Russ McCracken: HLA-DM (original) (raw)
This web page was produced as an assignment for an undergraduate course at Davidson College
HLA-DM
Introduction
The ability of the immune system to recognize and defend against the introduction of foreign antigens is partially dependent upon the ability of the host's major histocompatability complex (MHC) class II, which presents peptides degraded in intracellular vesicles to circulating CD4 T cells. HLA-DM is an immunological protein vital to the proper loading and presentation of these peptides in macrophages, immature dendritic cells, B cells, and other antigen presenting cells. The genes for this MHC II-like molecule are located in the MHC II region of human chromosome 6 (Janeway et al., 2005). This region contains intron, exon, and promoter sequences that encode for both α and β subunits similar to those comprising MHC II (Busch et al., 1998). However, the major difference between these two molecules is that HLA-DM has a closed binding groove and therefore lacks the ability to bind peptides; instead, it interacts with MHC II to catalyze antigen peptide binding and realease (Zarutskie et al., 2001). Following transcription and translation, the HLA-DM subunits are assembled in the endoplasmic reticulum and directed to the endosomal MHC II compartment (MIIC) by the tyrosine-based motif, YTPL, which is present in the HLA-DMβ cytoplasmic tail. MIIC will eventually fuse with a second endosomal vesicle containing an MHC II:invariant chain complex, at which time HLA-DM will begin its role as an MHC II peptide exchange molecule (Marks et al., 1995).
Figure 1. Jmol image of HLA-DM. Image from the Protein Source Data Bank.

Figure 2. HLA-DM α and β subunits forming a closed peptide binding groove. Image from the Protein Source Data Bank.
The MHC II : Invariant Chain Complex
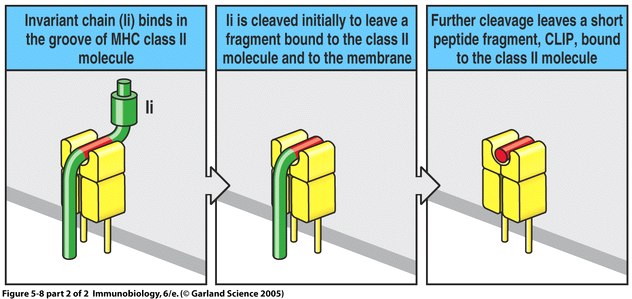
In order to understand the role of HLA-DM in peptide exchange, one must first have an understanding of the MHC:invariant chain complex occuring in the endosomal vesicles seperate from MIIC. The invariant chain (Ii) is a protein which binds the peptide binding groove of MHC II immediately following its assembly in the endoplasmic reticulum. This provides for the preservation of MHC until the vesicle fuses with MIIC and antigen peptides are introduced. Once the MHC:Ii vesicle fuses with MIIC, the portions of Ii outside the MHC II binding groove are cleaved by cathepsin to leave CLIP (class II-associated invariant chain peptide). At this time, HLA-DM is free to begin functioning (Janeway et al., 2005).
Figure 3. The binding and subsequent cleavage of the invariant chain to leave CLIP. Image from Janeway et al., 2005.
HLA-DM Function
HLA-DM has three main functions: it causes the dissociation of CLIP from the peptide binding groove of MHC II, stabalizes and prevents degradation of the empty MHC II, and facilitates the binding of antigen fragments to the open, stabalized binding groove (Janeway et al., 2005). Through a combination of these three roles, HLA-DM actively regulates which peptides are allowed to bind to MHC II and present on the antigen presenting cell surface through a process known as peptide editing (Anderson and Gorski, 2003). The peptide editing mechanism works to ensure the presentation of only the protein fragments most relevant for eliciting an immune response. HLA-DM relies on the total peptide binding energy as well as interaction with HLA-DR of MHC II as its main methods of selection for certain peptide fragments (Ullrich, 1997). High affinity peptides with high binding energy are more stable in the MHC binding groove while HLA-DM is present (Anderson and Gorski, 2003). Furthermore, HLA-DM binding to MHC II molecules as well as catalytic peptide exchange are increased when the pH of the endosomal compartment is in the range of 4.5-5.5 (Busch et al., 1998).
Studies of the interaction between HLA-DM and DR have shown that although they are not directly complexed, these molecules present their hydrophobic domains to one another, effectively shielding these domains from the surrounding aqueous environment. Contact of this nature adds stability to the DM-DR conformation, allowing HLA-DM to exchange peptides more easily. This concept explains the heightened preference of HLA-DM for the MHC II:CLIP complex which has a larger hydrophobic surface area than the MHC II:antigen peptide complex, thus more strongly attracting and holding on to HLA-DM for CLIP release and MHC stabalization. This hydophobic domain presentation explains the increased catalytic activity of HLA-DM at pH 4.5-5.5 because within this range there is a uncovering of additional domains on both HLA-DM and MHC II (Ullrich et al., 1997).
The factors regulating the stability of DM and MHC II along with the disruption of hydrogen bonds along the binding groove to cause peptide release are essential to HLA-DM's role as a peptide editor (Belmares et al., 2002). This process is conducted in the early endosomal stage, and is dependent upon the ratio of HLA-DM to HLA-DO, a DM negative regulator (Pathak et al., 2001). DO binds to DM and inhibits proper functioning in peptide exchange (Janeway et al., 2005).
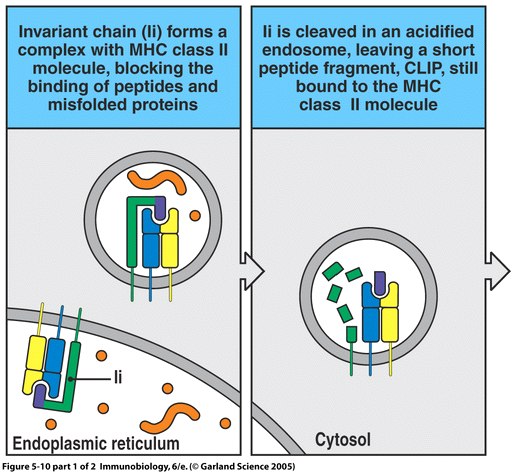
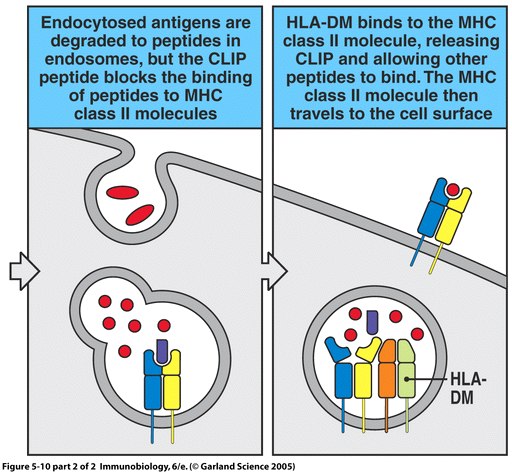
Figure 4. The process of MHC II peptide loading involves Ii chain binding, antigen protein introduction, and HLA-DM peptide editing. Image from Janeway et al., 2005.
HLA-DM Deficiency
Zarutskie et al. (2001) report that when HLA-DM is absent or rendered non-functional due to mutation, there is an observed increase in MHC II:CLIP complex presented on the antigen presenting cell surface. A finding such as this suggests much about the narrow role of HLA-DM as a protein exchange molecule rather than one involved in MHC transport to the cell surface. More importantly, however, a deficiency such as this holds severe consequences for the immune system’s ability to recognize and express the presence of foreign, potentially harmful molecules within the body. Non-functional HLA-DM is unable to dissociate CLIP from MHC II which is therefore not give a chance to bind with antigen peptide and present it to CD4 T cells to elicit an immune response.
No known diseases of the body directly target the functioning of HLA-DM as a method through which to escape immune system detection; however, there are specific instances where HLA-DM is affected or altered along a more general pathway of immune suppression. One example of such an occurrence is illustrated with infection by Herpes Simplex Virus Type I (HSV-1). This virus strives to alter the MHC II pathway to the point that its B cells can no longer present the viral peptide, preventing the activation of helper T cells. Specifically, this virus reduces the production of Ii and its viral envelope glycoprotein B (gB) disrupts proper distribution of HLA-DM, as well as other immunological proteins. Glycoprotein B binds HLA-DR of the MHC II peptide groove, which prevents MHC migration to the cell surface, and disables peptide editing by HLA-DM (Neumann et al., 2003). In this case, the absence of other components in the presentation process inhibit the functioning of HLA-DM. Furthermore, although still debated in the immunological community, it is thought that HLA-DM peptide editing may in specific instances lead to the activation of autoreactive T cells. Lich et al. (2003) suggest that changing HLA-DM concentration levels within antigen presenting cells may cause autoantigens to present on the cell surface, potentially leading to autoimmune disorders such as type I diabetes.
Drug Interaction
A search of OMIM revealed no indication of drug interactions with HLA-DM.
References
Anderson, MW and Gorski, J (2003) Cutting Edge: TCR Contacts as Anchors: Effects on Affinity and HLA-DM Stability. The Journal of Immunology. 171, 5683-5687.
Belmares, MP; Busch, R; Wucherpfennig, KW; McConnell, HM; Mellins, ED (2002) Structural Factors Contributing to DM Susceptibility of MHC Class II/Peptide Complexes. The Journal of Immunology. 169, 5109-5117.
Busch, R; Reich, Z; Zaller, DM; Sloan, V; Mellins, ED (1998) Secondary Structure Composition and pH-dependent Conformational Changes of Soluble Recombinant HLA-DM. The Journal of Biological Chemistry. 273, 27557-27564.
Janeway, CA; Travers, P; Walport, M; Schlomchik, MJ (2005) Immunobiology. Garland Science: New York, NY: 178-181.
Lich, JD; Jayne, JA; Zhou, D; Elliott, JF; Blum, JS (2003) Editing of an Immunodominant Epitope of Glutamate Decarboxylase by HLA-DM. The Journal of Immunology. 171, 853-859.
Marks, MS; Roche, PA; Donselaar, E; Woodruff, L; Peters, PJ; Bonifacino, JS (1995) A Lysosomal Targetting Signal in the Cytoplasmic Tail of the β Chain Directs HLA-DM to MHC Class II Compartments. The Journal of Cell Biology. 131, 351-369.
Neumann, J; Eis-Hubinger, AM; Koch, N (2003) Herpes Simplex Virus Type 1 Targets the MHC Class II Processing Pathway for Immune Evasion. The Journal of Immunology. 171, 3075-3083.
OMIM: http://www.ncbi.nlm.nih.gov/omim/ . Accessed on March 15, 2006.
Pathak, SS; Lich, JD; Blum, JS (2001) Cutting Edge: Editing of Recycling Class II:Peptide Complexes by HLA-DM. The Journal of Immunology. 167, 632-635.
Protein Source Data Bank: http://www.rcsb.org/pdb/explore.do?structureId=1HDM. Accessed on March 16, 2006.
Ullrich, HJ; Doring, K; Gruneberg, U; Jahnig, F; Trowsdale, J; Marieke Van Ham, S (1997) Interaction between HLA-DM and HLA-DR Involves Regions that Undergo Conformational CHanges at Lysosomal pH. Proceedings of the National Academy of Science of the United States of America. 94, 13163-13168.
Zarutskie, JA; Busch, R; Zavala-Ruiz, Z; Rushe, M, Mellins, ED; Stern, LJ (2001) The Kinetic Basis of Peptide Exchange Catalysis by HLA-DM. Proceedings of the National Academy of Sciences of the United States of America. 98, 12450-12455.
© Copyright 2006 Department of Biology, Davidson College, Davidson, NC 28036
Send comments, questions, and suggestions to: rumccracken@davidson.edu