Toxic Epidermal Necrolysis (TEN): Background, Pathophysiology, Etiology (original) (raw)
Background
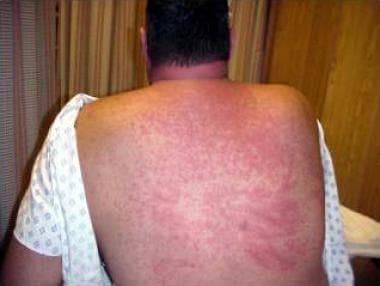
Toxic epidermal necrolysis (TEN) is a potentially life-threatening dermatologic disorder characterized by widespread erythema, necrosis, and bullous detachment of the epidermis and mucous membranes, resulting in exfoliation and possible sepsis and/or death. Mucous membrane involvement can result in gastrointestinal hemorrhage, respiratory failure, ocular abnormalities, and genitourinary complications.
Diffuse maculopapular rash in toxic epidermal necrolysis (TEN).
TEN is most commonly drug induced. However, the disorder occasionally has other potential etiologies, including infection including COVID-19, [1, 2] malignancy, and vaccinations, including following COVID-19 vaccination. [3] TEN is idiosyncratic, and its occurrence is not easily predicted.
Some authors believe that Stevens-Johnson syndrome (SJS; also known as erythema multiforme major) is a manifestation of the same process involved in TEN, with the latter involving more extensive necrotic epidermal detachment. TEN involves more than 30% of the body surface, whereas SJS involves less than 10%.
A classification system, based largely on the extent of epidermal detachment and morphology of the skin lesions, aids in differentiating opposite spectrums of the same disease entity. [4] This system comprises the following:
- TEN with spots
- TEN without spots
- Overlap Stevens-Johnson syndrome and TEN (SJS-TEN)
TEN with spots is defined as widespread, irregularly shaped erythematous or purpuric macules with blistering that occurs on all or part of the macule. Blisters become more confluent and result in detachment of the epidermis and erosions on greater than 30% of the body surface area. Mucosal surfaces are usually involved.
TEN without spots is defined as widespread, large areas of erythema with no discrete lesions. Epidermal detachment is greater than 10% of the body surface area. Mucosal surfaces are usually involved.
Overlap Stevens-Johnson syndrome and TEN (SJS-TEN) is characterized by widespread, irregularly shaped erythematous or purpuric macules with blistering that occurs on all or part of the macule. Blisters become confluent and result in detachment of the epidermis and erosions on 10-29% of the body surface area.
TEN is a clinical diagnosis, confirmed by histopathologic analysis of lesional skin. The mainstay of treatment is supportive care until the epithelium regenerates. Early transfer of patients to a burn or intensive care unit has been shown to reduce the risk of infection, mortality rate, and length of hospitalization.
Historical background
Alan Lyell provided an early description of TEN in 1956, describing the condition as "an eruption resembling scalding of the skin." [5] This dermatologic condition is characterized by extensive epidermal loss suggestive of severe scalding. In that same year, Lang and Walker reported a case of TEN. [6] The disorder was originally described by Debre et al in 1939 in French as l'erythrodermie bulleuses avec epidermolyse. [7]
Lyell later reclassified the conditions of 2 of his patients as having staphylococcal scalded skin syndrome, [8] which is due to Staphylococcus aureus infection rather than to a probable drug hypersensitivity-type reaction. Histopathologic analysis of the skin remains the main tool for discrimination between the two conditions.
Patient education
Patients who have had TEN must be counseled regarding the likely causative medication or agent, and they must be advised to avoid these medications and those of the same or similar classes in the future. Cross-reactivity may occur with agents that chemically resemble the causative agent. Patients must call a pharmacist whenever they start a new prescription.
The patient should be educated on any medical treatment that is still necessary and on sun protection to prevent post-inflammatory hyperpigmentation. There are several long-term complications in patients surviving TEN and the patient should be educated on the prognosis and the need for follow-up. At discharge, patients should be asked to complete the General Health Questionnaire-12 and scores of 2 or greater should trigger a referral to a psychiatrist or psychologist. Information on available support groups should be given to the patient. [9]
Genetic factors are suspected in drug-induced blistering disorders, and blood relatives of the patient also should not use the suspected drug.
For patient education information, see the Skin, Hair, and Nails Center, as well as Life-Threatening Skin Rashes.

Pathophysiology
The pathophysiology of TEN has not been fully elucidated; however, various theories have received wide acceptance. TEN is believed to be an immune-related cytotoxic reaction aimed at destroying keratinocytes that express a foreign antigen.
TEN mimics a hypersensitivity reaction, with its characteristic delayed reaction to an initial exposure and an increasingly rapid reaction with repeated exposure.
The widespread epidermolysis and blistering of TEN results from keratinocyte apoptosis—an organized series of biochemical reactions leading to cell changes and cell death. [10] However, the number of inflammatory T cells in the skin of patients with TEN is variable and perhaps too low to explain the widespread destruction. [11]
There is evidence supporting several immunopathologic pathways leading to keratinocyte apoptosis in TEN, including the following:
- Fas ligand activation on keratinocyte membranes leading to death receptor–mediated apoptosis [12]
- Release of destructive proteins (perforin and granzyme B) from cytotoxic T lymphocytes (CTLs) generated from an interaction with cells expressing major histocompatability complex (MHC) class I [13]
- Overproduction of T cell– and/or macrophage-derived cytokines (interferon-γ [INF-γ], tumor necrosis factor-α [TNF-α], and various interleukins) [14, 15]
- Drug-induced secretion of granulysin from CTLs, natural killer cells, and natural killer T cells [16]
Precisely how the inciting agent triggers the proposed pathways is yet to be elucidated.

Etiology
TEN can be induced by drugs or infection or can be idiopathic. Medications are the major precipitating cause. Numerous medications have been implicated, [17] including antibiotics, antiepileptic drugs, nonsteroidal anti-inflammatory drugs (NSAIDs), ampicillin, allopurinol, corticosteroids (topical and systemic), and the antiretroviral drugs nevirapine and abacavir. [18, 19] TEN usually occurs during the first course of therapy with a drug (ie, without sensitization).
Antibacterial drugs associated with TEN include the following:
- Sulfonamides (4.5 cases per million users per week)
- Chloramphenicol
- Macrolides (eg, azithromycin, clarithromycin, erythromycin, telithromycin) [20]
- Penicillins
- Quinolones (eg, ciprofloxacin, [21] trovafloxacin [22] )
Anticonvulsants associated with TEN include the following:
- Phenobarbital
- Phenytoin [23]
- Carbamazepine
- Valproic acid
- Lamotrigine
TEN in patients taking anticonvulsants has most often been reported within 2 months of starting the drug. However, some cases associated with long-term use have been reported.
NSAIDs associated with TEN include the following:
- Phenylbutazone and oxybutazone - Implicated most commonly, although they are no longer available in the United States
- Oxicams (eg, piroxicam, tenoxicam) - Implicated more often than other NSAIDs
- Ibuprofen
- Indomethacin
- Sulindac
- Tolmetin
With allopurinol, risk is not constant over time. Patients have a 5.5 relative risk. However, during the first 2 months of therapy, the relative risk is 52, and the long-term therapy risk is 0.5.
No laboratory test is able to confirm a specific drug etiology. A causal link is suggested when TEN occurs during the first 4 weeks of medication therapy, usually between 1 and 3 weeks. Drugs with longer half-lives and those with circulating active metabolites may result in more fulminant disease.
Infectious agents (ie, Mycoplasma pneumoniae, herpes virus, hepatitis A), immunizations (eg, meningococcal vaccine), and bone marrow or solid organ transplantation have also been associated with TEN. [24]

Epidemiology
In the United States, the annual frequency of TEN is reported to be 0.22-1.23 cases per 100,000 population. In the HIV-positive population, the incidence of TEN increases to 1 case per thousand per year. [25]
Worldwide, the average annual incidence of TEN is 0.4-1.3 cases per million population. [26] In 1992, the cumulative incidence of TEN and SJS in Germany was 1.9 cases per million population. A French survey of dermatologists and health care facilities reported an annual incidence of 1 case per million population.
Race-, sex-, and age-related demographics
A genetic predilection toward carbamazepine-induced TEN has been observed in HLA-B*1502–positive patients in mainland southeast Asian people from China to India. The US Food and Drug Administration recommends screening for the HLA-B*1502 allele before initiating carbamazepine in patients of Asian ancestry. [27] The HLA-B*58:01 allele is associated with allopurinol-induced TEN in Han Chinese people. HLA-A*66:01, HLA-B*44:03, and HLA-C*12:03 have been associated with cold medicine-TEN with severe ocular complications in patients who had taken cold medicines in the 1-14 days prior to onset of the disease. The HLA-B*44:03 (odds ratio, 5.50) and HLA-C*12:03 (OR, 8.79) alleles were associated with a less-robust tisk of cold medicine-TEN only in European patients. [28]
Registration databases from Tawain, Thailand, Japan, Malaysia, Singapore, Hong Kong, the Philippines, and mainland China (Fujian) identified that from 1998-2017, greater than 50% of cases of SJS/TENS involved carbamazepine, allopurinol, phenytoin, lamotrigine, and sulfamethoxazole. Oxacarbazepine, sulfasalazine, COX-II inhibitors, and strontium ranelate (not approved by the Food and Drug Administration but used in more than 70 countries as a treatment option for postmenopausal osteoporosis) have been identified as potential new causes of SJS/TENS in Asian patients. Other medications known to cause SJS/TENS such as oseltamavir, terbinafine, and sorafenib, were not associated with any cases in Asian patients enrolled in these databases. [29]
For unclear reasons, TEN appears to have a predilection for females. The female-to-male ratio is 1.5:1. [30]
TEN may occur in all age groups; however, the mean age of patients with TEN is reported to be between 46 and 63 years. Infection is more commonly implicated as an etiology in children, whereas medication exposure is more common in adults. Elderly persons may be at greater risk because of their tendency to use multiple medications.

Prognosis
The estimated mortality associated with TEN varies widely in different reports, from 10-70%. Outcome depends in part on the quality of care and the rapidity with which treatment is initiated.
The re-epitheliazation period lasts between 1 and 3 weeks, depending on the extent and severity of the clinical picture. [31]
Septicemia and multisystem organ failure are the primary causes of death. Epithelial loss results in vulnerability to bacterial and fungal infections. Sloughing of stratified epithelium of mucosal membranes can result in GI hemorrhage, respiratory failure, ocular abnormalities, and genitourinary lesions. Significant fluid loss from extensive skin exfoliation and an inability to tolerate oral intake can lead to hypovolemia, acute tubular necrosis, and shock.
Age, extent of epidermal involvement, and serum urea level are said to be the most important prognostic factors in TEN. [32] Mortality rates in children are much lower than in adults. [33] Elderly patients have a poor prognosis.
Other negative prognostic factors include the following:
- Elevated blood urea nitrogen (BUN) and serum creatinine levels
- Respiratory failure
- Multiple drugs
- Thrombocytopenia
- Lymphopenia
- Neutropenia
- Leukopenia
- Sepsis
Severity-of-illness score
A severity-of-illness score that estimates the risk of death in TEN (SCORTEN) has been developed and validated. [34] Each of the following independent prognostic factors is given a score of 1:
- Age >40 years
- Heart rate >120 beats per minute
- Cancer or hematologic malignancy
- Involved body surface area >10%
- Blood urea nitrogen level >10 mmol/L (28 mg/dL)
- Serum bicarbonate level < 20 mmol/L (20 mEq/L)
- Blood glucose level >14 mmol/L (252 mg/dL)
The number of positive criteria and the corresponding mortality rates are as follows:
- 0: 1 to 3%
- 2: 12%
- 3: 35%
- 4: 58%
- 5 or more: 90%
Sequelae
Major sequelae are generally limited to the affected organ systems (ie, the skin and mucosal membranes).
Cutaneous sequelae of TEN include the following:
- Changes in skin pigment (hypopigmentation or hyperpigmentation; sun exposure must be avoided for several months because ultraviolet light can worsen hyperpigmentation; sunblock is recommended)
- Nail loss and nail dystrophy
- Hypohidrosis (inability to sweat)
- Scarring, alopecia, and hypertrophic scarring
- Dermal desiccation, causing deep dermal wounds
- Chronic xerostomia
- Esophageal strictures
- Vulvovaginal synechiae
- Phimosis [35]
- Chronic erosion of the mouth and genitalia
Ocular complications generally result from abnormal keratinization of the tarsal conjunctiva. A Sjogrenlike syndrome with decreased lacrimal secretion causes dry eye and predisposes to corneal abrasions and corneal scarring with neovascularization. In addition, patients have been reported to have palpebral synechiae, entropion, or symblepharon (adhesion of the eyelids). [36, 37]
A study by Power and colleagues found that 50% of patients with TEN developed ocular complications. [38] Patients treated with steroids fared no better than those treated without steroids. Therefore, TEN remains a common cause of visual loss in a significant number of patients. Ultimately, 5-9% of patients can become blind as a result of some of these complications.
Up to 28% of women with vaginal involvement in the acute phase suffered from long-term sequelae, including burning sensation, dyspareunia, and postcoital bleeding from adhesions and stenosis. Male genitalia synechiae may require circumcision. [39]

- Aulakh S, Arora R, Sarangal R, Chopra D. Increased Predisposition of SJS TEN in COVID-19 Patients, Presenting as Post COVID Complication: Report of Two Cases. Indian Dermatol Online J. 2022 Mar-Apr. 13 (2):237-239. [QxMD MEDLINE Link].
- Jouhar L, Yahya M, Elsiddiq S. Toxic Epidermal Necrolysis associated with COVID-19 infection: A case report. Clin Case Rep. 2022 Mar. 10 (3):e05565. [QxMD MEDLINE Link].
- Mardani M, Mardani S, Asadi Kani Z, Hakamifard A. An extremely rare mucocutaneous adverse reaction following COVID-19 vaccination: Toxic epidermal necrolysis. Dermatol Ther. 2022 May. 35 (5):e15416. [QxMD MEDLINE Link].
- Bastuji-Garin S, Rzany B, Stern RS, Shear NH, Naldi L, Roujeau JC. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol. 1993 Jan. 129(1):92-6. [QxMD MEDLINE Link].
- LYELL A. Toxic epidermal necrolysis: an eruption resembling scalding of the skin. Br J Dermatol. 1956 Nov. 68(11):355-61. [QxMD MEDLINE Link].
- LANG R, WALKER J. An unusual bullous eruption. S Afr Med J. 1956 Feb 4. 30(5):97-8. [QxMD MEDLINE Link].
- Debre R, Lemy M, Lamotte M. L'erythrodermie bulleuses avec epidermolyse. Bull Soc Pediatr (Paris). 1939;37:231-238:
- Lyell A. Toxic epidermal necrolysis (the scalded skin syndrome): a reappraisal. Br J Dermatol. 1979 Jan. 100(1):69-86. [QxMD MEDLINE Link].
- Dodiuk-Gad RP, Chung WH, Valeyrie-Allanore L, Shear NH. Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: An Update. Am J Clin Dermatol. 2015 Dec. 16 (6):475-93. [QxMD MEDLINE Link].
- Pereira FA, Mudgil AV, Rosmarin DM. Toxic epidermal necrolysis. J Am Acad Dermatol. 2007 Feb. 56(2):181-200. [QxMD MEDLINE Link].
- Nagy N, McGrath JA. Blistering skin diseases: a bridge between dermatopathology and molecular biology. Histopathology. 2010 Jan. 56(1):91-9. [QxMD MEDLINE Link].
- Abe R, Shimizu T, Shibaki A, Nakamura H, Watanabe H, Shimizu H. Toxic epidermal necrolysis and Stevens-Johnson syndrome are induced by soluble Fas ligand. Am J Pathol. 2003 May. 162(5):1515-20. [QxMD MEDLINE Link]. [Full Text].
- Posadas SJ, Padial A, Torres MJ, Mayorga C, Leyva L, Sanchez E, et al. Delayed reactions to drugs show levels of perforin, granzyme B, and Fas-L to be related to disease severity. J Allergy Clin Immunol. 2002 Jan. 109(1):155-61. [QxMD MEDLINE Link].
- Paul C, Wolkenstein P, Adle H, Wechsler J, Garchon HJ, Revuz J, et al. Apoptosis as a mechanism of keratinocyte death in toxic epidermal necrolysis. Br J Dermatol. 1996 Apr. 134(4):710-4. [QxMD MEDLINE Link].
- Nassif A, Moslehi H, Le Gouvello S, Bagot M, Lyonnet L, Michel L, et al. Evaluation of the potential role of cytokines in toxic epidermal necrolysis. J Invest Dermatol. 2004 Nov. 123(5):850-5. [QxMD MEDLINE Link].
- Chung WH, Hung SI, Yang JY, Su SC, Huang SP, Wei CY, et al. Granulysin is a key mediator for disseminated keratinocyte death in Stevens-Johnson syndrome and toxic epidermal necrolysis. Nat Med. 2008 Dec. 14(12):1343-50. [QxMD MEDLINE Link].
- Roujeau JC, Kelly JP, Naldi L, Rzany B, Stern RS, Anderson T, et al. Medication use and the risk of Stevens-Johnson syndrome or toxic epidermal necrolysis. N Engl J Med. 1995 Dec 14. 333(24):1600-7. [QxMD MEDLINE Link].
- Das S, Roy AK, Biswas I. A six-month prospective study to find out the treatment outcome, prognosis and offending drugs in toxic epidermal necrolysis from an urban institution in kolkata. Indian J Dermatol. 2013 May. 58(3):191-3. [QxMD MEDLINE Link]. [Full Text].
- Thammakumpee J, Yongsiri S. Characteristics of toxic epidermal necrolysis and stevens-johnson syndrome: a 5-year retrospective study. J Med Assoc Thai. 2013 Apr. 96(4):399-406. [QxMD MEDLINE Link].
- Pejčić AV. Stevens-Johnson syndrome and toxic epidermal necrolysis associated with the use of macrolide antibiotics: a review of published cases. Int J Dermatol. 2021 Jan. 60 (1):12-24. [QxMD MEDLINE Link].
- Moshfeghi M, Mandler HD. Ciprofloxacin-induced toxic epidermal necrolysis. Ann Pharmacother. 1993 Dec. 27(12):1467-9. [QxMD MEDLINE Link].
- Ernst ME, Ernst EJ, Klepser ME. Levofloxacin and trovafloxacin: the next generation of fluoroquinolones?. Am J Health Syst Pharm. 1997 Nov 15. 54(22):2569-84. [QxMD MEDLINE Link].
- Creamer JD, Whittaker SJ, Kerr-Muir M, Smith NP. Phenytoin-induced toxic epidermal necrolysis: a case report. Clin Exp Dermatol. 1996 Mar. 21(2):116-20. [QxMD MEDLINE Link].
- Chahal D, Aleshin M, Turegano M, Chiu M, Worswick S. Vaccine-induced toxic epidermal necrolysis: A case and systematic review. Dermatol Online J. 2018 Jan 15. 24 (1):[QxMD MEDLINE Link].
- Lissia M, Mulas P, Bulla A, Rubino C. Toxic epidermal necrolysis (Lyell's disease). Burns. 2010 Mar. 36(2):152-63. [QxMD MEDLINE Link].
- Abood GJ, Nickoloff BJ, Gamelli RL. Treatment strategies in toxic epidermal necrolysis syndrome: where are we at?. J Burn Care Res. 2008 Jan-Feb. 29(1):269-76. [QxMD MEDLINE Link].
- Ferrell PB Jr, McLeod HL. Carbamazepine, HLA-B*1502 and risk of Stevens-Johnson syndrome and toxic epidermal necrolysis: US FDA recommendations. Pharmacogenomics. 2008 Oct. 9(10):1543-6. [QxMD MEDLINE Link]. [Full Text].
- Dunn J. Genetics and Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis: What Have We Learned?. JAMA Ophthalmol. 2017 Apr 1. 135 (4):361-362. [QxMD MEDLINE Link].
- Wang YH, Chen CB, Tassaneeyakul W, Saito Y, et al. The Medication Risk of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in Asians: The Major Drug Causality and Comparison With the US FDA Label. Clin Pharmacol Ther. 2019 Jan. 105 (1):112-120. [QxMD MEDLINE Link].
- Struck MF, Hilbert P, Mockenhaupt M, Reichelt B, Steen M. Severe cutaneous adverse reactions: emergency approach to non-burn epidermolytic syndromes. Intensive Care Med. 2010 Jan. 36(1):22-32. [QxMD MEDLINE Link].
- Estrella-Alonso A, Aramburu JA, González-Ruiz MY, Cachafeiro L, Sánchez MS, Lorente JA. Toxic epidermal necrolysis: a paradigm of critical illness. Rev Bras Ter Intensiva. 2017 Oct-Dec. 29 (4):499-508. [QxMD MEDLINE Link].
- Revuz J, Roujeau JC, Guillaume JC, Penso D, Touraine R. Treatment of toxic epidermal necrolysis. Créteil's experience. Arch Dermatol. 1987 Sep. 123(9):1156-8. [QxMD MEDLINE Link].
- Koh MJ, Tay YK. An update on Stevens-Johnson syndrome and toxic epidermal necrolysis in children. Curr Opin Pediatr. 2009 Aug. 21(4):505-10. [QxMD MEDLINE Link].
- Bastuji-Garin S, Fouchard N, Bertocchi M, Roujeau JC, Revuz J, Wolkenstein P. SCORTEN: a severity-of-illness score for toxic epidermal necrolysis. J Invest Dermatol. 2000 Aug. 115(2):149-53. [QxMD MEDLINE Link].
- Aziza Y, Harada K, Ueta M, Fukuoka H, Kinoshita S, Sotozono C. Challenges in the management of bilateral eyelid closure in Stevens-Johnson Syndrome. Am J Ophthalmol Case Rep. 2022 Jun. 26:101473. [QxMD MEDLINE Link].
- Magina S, Lisboa C, Leal V, Palmares J, Mesquita-Guimarães J. Dermatological and ophthalmological sequels in toxic epidermal necrolysis. Dermatology. 2003. 207(1):33-6. [QxMD MEDLINE Link].
- Aziza Y, Harada K, Ueta M, Fukuoka H, Kinoshita S, Sotozono C. Challenges in the management of bilateral eyelid closure in Stevens-Johnson Syndrome. Am J Ophthalmol Case Rep. 2022 Jun. 26:101473. [QxMD MEDLINE Link].
- Power WJ, Ghoraishi M, Merayo-Lloves J, Neves RA, Foster CS. Analysis of the acute ophthalmic manifestations of the erythema multiforme/Stevens-Johnson syndrome/toxic epidermal necrolysis disease spectrum. Ophthalmology. 1995 Nov. 102(11):1669-76. [QxMD MEDLINE Link].
- Noe MH, Micheletti RG. Diagnosis and management of Stevens-Johnson syndrome/toxic epidermal necrolysis. Clin Dermatol. 2020 Nov - Dec. 38 (6):607-612. [QxMD MEDLINE Link].
- Brown CS, Defazio JR, An G, O'Connor A, Whitcomb E, Hart J, et al. Toxic Epidermal Necrolysis with Gastrointestinal Involvement: A Case Report and Review of the Literature. J Burn Care Res. 2017 Jan/Feb. 38 (1):e450-e455. [QxMD MEDLINE Link].
- Barrera JE, Meyers AD, Hartford EC. Hypopharyngeal stenosis and dysphagia complicating toxic epidermal necrolysis. Arch Otolaryngol Head Neck Surg. 1998 Dec. 124(12):1375-6. [QxMD MEDLINE Link].
- Bachot N, Roujeau JC. Differential diagnosis of severe cutaneous drug eruptions. Am J Clin Dermatol. 2003. 4(8):561-72. [QxMD MEDLINE Link].
- Szatkowski J, Schwartz RA. Acute generalized exanthematous pustulosis (AGEP): A review and update. J Am Acad Dermatol. 2015 Nov. 73 (5):843-8. [QxMD MEDLINE Link].
- Abadías-Granado I, Palma-Ruiz AM, Cerro PA, Morales-Callaghan AM, et al. Generalized pustular figurate erythema first report in two COVID-19 patients on hydroxychloroquine. J Eur Acad Dermatol Venereol. 2021 Jan. 35 (1):e5-e7. [QxMD MEDLINE Link].
- Schwartz RA, Janniger CK. Generalized pustular figurate erythema: A newly delineated severe cutaneous drug reaction linked with hydroxychloroquine. Dermatol Ther. 2020 May. 33 (3):e13380. [QxMD MEDLINE Link].
- Patel S, John AM, Handler MZ, Schwartz RA. Fixed Drug Eruptions: An Update, Emphasizing the Potentially Lethal Generalized Bullous Fixed Drug Eruption. Am J Clin Dermatol. 2020 Jun. 21 (3):393-399. [QxMD MEDLINE Link].
- Stingeni L, Bianchi L, Tramontana M, Pigatto PD, Patruno C, Corazza M, et al. Skin tests in the diagnosis of adverse drug reactions. G Ital Dermatol Venereol. 2020 Oct. 155 (5):602-621. [QxMD MEDLINE Link].
- Avakian R, Flowers FP, Araujo OE, Ramos-Caro FA. Toxic epidermal necrolysis: a review. J Am Acad Dermatol. 1991 Jul. 25(1 Pt 1):69-79. [QxMD MEDLINE Link].
- Fromowitz JS, Ramos-Caro FA, Flowers FP. Practical guidelines for the management of toxic epidermal necrolysis and Stevens-Johnson syndrome. Int J Dermatol. 2007 Oct. 46(10):1092-4. [QxMD MEDLINE Link].
- Sharma S, Singh S, Basu S, Shanbhag SS. Chronic Ocular Sequelae and Subsequent Surgical Interventions in Stevens-Johnson Syndrome After Amniotic Membrane Transplantation. Cornea. 2022 May 1. 41 (5):632-634. [QxMD MEDLINE Link].
- Endorf FW, Cancio LC, Gibran NS. Toxic epidermal necrolysis clinical guidelines. J Burn Care Res. 2008 Sep-Oct. 29(5):706-12. [QxMD MEDLINE Link].
- Garcia-Doval I, LeCleach L, Bocquet H, Otero XL, Roujeau JC. Toxic epidermal necrolysis and Stevens-Johnson syndrome: does early withdrawal of causative drugs decrease the risk of death?. Arch Dermatol. 2000 Mar. 136(3):323-7. [QxMD MEDLINE Link].
- Palmieri TL, Greenhalgh DG, Saffle JR, Spence RJ, Peck MD, Jeng JC, et al. A multicenter review of toxic epidermal necrolysis treated in U.S. burn centers at the end of the twentieth century. J Burn Care Rehabil. 2002 Mar-Apr. 23(2):87-96. [QxMD MEDLINE Link].
- Woolum JA, Bailey AM, Baum RA, Metts EL. A Review of the Management of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis. Adv Emerg Nurs J. 2019 Jan/Mar. 41 (1):56-64. [QxMD MEDLINE Link].
- Papp A, Sikora S, Evans M, Song D, Kirchhof M, Miliszewski M, et al. Treatment of toxic epidermal necrolysis by a multidisciplinary team. A review of literature and treatment results. Burns. 2018 Jun. 44 (4):807-815. [QxMD MEDLINE Link].
- Ririe MR, Blaylock RC, Morris SE, Jung JY. Intravenous immune globulin therapy for Stevens-Johnson syndrome/toxic epidermal necrolysis complicated by hemolysis leading to pigment nephropathy and hemodialysis. J Am Acad Dermatol. 2013 May 11. [QxMD MEDLINE Link].
- Chaidemenos GC, Chrysomallis F, Sombolos K, Mourellou O, Ioannides D, Papakonstantinou M. Plasmapheresis in toxic epidermal necrolysis. Int J Dermatol. 1997 Mar. 36(3):218-21. [QxMD MEDLINE Link].
- Kohanim S, Palioura S, Saeed HN, Akpek EK, et al. Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis--A Comprehensive Review and Guide to Therapy. I. Systemic Disease. Ocul Surf. 2016 Jan. 14 (1):2-19. [QxMD MEDLINE Link].
- Seminario-Vidal L, Kroshinsky D, Malachowski SJ, Sun J, et al. Society of Dermatology Hospitalists supportive care guidelines for the management of Stevens-Johnson syndrome/toxic epidermal necrolysis in adults. J Am Acad Dermatol. 2020 Jun. 82 (6):1553-1567. [QxMD MEDLINE Link].
- Sims JR, Kozlova A, Ostrovsky A. Modified technique for sutureless amniotic membrane transplantation in acute Stevens-Johnson syndrome using fibrin sealant. Ocul Surf. 2022 May 26. [QxMD MEDLINE Link].
- Mereniuk A, Jaque A, Jeschke MG, Shear NH. Toxic Epidermal Necrolysis Spectrum Management at Sunnybrook Health Sciences Centre: Our Multidisciplinary Approach After Review of the Current Evidence. J Cutan Med Surg. 2018 Mar/Apr. 22 (2):213-219. [QxMD MEDLINE Link].
- McPherson T, Exton LS, Biswas S, Creamer D, Dziewulski P, Newell L, et al. British Association of Dermatologists' guidelines for the management of Stevens-Johnson syndrome/toxic epidermal necrolysis in children and young people, 2018. Br J Dermatol. 2019 Jul. 181 (1):37-54. [QxMD MEDLINE Link].
- O’Brien KF, Saardi KM, Johnson LS, Shupp JW, Rodic N, Pasieka HB. Recommendations for Prevention of Drug Re-Exposure in Toxic Epidermal Necrolysis. J Drugs Dermatol. 2019 Oct 1. 18 (10):1049-1052. [QxMD MEDLINE Link].
- Gupta LK, Martin AM, Agarwal N, D'Souza P, Das S, Kumar R, et al. Guidelines for the management of Stevens-Johnson syndrome/toxic epidermal necrolysis: An Indian perspective. Indian J Dermatol Venereol Leprol. 2016 Nov-Dec. 82 (6):603-625. [QxMD MEDLINE Link].
- Picard E, Gillis D, Klapholz L, Schreiber L, Engelhard D. Toxic epidermal necrolysis associated with Klebsiella pneumoniae sepsis. Pediatr Dermatol. 1994 Dec. 11(4):331-4. [QxMD MEDLINE Link].
- Jouhar L, Yahya M, Elsiddiq S. Toxic Epidermal Necrolysis associated with COVID-19 infection: A case report. Clin Case Rep. 2022 Mar. 10 (3):e05565. [QxMD MEDLINE Link].
- Mardani M, Mardani S, Asadi Kani Z, Hakamifard A. An extremely rare mucocutaneous adverse reaction following COVID-19 vaccination: Toxic epidermal necrolysis. Dermatol Ther. 2022 May. 35 (5):e15416. [QxMD MEDLINE Link].
- Aulakh S, Arora R, Sarangal R, Chopra D. Increased Predisposition of SJS TEN in COVID-19 Patients, Presenting as Post COVID Complication: Report of Two Cases. Indian Dermatol Online J. 2022 Mar-Apr. 13 (2):237-239. [QxMD MEDLINE Link].
Author
Coauthor(s)
Robert A Schwartz, MD, MPH Professor and Head of Dermatology, Professor of Pathology, Professor of Pediatrics, Professor of Medicine, Rutgers New Jersey Medical School
Robert A Schwartz, MD, MPH is a member of the following medical societies: Alpha Omega Alpha, American Academy of Dermatology, New York Academy of Medicine, Royal College of Physicians of Edinburgh, Sigma Xi, The Scientific Research Honor Society
Disclosure: Nothing to disclose.
Chief Editor
Michael Stuart Bronze, MD David Ross Boyd Professor and Chairman, Department of Medicine, Stewart G Wolf Endowed Chair in Internal Medicine, Department of Medicine, University of Oklahoma Health Science Center; Master of the American College of Physicians; Fellow, Infectious Diseases Society of America; Fellow of the Royal College of Physicians, London
Michael Stuart Bronze, MD is a member of the following medical societies: Alpha Omega Alpha, American College of Physicians, American Medical Association, Association of Professors of Medicine, Infectious Diseases Society of America, Oklahoma State Medical Association, Southern Society for Clinical Investigation
Disclosure: Nothing to disclose.
Additional Contributors
Victor Cohen, PharmD Clinical Coordinator, Department of Emergency Medicine, Maimonides Medical Center, Assistant Professor, Division of Pharmacy Practice, Schwartz College of Pharmacy and Health Sciences
Victor Cohen, PharmD is a member of the following medical societies: American Association of Colleges of Pharmacy, American College of Clinical Pharmacy, American Society of Health-System Pharmacists, Society of Critical Care Medicine
Disclosure: Nothing to disclose.
Acknowledgements
Theodore J Gaeta, DO, MPH, FACEP Clinical Associate Professor, Department of Emergency Medicine, Joan and Sanford Weill Medical College at Cornell University; Vice Chairman and Program Director of Emergency Medicine Residency Program, Department of Emergency Medicine, New York Methodist Hospital; Academic Chair, Adjunct Professor, Department of Emergency Medicine, St George's University School of Medicine
Theodore J Gaeta, DO, MPH, FACEP is a member of the following medical societies: Alliance for Clinical Education, American College of Emergency Physicians, Clerkship Directors in Emergency Medicine, Council of Emergency Medicine Residency Directors, New York Academy of Medicine, and Society for Academic Emergency Medicine
Disclosure: Nothing to disclose.
Gregory P Garra, DO Clinical Assistant Professor, Department of Emergency Medicine, Stony Brook University School of Medicine; Residency Program Director, Department of Emergency Medicine, Stony Brook University Hospital
Gregory P Garra, DO is a member of the following medical societies: American College of Emergency Physicians and Society for Academic Emergency Medicine
Disclosure: Nothing to disclose.
Fred A Lopez, MD Associate Professor and Vice Chair, Department of Medicine, Assistant Dean for Student Affairs, Louisiana State University School of Medicine
Fred A Lopez, MD is a member of the following medical societies: Alpha Omega Alpha, American College of Physicians-American Society of Internal Medicine, Infectious Diseases Society of America, and Louisiana State Medical Society
Disclosure: Nothing to disclose.
Mark L Plaster, MD, JD Executive Editor, Emergency Physicians Monthly
Mark L Plaster, MD, JD is a member of the following medical societies: American Academy of Emergency Medicine and American College of Emergency Physicians
Disclosure: M L Plaster Publishing Co LLC Ownership interest Management position
Jennifer Stellke, DO Resident Physician, Department of Emergency Medicine, Stony Brook University Medical Center
Disclosure: Nothing to disclose.
Francisco Talavera, PharmD, PhD Adjunct Assistant Professor, University of Nebraska Medical Center College of Pharmacy; Editor-in-Chief, Medscape Drug Reference
Disclosure: Medscape Salary Employment
Charles V Sanders, MD Edgar Hull Professor and Chairman, Department of Internal Medicine, Professor of Microbiology, Immunology and Parasitology, Louisiana State University School of Medicine at New Orleans; Medical Director, Medicine Hospital Center, Charity Hospital and Medical Center of Louisiana at New Orleans; Consulting Staff, Ochsner Medical Center
Charles V Sanders, MD is a member of the following medical societies: Alliance for the Prudent Use of Antibiotics, Alpha Omega Alpha, American Association for the Advancement of Science, American Association of University Professors, American Clinical and Climatological Association, American College of Physician Executives, American College of Physicians, American Federation for Medical Research, American Foundation for AIDS Research, American GeriatricsSociety, American Lung Association, American Medical Association, American Society for Microbiology, American Thoracic Society, American Venereal Disease Association, Association for Professionals in Infection Control and Epidemiology, Association of American Medical Colleges, Association of American Physicians, Association of Professors of Medicine, Infectious Disease Society for Obstetrics and Gynecology, Infectious Diseases Societyof America, Louisiana State Medical Society, Orleans Parish Medical Society, Royal Society of Medicine, Sigma Xi, Society of General Internal Medicine, Southeastern Clinical Club, Southern Medical Association, Southern Society for Clinical Investigation, and Southwestern Association of Clinical Microbiology
Disclosure: Baxter International and Johnson & Johnson Royalty Other