Chronic Lymphocytic Leukemia (CLL): Practice Essentials, Pathophysiology, Etiology (original) (raw)
Practice Essentials
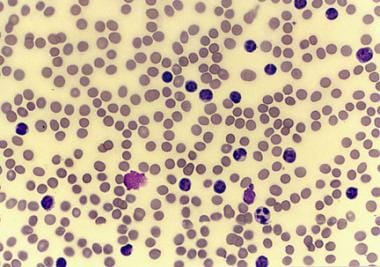
Chronic lymphocytic leukemia (CLL) is a monoclonal disorder characterized by a progressive proliferation and accumulation of mature yet functionally incompetent lymphocytes. The histologic sample in the image below portrays the appearance of these lymphocytes. CLL is the most common form of leukemia found in adults in Western countries. [1] Some patients die within several years of diagnosis, usually due to complications from CLL, but most patients survive for at least five years. [2]
Peripheral smear from a patient with chronic lymphocytic leukemia, small lymphocytic variety.
Signs and symptoms
Patients with CLL present with a wide range of signs and symptoms. The onset is insidious, and it is not unusual for CLL to be discovered incidentally when a blood cell count is performed for another reason; 25-50% of patients will be asymptomatic at time of presentation.
Signs and symptoms include the following:
- Enlarged lymph nodes, liver, or spleen
- Recurrent infections
- Loss of appetite or early satiety
- Abnormal bruising (late-stage sign)
- Fatigue
- Night sweats
See Presentation for more detail.
Diagnosis
Patients with CLL typically have a higher-than-normal white blood cell count, which is determined by a complete blood count (CBC). The most recent guidelines from the International Workshop for CLL (iwCLL) state that the diagnosis requires the presence of at least 5000 B-lymphocytes/μL for at least 3 months. [3] The clonality of the B-lymphocytes must be confirmed with flow cytometry. Other tests that may be helpful for diagnosis include bone marrow biopsy and ultrasonography of the liver and spleen. Immunoglobulin testing may be indicated for patients who develop repeated infections.
Staging
Two staging systems are used for CLL: Rai and Binet.
The Rai staging system categorizes patients into low-, intermediate-, and high-risk groups, as follows:
- Low risk (formerly stage 0) – Lymphocytosis in the blood and marrow only (25% of presenting population) [4]
- Intermediate risk (formerly stages I and II) – Lymphocytosis with enlarged nodes in any site or splenomegaly and/or hepatomegaly (50% of presentations)
- High risk (formerly stages III and IV) – Lymphocytosis with disease-related anemia (hemoglobin < 11 g/dL) or thrombocytopenia (platelets < 100 × 109/L) (25% of all patients)
The Binet staging system categorizes patients according to the number of lymph node groups involved. The areas of lymph node involvement considered are the head and neck, including the Waldeyer ring; axillae; groins; a palpable spleen; and a palpable liver. [2]
- Stage A – Hemoglobin 10 g/dL or higher, platelets 100 × 109/L or higher, and up to two of the above areas involved.
- Stage B – Hemoglobin and platelet levels as in stage A and three or more of the above lymph node areas involved
- Stage C – Hemoglobin less than 10 g/dL and/or platelets less than 100 × 109/L
See Workup for more detail.
Management
Patients with early-stage CLL, including low Binet or Rai stages, are not treated with chemotherapy until they become symptomatic or display evidence of rapid progression of disease. Early initiation of chemotherapy has failed to show survival benefit in CLL. [2]
Although combination chemotherapy regimens, including the nucleoside analogue fludarabine, were once the most commonly used first-line therapy in CLL, non-chemotherapy regimens (eg, with Bruton tyrosine kinase [BTK] inhibitors) are currently preferred in most cases. Treatment selection takes into account the molecular and genetic characteristics of the disease and may include the following agents, as monotherapy or in combination [5] :
- Acalabrutinib
- Obinutuzumab
- Venetoclax
- Ibrutinib
- Zanubrutinib
- Rituximab (in combination therapies)
Fludarabine, cyclophosphamide, and rituximab (FCR) was previously a recommended first-line treatment for fit, young patients (< 60 years). [6, 7, 4] However, a 2019 clinical trial called this recommendation into question; it showed superior progression-free and overall survival with ibrutinib-rituximab compared with FCR, in particular in patients without the IGHV mutation, and lower rates of serious infectious complications with ibrutinib-rituximab. [8]
Allogeneic stem cell transplantation is the only known curative therapy for CLL. It should be discussed with patients as a treatment option at the point of first or second relapse. [2]
See Treatment and Medication for more detail.
For patient education information, see Leukemia and Living With Chronic Lymphocytic Leukemia.

Pathophysiology
The cells of origin in most cases of CLL are clonal B cells arrested in the B-cell differentiation pathway, intermediate between pre-B cells and mature B cells. Morphologically, in the peripheral blood, these cells resemble mature lymphocytes.
CLL B-lymphocytes typically show B-cell surface antigens, as demonstrated by CD19, CD20dim, CD21, and CD23 monoclonal antibodies. In addition, they express CD5, which is more typically found on T cells. Because normal CD5+ B cells are present in the mantle zone of lymphoid follicles, B-cell CLL is most likely a malignancy of a mantle zone–based subpopulation of anergic self-reactive cells devoted to the production of polyreactive natural autoantibodies.
CLL B-lymphocytes express extremely low levels of surface membrane immunoglobulin, most often immunoglobulin M (IgM) or IgM/IgD and IgD. Additionally, they also express extremely low levels of a single immunoglobulin light chain (kappa or lambda).
An abnormal karyotype is observed in the majority of patients with CLL. The most common abnormality is deletion of 13q, which occurs in over 50% of patients. Individuals showing 13q14 abnormalities have a relatively benign disease that usually manifests as stable or slowly progressive isolated lymphocytosis.
Deletion of 11q and 17p as well as trisomy 12 have also been reported, albeit less commonly. The presence of trisomy 12, which is observed in 15% of CLL patients, is associated with atypical morphology and progressive disease. Deletion in the short arm of chromosome 17 has been associated with rapid progression, short remission, and decreased overall survival. 17p13 deletions are associated with loss of function of the tumor suppressor gene p53. Deletions of bands 11q22-q23, observed in 19% of patients, are associated with extensive lymph node involvement, aggressive disease, and shorter survival [2] .
More sensitive techniques have demonstrated abnormalities of chromosome 12. About 40-50% of patients demonstrate no chromosomal abnormalities on conventional cytogenetic studies. However, 80% of patients will have abnormalities detectable by fluorescence in situ hybridization (FISH). Approximately 2-5% of patients with CLL exhibit a T-cell phenotype.
The proto-oncogene Bcl2 is overexpressed in B-cell CLL. [9] The proto-oncogene Bcl2 is a known suppressor of apoptosis (programmed cell death), resulting in a long life for the involved cells. Despite the frequent overexpression of Bcl-2 protein, genetic translocations that are known to result in the overexpression of Bcl2, such as t(14;18), are not found in patients with CLL. Analysis of the molecular pathophysiology of CLL allows the development of therapies that target leukemic cells with these genetic changes. Venetoclax, which is one of the first-line treatment options for CLL, is designed to block the Bcl2 protein.
Studies have shown that this upregulation in Bcl2 is related to deletions of band 13q14. Two genes, named miRNA15a and miRNA16-1, are located at 13q14 and have been shown to encode not for proteins, but rather for a regulatory RNA called microRNA (miRNA). [10, 11] These miRNA genes belong to a family of highly conserved noncoding genes throughout the genome whose transcripts inhibit gene expression by causing degradation of mRNA or by blocking transcription of mRNA.
Deletions of miRNA15a and miRNA16-1 lead to overexpression of Bcl2 through loss of downregulating miRNAs. Genetic analyses have demonstrated deletion or downregulation of these miRNA genes in 70% of CLL cases. [12]
A study of the CLL genome by Wang et al discovered splicing factor 3b (SF3B1) mutations affecting pre-mRNA in 15% of sampled cells. [13] SF3B1 mutations are also found in 20% of myelodysplastic syndrome cases. [14] In future this may provide a therapeutic target.
Investigations have also identified a number of high-risk genetic features and markers, including the following:
- Germline immunoglobulin variable heavy chain (IgVH)
- IgVH V3-21 gene usage
- Increased CD38 expression
- Increased Zap70 expression
- Elevated serum beta-2-microglobulin levels
- Increased serum thymidine kinase activity
- Short lymphocyte doubling time (< 6 months)
- Increased serum levels of soluble CD23
These features have been associated with rapid progression, short remission, resistance to treatment, and shortened overall survival in patients with CLL.
Germline IgVH has been shown to indicate a poor prognosis. Studies have shown that these patients also have earlier progression of CLL after treatment with chemotherapy. Zeta-associated peptide of 70 kilodaltons (Zap70) is a cytoplasmic tyrosine kinase whose expression has also been associated with a poor prognosis. Cells with germline IgVH often have an increased expression of Zap70; however, studies have shown discordance rates of 10-20% between IgVH mutational status and Zap70 expression levels. Elevated levels of Zap70 are believed to decrease the threshold for signaling through Bcl2, thereby facilitating the antiapoptotic effects of Bcl2.

Etiology
As is the case with most malignancies, the exact cause of CLL is uncertain. CLL is an acquired disorder, and reports of truly familial cases are exceedingly rare. [15] A meta-analysis of four genome-wide association studies that included 3100 cases of CLL found multiple risk loci. Several of those loci are in close proximity to genes involved in apoptosis, suggesting a plausible underlying biological mechanism. [16]

Epidemiology
United States Statistics
The American Cancer Society estimates that 18,740 new cases of CLL will be diagnosed in the United States in 2023. [17] The true incidence in the US is unknown and is likely higher, as estimates of CLL incidence come from tumor registries, and many cases are not reported. Although the incidence of CLL has been stable over the last two decades, mortality has been steadily declining. [2]
International Statistics
Although the incidence of CLL in Western countries is similar to that of the United States, CLL is extremely rare in Asian countries (eg, China, Japan), where it is estimated to comprise only 10% of all leukemias. However, underreporting and incomplete registries may significantly underestimate the true incidence of CLL in those countries.
Race-, sex-, and age-related demographics
The incidence of CLL is higher in Whites than in Blacks. The incidence of CLL is higher in males than in females, with a male-to-female ratio of 1.9:1. [2] CLL is a disease that primarily affects the elderly, with the median age at diagnosis being 70 years. [18] In familial CLL (ie, disease in patients with at least one first-degree relative with CLL), median age at diagnosis is 57 years. [19]

Prognosis
The prognosis of patients with CLL varies widely at diagnosis. Some patients die rapidly, within 2-3 years of diagnosis, because of complications from CLL. In most patients CLL initially has a relatively benign course, but eventually enters a progressive, treatment-resistant phase. During this later phase, morbidity is considerable, both from the disease and from complications of therapy. [20, 21]
With the advances in treatment of CLL in recent decades, prolonged survival is possible. However, treatment rarely cures CLL, and mortality rates in patients with CLL remain significantly higher than in the general population. [22]
Prognosis depends on the disease stage at diagnosis as well as the presence or absence of high-risk markers (see Pathophysiology). Given the recent advancements in CLL treatment, the Rai and Binet staging systems do not provide sufficient utility to estimate prognosis. The most accurate prognostic score currently utilized by oncologists is the CLL International Prognostic Index (CLL-IPI), which relies on five independent prognostic factors [2] :
- Patient age
- Clinical stage (Rai or Binet)
- Serum B2 microglobulin level
- Mutational status of immunoglobulin heavy chain variable (IGVH)
- 17p deletion and/or TP53 mutation
Patients are deemed low-, intermediate-, high-, or very high risk depending on the presence or absence of the prognostic factors (see the CLL-IPI calculator). Low-risk patients have over a 90% chance of 5-year overall survival and, therefore, treatment is not recommended. Intermediate-risk patients have a nearly 90% chance of 5-year overall survival and are treated only if they are symptomatic. High-risk patients are managed similarly to intermediate-risk patients, but they have a slightly lower chance of 5-year overall survival at 63%. Very high-risk patients are treated with targeted agents (see Treatment and Medication.

- Nabhan C, Rosen ST. Chronic lymphocytic leukemia: a clinical review. JAMA. 2014 Dec 3. 312 (21):2265-76. [QxMD MEDLINE Link].
- Hallek M, Al-Sawaf O. Chronic lymphocytic leukemia: 2022 update on diagnostic and therapeutic procedures. Am J Hematol. 2021 Dec 1. 96 (12):1679-1705. [QxMD MEDLINE Link].
- Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Döhner H, et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood. 2018 Jun 21. 131 (25):2745-2760. [QxMD MEDLINE Link].
- PDQ Adult Treatment Editorial Board. Chronic Lymphocytic Leukemia Treatment (PDQ®): Health Professional Version. PDQ Cancer Information Summaries. Bethesda, MD: National Cancer Institute; June 20, 2023. [Full Text].
- [Guideline] National Comprehensive Cancer Network. Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma. NCCN. Available at https://www.nccn.org/professionals/physician_gls/pdf/cll.pdf. Version 1.2023 — August 30, 2022; Accessed: January 12, 2023.
- Klepfish A, Gilles L, Ioannis K, Eliezer R, Ami S. Enhancing the action of rituximab in chronic lymphocytic leukemia by adding fresh frozen plasma: complement/rituximab interactions & clinical results in refractory CLL. Ann N Y Acad Sci. 2009 Sep. 1173:865-73. [QxMD MEDLINE Link].
- Badoux XC, Keating MJ, Wang X, et al. Fludarabine, cyclophosphamide and rituximab chemoimmunotherapy is highly effective treatment for relapsed patients with CLL. Blood. 2011 Jan 18. [QxMD MEDLINE Link].
- Shanafelt TD, Wang XV, Kay NE, Hanson CA, O'Brien S, Barrientos J, et al. Ibrutinib-Rituximab or Chemoimmunotherapy for Chronic Lymphocytic Leukemia. N Engl J Med. 2019 Aug 1. 381 (5):432-443. [QxMD MEDLINE Link].
- Zenz T, Benner A, Duhrsen U, Durig J, Dohner H, Siffert W, et al. BCL2-938C>A polymorphism and disease progression in chronic lymphocytic leukemia. Leuk Lymphoma. 2009 Sep 11. 1-6. [QxMD MEDLINE Link].
- Nicoloso MS, Kipps TJ, Croce CM, Calin GA. MicroRNAs in the pathogeny of chronic lymphocytic leukaemia. Br J Haematol. 2007 Dec. 139(5):709-16. [QxMD MEDLINE Link]. [Full Text].
- Hanlon K, Rudin CE, Harries LW. Investigating the targets of MIR-15a and MIR-16-1 in patients with chronic lymphocytic leukemia (CLL). PLoS One. 2009 Sep 25. 4(9):e7169. [QxMD MEDLINE Link]. [Full Text].
- Fabbri M, Bottoni A, Shimizu M, et al. Association of a microRNA/TP53 feedback circuitry with pathogenesis and outcome of B-cell chronic lymphocytic leukemia. JAMA. 2011 Jan 5. 305(1):59-67. [QxMD MEDLINE Link].
- Wang L, Lawrence MS, Wan Y, et al. SF3B1 and other novel cancer genes in chronic lymphocytic leukemia. N Engl J Med. 2011 Dec 29. 365(26):2497-506. [QxMD MEDLINE Link]. [Full Text].
- Malcovati L, Stevenson K, Papaemmanuil E, et al. SF3B1-mutant MDS as a distinct disease subtype: a proposal from the International Working Group for the Prognosis of MDS. Blood. 2020;136(2):157-170. Blood. 2021 May 27. 137 (21):3003. [QxMD MEDLINE Link]. [Full Text].
- Slager SL, Kay NE. Familial Chronic Lymphocytic Leukemia: What Does it Mean to Me?. Clin Lymphoma Myeloma. 2009 Sep 1. 9:S194-S197. [QxMD MEDLINE Link].
- Berndt SI, Camp NJ, Skibola CF, Vijai J, Wang Z, et al. Meta-analysis of genome-wide association studies discovers multiple loci for chronic lymphocytic leukemia. Nat Commun. 2016 Mar 9. 7:10933. [QxMD MEDLINE Link].
- Cancer Facts & Figures 2023. American Cancer Society. Available at https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2023/2023-cancer-facts-and-figures.pdf. Accessed: January 12, 2023.
- Cancer Stat Facts: Leukemia — Chronic Lymphocytic Leukemia (CLL). National Cancer Institute Surveillance, Epidemiology, and End Results Program. Available at https://seer.cancer.gov/statfacts/html/clyl.html. Accessed: January 12, 2023.
- Benintende G, Innocenti I, Fresa A, Autore F, Tomasso A, Piciocchi A, et al. Clinical and molecular features of familial chronic lymphocytic leukemia: a pilot monocentric study. Haematologica. 2022 Dec 22. [QxMD MEDLINE Link].
- Rai KR, Barrientos JC. Chronic lymphocytic leukemia. Bast RC Jr, Croce CM, Hait WN, et al, eds. Holland-Frei Cancer Medicine. 9th ed. Hoboken, NJ: John Wiley & Sons, Inc; 2017. 1595-1604.
- Kristinsson SY, Dickman PW, Wilson WH, et al. Improved survival in chronic lymphocytic leukemia in the past decade: a population-based study including 11,179 patients diagnosed between 1973-2003 in Sweden. Haematologica. 2009 Sep. 94(9):1259-65. [QxMD MEDLINE Link]. [Full Text].
- van der Straten L, Levin MD, Visser O, Posthuma EFM, Doorduijn JK, Kater AP, et al. Conditional relative survival among patients with chronic lymphocytic leukaemia: A population-based study in the Netherlands. EJHaem. 2022 Feb. 3 (1):180-183. [QxMD MEDLINE Link]. [Full Text].
- Jacque N, Leblond V. [Chronic lymphocytic leukemia]. Presse Med. 2019 Jul-Aug. 48 (7-8 Pt 1):807-815. [QxMD MEDLINE Link].
- Maurer MJ, Cerhan JR, Katzmann JA, et al. Monoclonal and polyclonal serum free light chains and clinical outcome in chronic lymphocytic leukemia. Blood. 2011 Sep 8. 118(10):2821-6. [QxMD MEDLINE Link]. [Full Text].
- Clay-Gilmour AI, Rishi AR, Goldin LR, et. al. Association of elevated serum free light chains with chronic lymphocytic leukemia and monoclonal B-cell lymphocytosis. Blood Cancer J. 2019 Aug 5. 9 (8):59. [QxMD MEDLINE Link].
- [Guideline] Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Döhner H, et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood. 2018 Jun 21. 131 (25):2745-2760. [QxMD MEDLINE Link]. [Full Text].
- Bouley J, Deriano L, Delic J, Merle-Béral H. New molecular markers in resistant B-CLL. Leuk Lymphoma. 2006 May. 47(5):791-801. [QxMD MEDLINE Link].
- Parikh SA, Strati P, Tsang M, West CP, Shanafelt TD. Should IGHV status and FISH testing be performed in all CLL patients at diagnosis? A systematic review and meta-analysis. Blood. 2016 Feb 3. 39 (8A):935-7. [QxMD MEDLINE Link].
- Hillmen P, Skotnicki AB, Robak T, et al. Alemtuzumab compared with chlorambucil as first-line therapy for chronic lymphocytic leukemia. J Clin Oncol. 2007 Dec 10. 25(35):5616-23. [QxMD MEDLINE Link].
- Montillo M, Tedeschi A, Miqueleiz S, et al. Alemtuzumab as consolidation after a response to fludarabine is effective in purging residual disease in patients with chronic lymphocytic leukemia. J Clin Oncol. 2006 May 20. 24(15):2337-42. [QxMD MEDLINE Link]. [Full Text].
- Moreton P, Kennedy B, Lucas G, et al. Eradication of minimal residual disease in B-cell chronic lymphocytic leukemia after alemtuzumab therapy is associated with prolonged survival. J Clin Oncol. 2005 May 1. 23(13):2971-9. [QxMD MEDLINE Link]. [Full Text].
- Visone R, Veronese A, Rassenti LZ, et al. miR-181b is a biomarker of disease progression in chronic lymphocytic leukemia. Blood. 2011 Sep 15. 118(11):3072-9. [QxMD MEDLINE Link]. [Full Text].
- International CLL-IPI working group. An international prognostic index for patients with chronic lymphocytic leukaemia (CLL-IPI): a meta-analysis of individual patient data. Lancet Oncol. 2016 Jun. 17 (6):779-790. [QxMD MEDLINE Link].
- Bosch F, Ferrer A, Villamor N, et al. Fludarabine, cyclophosphamide, and mitoxantrone as initial therapy of chronic lymphocytic leukemia: high response rate and disease eradication. Clin Cancer Res. 2008 Jan 1. 14(1):155-61. [QxMD MEDLINE Link].
- Byrd JC, Gribben JG, Peterson BL, et al. Select high-risk genetic features predict earlier progression following chemoimmunotherapy with fludarabine and rituximab in chronic lymphocytic leukemia: justification for risk-adapted therapy. J Clin Oncol. 2006 Jan 20. 24(3):437-43. [QxMD MEDLINE Link].
- Seymour JF. CLL12: a positive answer to a poorly phrased question. Blood. 2022 Jan 13. 139 (2):151-152. [QxMD MEDLINE Link]. [Full Text].
- Wierda WG, O'Brien S, Wang X, et al. Multivariable model for time to first treatment in patients with chronic lymphocytic leukemia. J Clin Oncol. 2011 Nov 1. 29(31):4088-95. [QxMD MEDLINE Link].
- Knauf WU, Lissitchkov T, Aldaoud A, Liberati AM, Loscertales J, Herbrecht R, et al. Bendamustine compared with chlorambucil in previously untreated patients with chronic lymphocytic leukaemia: updated results of a randomized phase III trial. Br J Haematol. 2012 Oct. 159(1):67-77. [QxMD MEDLINE Link].
- Fischer K, Cramer P, Busch R, et al. Bendamustine combined with rituximab in patients with relapsed and/or refractory chronic lymphocytic leukemia: a multicenter phase II trial of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol. 2011 Sep 10. 29(26):3559-66. [QxMD MEDLINE Link].
- Castellino AM. 'Possibility of Cure' for Some Patients With CLL. Medscape Medical News. Available at https://www.medscape.com/viewarticle/918027. September 10, 2019; Accessed: January 12, 2023.
- St-Pierre F, Ma S. Use of BTK Inhibitors in Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma (CLL/SLL): A Practical Guidance. Blood Lymphat Cancer. 2022. 12:81-98. [QxMD MEDLINE Link]. [Full Text].
- Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013 Jul 4. 369(1):32-42. [QxMD MEDLINE Link]. [Full Text].
- Chustecka Z. Ibrutinib in CLL: indication expanded, benefit confirmed. Medscape Medical News. Available at http://www.medscape.com/viewarticle/828980. July 28, 2014; Accessed: January 12, 2023.
- Byrd JC, Brown JR, O'Brien S, Barrientos JC, Kay NE, Reddy NM, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014 Jul 17. 371 (3):213-23. [QxMD MEDLINE Link]. [Full Text].
- Burger JA, Tedeschi A, Barr PM, Robak T, Owen C, Ghia P, et al. Ibrutinib as Initial Therapy for Patients with Chronic Lymphocytic Leukemia. N Engl J Med. 2015 Dec 17. 373 (25):2425-37. [QxMD MEDLINE Link].
- Langerbeins P, Zhang C, Robrecht S et. al. The CLL12 trial: ibrutinib vs placebo in treatment-naïve, early-stage chronic lymphocytic leukemia. Blood. 2022 Jan 13. 139 (2):177-187. [QxMD MEDLINE Link].
- Seymour JF. CLL12: a positive answer to a poorly phrased question. Blood. 2022 Jan 13. 139 (2):151-152. [QxMD MEDLINE Link].
- Dickerson T, Wiczer T, Waller A, Philippon J, Porter K, Haddad D, et al. Hypertension and Incident Cardiovascular Events Following Ibrutinib Initiation. Blood. 2019 Oct 3. 118 (8):2062-8. [QxMD MEDLINE Link]. [Full Text].
- Salem JE, Manouchehri A, Bretagne M, Lebrun-Vignes B, Groarke JD, Johnson DB, et al. Cardiovascular Toxicities Associated With Ibrutinib. J Am Coll Cardiol. 2019 Oct 1. 74 (13):1667-1678. [QxMD MEDLINE Link].
- Burger JA. Treatment of Chronic Lymphocytic Leukemia. N Engl J Med. 2020 Jul 30. 383 (5):460-473. [QxMD MEDLINE Link]. [Full Text].
- Puła B, Gołos A, Górniak P, Jamroziak K. Overcoming Ibrutinib Resistance in Chronic Lymphocytic Leukemia. Cancers (Basel). 2019 Nov 21. 11 (12):[QxMD MEDLINE Link]. [Full Text].
- Hoang, T., Cheung, M., Chan, K., et al. The Impact of Drug Interactions on Outcomes in Ibrutinib-Treated Patients with Chronic Lymphocytic Leukemia in Routine Clinical Care: A Population-Based Cohort Study. American Society of Hematology. Available at https://ash.confex.com/ash/2022/webprogram/Paper158768.html. December 12, 2022; Accessed: January 12, 2023.
- Sharman JP, Egyed M, Jurczak W, et al. Efficacy and safety in a 4-year follow-up of the ELEVATE-TN study comparing acalabrutinib with or without obinutuzumab versus obinutuzumab plus chlorambucil in treatment-naïve chronic lymphocytic leukemia. Leukemia. 2022 Jan 1. [QxMD MEDLINE Link]. [Full Text].
- Project Orbis: FDA approves acalabrutinib for CLL and SLL. U.S. Food & Drug Administration. Available at https://www.fda.gov/drugs/resources-information-approved-drugs/project-orbis-fda-approves-acalabrutinib-cll-and-sll. November 21, 2019; Accessed: January 12, 2023.
- Byrd JC, Woyach JA, Furman RR, Martin P, O'Brien S, Brown JR, et al. Acalabrutinib in treatment-naive chronic lymphocytic leukemia. Blood. 2021 Jun 17. 137 (24):3327-3338. [QxMD MEDLINE Link].
- Real-World Study Shows Patients Treated with IMBRUVICA® (ibrutinib) Were Less Likely to Initiate a Next-Line Treatment than Patients on Acalabrutinib in First-line Chronic Lymphocytic Leukemia. Johnson & Johnson. December 12, 2022. Available at https://www.jnj.com/real-world-study-shows-patients-treated-with-imbruvica-ibrutinib-were-less-likely-to-initiate-a-next-line-treatment-than-patients-on-acalabrutinib-in-first-line-chronic-lymphocytic-leukemia.
- McKnight P. FDA Oks Zanubrutinib (Brukinsa) for CLL or SLL. Medscape Medical News. Available at https://www.medscape.com/viewarticle/987252. January 20, 2023; Accessed: January 27, 2023.
- Brown JR, Eichhorst B, Hillmen P, Jurczak W, Kaźmierczak M, Lamanna N, et al. Zanubrutinib or Ibrutinib in Relapsed or Refractory Chronic Lymphocytic Leukemia. N Engl J Med. 2022 Dec 13. [QxMD MEDLINE Link].
- Mato AR, Shah NN, Jurczak W, Cheah CY, Pagel JM, Woyach JA, et al. Pirtobrutinib in relapsed or refractory B-cell malignancies (BRUIN): a phase 1/2 study. Lancet. 2021 Mar 6. 397 (10277):892-901. [QxMD MEDLINE Link].
- Mato AR, Woyach JA, Brown JR, Ghia P, Patel K, Eyre TA, et al. Pirtobrutinib after a Covalent BTK Inhibitor in Chronic Lymphocytic Leukemia. N Engl J Med. 2023 Jul 6. 389 (1):33-44. [QxMD MEDLINE Link].
- Venclexta (venetoclax) [package insert]. North Chicago, IL: AbbVie, Inc. May 2019. Available at [Full Text].
- FDA approves venetoclax for CLL and SLL. U.S. Food & Drug Administration. Available at https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-venetoclax-cll-and-sll. May 15, 2019; Accessed: September 13, 2019.
- FDA approves venetoclax for CLL and SLL. U.S. Food Drug Administration. Available at https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-venetoclax-cll-and-sll. May 15, 2019; Accessed: November 7, 2022.
- Seymour JF, Kipps TJ, Eichhorst B, Hillmen P, D'Rozario J, Assouline S, et al. Venetoclax-Rituximab in Relapsed or Refractory Chronic Lymphocytic Leukemia. N Engl J Med. 2018 Mar 22. 378 (12):1107-1120. [QxMD MEDLINE Link].
- Kater AP, Seymour JF, Hillmen P, Eichhorst B, Langerak AW, Owen C, et al. Fixed Duration of Venetoclax-Rituximab in Relapsed/Refractory Chronic Lymphocytic Leukemia Eradicates Minimal Residual Disease and Prolongs Survival: Post-Treatment Follow-Up of the MURANO Phase III Study. J Clin Oncol. 2019 Feb 1. 37 (4):269-277. [QxMD MEDLINE Link]. [Full Text].
- Jain N, Keating M, Thompson P, et al. Ibrutinib and Venetoclax for First-Line Treatment of CLL. N Engl J Med. 2019 May 30. 380 (22):2095-2103. [QxMD MEDLINE Link].
- Kater AP, et al; GLOW Investigators. Fixed-Duration Ibrutinib-Venetoclax in Patients with Chronic Lymphocytic Leukemia and Comorbidities. N Engl J Med Evidence. May 13, 2022. 1(7):[Full Text].
- Three-Drug Combination Therapy Effective in Patients with High-Risk CLL, Trial Shows. Dana-Farber Cancer Institute. December 10, 2022. Available at https://www.dana-farber.org/newsroom/news-releases/2022/three-drug-combination-therapy-effective-in-patients-with-high-risk-cll--trial-shows/.
- Awan FT, Hillmen P, Hellmann A, Robak T, Hughes SG, Trone D, et al. A randomized, open-label, multicentre, phase 2/3 study to evaluate the safety and efficacy of lumiliximab in combination with fludarabine, cyclophosphamide and rituximab versus fludarabine, cyclophosphamide and rituximab alone in subjects with relapsed chronic lymphocytic leukaemia. Br J Haematol. 2014 Nov. 167 (4):466-77. [QxMD MEDLINE Link].
- Goede V, Fischer K, Busch R, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014 Mar 20. 370 (12):1101-10. [QxMD MEDLINE Link]. [Full Text].
- McCall B. Obinutuzumab Active in Elderly Chronic Lymphocytic Leukemia. Medscape Medical News from the 18th Congress of the European Hematology Association (EHA). Available at http://www.medscape.com/viewarticle/806700. June 21, 2013; Accessed: January 12, 2023.
- Moreno C, Greil R, Demirkan F, Tedeschi A, Anz B, Larratt L, et al. Ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab in first-line treatment of chronic lymphocytic leukaemia (iLLUMINATE): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019 Jan. 20 (1):43-56. [QxMD MEDLINE Link].
- Lemery SJ, Zhang J, Rothmann MD, Yang J, Earp J, Zhao H. U.S. Food and Drug Administration approval: ofatumumab for the treatment of patients with chronic lymphocytic leukemia refractory to fludarabine and alemtuzumab. Clin Cancer Res. 2010 Sep 1. 16(17):4331-8. [QxMD MEDLINE Link].
- Nelson R. FDA Grants Full Approval to Ofatumumab (Arzerra) for CLL. Medscape Medical News. Available at http://www.medscape.com/viewarticle/823837. April 17, 2014; Accessed: January 12, 2023.
- Furman RR, Sharman JP, Coutre SE, Cheson BD, Pagel JM, Hillmen P, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014 Mar 13. 370(11):997-1007. [QxMD MEDLINE Link]. [Full Text].
- van Oers MH, Kuliczkowski K, Smolej L, Petrini M, Offner F, Grosicki S, et al. Ofatumumab maintenance versus observation in relapsed chronic lymphocytic leukaemia (PROLONG): an open-label, multicentre, randomised phase 3 study. Lancet Oncol. 2015 Oct. 16 (13):1370-9. [QxMD MEDLINE Link].
- Robak, T, Warzocha, K, Babu, KG, et al. Health-Related Quality of Life and Patient-Reported Outcomes in Patients Receiving Ofatumumab in Combination with Fludarabine and Cyclophosphamide (FC) Versus FC Alone in the Complement 2 Trial. Blood. 2015. 126(23):5288. [Full Text].
- Novartis Withdraws Chronic Leukemia Drug Arzerra From Non-U.S. Markets. FDA News. Available at https://www.fdanews.com/articles/185419-novartis-withdraws-chronic-leukemia-drug-arzerra-from-non-us-markets. January 30, 2018; Accessed: January 12, 2023.
- Nabhan C, Coutré S, Hillmen P. Minimal residual disease in chronic lymphocytic leukaemia: is it ready for primetime?. Br J Haematol. 2007 Feb. 136(3):379-92. [QxMD MEDLINE Link].
- Sayala HA, Rawstron AC, Hillmen P. Minimal residual disease assessment in chronic lymphocytic leukaemia. Best Pract Res Clin Haematol. 2007 Sep. 20(3):499-512. [QxMD MEDLINE Link].
- Parikh SA, Keating MJ, O'Brien S, et al. Frontline chemoimmunotherapy with fludarabine, cyclophosphamide, alemtuzumab, and rituximab for high-risk chronic lymphocytic leukemia. Blood. 2011 Aug 25. 118(8):2062-8. [QxMD MEDLINE Link].
- Badoux XC, Keating MJ, Wang X, et al. Cyclophosphamide, fludarabine, alemtuzumab, and rituximab as salvage therapy for heavily pretreated patients with chronic lymphocytic leukemia. Blood. 2011 Aug 25. 118(8):2085-93. [QxMD MEDLINE Link].
- Skoetz N, Bauer K, Elter T, Monsef I, Roloff V, Hallek M, et al. Alemtuzumab for patients with chronic lymphocytic leukaemia. Cochrane Database Syst Rev. 2012 Feb 15. CD008078. [QxMD MEDLINE Link]. [Full Text].
- Mougalian SS, O'Brien S. Adverse prognostic features in chronic lymphocytic leukemia. Oncology (Williston Park). 2011 Jul. 25(8):692-6, 699. [QxMD MEDLINE Link].
- Woyach JA, Ruppert AS, Heerema NA, Peterson BL, Gribben JG, Morrison VA, et al. Chemoimmunotherapy With Fludarabine and Rituximab Produces Extended Overall Survival and Progression-Free Survival in Chronic Lymphocytic Leukemia: Long-Term Follow-Up of CALGB Study 9712. J Clin Oncol. 2011 Apr 1. 29(10):1349-55. [QxMD MEDLINE Link].
- Robak T, Dmoszynska A, Solal-Celigny P, et al. Rituximab plus fludarabine and cyclophosphamide prolongs progression-free survival compared with fludarabine and cyclophosphamide alone in previously treated chronic lymphocytic leukemia. J Clin Oncol. 2010 Apr 1. 28(10):1756-65. [QxMD MEDLINE Link].
- Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010 Oct 2. 376(9747):1164-74. [QxMD MEDLINE Link].
- Elter T, Gercheva-Kyuchukova L, Pylylpenko H, Robak T, Jaksic B, Rekhtman G, et al. Fludarabine plus alemtuzumab versus fludarabine alone in patients with previously treated chronic lymphocytic leukaemia: a randomised phase 3 trial. Lancet Oncol. 2011 Dec. 12 (13):1204-13. [QxMD MEDLINE Link]. [Full Text].
- Badoux XC, Keating MJ, Wang X, O'Brien SM, Ferrajoli A, Faderl S, et al. Cyclophosphamide, fludarabine, alemtuzumab, and rituximab as salvage therapy for heavily pretreated patients with chronic lymphocytic leukemia. Blood. 2011 Aug 25. 118 (8):2085-93. [QxMD MEDLINE Link]. [Full Text].
- Goede V, Fischer K, Busch R, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014 Mar 20. 370 (12):1101-10. [QxMD MEDLINE Link]. [Full Text].
- Chustecka Z. Idelalisib (Zydelig) approved for CLL and lymphoma. Medscape Medical News. July 23, 2014. [Full Text].
- FDA news release: FDA approves Zydelig for three types of blood cancers. US Food and Drug Administration. July 23, 2014. Available at http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm406387.htm.
- Flinn IW, Hillmen P, Montillo M, Nagy Z, Illés Á, Etienne G, et al. The phase 3 DUO trial: duvelisib vs ofatumumab in relapsed and refractory CLL/SLL. Blood. 2018 Dec 6. 132 (23):2446-2455. [QxMD MEDLINE Link].
- Gribben JG, Jurczak W, Jacobs RW, et al. Umbralisib Plus Ublituximab (U2) Is Superior to Obinutuzumab Plus Chlorambucil (O+Chl) in Patients with Treatment Naïve (TN) and Relapsed/Refractory (R/R) Chronic Lymphocytic Leukemia (CLL): Results from the Phase 3 Unity-CLL Study. Blood. November 5, 2020. 136 suppl 1:37-39. [Full Text].
- Ukoniq (umbralisib): Drug Safety Communication - FDA Approval of Lymphoma Medicine is Withdrawn Due to Safety Concerns. U.S. Food & Drug Administration. Available at https://www.fda.gov/safety/medical-product-safety-information/ukoniq-umbralisib-drug-safety-communication-fda-approval-lymphoma-medicine-withdrawn-due-safety. June 1, 2022;
- González-Rodríguez AP, Payer AR, Acebes-Huerta A, Huergo-Zapico L, Villa-Alvarez M, Gonzalez-García E, et al. Lenalidomide and chronic lymphocytic leukemia. Biomed Res Int. 2013. 2013:932010. [QxMD MEDLINE Link]. [Full Text].
- REVLIMID [lenalidomide] [package insert]. Summit, NJ: Celgene Corporation. 11/2013. Available at [Full Text].
- Feins S, Kong W, Williams EF, Milone MC, Fraietta JA. An introduction to chimeric antigen receptor (CAR) T-cell immunotherapy for human cancer. Am J Hematol. 2019 May. 94 (S1):S3-S9. [QxMD MEDLINE Link]. [Full Text].
- Gauthier J, Hirayama AV, Purushe J, Hay KA, Lymp J, Li DH, et al. Feasibility and efficacy of CD19-targeted CAR T cells with concurrent ibrutinib for CLL after ibrutinib failure. Blood. 2020 May 7. 135 (19):1650-1660. [QxMD MEDLINE Link].
- Melenhorst JJ, Chen GM, Wang M, et al. Decade-long leukaemia remissions with persistence of CD4+ CAR T cells. Nature. 2022 Feb 2. [QxMD MEDLINE Link]. [Full Text].
- Eichhorst BF, Busch R, Stilgenbauer S, et al. First-line therapy with fludarabine compared with chlorambucil does not result in a major benefit for elderly patients with advanced chronic lymphocytic leukemia. Blood. 2009 Oct 15. 114(16):3382-91. [QxMD MEDLINE Link].
- Laurenti L, Innocenti I, Autore F, et al. Chlorambucil plus rituximab as front-line therapy for elderly and/or unfit chronic lymphocytic leukemia patients: correlation with biologically-based risk stratification. Haematologica. 2017 Sep. 102 (9):e352-e355. [QxMD MEDLINE Link]. [Full Text].
- Woyach JA, Ruppert AS, Rai K, Lin TS, Geyer S, Kolitz J, et al. Impact of Age on Outcomes After Initial Therapy With Chemotherapy and Different Chemoimmunotherapy Regimens in Patients With Chronic Lymphocytic Leukemia: Results of Sequential Cancer and Leukemia Group B Studies. J Clin Oncol. 2012 Dec 10. [QxMD MEDLINE Link].
- Gribben JG, Hosing C, Maloney DG. Stem cell transplantation for indolent lymphoma and chronic lymphocytic leukemia. Biol Blood Marrow Transplant. 2011 Jan. 17(1 Suppl):S63-70. [QxMD MEDLINE Link].
- Michallet M, Dreger P, Sutton L, et al. Autologous hematopoietic stem cell transplantation in chronic lymphocytic leukemia: results of European intergroup randomized trial comparing autografting versus observation. Blood. 2011 Feb 3. 117(5):1516-1521. [QxMD MEDLINE Link].
- Hodgson K, Ferrer G, Montserrat E, Moreno C. Chronic lymphocytic leukemia and autoimmunity: a systematic review. Haematologica. 2011 May. 96(5):752-61. [QxMD MEDLINE Link]. [Full Text].
- Raanani P, Gafter-Gvili A, Paul M, Ben-Bassat I, Leibovici L, Shpilberg O. Immunoglobulin prophylaxis in chronic lymphocytic leukemia and multiple myeloma: systematic review and meta-analysis. Leuk Lymphoma. 2009 May. 50(5):764-72. [QxMD MEDLINE Link].
- Koehrer S, Keating MJ, Wierda WG. Eltrombopag, a second-generation thrombopoietin receptor agonist, for chronic lymphocytic leukemia-associated ITP. Leukemia. 2010 May. 24(5):1096-8. [QxMD MEDLINE Link].
- Borthakur G, O'Brien S, Wierda WG, et al. Immune anaemias in patients with chronic lymphocytic leukaemia treated with fludarabine, cyclophosphamide and rituximab--incidence and predictors. Br J Haematol. 2007 Mar. 136(6):800-5. [QxMD MEDLINE Link].
- Moulin B, Ronco PM, Mougenot B, Francois A, Fillastre JP, Mignon F. Glomerulonephritis in chronic lymphocytic leukemia and related B-cell lymphomas. Kidney Int. 1992 Jul. 42(1):127-35. [QxMD MEDLINE Link].
- Arora S, Levitan D, Regmi N, Sidhu G, Gupta R, Nicastri AD, et al. Cryoglobulinemia in a patient with chronic lymphocytic leukemia - A case report and review of literature of renal involvement in CLL. Blood Cells Mol Dis. 2016 Sep. 60:7-11. [QxMD MEDLINE Link].
- [Guideline] Eichhorst B, Robak T, Montserrat E, Ghia P, Niemann CU, Kater AP, et al. Chronic lymphocytic leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021 Jan. 32 (1):23-33. [QxMD MEDLINE Link]. [Full Text].
- Tam CS, Trotman J, Opat S, Burger JA, Cull G, Gottlieb D, et al. Phase 1 study of the selective BTK inhibitor zanubrutinib in B-cell malignancies and safety and efficacy evaluation in CLL. Blood. 2019 Sep 12. 134 (11):851-859. [QxMD MEDLINE Link].
- Condoluci A, Terzi di Bergamo L, Langerbeins P, et al. International Prognostic Score for Asymptomatic Early-stage Chronic Lymphocytic Leukemia. Blood. 2020 Apr 8. [QxMD MEDLINE Link]. [Full Text].
- Rai KR, Sawitsky A, Cronkite EP, et al. Clinical staging of chronic lymphocytic leukemia. Blood. 1975 Aug. 46(2):219-34. [QxMD MEDLINE Link]. [Full Text].
- Robak T, Jamroziak K, Gora-Tybor J, et al. Comparison of cladribine plus cyclophosphamide with fludarabine plus cyclophosphamide as first-line therapy for chronic lymphocytic leukemia: a phase III randomized study by the Polish Adult Leukemia Group (PALG-CLL3 Study). J Clin Oncol. 2010 Apr 10. 28(11):1863-9. [QxMD MEDLINE Link].
- Chustecka Z. Ibrutinib (Imbruvica) Approved for CLL in US. Medscape [serial online]. Available at http://www.medscape.com/viewarticle/820537. February 13, 2014; Accessed: January 12, 2023.
- Nelson R. FDA Gives Arzerra Breakthrough Therapy Designation for CLL. Medscape Medical News. Available at http://www.medscape.com/viewarticle/811307. September 19, 2013; Accessed: January 12, 2023.
- Shanafelt T, Lanasa MC, Call TG, et al. Ofatumumab-based chemoimmunotherapy is effective and well tolerated in patients with previously untreated chronic lymphocytic leukemia (CLL). Cancer. 2013 Aug 6. [QxMD MEDLINE Link].
- Hillmen P, Rawstron AC, Brock K, Muñoz-Vicente S, Yates FJ, Bishop R, et al. Ibrutinib Plus Venetoclax in Relapsed/Refractory Chronic Lymphocytic Leukemia: The CLARITY Study. J Clin Oncol. 2019 Jul 11. JCO1900894. [QxMD MEDLINE Link].
- Smith J. FDA approves ibrutinib plus obinutuzumab for CLL/SLL. MDedge. Available at https://www.mdedge.com/hematology-oncology/article/193367/cll/fda-approves-ibrutinib-plus-obinutuzumab-cll/sll. January 28, 2019; Accessed: November 7, 2022.
Author
Mohammad Muhsin Chisti, MD, FACP Associate Professor of Medicine (Hematology and Oncology), Oakland University William Beaumont School of Medicine; Medical Director of Research, Karmanos Cancer Institute
Mohammad Muhsin Chisti, MD, FACP is a member of the following medical societies: American College of Physicians, American Medical Association, American Society of Clinical Oncology, American Society of Hematology, Medical Society of the State of New York
Disclosure: Nothing to disclose.
Coauthor(s)
Bilal M Ali, BS MD Candidate, Oakland University William Beaumont School of Medicine
Disclosure: Nothing to disclose.
Mariam Aoun, MD Resident Physician, Department of Internal Medicine, Beaumont Hospital – Royal Oak
Disclosure: Nothing to disclose.
Specialty Editor Board
Mary L Windle, PharmD Adjunct Associate Professor, University of Nebraska Medical Center College of Pharmacy; Editor-in-Chief, Medscape Drug Reference
Disclosure: Nothing to disclose.
Chief Editor
Emmanuel C Besa, MD Professor Emeritus, Department of Medicine, Division of Hematologic Malignancies and Hematopoietic Stem Cell Transplantation, Kimmel Cancer Center, Jefferson Medical College of Thomas Jefferson University
Emmanuel C Besa, MD is a member of the following medical societies: American Association for Cancer Education, American Society of Clinical Oncology, American College of Clinical Pharmacology, American Federation for Medical Research, American Society of Hematology, New York Academy of Sciences
Disclosure: Nothing to disclose.
Additional Contributors
Haleem J Rasool, MD, FACP Chair, Department of Oncology, Mayo Clinic Health System, La Crosse, WI
Haleem J Rasool, MD, FACP is a member of the following medical societies: American College of Physicians, American Society of Clinical Oncology, American Society of Hematology
Disclosure: Nothing to disclose.
Delong Liu, MD, PhD Professor of Medicine, Division of Oncology/Hematology, New York Medical College; Chief of Hematology, Phelps Memorial Hospital Center; Director of Non-ablative Allogeneic Stem Cell Transplantation Program, Westchester Medical Center; Editor-in-Chief, Journal of Hematology and Oncology
Delong Liu, MD, PhD is a member of the following medical societies: American Society of Hematology, American Society of Clinical Oncology
Disclosure: Nothing to disclose.
Samir C Patel, MD Fellow, Department of Hematology and Medical Oncology, Metropolitan Hospital, New York Medical College
Disclosure: Nothing to disclose.
Acknowledgements
Michael Perry, MD, MS, MACP† Former Nellie B Smith Chair of Oncology Emeritus, Former Director, Division of Hematology and Medical Oncology, Former Deputy Director, Ellis Fischel Cancer Center, University of Missouri-Columbia School of Medicine
Clarence Sarkodee-Adoo, MD Consulting Staff, Department of Bone Marrow Transplantation, City of Hope Samaritan BMT Program
Disclosure: Takeda Millenium Honoraria Speaking and teaching
Francisco Talavera, PharmD, PhD Adjunct Assistant Professor, University of Nebraska Medical Center College of Pharmacy; Editor-in-Chief, Medscape Drug Reference
Disclosure: Medscape Salary Employment