Rickets: Practice Essentials, Pathophysiology, Epidemiology (original) (raw)
Overview
Practice Essentials
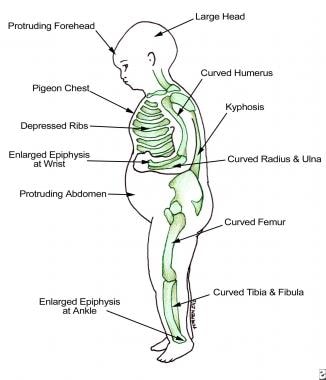
Rickets is a disease of growing bone that is unique to children and adolescents. It is caused by a failure of osteoid to calcify in a growing person. Failure of osteoid to calcify in adults is called osteomalacia. The image below illustrates findings in a patient with rickets.
Findings in patients with rickets.
See 23 Hidden Clues to Diagnosing Nutritional Deficiencies, a Critical Images slideshow, to help identify clues to conditions associated with malnutrition.
Vitamin D deficiency rickets occurs when the metabolites of vitamin D are deficient. Less commonly, a dietary deficiency of calcium or phosphorus may also produce rickets. Vitamin D-3 (cholecalciferol) is formed in the skin from a derivative of cholesterol under the stimulus of ultraviolet-B light. Ultraviolet light or cod liver oil was the only significant source of vitamin D until early in the 20th century when ergosterol (vitamin D-2) was synthesized from irradiated plant steroids.
During the Industrial Revolution, rickets appeared in epidemic form in temperate zones where the pollution from factories blocked the sun’s ultraviolet rays. Thus, rickets was probably the first childhood disease caused by environmental pollution. In the United Kingdom during the 17th century, an estimated 2-8% of deaths in urban areas were attributed to rickets, which became known as the “English disease.” [1]
Natural nutritional sources of vitamin D are limited primarily to fatty, ocean-going fish. In the United States, dairy milk is fortified with vitamin D (400 IU/L). Human milk contains little vitamin D, generally less than 20-40 IU/L. Therefore, infants who are breastfed are at risk for rickets, especially those who receive no oral supplementation and those who have darkly pigmented skin, which blocks penetration of ultraviolet light.
Rickets may lead to skeletal deformity and short stature. In females, pelvic distortion from rickets may cause problems with childbirth later in life. Severe rickets has been associated with respiratory failure in children.
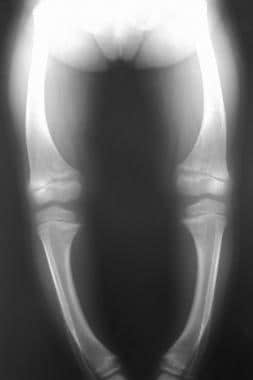
Findings in rickets are illustrated in the image below.
Radiograph in a 4-year-old girl with rickets depicts bowing of the legs caused by loading.

Pathophysiology
Cholecalciferol (ie, vitamin D-3) is formed in the skin from 5-dihydrotachysterol. This steroid undergoes hydroxylation in 2 steps. The first hydroxylation occurs at position 25 in the liver, producing calcidiol (25-hydroxycholecalciferol), which circulates in the plasma as the most abundant of the vitamin D metabolites and is thought to be a good indicator of overall vitamin D status.
The second hydroxylation step occurs in the kidney at the 1 position, where it undergoes hydroxylation to the active metabolite calcitriol (1,25-dihydroxycholecalciferol). This cholecalciferol, which circulates in the bloodstream in minute amounts, is not technically a vitamin but a hormone.
Calcitriol acts at 3 known sites to tightly regulate calcium metabolism: (1) it promotes absorption of calcium and phosphorus from the intestine; (2) it increases reabsorption of phosphate in the kidney; and, (3) it acts on bone to release calcium and phosphate. Calcitriol may also directly facilitate calcification. These actions result in an increase in the concentrations of calcium and phosphorus in extracellular fluid.
This increase of calcium and phosphorus in extracellular fluid, in turn, leads to the calcification of osteoid, primarily at the metaphyseal growing ends of bones but also throughout all osteoid in the skeleton. Parathyroid hormone facilitates the 1-hydroxylation step in vitamin D metabolism.
In the vitamin D deficiency state, hypocalcemia develops, which stimulates excess secretion of parathyroid hormone. In turn, renal phosphorus loss is enhanced, further reducing deposition of calcium in the bone.
Excess parathyroid hormone also produces changes in the bone similar to those occurring in hyperparathyroidism. Early in the course of rickets, the calcium concentration in the serum decreases. After the parathyroid response, the calcium concentration usually returns to the reference range, though phosphorus levels remain low. Alkaline phosphatase, which is produced by overactive osteoblast cells, leaks into the extracellular fluids, so that its concentration rises to anywhere from moderate elevation to very high levels.
Intestinal malabsorption of fat and diseases of the liver or kidney may produce the clinical and secondary biochemical picture of nutritional rickets. In such cases, disturbance in calcium homeostasis may be the consequence of renal excretion or may result from intestinal losses, as dietary calcium forms insoluble soaps with malabsorbed fats. Anticonvulsant drugs (eg, phenobarbital, phenytoin) accelerate metabolism of calcidiol, which may lead to insufficiency and rickets, particularly in children who have darkly pigmented skin and those who are kept primarily indoors (eg, children who are institutionalized).
Calcium and vitamin D intakes are low in infants who are fed vegan diets, particularly in those who are lactovegans, and monitoring of their vitamin D status is essential. [2]
Studies have noted that disorders of increased fibroblast growth factor 23 (FGF-23) function are associated with rickets. [3]

Epidemiology
United States statistics
In the United States, vitamin D deficiency rickets does not generally occur in infants fed proprietary infant formulas, because both formula and cow milk sold in the United States contain 400 IU of vitamin D per liter. Accordingly, except in pediatric patients with chronic malabsorption syndromes or end-stage renal disease, nearly all cases of rickets occur in breastfed infants who have dark skin and receive no vitamin D supplementation.
International statistics
The incidence of rickets in Europe is similar to that in the United States. In sunny areas, such as in the Middle East, rickets may occur when infants are bundled in clothing and are not exposed to sunlight. In some parts of Africa, deficiency of calcium, phosphorus, or both in the diet may also lead to rickets, especially in societies where corn is predominant in the diet.
The frequency of rickets has been increasing internationally. Possible reasons include recommendations for children to wear sunscreen while outdoors and a tendency for children to spend more time indoors, watching television or playing electronic games, instead of playing outdoors. [4]
Sodri et al reported the case of a 22-month-old girl in Malaysia with nutritional rickets, who presented with abnormal gait and bowing of the legs during the coronavirus disease 2019 (COVID-19) pandemic. Because of lockdowns related to COVID, she had minimal exposure to sunlight. In addition, her calcium intake was poor. [5]

- Newton G. Diagnosing rickets in early modern England: statistical evidence and social response. Soc Hist Med. 2022 May. 35 (2):566-88. [QxMD MEDLINE Link]. [Full Text].
- Zmora E, Gorodischer R, Bar-Ziv J. Multiple nutritional deficiencies in infants from a strict vegetarian community. Am J Dis Child. 1979 Feb. 133(2):141-4. [QxMD MEDLINE Link].
- McKay CP, Portale A. Emerging topics in pediatric bone and mineral disorders 2008. Semin Nephrol. 2009 Jul. 29(4):370-8. [QxMD MEDLINE Link].
- Lowdon J. Rickets: concerns over the worldwide increase. J Fam Health Care. 2011 Mar-Apr. 21(2):25-9. [QxMD MEDLINE Link].
- Sodri NI, Mohamed-Yassin MS, Mohd Nor NS, Ismail IA. Rickets due to severe vitamin D and calcium deficiency during the COVID-19 pandemic in Malaysia. Am J Case Rep. 2021 Nov 1. 22:e934216. [QxMD MEDLINE Link]. [Full Text].
- Chapman T, Sugar N, Done S, Marasigan J, Wambold N, Feldman K. Fractures in infants and toddlers with rickets. Pediatr Radiol. 2010 Jul. 40(7):1184-9. [QxMD MEDLINE Link].
- Shah BR, Finberg L. Single-day therapy for nutritional vitamin D-deficiency rickets: a preferred method. J Pediatr. 1994 Sep. 125(3):487-90. [QxMD MEDLINE Link].
- Dabas A, Dabas V, Dabla PK, et al. Daily versus weekly oral vitamin D3 therapy for nutritional rickets in Indian children: A randomized controlled open-label trial. Br J Nutr. 2022 May 13. 1-23. [QxMD MEDLINE Link].
- Thacher TD, Smith L, Fischer PR, Isichei CO, Cha SS, Pettifor JM. Optimal Dose of Calcium for Treatment of Nutritional Rickets: A Randomized Controlled Trial. J Bone Miner Res. 2016 Nov. 31 (11):2024-2031. [QxMD MEDLINE Link].
- Casey CF, Slawson DC, Neal LR. VItamin D supplementation in infants, children, and adolescents. Am Fam Physician. 2010 Mar 15. 81(6):745-8. [QxMD MEDLINE Link].
- Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Ross AC, Taylor CL, Yaktine AL, Del Valle HB eds. Dietary Reference Intakes for Calcium and Vitamin D. Washington DC: National Academies Press; 2011.
- Pramyothin P, Holick MF. Vitamin D supplementation: guidelines and evidence for subclinical deficiency. Curr Opin Gastroenterol. 2012 Mar. 28(2):139-50. [QxMD MEDLINE Link].
- Braegger C, Campoy C, Colomb V, et al. Vitamin D in the healthy European paediatric population. J Pediatr Gastroenterol Nutr. 2013 Jun. 56(6):692-701. [QxMD MEDLINE Link].
- Greer FR. Issues in establishing vitamin D recommendations for infants and children. Am J Clin Nutr. 2004 Dec. 80(6 Suppl):1759S-62S. [QxMD MEDLINE Link].
- [Guideline] Wagner CL, Greer FR. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008 Nov. 122(5):1142-52. [QxMD MEDLINE Link].
- Findings in patients with rickets.
- Anteroposterior and lateral radiographs of the wrist of an 8-year-old boy with rickets demonstrates cupping and fraying of the metaphyseal region.
- Radiographs of the knee of a 3.6-year-old girl with hypophosphatemia depict severe fraying of the metaphysis.
- Radiograph in a 4-year-old girl with rickets depicts bowing of the legs caused by loading.


Author
Steven M Schwarz, MD, FAAP, FACN, AGAF Professor of Pediatrics, Children's Hospital at Downstate, State University of New York Downstate Medical Center
Steven M Schwarz, MD, FAAP, FACN, AGAF is a member of the following medical societies: American Academy of Pediatrics, American College of Nutrition, American Association for Physician Leadership, New York Academy of Medicine, Gastroenterology Research Group, American Gastroenterological Association, American Pediatric Society, North American Society for Pediatric Gastroenterology, Hepatology and Nutrition, Society for Pediatric Research
Disclosure: Nothing to disclose.
Coauthor(s)
Frank R Greer, MD Professor of Pediatrics, University of Wisconsin School of Medicine; Professor (Affiliate) of Nutritional Sciences, University of Wisconsin College of Agriculture and Life Sciences; Attending Physician in Neonatology, Center for Perinatal Care, Meriter Hospital
Frank R Greer, MD is a member of the following medical societies: American Academy of Pediatrics, American Pediatric Society
Disclosure: Nothing to disclose.
Laurence Finberg, MD Clinical Professor, Department of Pediatrics, University of California, San Francisco, School of Medicine and Stanford University School of Medicine
Laurence Finberg, MD is a member of the following medical societies: American Medical Association
Disclosure: Nothing to disclose.
Specialty Editor Board
Mary L Windle, PharmD Adjunct Associate Professor, University of Nebraska Medical Center College of Pharmacy; Editor-in-Chief, Medscape Drug Reference
Disclosure: Nothing to disclose.
Chief Editor
Jatinder Bhatia, MBBS, FAAP Professor of Pediatrics, Medical College of Georgia, Georgia Regents University; Chief, Division of Neonatology, Director, Fellowship Program in Neonatal-Perinatal Medicine, Director, Transport/ECMO/Nutrition, Vice Chair, Clinical Research, Department of Pediatrics, Children's Hospital of Georgia
Jatinder Bhatia, MBBS, FAAP is a member of the following medical societies: Academy of Nutrition and Dietetics, American Academy of Pediatrics, American Association for the Advancement of Science, American Pediatric Society, American Society for Nutrition, American Society for Parenteral and Enteral Nutrition, Society for Pediatric Research, Southern Society for Pediatric Research
Disclosure: Serve(d) as a director, officer, partner, employee, advisor, consultant or trustee for: Nestle
Serve(d) as a speaker or a member of a speakers bureau for: Nestle
Received income in an amount equal to or greater than $250 from: Nestle.
Additional Contributors
Steven M Schwarz, MD, FAAP, FACN, AGAF Professor of Pediatrics, Children's Hospital at Downstate, State University of New York Downstate Medical Center
Steven M Schwarz, MD, FAAP, FACN, AGAF is a member of the following medical societies: American Academy of Pediatrics, American College of Nutrition, American Association for Physician Leadership, New York Academy of Medicine, Gastroenterology Research Group, American Gastroenterological Association, American Pediatric Society, North American Society for Pediatric Gastroenterology, Hepatology and Nutrition, Society for Pediatric Research
Disclosure: Nothing to disclose.