa and rise of Rhizaria, an emerging supergroup of amoeboid eukaryotes. Proc. Natl. Acad. Sci. USA, 101, 8066-8071.
Nowack, E.C., Melkonian, M. and Glockner, G. (2008) Chromatophore genome sequence of Paulinella sheds light on acquisition of photosynthesis by eukaryotes. Curr. Biol., 18, 410-418.
Okamoto, N. and Inouye, I. (2005) A secondary symbiosis in progress? Science, 310, 287.
Patron, N.J., Inagaki, Y. and Keeling, P.J. (2007) Multiple gene phylogenies support the monophyly of cryptomonad and haptophyte host lineages. Curr. Biol., 17, 887-891.
Patron, N.J., Rogers, M.B. and Keeling, P.J. (2004) Gene replacement of fructose-1,6-bisphosphate aldolase (FBA) supports a single photosynthetic ancestor of chromalveolates. Eukaryot. Cell, 3, 1169-1175.
Polet, S., Berney, C., Fahrni, J. and Pawlowski, J. (2004) Small-subunit ribosomal RNA gene sequences of Phaeodarea challenge the monophyly of Haeckel's Radiolaria. Protist, 155, 53-63.
Ragan, M.A., Goggin, C.L., Cawthorn, R.J., Cerenius, L., Jamieson, A.V., Plourde, S.M., Rand, T.G., Soderhall, K. and Gutell, R.R. (1996) A novel clade of protistan parasites near the animal-fungal divergence. Proc. Natl. Acad. Sci. USA, 93, 11907-11912.
Ralph, S.A., Van Dooren, G.G., Waller, R.F., Crawford, M.J., Fraunholz, M.J., Foth, B.J., Tonkin, C.J., Roos, D.S. and McFadden, G.I. (2004) Tropical infectious diseases: Metabolic maps and functions of the Plasmodium falciparum apicoplast. Nat. Rev. Microbiol., 2, 203-216.
Reichert, A.S. and Neupert, W. (2004) Mitochondriomics or what makes us breathe. Trends Genet., 20, 555-562.
Reyes-Prieto, A., Weber, A.P. and Bhattacharya, D. (2007) The origin and establishment of the plastid in algae and plants. Annu. Rev. Genet., 41, 147-168.
Rice, D.W. and Palmer, J.D. (2006) An exceptional horizontal gene transfer in plastids: gene replacement by a distant bacterial paralog and evidence that haptophyte and cryptophyte plastids are sisters. BMC Biol., 4, 31.
Richards, T.A. and Cavalier-Smith, T. (2005) Myosin domain evolution and the primary divergence of eukaryotes. Nature, 436, 1113-1118.
Rodriguez-Ezpeleta, N., Brinkmann, H., Burey, S.C., Roure, B., Burger, G., Loffelhardt, W., Bohnert, H.J., Philippe, H. and Lang, B.F. (2005) Monophyly of primary photosynthetic eukaryotes: green plants, red algae, and glaucophytes. Curr Biol, 15, 1325-1330.
Rodriguez-Ezpeleta, N., Brinkmann, H., Burger, G., Roger, A.J., Gray, M.W., Philippe, H. and Lang, B.F. (2007) Toward resolving the eukaryotic tree: the phylogenetic positions of jakobids and cercozoans. Curr. Biol., 17, 1420-1425.
Roger, A.J. (1999) Reconstructing early events in eukaryotic evolution. Am. Nat., 154, S146-S163.
Rogers, M.B., Gilson, P.R., Su, V., McFadden, G.I. and Keeling, P.J. (2007) The complete chloroplast genome of the chlorarachniophyte Bigelowiella natans: evidence for independent origins of chlorarachniophyte and euglenid secondary endosymbionts. Mol. Biol. Evol., 24, 54-62.
Rohdich, F., Kis, K., Bacher, A. and Eisenreich, W. (2001) The non-mevalonate pathway of isoprenoids: genes, enzymes and intermediates. Curr. Opin. Chem. Biol., 5, 535-540.
Ruiz-Trillo, I., Lane, C.E., Archibald, J.M. and Roger, A.J. (2006) Insights into the evolutionary origin and genome architecture of the unicellular opisthokonts Capsaspora owczarzaki and Sphaeroforma arctica. J. Eukaryot. Microbiol., 53, 379-384.
Rumpho, M.E., Worful, J.M., Lee, J., Kannan, K., Tyler, M.S., Bhattacharya, D., Moustafa, A. and Manhart, J.R. (2008) Horizontal gene transfer of the algal nuclear gene psbO to the photosynthetic sea slug Elysia chlorotica. Proc. Natl. Acad. Sci. USA, 105, 17867-17871.
Simpson, A.G. (2003) Cytoskeletal organization, phylogenetic affinities and systematics in the contentious taxon Excavata (Eukaryota). Int. J. Syst. Evol. Microbiol., 53, 1759-1777.
Simpson, A.G., Inagaki, Y. and Roger, A.J. (2006) Comprehensive multigene phylogenies of excavate protists reveal the evolutionary positions of "primitive" eukaryotes. Mol. Biol. Evol., 23, 615-625.
Simpson, A.G. and Patterson, D.J. (2001) On core jakobids and excavate taxa: the ultrastructure of Jakoba incarcerata. J. Eukaryot. Microbiol., 48, 480-492.
Simpson, A.G. and Roger, A.J. (2002) Eukaryotic evolution: getting to the root of the problem. Curr Biol, 12, R691-693.
Simpson, A.G., Roger, A.J., Silberman, J.D., Leipe, D.D., Edgcomb, V.P., Jermiin, L.S., Patterson, D.J. and Sogin, M.L. (2002) Evolutionary history of "early-diverging" eukaryotes: the excavate taxon Carpediemonas is a close relative of Giardia. Mol. Biol. Evol., 19, 1782-1791.
Simpson, A.G.B. and Patterson, D.J. (1999) The ultrastructure of Carpediemonas membranifera (Eukaryota) with reference to the "Excavate hypothesis". Eur. J. Protistol., 35, 353-370.
Sogin, M.L. (1989) Evolution of eukaryotic microorganisms and their small subunit ribosomal RNAs. Amer. Zool., 29, 487-499.
Sogin, M.L., Elwood, H.J. and Gunderson, J.H. (1986) Evolutionary diversity of eukaryotic small-subunit rRNA genes. Proc. Natl. Acad. Sci. USA, 83, 1383-1387.
Stanier, R.Y. (1970) Some aspects of the biology of cells and their possible evolutionary significance. Symp. Soc. Gen. Mircrobiol., 20, 1-38.
Stechmann, A. and Cavalier-Smith, T. (2002) Rooting the eukaryote tree by using a derived gene fusion. Science, 297, 89-91.
Stechmann, A. and Cavalier-Smith, T. (2003) The root of the eukaryote tree pinpointed. Curr. Biol., 13, R665-666.
Steenkamp, E.T., Wright, J. and Baldauf, S.L. (2006) The protistan origins of animals and fungi. Mol. Biol. Evol., 23, 93-106.
Taylor, F.J. (1978) Problems in the development of an explicit hypothetical phylogeny of the lower eukaryotes. Biosystems, 10, 67-89.
Tovar, J., Fischer, A. and Clark, C.G. (1999) The mitosome, a novel organelle related to mitochondria in the amitochondrial parasite Entamoeba histolytica. Mol. Microbiol., 32, 1013-1021.
Turner, S. (1997) Molecular systematics of oxygenic photosynthetic bacteria. Pl. Syst. Evol. [Suppl.], 11, 13-52.
Turner, S., Pryer, K.M., Miao, V.P. and Palmer, J.D. (1999) Investigating deep phylogenetic relationships among cyanobacteria and plastids by small subunit rRNA sequence analysis. J. Eukaryot. Microbiol., 46, 327-338.
van der Giezen, M. and Tovar, J. (2005) Degenerate mitochondria. EMBO Rep., 6, 525-530.
van der Giezen, M., Tovar, J. and Clark, C.G. (2005) Mitochondrion-derived organelles in protists and fungi. Int. Rev. Cytol., 244, 175-225.
Wainright, P.O., Hinkle, G., Sogin, M.L. and Stickel, S.K. (1993) Monophyletic origins of the metazoa: an evolutionary link with fungi. Science, 260, 340-342.
Williams, B.A.P. and Keeling, P.J. (2003) Cryptic organelles in parasitic protists and fungi. Adv. Parasitol., 54, 9-67.
Wilson, R.J. (2002) Progress with parasite plastids. J. Mol. Biol., 319, 257-274.
Woese, C.R. (1987) Bacterial evolution. Microbiol. Rev., 51, 221-271.
Woese, C.R. and Fox, G.E. (1977) Phylogenetic structure of the prokaryotic domain: the primary kingdoms. Proc. Natl. Acad. Sci. USA, 74, 5088-5090.
Woese, C.R., Kandler, O. and Wheelis, M.L. (1990) Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc. Natl. Acad. Sci. USA, 87, 4576-4579.
Yoon, H.S., Hackett, J.D., Pinto, G. and Bhattacharya, D. (2002) A single, ancient origin of the plastid in the Chromista. Proc. Natl. Acad. Sci. USA, 99, 15507-15512.