Gonadotropin-Releasing Hormone Deficiency in Adults: Practice Essentials, Background, Pathophysiology (original) (raw)
Overview
Practice Essentials
Gonadotropin-releasing hormone (GnRH) is a neurohormone central to initiation of the reproductive hormone cascade. Pulsatile secretion of GnRH from the hypothalamus is key in establishing and maintaining normal gonadal function. Failure of this release results in isolated GnRH deficiency that can be distinguished by partial or complete lack of GnRH–induced luteinizing hormone (LH) pulses, normalization with pulsatile GnRH replacement therapy, and otherwise normal hypothalamic-pituitary neuroanatomy and neurophysiology.
Signs and symptoms
Most physical findings are related to failure of sexual maturation. Affected patients have eunuchoidal body habitus, with arm-span greater than height by 5 cm or more. Secondary sexual characteristics are often absent. Women have little or no breast development, and men have little or no facial hair.
See Presentation for more detail.
Diagnosis
Laboratory studies
Most patients have low serum levels of basal gonadotropins, estrogen/testosterone, and poor response to GnRH stimulation. The GnRH stimulation test using a synthetic GnRH analog, such as buserelin, has been used to differentiate males with gonadotropin deficiency from those with delayed puberty.
Imaging studies
Magnetic resonance imaging appears to be the single best study for the diagnosis of Kallmann syndrome and exclusion of other central nervous system disorders associated with hypogonadotropic hypogonadism.
See Workup for more detail.
Management
The choice of therapy depends on the patient's desire to achieve one or more of the following:
- Secondary sex characteristics
- Fertility
- Bone and muscle mass
See Treatment and Medication for more detail.

Background
Clinicians and scientists have long been intrigued by the findings of olfactory disturbances and concomitant reproductive dysfunction. In 1856, Spanish pathologist Maestre de San Juan noted the association between the failure of testicular development and the absence of the olfactory bulbs. However, the syndrome comprising complete GnRH deficiency and lack of olfactory senses is named Kallmann syndrome (KS) after the American geneticist Kallmann.
In 1944, Kallmann, Schoenfeld, and Barrera were the first to identify a genetic basis to this disorder. [1, 2] In 1954, de Morsier connected the syndrome of hypogonadism and anosmia with the abnormal development of the anterior portion of the brain. [3] KS is a rare disorder that occurs in both sexes. In contrast to KS, GnRH deficiency leading to hypogonadotropic hypogonadism with an intact sense of smell is termed idiopathic hypogonadotropic hypogonadism (IHH). IHH results from dysfunction of GnRH neurons that have developed and migrated properly, whereas KS is caused by defective migration of GnRH neurons to their proper position in the hypothalamus during fetal development. [4]

Pathophysiology
Gonadotropin-releasing hormone neurons
A fundamental understanding of the anatomy, biochemistry, ontogeny, and physiology of GnRH neurons aids in understanding the pathophysiology, diagnosis, and treatment of KS and idiopathic hypogonadotropic hypogonadism (IHH).
Gonadotropin-releasing hormone and gonadotropin-releasing hormone receptors
The decapeptide GnRH is derived from posttranslation processing of a tripartite 92–amino acid (AA) pre-pro-GnRH. The first 23 AA is a signal peptide and the last 56 AA is known as GnRH–associated protein (GAP). GnRH is encoded from a single gene located on the short arm of chromosome 8. Serum levels of GnRH are difficult to obtain due to its short half-life (2-4 min) and complete confinement to the hypophyseal-portal blood supply. Due to the small structure and ease of mutation of GnRH, chemists have created several clinically useful GnRH analogs. GnRH binds with high affinity to cell surface LH and follicle stimulating hormone (FSH) receptors located on the pituitary gonadotrophs. These 7-transmembrane, cell surface G protein-coupled receptors activate phospholipase C (PLC).
PLC leads to the activation of several second messenger molecules, the most important being diacylglycerol (DG) and inositol 1,4,5-trisphosphate (IP3). In turn, DG activates protein kinase C and causes IP3 -stimulated release of calcium ions from intracellular pools. The result is the synthesis and release of FSH and LH from the pituitary gonadotrophs. The released gonadotropins stimulate the gonads to produce steroid hormones, and in the testes, to produce sperm or in the ovaries, to release oocytes. Mutated GnRH receptors (GnRH-R), as predicted by the biochemistry, could result in the clinical manifestations of isolated gonadotropin deficiency. Many factors interact to regulate the synthesis and secretion of GnRH, and to regulate the translation of GnRH receptors; the review of this regulation is beyond the scope of this article.
Ontogeny and function
During fetal development, the migration of GnRH neurons follows a precise path from the olfactory placode to the preoptic area of the hypothalamus in mammals. The olfactory placode is composed of thickened ectoderm that is lateral to the head of the developing embryo and invaginates to form simple olfactory pits on either side of the nasal septum. The lateral epithelium of the olfactory pits gives rise to the olfactory nerves. The medial portion develops into the site of initial GnRH appearance and the terminal nerves. The terminal nerves, ganglionated cranial nerves for which the exact function is unknown, enter the forebrain and serve as a highway for the GnRH neuronal migration. In humans, GnRH neuron migration begins in the 6th week of embryonic development.
Migrating GnRH neurons do not contain neurosecretory vesicles until they reach the area of the arcuate nucleus in the hypothalamus. For this reason, neurons that do not reach the forebrain are unable to secrete GnRH. GnRH neurons have been identified in the fetal hypothalamus at 9 weeks' gestation and are connected to the pituitary portal system by 16 weeks' gestation. At 10 weeks' gestation, gonadotropes are detectable in the pituitary, and by the 12th week, FSH and LH are measurable in the bloodstream. Fetal peripheral blood levels of gonadotropins peak during the second trimester of pregnancy and decrease by term as the negative feedback mechanism develops.
LH pulsatility, which can be measured in the bloodstream, is determined by the precise frequency and amplitude of pulsatile GnRH release; thus, serum LH is used as a marker of GnRH pulsatility. [5]
GnRH is secreted during the neonatal period, resulting in pulsatile LH and FSH secretion, which decreases by age 6 months in boys and by age 1-2 years in girls until puberty. Before the initiation of puberty, GnRH is still secreted in a pulsatile fashion but at reduced amplitude and frequency. The hypothalamic pulse generator, the master regulator of GnRH secretion, is likely suppressed by a mechanism that inhibits GnRH release but not its synthesis.
This theory has been demonstrated in primates, in which GnRH messenger RNA (mRNA) and proteins are abundant in the hypothalamus during an equivalent developmental stage.
The pubertal period is characterized by a predominantly nocturnal increase in both the amplitude and frequency of GnRH–induced LH pulses. Sex steroids are secreted from the gonads in response to this nocturnal increase in gonadotropins. Gonadotropins continue to be secreted in a pulsatile fashion, under the control of pulsatile GnRH release, during adulthood. The mechanism that awakens the pubertal surge of more robust GnRH secretion is not completely understood. Metabolic cues, steroid hormones, neurosteroids, growth factors, and neurotransmitter systems have been implicated, including glutamate, gamma-aminobutyric acid, neuropeptide Y (NPY), opioids, leptin, kisspeptin, and estradiol. [6]
Most studies in males have shown LH pulses to occur every 2 hours; in females, LH (and thus GnRH) pulse frequency varies throughout the menstrual cycle. In the early follicular phase, LH pulse frequency is every 90 minutes and increases to every 60 minutes by the late follicular phase. The LH "surge" that triggers ovulation occurs due to a "switch" from negative to positive feedback of estrogen at the pituitary, leading to a brief burst of pulsatile LH release, which stimulates ovulation. [7] Following ovulation, LH pulse frequency decreases, with frequency ranging from every 4-8 hours during the luteal phase.
Studying gonadotropin-releasing hormone secretion
Studying GnRH physiology in humans and animal models has been challenging. GnRH itself is almost entirely confined to the portal blood supply of the pituitary, thus direct sampling in humans is not feasible, and difficult if not impossible in animal models. Measurements of GnRH in the periphery are inaccurate because of its rapid 2-minute to 4-minute half-life. Much of the information known about GnRH has come from animal studies.
Belchetz and coworkers in the 1970s demonstrated in rhesus monkeys that pulsatile release of GnRH is required for maintaining gonadotrope function. [8] The researchers were able to differentiate between episodic and continuous stimulation by GnRH causing maintenance and desensitization, respectively, of the gonadotrope response.
Another model developed to study GnRH neuron function is immortalized GnRH cell lines. Interestingly, implantation of these cells into the hypothalami of female GnRH–deficient mice restores normal estrus (equivalent of menstrual) cycles. Immortalized GnRH cell lines in culture have provided an important in vitro tool for studying reproductive neuroendocrine function. In vivo studies of GnRH neuron function have also been possible since development of transgenic mouse models in which GnRH neurons are labeled with green fluorescent protein (GnRH-GFP mouse). [9] This model allows GnRH neurons to be visualized in vivo in hypothalamic sections. Studies from this model are elucidating the complex physiology of GnRH neurons, including neuronal firing patterns, neuronal inputs, migratory patterns, and intracellular signaling systems.
Human studies have been limited to frequent sampling studies in healthy and diseased models, the use of pharmacological probes, and genetic studies. As in animals, LH has long been used as a marker of GnRH pulse activity in humans. Most recently, the glycoprotein free alpha subunit (FAS) has been used as a marker due to its correlation with LH. FAS is useful in tracking GnRH because of its 12-minute to 15-minute half-life. In addition to LH and FAS, an estimate of endogenous GnRH can be obtained using GnRH antagonists as probes. Administering a GnRH antagonist induces a GnRH receptor blockade so that the amount of GnRH present is inversely proportional to the amount of LH inhibitor.

Etiology
GnRH deficiency is inherited through autosomal dominant, autosomal recessive, and X-linked transmissions. However, more than two thirds of cases are sporadic. In fact, only 30% of cases of GnRH deficiency are due to mutations in known genes.
Evidence suggests that most familial cases of GnRH deficiency are controlled by autosomal inheritance. In a study of 106 patients with GnRH deficiency at Massachusetts General Hospital, only 21% of familial cases were X-linked. [10] Using isolated congenital anosmia as a marker for KS, X-linked and autosomal recessive transmission was 18% and 32%, respectively. Autosomal dominance accounted for 50% of cases. When delayed puberty was included in the phenotypic analysis, X-linked cases accounted for 11% of cases, whereas autosomal recessive and autosomal dominant cases were 25% and 64%, respectively.
KAL1 gene
The KAL1 gene, described in 1991, is an example of an X-linked gene that encodes anosmin 1, an extracellular glycoprotein that is similar in amino acid structure to molecules involved in neural development, such as protease inhibitors, neurophysins, and neural cell adhesion molecules. [11] Anosmin 1 appears to be important to the migration of the GnRH neurons to their resting place in the hypothalamus. The KAL1 gene is located on the short arm of the X chromosome at Xp22.3. Approximately 10-20% of males with KS have KAL1 gene mutations, and the phenotypes associated with this mutation tends to be more severe and less variable compared to other KS mutations. KAL1 mutations are inherited in an X-linked recessive pattern and produce a syndrome of short stature, intellectual disability, ichthyosis, chondroplasia punctata, and KS.
Most of the data on the KAL1 gene come from studies in chickens. The timing of KAL1 expression in the chicken has aided in understanding the migration defects of GnRH neurons in human KS. KAL1 is expressed in 2 distinctly different periods of embryonic development. KAL1 expression is found in limb buds, facial mesenchyme, and the neurons innervating the extrinsic eye muscles during embryonic development. By embryonic day 5 (of a 21 day incubation period of a chicken), GnRH neurons migrate along the olfactory nerve and penetrate the olfactory bulb by embryonic day 7-8. KAL1 expression is increased in the olfactory bulb by embryonic day 7-8. At embryonic day 9-10, KAL1 expression is up-regulated as synapses are formed between the olfactory nerve and the mitral cell layer.
Studies have demonstrated that neural migration is controlled by factors intrinsic to the olfactory epithelium. When the olfactory placode is destroyed in the chick, KAL1 expression continues in the olfactory bulb, suggesting that KAL1 expression and olfactory nerve innervation are independent of one another. In humans, KAL1 transcripts are not identified at the time of olfactory nerve migration, again suggesting independence between KAL1 expression and olfactory nerve migration. In KS, a defect in neuronal interaction, rather than neural migration, has been suggested. In a study of a 19-week fetus with X-linked KS, the olfactory nerves were shown to have arrested within the meninges, whereas the GnRH neurons were arrested in the forebrain, never reaching the hypothalamus. Both groups of neurons passed through the cribriform plate but arrested prematurely. The KAL1 gene may play a later role, such as controlling the penetration of GnRH neurons into the olfactory bulb.
Without KAL1 and without functioning synaptic connections, the olfactory nerve might atrophy and degenerate, causing the defective GnRH migration.
The KAL1 gene may also play a role in the development of other tissues, such as facial mesenchyme, fibrous and perichondral cells, blood vessels, renal glomeruli, and developing limb buds. In humans, defective KAL1 expression in the cerebellum may be linked to nystagmus and ataxia observed in some patients with KS.
Fibroblast growth factor receptor 1 and fibroblast growth factor 8
There are 2 KS-related loci, KAL1 and KAL2. The former encodes anosmin and is described above. KAL–2 encodes the fibroblast growth factor receptor 1 (FGFR1). Approximately 10% of patients with KS have loss-of-function mutations in FGFR1. [12] The KAL2- associated disorder is inherited in an autosomal dominant manner. The clinical phenotype ranges from severe KS to delayed puberty. [13] Associated features include cleft palate, hearing loss, agenesis of the corpus callosum, and fusion of metacarpal bones. In affected individuals, the lack of smell has a variable penetrance. [14] Anosmin, a product of KAL1 gene, interacts and enhances the signaling of FGFR1. [15] Thus, in FGFR1 heterozygous affected women, the KAL gene, by escaping X-inactivation, may rescue FGFR1 signaling. [16] This effect of X-inactivation likely explains why this condition is more prevalent in males.
In addition to FGFR1, fibroblast growth factor 8 (FGF8) gene mutations have also been associated with KS and IHH, with varying degrees of olfactory and reproductive function. [17] Interestingly, a mouse model of FGF8 deficiency lacks both hypothalamic GnRH neurons and olfactory bulbs, suggesting a role for FGF8 in olfactory and GnRH neuron migration. [18]
Prokineticin 2 and prokineticin 2 receptor genes
Prokineticin 2 (PROK2) and its receptor (PROKR2) are a ligand-receptor pair involved in the development of the olfactory bulbs and GnRH neuron migration. Neurogenesis persists in the olfactory bulb of the adult mammalian brain due to the chemoattractant effect of prokineticin 2 (PROK2). In PROK2 -deficient and PROKR2 -deficient mice, there is a significant reduction in olfactory bulb size and impaired neuronal migration. [19, 20] Mutations in PROK2 and in the receptor (PROKR2) gene have been associated with the development of KS and normosmic IHH, with variable phenotypic severities. [21, 22] In one series, 9% of patients with KS had mutations in either PROK2 or PROKR2. [23] Accompanying phenotypic features include fibrous dysplasia, synkinesia, and epilepsy.
G protein-coupled receptor 54
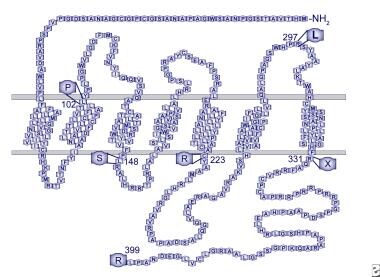
G protein-coupled receptor 54 (GPR54) binds to kisspeptin and its derivatives. This receptor is widely expressed throughout the brain. It has been shown that in a large consanguineous Saudi family with 6 individuals with IHH, a homozygous single nucleotide change in exon 3 of GPR54 was found in all 6 affected individuals, resulting in substitution of a serine for the normal leucine in the second intracellular loop of the receptor (L148S). See the image below.
Human GPR54 receptor model. Mutations identified in patients with idiopathic hypogonadotropic hypogonadism are indicated.
This change did not occur in the homozygous state in any unaffected family members and was not identified in any controls. This 7-transmembrane domain receptor shares highest homology, about 45%, with the galanin subfamily of receptors. The amino acid sequence is highly conserved across species, with 95% homology between the rat and mouse and 82% between mouse and human (98% in the transmembrane domains). [24]
A GPR54-deficient mouse model resulted in a phenotype similar to that seen in humans with KS. These mice have normal hypothalamic GnRH content, but develop IHH that is responsive to GnRH therapy, suggesting that. GnRH neurons continue to synthesize GnRH, but that GPR54 is necessary for GnRH processing and/or secretion. The ligand for GPR54 has been identified as the 54 amino acid protein metastatin. Kisspeptin, a 145-amino acid precursor, gives rise to metastin after cleavage. GPR54 activation advances puberty in rodents and overcomes amenorrhea that is due to starvation or leptin deficiency. Thus, the kisspeptin/metastin/GPR54 system is clearly a major gatekeeper of the pubertal process. [25] Furthermore, the kisspeptin/metastin/GPR54 system plays a major role in the sexual differentiation of the brain and sexual behavior. [26]
Of note, no mutations responsible for KS/IHH have been reported in the KISS1 gene, the gene encoding kisspeptin itself.
Gonadotropin-releasing hormone receptor and gonadotropin-releasing hormone 1
The GnRH receptor is a G protein–coupled receptor, which activates phospholipase C, ultimately mobilizing intracellular calcium. Mutations in the GnRH receptor (GnRHR) have been described in families with hypogonadotropic hypogonadism. One case reports phenotypically normal parents heterozygous for a GnRHR mutation who had a son with normal puberty and normal olfaction but with small (8-mL) testes and an abnormal semen analysis. Their daughter had primary amenorrhea and was infertile. LH pulse frequency was normal but with low amplitude pulsation.
Other reports describe GnRHR mutations causing hypogonadotropic hypogonadism that presents with complete gonadotropin deficiency. An example is a male patient seeking treatment for delayed puberty who presented with no secondary sexual characteristics, cryptorchid testes, low gonadotropins, and low testosterone. The patient did not respond to exogenous GnRH, but treatment with gonadotropins corrected testicular growth and descent, confirming a defect at the level of the GnRHR.
Recently, homozygous mutations in GNRH1, the genetic precursor to GnRH, have been shown to be a rare cause of normosmic IHH. The GNRH1 mutation is inherited in an autosomal recessive pattern. Administration of exogenous pulsatile GnRH restores the hypothalamic-pituitary-gonadal axis in these patients. [27]
DAX1 gene
Adrenal hypoplasia congenita arises from X-linked or autosomal recessive syndromes and presents in infancy with primary adrenal insufficiency. Treatable with steroids, it has resulted in affected adults developing hypogonadotropic hypogonadism. A pituitary origin for one group with hypogonadotropic hypogonadism has been suggested by the failed attempts in those patients to stimulate LH and FSH with pulsatile GnRH. A smaller group has had gonadotropin responses to GnRH therapy, characterizing a hypothalamic-versus-pituitary defect.
The DAX1 gene has been identified at Xp21 as the gene responsible for adrenal hypoplasia congenita. As with the KAL gene, there is a growing body of evidence that DAX mutations result in a wide phenotypic range. These data suggest that DAX1 mutations impair gonadotropin production via defects at the levels of both the pituitary and the hypothalamus. One suggested role for DAX1 is as a "brake" for normal male maturation, while also being necessary for normal adrenal and hypothalamic/pituitary development. DAX1 has been shown to block steroidogenesis in adrenal cells by transcriptional repression. Indeed, loss of function of this repressor may lead to a host of adrenal, hypothalamic, and pituitary abnormalities.
Additionally, steroidogenic factor 1 (SF-1), a nuclear hormone receptor for DAX1 (dosage-sensitive sex reversal, adrenal hypoplasia critical region, on chromosome X, gene 1), plays a regulatory role in adrenal development and development of the hypothalamic-pituitary-gonadal axis. [28] Specifically, SF-1 regulates expression of the p450 steroid hydroxylase genes in the gonads and the adrenal cortex, Mullerian Inhibitory Substance (MIS), the alpha subunit of the gonadotropins, and the beta subunit of LH.
Leptin and leptin receptor
Mutations in either leptin, a cytokine secreted from adipocytes that serves as a central satiety signal and a permissive signal to the reproductive system, or the leptin receptor lead to normosmic hypogonadotropic hypogonadism. Patients with this rare disorder fail to progress through puberty without exogenous leptin administration. The major associated phenotypic feature is obesity due to hyperphagia, which is also attenuated by leptin treatment. [29]
TAC3 and TACR3
Recently, analysis of single nucleotide polymorphisms (SNPs) among families with multiple members affected by IHH have identified autosomal recessive mutations in TAC3 and its receptor, TACR3, as another cause of IHH. [30] TAC3 encodes for neurokinin B, which is the ligand for the neurokinin-3 receptor (TACR3), Patients with mutations in TAC3 or TACR3 have isolated IHH without other phenotypic features, suggesting TAC3 and TACR3 function specifically to promote GnRH release. In fact, neurokinin B is found co-localized with kisspeptin and dynorphin in neurons of the arcuate nucleus of the hypothalamus. These neurons project to the median eminence and are closely opposed to GnRH neurons. Further, GnRH neurons have been shown to express TACR3.
Communication between GnRH neurons and neurons co-expressing kisspeptin, dynorphin, and neurokinin B has been proposed to represent the "GnRH pulse generator." [12] A study by Nagae et al provided direct evidence in rats that kisspeptin/neurokinin B/dynorphin A (KNDy) neurons in the arcuate nucleus of the hypothalamus generate GnRH pulses. [31]
NELF
Nasal epithelial LHRH factor (NELF) is involved in GnRH and olfactory neuronal development and has been implicated in rare cases of IHH. NELF co-localizes with GnRH in stem cells of the olfactory system. Heterozygous mutations have been identified in only 2 reported cases of IHH; thus, the role of NELF as a genetic cause of IHH has not been fully elucidated. [32]
Others
Advances in molecular genetics have lead to the discovery of several additional candidate genes for KS and IHH, and the future holds much more to be discovered in this area. These include SEMA3A, a semaphorin protein family member that is necessary for GnRH neuron development due to its role as a guidance cue for GnRH neuron migration. Lack of SEMA3A signaling in mice causes hypogonadal hypogonadism, and this mutation has been described in one case of human KS. [33] Missense mutations in WDR11, a gene involved in olfactory neuron development and human puberty, have also recently been described in patients with KS and IHH. [34]
Although most cases of IHH have been attributed to single gene defects, Pitteloud et al reported 2 families with this condition but with 2 different gene mutations. [22] With oligogenic mutations resulting in compound heterozygotes, synergistic effects of the mutated genes are hypothesized to result in hypothalamic hypogonadism. Since this initial finding by Pitteloud, several additional cases of oligogenic mutations have been identified in patients with KS and normosmic IHH. Mutations of PROKR2 + GPR54, PROKR2 + GnRHR, PROKR2 + KAL1, PROKR2 + FGFR1, PROKR2 + PROK2, FGFR1 + NELF, FGFR1 + GnRHR, and FGFR1 + FGF8 have been identified.
Interestingly, in addition, one patient normosmic IHH and 3 different mutations has been identified to date (PROKR2, GnRHR, and FGFR1). [35, 21] Furthermore, a study of a large cohort of patients suggests that oligogenicity is the norm in KS and IHH, rather than monogenicity. [36] With the advanced technology available for genetic analysis and with the identification of the human genome, scientists are constantly shedding new light on the complex genetic transmission of KS and IHH. This oligogenic model may explain the phenotypic variability observed within and across families with single gene defects.
Furthermore, cases of adult-onset and reversible IHH suggest that not only are genetic abnormalities involved in the pathogenesis of this disorder but that nongenetic factors may also contribute, such as hormonal and/or environmental factors. These have yet to be elucidated but research is ongoing.
An analysis of a cohort of 81 Greek isolated GnRH deficiency patients found the prevalence of normosmic idiopathic hypogonadotropic hypogonadism higher than Kallmann syndrome (67% to 33%) and putative causal genetic change was discovered in approximately 21% of the cohort. [37]

Epidemiology
United States statistics
The incidence of KS in the United States is 1 case per 10,000 men and 1 case per 50,000 women. The incidence of normosmic IHH is also rare and is estimated to be around 1 case in 70,000 to 1 case in 100,000 individuals.
International statistics
By examining military records, the incidence of KS has been estimated to be between 1 case per 86,000 in Sardinia and 1 case in 10,000 in France. [38]
Race-, sex-, and age-related demographics
Race
Race is not a factor in incidence.
Sex
In a referral population at Massachusetts General Hospital over a 20-year period, the male-to-female ratio was 3.9 to 1. [10]
A spectrum of GnRH deficiency, with various secretory patterns ranging from complete lack of LH pulsatility to diminished pulse amplitude similar to early puberty, occurs in both men and women, contributing to the clinical heterogeneity of the disorder. This suggests that multiple genetic determinants may control the expression of GnRH secretion.
Age
The disease comes to attention when the patient fails to begin puberty and does not develop secondary sexual characteristics.

Prognosis
Morbidity/mortality
These patients are not known to have an increased mortality rate; however, prolonged deficiency in gonadal hormones contributes to increased morbidity and may contribute to the aging process.

Patient Education

- Kallman FJ, Schoenfeld WA. The genetic aspects of primary eunuchoidism. Am J Ment Def. 1944. 158:203-236.
- Cariboni A, André V, Chauvet S, Cassatella D, Davidson K, Caramello A, et al. Dysfunctional SEMA3E signaling underlies gonadotropin-releasing hormone neuron deficiency in Kallmann syndrome. J Clin Invest. 2015 Jun. 125 (6):2413-28. [QxMD MEDLINE Link].
- deMorsier G. Etudes sur les dysraphies cranio-encephaliques. Agenesie des lobes olfactifs (telencephaloschizis lateral) et des comissures calleuse et anterieure (telencephaloschizis median). La dysplasie olfacto-genitale. Schweiz Arch Neurol Neurochir Psychiatr. 1954. 74:309.
- Choi JH, Balasubramanian R, Lee PH, Shaw ND, Hall JE, Plummer L, et al. Expanding the Spectrum of Founder Mutations Causing Isolated Gonadotropin-Releasing Hormone Deficiency. J Clin Endocrinol Metab. 2015 Oct. 100 (10):E1378-85. [QxMD MEDLINE Link].
- Wildt L, Schwilden H, Wesner G. The pulsatile pattern of gonadotropin secretion and follicular development during the menstrual cycle and in women with hypothalamic and hypoandrogenic amenorrhea. Leyendecker G, et al, eds. Brain and Pituitary Peptides. New York, NY: Karger Publishers; 1983. 11-27.
- Plant TM. Hypothalamic control of the pituitary-gonadal axis in higher primates: key advances over the last two decades. J Neuroendocrinol. 2008 Jun. 20(6):719-26. [QxMD MEDLINE Link].
- Foster DL, Karsch FJ. Development of the mechanism regulating the preovulatory surge of luteinizing hormone in sheep. Endocrinology. 1975 Nov. 97(5):1205-9. [QxMD MEDLINE Link].
- Belchetz PE, Plant TM, Nakai Y, Keogh EJ, Knobil E. Hypophysial responses to continuous and intermittent delivery of hypopthalamic gonadotropin-releasing hormone. Science. 1978 Nov 10. 202(4368):631-3. [QxMD MEDLINE Link].
- Suter KJ, Song WJ, Sampson TL, et al. Genetic targeting of green fluorescent protein to gonadotropin-releasing hormone neurons: characterization of whole-cell electrophysiological properties and morphology. Endocrinology. 2000 Jan. 141(1):412-9. [QxMD MEDLINE Link].
- Waldstreicher J, Seminara SB, Jameson JL, et al. The genetic and clinical heterogeneity of gonadotropin-releasing hormone deficiency in the human. J Clin Endocrinol Metab. 1996 Dec. 81(12):4388-95. [QxMD MEDLINE Link].
- Franco B, Guioli S, Pragliola A, et al. A gene deleted in Kallmann's syndrome shares homology with neural cell adhesion and axonal path-finding molecules. Nature. 1991 Oct 10. 353(6344):529-36. [QxMD MEDLINE Link].
- Topaloglu AK, Kotan LD. Molecular causes of hypogonadotropic hypogonadism. Curr Opin Obstet Gynecol. 2010 Aug. 22(4):264-70. [QxMD MEDLINE Link].
- Pitteloud N, Meysing A, Quinton R, et al. Mutations in fibroblast growth factor receptor 1 cause Kallmann syndrome with a wide spectrum of reproductive phenotypes. Mol Cell Endocrinol. 2006 Jul 25. 254-255:60-9. [QxMD MEDLINE Link].
- Massin N, Pecheux C, Eloit C, et al. X chromosome-linked Kallmann syndrome: clinical heterogeneity in three siblings carrying an intragenic deletion of the KAL-1 gene. J Clin Endocrinol Metab. 2003 May. 88(5):2003-8. [QxMD MEDLINE Link].
- Cadman SM, Kim SH, Hu Y, et al. Molecular pathogenesis of Kallmann's syndrome. Horm Res. 2007. 67(5):231-42. [QxMD MEDLINE Link].
- Gonzalez-Martinez D, Hu Y, Bouloux PM. Ontogeny of GnRH and olfactory neuronal systems in man: novel insights from the investigation of inherited forms of Kallmann's syndrome. Front Neuroendocrinol. 2004 Jul. 25(2):108-30. [QxMD MEDLINE Link].
- Trarbach EB, Abreu AP, Silveira LF, et al. Nonsense mutations in FGF8 gene causing different degrees of human gonadotropin-releasing deficiency. J Clin Endocrinol Metab. 2010 Jul. 95(7):3491-6. [QxMD MEDLINE Link].
- Falardeau J, Chung WC, Beenken A, et al. Decreased FGF8 signaling causes deficiency of gonadotropin-releasing hormone in humans and mice. J Clin Invest. 2008 Aug. 118(8):2822-31. [QxMD MEDLINE Link].
- Ng KL, Li JD, Cheng MY, et al. Dependence of olfactory bulb neurogenesis on prokineticin 2 signaling. Science. 2005 Jun 24. 308(5730):1923-7. [QxMD MEDLINE Link].
- Prosser HM, Bradley A, Caldwell MA. Olfactory bulb hypoplasia in Prokr2 null mice stems from defective neuronal progenitor migration and differentiation. Eur J Neurosci. 2007 Dec. 26(12):3339-44. [QxMD MEDLINE Link].
- Dode C, Teixeira L, Levilliers J, et al. Kallmann syndrome: mutations in the genes encoding prokineticin-2 and prokineticin receptor-2. PLoS Genet. 2006 Oct 20. 2(10):e175. [QxMD MEDLINE Link].
- Pitteloud N, Zhang C, Pignatelli D, et al. Loss-of-function mutation in the prokineticin 2 gene causes Kallmann syndrome and normosmic idiopathic hypogonadotropic hypogonadism. Proc Natl Acad Sci U S A. 2007 Oct 30. 104(44):17447-52. [QxMD MEDLINE Link].
- Dode C, Teixeira L, Levilliers J, et al. Kallmann syndrome: mutations in the genes encoding prokineticin-2 and prokineticin receptor-2. PLoS Genet. 2006 Oct 20. 2(10):e175. [QxMD MEDLINE Link].
- Seminara SB, Hayes FJ, Crowley WF Jr. Gonadotropin-releasing hormone deficiency in the human (idiopathic hypogonadotropic hypogonadism and Kallmann's syndrome): pathophysiological and genetic considerations. Endocr Rev. 1998 Oct. 19(5):521-39. [QxMD MEDLINE Link].
- Navarro VM, Fernandez-Fernandez R, Castellano JM, et al. Advanced vaginal opening and precocious activation of the reproductive axis by KiSS-1 peptide, the endogenous ligand of GPR54. J Physiol. 2004 Dec 1. 561:379-86. [QxMD MEDLINE Link].
- Kauffman AS, Park JH, McPhie-Lalmansingh AA, et al. The kisspeptin receptor GPR54 is required for sexual differentiation of the brain and behavior. J Neurosci. 2007 Aug 15. 27(33):8826-35. [QxMD MEDLINE Link].
- Bouligand J, Ghervan C, Tello JA, Brailly-Tabard S, Salenave S, Chanson P. Isolated familial hypogonadotropic hypogonadism and a GNRH1 mutation. N Engl J Med. 2009 Jun 25. 360(26):2742-8. [QxMD MEDLINE Link].
- Santhakumar A, Balasubramaniam R, Miller M, Quinton R. Reversal of Isolated Hypogonadotropic Hypogonadism: long-term integrity of hypothalamo-pituitary-testicular axis in two men is dependent on intermittent androgen exposure. Clin Endocrinol (Oxf). 2013 Oct 7. [QxMD MEDLINE Link].
- Farooqi IS, Jebb SA, Langmack G, et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med. 1999 Sep 16. 341(12):879-84. [QxMD MEDLINE Link].
- Topaloglu AK, Reimann F, Guclu M, et al. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nat Genet. 2009 Mar. 41(3):354-8. [QxMD MEDLINE Link].
- Nagae M, Uenoyama Y, Okamoto S, et al. Direct evidence that KNDy neurons maintain gonadotropin pulses and folliculogenesis as the GnRH pulse generator. Proc Natl Acad Sci U S A. 2021 Feb 2. 118 (5):[QxMD MEDLINE Link]. [Full Text].
- Miura K, Acierno JS Jr, Seminara SB. Characterization of the human nasal embryonic LHRH factor gene, NELF, and a mutation screening among 65 patients with idiopathic hypogonadotropic hypogonadism (IHH). J Hum Genet. 2004. 49(5):265-8. [QxMD MEDLINE Link].
- Cariboni A, Davidson K, Rakic S, et al. Defective gonadotropin-releasing hormone neuron migration in mice lacking SEMA3A signalling through NRP1 and NRP2: implications for the aetiology of hypogonadotropic hypogonadism. Hum Mol Genet. 2011 Jan 15. 20(2):336-44. [QxMD MEDLINE Link].
- Kim HG, Ahn JW, Kurth I, et al. WDR11, a WD protein that interacts with transcription factor EMX1, is mutated in idiopathic hypogonadotropic hypogonadism and Kallmann syndrome. Am J Hum Genet. 2010 Oct 8. 87(4):465-79. [QxMD MEDLINE Link]. [Full Text].
- Pitteloud N, Durrani S, Raivio T, Sykiotis GP. Complex genetics in idiopathic hypogonadotropic hypogonadism. Front Horm Res. 2010. 39:142-53. [QxMD MEDLINE Link].
- Sykiotis GP, Plummer L, Hughes VA, et al. Oligogenic basis of isolated gonadotropin-releasing hormone deficiency. Proc Natl Acad Sci U S A. 2010 Aug 24. 107(34):15140-4. [QxMD MEDLINE Link]. [Full Text].
- Stamou MI, Varnavas P, Kentrou M, Adamidou F, Voutetakis A, Jing J, et al. Isolated GnRH Deficiency: Genotypic and Phenotypic Characteristics of the Genetically Heterogeneous Greek Population. Eur J Endocrinol. 2016 Nov 24. [QxMD MEDLINE Link].
- Filippi G. Klinefelter's syndrome in Sardinia. Clinical report of 265 hypogonadic males detected at the time of military check-up. Clin Genet. 1986 Oct. 30(4):276-84. [QxMD MEDLINE Link].
- Silveira LF, Latronico AC. Approach to the patient with hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2013 May. 98(5):1781-8. [QxMD MEDLINE Link].
- Colquhoun-Kerr JS, Gu WX, Jameson JL, Withers S, Bode HH. X-linked Kallmann syndrome and renal agenesis occurring together and independently in a large Australian family. Am J Med Genet. 1999 Mar 5. 83(1):23-7. [QxMD MEDLINE Link].
- Jongmans MC, van Ravenswaaij-Arts CM, et al. CHD7 mutations in patients initially diagnosed with Kallmann syndrome--the clinical overlap with CHARGE syndrome. Clin Genet. 2009 Jan. 75(1):65-71. [QxMD MEDLINE Link]. [Full Text].
- Teixeira L, Guimiot F, Dode C, et al. Defective migration of neuroendocrine GnRH cells in human arrhinencephalic conditions. J Clin Invest. 2010 Oct 1. 120(10):3668-72. [QxMD MEDLINE Link]. [Full Text].
- Shiraishi K, Naito K. Fertile eunuch syndrome with the mutations (Trp8Arg and Ile15Thr) in the beta subunit of luteinizing hormone. Endocr J. 2003 Dec. 50(6):733-7. [QxMD MEDLINE Link].
- Makler A, Glezerman M, Lunenfeld B. The fertile eunuch syndrome. An isolated leydig-cell failure?. Andrologia. 1977 Apr-Jun. 9(2):163-70. [QxMD MEDLINE Link].
- Pitteloud N, Boepple PA, DeCruz S, et al. The fertile eunuch variant of idiopathic hypogonadotropic hypogonadism: spontaneous reversal associated with a homozygous mutation in the gonadotropin-releasing hormone receptor. J Clin Endocrinol Metab. 2001 Jun. 86(6):2470-5. [QxMD MEDLINE Link].
- Roth MY, Lin K, Bay K, Amory JK, Anawalt BD, Matsumoto AM, et al. Serum insulin-like factor 3 is highly correlated with intratesticular testosterone in normal men with acute, experimental gonadotropin deficiency stimulated with low-dose human chorionic gonadotropin: a randomized, controlled trial. Fertil Steril. 2013 Jan. 99(1):132-9. [QxMD MEDLINE Link]. [Full Text].
- Wilson DA, Hofman PL, Miles HL, et al. Evaluation of the buserelin stimulation test in diagnosing gonadotropin deficiency in males with delayed puberty. J Pediatr. 2006 Jan. 148(1):89-94. [QxMD MEDLINE Link].
- Sun QH, Zheng Y, Zhang XL, Mu YM. Role of Gonadotropin-releasing Hormone Stimulation Test in Diagnosing Gonadotropin Deficiency in Both Males and Females with Delayed Puberty. Chin Med J (Engl). 2015 20th Sep. 128 (18):2439-2443. [QxMD MEDLINE Link].
- Mishell DR Jr, Nakamura RM, Crosignani PG, et al. Serum gonadotropin and steroid patterns during the normal menstrual cycle. Am J Obstet Gynecol. 1971 Sep. 111(1):60-5. [QxMD MEDLINE Link].
- Stevenson JC. Metabolic effects of the menopause and oestrogen replacement. Baillieres Clin Obstet Gynaecol. 1996 Sep. 10(3):449-67. [QxMD MEDLINE Link].
- Jewelewicz R. New developments in topical estrogen therapy. Fertil Steril. 1997 Jan. 67(1):1-12. [QxMD MEDLINE Link].
- Quinton R, Cheow HK, Tymms DJ, et al. Kallmann's syndrome: is it always for life?. Clin Endocrinol (Oxf). 1999 Apr. 50(4):481-5. [QxMD MEDLINE Link].
- Raivio T, Falardeau J, Dwyer A, et al. Reversal of idiopathic hypogonadotropic hypogonadism. N Engl J Med. 2007 Aug 30. 357(9):863-73. [QxMD MEDLINE Link].
- Ioannidou-Kadis S, Wright PJ, Neely RD, Quinton R. Complete reversal of adult-onset isolated hypogonadotropic hypogonadism with clomiphene citrate. Fertil Steril. 2006 Nov. 86(5):1513.e5-9. [QxMD MEDLINE Link].
- Adams JM, Taylor AE, Schoenfeld DA, Crowley WF Jr, Hall JE. The midcycle gonadotropin surge in normal women occurs in the face of an unchanging gonadotropin-releasing hormone pulse frequency. J Clin Endocrinol Metab. 1994 Sep. 79(3):858-64. [QxMD MEDLINE Link].
- Angelopoulos N, Goula A, Tolis G. The role of luteinizing hormone activity in controlled ovarian stimulation. J Endocrinol Invest. 2005 Jan. 28(1):79-88. [QxMD MEDLINE Link].
- Apter D, Cacciatore B, Alfthan H, Stenman UH. Serum luteinizing hormone concentrations increase 100-fold in females from 7 years to adulthood, as measured by time-resolved immunofluorometric assay. J Clin Endocrinol Metab. 1989 Jan. 68(1):53-7. [QxMD MEDLINE Link].
- Arimura A, Kastin AJ, Gonzalez-Barcena D, et al. Disappearance of LH-releasing hormone in man as determined by radioimmunoassay. Clin Endocrinol (Oxf). 1974 Oct. 3(4):421-5. [QxMD MEDLINE Link].
- Backstrom CT, McNeilly AS, Leask RM, Baird DT. Pulsatile secretion of LH, FSH, prolactin, oestradiol and progesterone during the human menstrual cycle. Clin Endocrinol (Oxf). 1982 Jul 1. 17(1):29-42. [QxMD MEDLINE Link].
- Baker HW, Santen RJ, Burger HG, et al. Rhythms in the secretion of gonadotropins and gonadal steroids. J Steroid Biochem. 1975 May. 6(5):793-801. [QxMD MEDLINE Link].
- Bardin CW, Ross GT, Rifkind AB, Cargille CM, Lipsett MB. Studies of the pituitary-Leydig cell axis in young men with hypogonadotropic hypogonadism and hyposmia: comparison with normal men, prepuberal boys, and hypopituitary patients. J Clin Invest. 1969 Nov. 48(11):2046-56. [QxMD MEDLINE Link].
- Barkan AL, Reame NE, Kelch RP, Marshall JC. Idiopathic hypogonadotropic hypogonadism in men: dependence of the hormone responses to gonadotropin-releasing hormone (GnRH) on the magnitude of the endogenous GnRH secretory defect. J Clin Endocrinol Metab. 1985 Dec. 61(6):1118-25. [QxMD MEDLINE Link].
- Barnhart KM, Mellon PL. The orphan nuclear receptor, steroidogenic factor-1, regulates the glycoprotein hormone alpha-subunit gene in pituitary gonadotropes. Mol Endocrinol. 1994 Jul. 8(7):878-85. [QxMD MEDLINE Link].
- Barron JL, Millar RP, Searle D. Metabolic clearance and plasma half-disappearance time of D-TRP6 and exogenous luteinizing hormone-releasing hormone. J Clin Endocrinol Metab. 1982 Jun. 54(6):1169-73. [QxMD MEDLINE Link].
- Bartley JA, Miller DK, Hayford JT, McCabe ER. Concordance of X-linked glycerol kinase deficiency with X-linked congenital adrenal hypoplasia. Lancet. 1982 Oct 2. 2(8301):733-6. [QxMD MEDLINE Link].
- Behre HM, Kliesch S, Leifke E, Link TM, Nieschlag E. Long-term effect of testosterone therapy on bone mineral density in hypogonadal men. J Clin Endocrinol Metab. 1997 Aug. 82(8):2386-90. [QxMD MEDLINE Link].
- Beighle C, Karp LE, Hanson JW, Hall JG, Hoehn H. Small structural changes of chromosome 8. Two cases with evidence for deletion. Hum Genet. 1977 Aug 31. 38(1):113-21. [QxMD MEDLINE Link].
- Bovet P, Reymond MJ, Rey F, Gomez F. Lack of gonadotropic response to pulsatile gonadotropin-releasing hormone in isolated hypogonadotropic hypogonadism associated to congenital adrenal hypoplasia. J Endocrinol Invest. 1988 Mar. 11(3):201-4. [QxMD MEDLINE Link].
- Boyar R, Perlow M, Hellman L, Kapen S, Weitzman E. Twenty-four hour pattern of luteinizing hormone secretion in normal men with sleep stage recording. J Clin Endocrinol Metab. 1972 Jul. 35(1):73-81. [QxMD MEDLINE Link].
- Boyar RM, Rosenfeld RS, Kapen S, et al. Human puberty. Simultaneous augmented secretion of luteinizing hormone and testosterone during sleep. J Clin Invest. 1974 Sep. 54(3):609-18. [QxMD MEDLINE Link].
- Boyar RM, Wu RH, Kapen S, Hellman L, Weitzman ED, Finkelstein JW. Clinical and laboratory heterogeneity in idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 1976 Dec. 43(6):1268-75. [QxMD MEDLINE Link].
- Brandenberger AW, Haenggi W, von Fischer B, Birkhaeuser MH. Kallmann syndrome and associated malformation of the uterus. Fertil Steril. 1994 Feb. 61(2):395-7. [QxMD MEDLINE Link].
- Bridges NA, Matthews DR, Hindmarsh PC, Brook CG. Changes in gonadotrophin secretion during childhood and puberty. J Endocrinol. 1994 Apr. 141(1):169-76. [QxMD MEDLINE Link].
- Brown DC, Stirling HF, Butler GE, Kelnar CJ, Wu FC. Differentiation of normal male prepuberty and hypogonadotrophic hypogonadism using an ultrasensitive luteinizing hormone assay. Horm Res. 1996. 46(2):83-7. [QxMD MEDLINE Link].
- Calof AL, Chikaraishi DM. Analysis of neurogenesis in a mammalian neuroepithelium: proliferation and differentiation of an olfactory neuron precursor in vitro. Neuron. 1989 Jul. 3(1):115-27. [QxMD MEDLINE Link].
- Cariboni A, Pimpinelli F, Colamarino S, et al. The product of X-linked Kallmann's syndrome gene (KAL1) affects the migratory activity of gonadotropin-releasing hormone (GnRH)-producing neurons. Hum Mol Genet. 2004 Nov 15. 13(22):2781-91. [QxMD MEDLINE Link].
- Castellano JM, Navarro VM, Fernandez-Fernandez R, et al. Changes in hypothalamic KiSS-1 system and restoration of pubertal activation of the reproductive axis by kisspeptin in undernutrition. Endocrinology. 2005 Sep. 146(9):3917-25. [QxMD MEDLINE Link].
- Castillo RH, Matteri RL, Dumesic DA. Luteinizing hormone synthesis in cultured fetal human pituitary cells exposed to gonadotropin-releasing hormone. J Clin Endocrinol Metab. 1992 Jul. 75(1):318-22. [QxMD MEDLINE Link].
- Clark SJ, Hauffa BP, Rodens KP, Styne DL, Kaplan SL, Grumbach MM. Hormone ontogeny in the ovine fetus: XIX: The effect of a potent luteinizing hormone-releasing factor agonist on gonadotropin and testosterone release in the fetus and neonate. Pediatr Res. 1989 Apr. 25(4):347-52. [QxMD MEDLINE Link].
- Clement K, Vaisse C, Lahlou N, et al. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature. 1998 Mar 26. 392(6674):398-401. [QxMD MEDLINE Link].
- Conn PM, Crowley WF Jr. Gonadotropin-releasing hormone and its analogs. Annu Rev Med. 1994. 45:391-405. [QxMD MEDLINE Link].
- Cortez AB, Galindo A, Arensman FW, Van Dop C. Congenital heart disease associated with sporadic Kallmann syndrome. Am J Med Genet. 1993 Jun 15. 46(5):551-4. [QxMD MEDLINE Link].
- Crowley WF Jr, Filicori M, Spratt DI, Santoro NF. The physiology of gonadotropin-releasing hormone (GnRH) secretion in men and women. Recent Prog Horm Res. 1985. 41:473-531. [QxMD MEDLINE Link].
- de Roux N, Young J, Misrahi M, et al. A family with hypogonadotropic hypogonadism and mutations in the gonadotropin-releasing hormone receptor. N Engl J Med. 1997 Nov 27. 337(22):1597-602. [QxMD MEDLINE Link].
- de Roux N, Young J, Misrahi M, Schaison G, Milgrom E. Loss of function mutations of the GnRH receptor: a new cause of hypogonadotropic hypogonadism. J Pediatr Endocrinol Metab. 1999 Apr. 12 Suppl 1:267-75. [QxMD MEDLINE Link].
- de Zegher F, Lagae L, Declerck D, Vinckier F. Kallmann syndrome and delayed puberty associated with agenesis of lateral maxillary incisors. J Craniofac Genet Dev Biol. 1995 Apr-Jun. 15(2):87-9. [QxMD MEDLINE Link].
- Dean JC, Johnston AW, Klopper AI. Isolated hypogonadotrophic hypogonadism: a family with autosomal dominant inheritance. Clin Endocrinol (Oxf). 1990 Mar. 32(3):341-7. [QxMD MEDLINE Link].
- Drenth J, Low BW, Richardson JS. The toxin-agglutinin fold. A new group of small protein structures organized around a four-disulfide core. J Biol Chem. 1980 Apr 10. 255(7):2652-5. [QxMD MEDLINE Link].
- Duke VM, Winyard PJ, Thorogood P, Soothill P, Bouloux PM, Woolf AS. KAL, a gene mutated in Kallmann's syndrome, is expressed in the first trimester of human development. Mol Cell Endocrinol. 1995 Apr 28. 110(1-2):73-9. [QxMD MEDLINE Link].
- Dungan HM, Clifton DK, Steiner RA. Minireview: kisspeptin neurons as central processors in the regulation of gonadotropin-releasing hormone secretion. Endocrinology. 2006 Mar. 147(3):1154-8. [QxMD MEDLINE Link].
- Edelman GM, Crossin KL. Cell adhesion molecules: implications for a molecular histology. Annu Rev Biochem. 1991. 60:155-90. [QxMD MEDLINE Link].
- Elmquist JK. Anatomic basis of leptin action in the hypothalamus. Front Horm Res. 2000. 26:21-41. [QxMD MEDLINE Link].
- Evans WS, Sollenberger MJ, Booth RA Jr. Contemporary aspects of discrete peak-detection algorithms. II. The paradigm of the luteinizing hormone pulse signal in women. Endocr Rev. 1992 Feb. 13(1):81-104. [QxMD MEDLINE Link].
- Fernald RD, White RB. Gonadotropin-releasing hormone genes: phylogeny, structure, and functions. Front Neuroendocrinol. 1999 Jul. 20(3):224-40. [QxMD MEDLINE Link].
- Filicori M, Butler JP, Crowley WF Jr. Neuroendocrine regulation of the corpus luteum in the human. Evidence for pulsatile progesterone secretion. J Clin Invest. 1984 Jun. 73(6):1638-47. [QxMD MEDLINE Link].
- Finkelstein JS, Klibanski A, Neer RM. Osteoporosis in men with idiopathic hypogonadotropic hypogonadism. Ann Intern Med. 1987 Mar. 106(3):354-61. [QxMD MEDLINE Link].
- Finkelstein JS, Klibanski A, Neer RM, et al. Increases in bone density during treatment of men with idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 1989 Oct. 69(4):776-83. [QxMD MEDLINE Link].
- Flanagan CA, Millar RP, Illing N. Advances in understanding gonadotrophin-releasing hormone receptor structure and ligand interactions. Rev Reprod. 1997 May. 2(2):113-20. [QxMD MEDLINE Link].
- Human GPR54 receptor model. Mutations identified in patients with idiopathic hypogonadotropic hypogonadism are indicated.
- KiSS-1 protein product model. Amino acids 1-19 are predicted to form a signal peptide. Proteolytic processing is predicted to produce kisspeptin-54, corresponding to amino acids 68-121. Shown is the C-terminal amidated decapeptide sequence, wherein biologic actively resides.


Author
Vaishali Popat, MD, MPH Clinical Investigator, Intramural Research Program in Reproductive and Adult Endocrinology, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health
Vaishali Popat, MD, MPH is a member of the following medical societies: American College of Physicians, Endocrine Society
Disclosure: Nothing to disclose.
Coauthor(s)
Karim Anton Calis, PharmD, MPH, FASHP, FCCP Clinical Professor, Medical College of Virginia, Virginia Commonwealth University School of Pharmacy; Clinical Professor, University of Maryland School of Pharmacy; Director of Clinical Research and Compliance, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health
Karim Anton Calis, PharmD, MPH, FASHP, FCCP is a member of the following medical societies: American College of Clinical Pharmacy, American Society of Health-System Pharmacists, Endocrine Society
Disclosure: Nothing to disclose.
Specialty Editor Board
Francisco Talavera, PharmD, PhD Adjunct Assistant Professor, University of Nebraska Medical Center College of Pharmacy; Editor-in-Chief, Medscape Drug Reference
Disclosure: Received salary from Medscape for employment. for: Medscape.
Chief Editor
Additional Contributors
Bruce A Meyer, MD, MBA Executive Vice President for Health System Affairs, Executive Director, Faculty Practice Plan, Professor, Department of Obstetrics and Gynecology, University of Texas Southwestern Medical School
Bruce A Meyer, MD, MBA is a member of the following medical societies: Medical Group Management Association, American College of Obstetricians and Gynecologists, American Association for Physician Leadership, American Institute of Ultrasound in Medicine, Association of Professors of Gynecology and Obstetrics, Massachusetts Medical Society, Society for Maternal-Fetal Medicine
Disclosure: Nothing to disclose.
Ziad Rafic Hubayter, MD, MPH, MBA Assistant Professor of Obstetrics and Gynecology, Division of Fertility and IVF, GW Medical Faculty Associates, George Washington University School of Medicine and Health Sciences
Ziad Rafic Hubayter, MD, MPH, MBA is a member of the following medical societies: American College of Obstetricians and Gynecologists, American Medical Association, American Society for Reproductive Medicine
Disclosure: Nothing to disclose.
Acknowledgements
The authors and editors of Medscape Reference gratefully acknowledge the contributions of previous authors James N Anasti, MD and Michael Cackovic, MD to the development and writing of this article.