Pediatric Right Bundle Branch Block: Background, Pathophysiology, Etiology (original) (raw)
Background
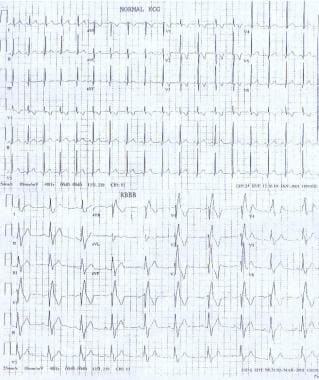
Right bundle branch block (RBBB) occurs when transmission of the electrical impulse is delayed or not conducted along the right bundle branch. Thus, the right ventricle depolarizes by means of cell-to-cell conduction that spreads from the interventricular septum and left ventricle to the right ventricle. This results in the characteristic electrocardiographic (ECG) pattern compared with a normal ECG as shown in the image below.
Pediatric Right Bundle Branch Block. These electrocardiographs demonstrate a normal sinus rhythm and a sinus rhythm with a right bundle branch block.

Pathophysiology
Knowledge of the anatomy and electrophysiology of cardiac conduction system from the atrioventricular (AV) junction to the Purkinje fibers is essential to understanding the pathophysiology of right bundle branch block (RBBB).
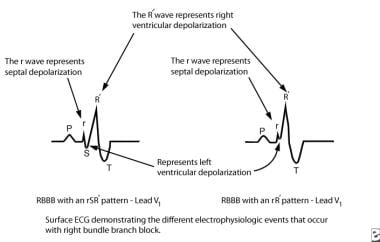
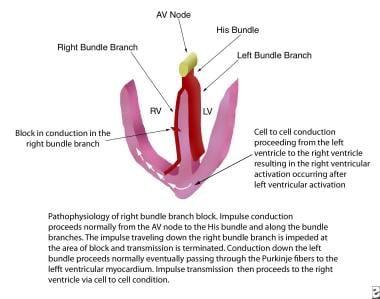
Right bundle branch block occurs when the electrical impulse from the bundle of His does not conduct along the right bundle branch. Conduction down the left bundle branch proceeds normally, and the interventricular septum and left ventricle rapidly depolarize in the normal fashion. Depolarization of the right ventricle occurs later and is comparatively slow, accounting for the electrocardiographic (ECG) findings in right bundle branch block; this may manifest either as rsR' or rR' pattern in the right chest leads (see the images below).
Pediatric Right Bundle Branch Block. These electrocardiographs demonstrate a normal sinus rhythm and a sinus rhythm with a right bundle branch block.
Pediatric Right Bundle Branch Block. This image depicts the electrophysiologic events of right bundle branch block. AV = atrioventricular.
Pediatric Right Bundle Branch Block. Pathophysiology of right bundle branch block is described. AV = atrioventricular; LV = left ventricular; RV = right ventricular.
Exercise-induced right bundle branch block has been rarely noted and appears to be benign. [1]
Embryology
The cardiac conduction system develops from rings of specialized tissue found in the embryonic heart tube. One theory describes four rings, each located between different segments of the heart tube. With looping and growth of the cardiac septi, the rings are brought together and develop into the sinus node, the AV node, and the penetrating bundle. Another theory describes a single ring of tissue located between the bulbus cordis and the primitive ventricle, which gives rise to the AV node, His bundle, right bundle branch, and left bundle branch.
Anatomy
The specialized conduction system of the heart is composed of cells that conduct electrical impulses faster than the surrounding myocardium. The conduction system can be divided into distinct anatomic segments, and each segment is described in sequence beginning at the AV junction and ending with the Purkinje fibers.
The AV junction can be divided into three regions as follows: transitional cell zone, AV node, and penetrating portion of the AV bundle (His bundle, common bundle).
The transitional cell zone is where the right atrium merges with the compact AV node by means of discrete atrial pathways termed the slow and fast pathways.
The next segment is the AV node, which lies anterior and superior to the ostium of the coronary sinus, directly above the insertion of the septal leaflet of the tricuspid valve. This area is located at the apex of the triangle of Koch, which is formed by the tricuspid annulus, the tendon of Todaro, and the ostium of the coronary sinus. Blood supply to the AV node is derived from the AV nodal artery, which is a branch of the right coronary artery in 85%-90% of individuals and a branch of the left circumflex coronary artery in 10%-15% of individuals.
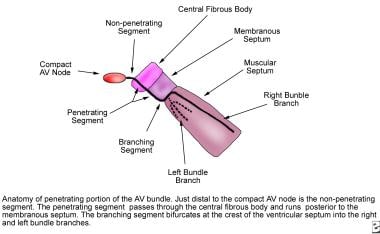
At the apex of the triangle of Koch, the compact AV node becomes the penetrating bundle of His. It penetrates the central fibrous body at the attachment of the tendon of Todaro, runs between the membranous septum and the muscular septum, and bifurcates at the crest of the muscular ventricular septum. The His bundle is divided into three anatomic segments. The proximal or nonpenetrating segment lies distal to the AV node and proximal to the central fibrous body. The middle or penetrating segment penetrates the central fibrous body and runs posterior to the membranous septum. The distal or branching segment bifurcates at the crest of the muscular septum into the right and left bundle branches (see the image below).
Pediatric Right Bundle Branch Block. Anatomy of the penetrating portion of the atrioventricular (AV) bundle is shown.
The right bundle branch, a direct continuation of the penetrating bundle of His, originates distal to the attachment of the septal leaflet of the tricuspid valve with the membranous septum and surfaces on the right ventricular septum just below the papillary muscle of the conus. It is unbranched and proceeds toward the apex of the right ventricle along the posterior margin of the septal band, courses through the moderator band to the base of the anterior papillary muscle, and proceeds to the right ventricular free wall.
The left bundle branch originates at the crest of the muscular ventricular septum just distal to the membranous septum. It arises in a fanlike fashion that descends inferiorly along the left ventricular septal surface beneath the noncoronary cusp of the aortic valve. The left bundle branch usually branches into three major fascicles. The anterior fascicle is directed to the base of the anterolateral papillary muscle, the posterior fascicle is directed to the base of the posteromedial papillary muscle, and, in 60% of hearts, a central fascicle proceeds to the midseptal region. When no central fascicle is present, as in 40% of hearts, the midseptal region is supplied by radiations from the anterior fascicle or the anterior and posterior fascicles.
At the terminal aspect of each bundle branch, Purkinje fibers are interlaced on the endocardial surface of both ventricles and tend to be concentrated at the tips of the papillary muscles.
For a discussion on the anatomy of subtypes, see below.
Electrophysiology of cardiac conduction
The heart is a two-step mechanical pump coordinated by precisely timed electrical impulses. For the pump to perform optimally, sequential depolarizations of the atria and then the ventricles allow atrial contraction to provide complete diastolic filling of the ventricles (AV synchrony). After the ventricles are filled, rapid activation of the ventricular myocardium permits a synchronized contraction to eject blood most effectively to the great vessels.
Normal cardiac conduction
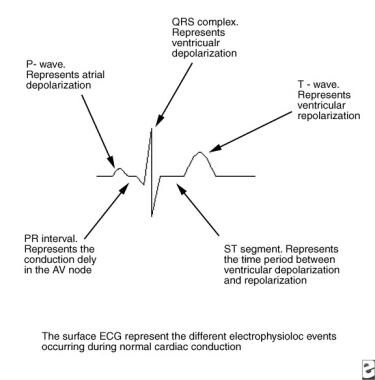
In normal cardiac conduction, electrical excitation of the heart proceeds in a sequential manner from the atria to the ventricles and is demonstrated on the surface ECG (see the image below).
Pediatric Right Bundle Branch Block. This image depicts the electrophysiologic sequence of events that occur in normal cardiac conduction. AV = atrioventricular.
The electrical impulse is generated in the sinus node and proceeds along proposed internodal conduction pathways to reach the AV node. As the impulse conducts through the AV node, conduction slows, allowing time for atrial contraction to occur before the ventricle is activated (PR segment). After the impulse passes through the compact AV node, it is rapidly conducted through the crux of the heart to the ventricles by means of the bundle of His (penetrating bundle) to the branching bundle, the bundle branches, the distal Purkinje fibers, and finally the ventricular myocardial cells (narrow QRS complex). When depolarization is complete, the ventricle repolarizes in preparation for conducting another impulse.
Types of right bundle branch block
Three types of right bundle branch block have been identified in electrophysiologic studies. Proximal, or central, right bundle branch block occurs when a conduction block is present just distal to the bundle of His in the superior aspect of the right bundle branch. This generally occurs when the proximal bundle is injured during surgery for lesions with an inlet or membranous ventricular septal defect (VSD).
Another type of right bundle branch block occurs when the impulse is interrupted between the proximal and distal aspects of the right bundle branch; this type is most commonly observed after surgical division of the moderator band during repair of tetralogy of Fallot.
Distal right bundle branch block is observed when distal ramifications of the right bundle are disrupted during right ventriculotomy or resection of muscle bundles in the right ventricular outflow tract. Regardless of the type of right bundle branch block, the ECG patterns remain similar.
Natural history
In general, surgically induced right bundle branch block results in no clinically significant acute hemodynamic consequences and has a benign course over the long term. In rare cases, a progression to complete heart block and sudden death is a concern, particularly if the right bundle branch block pattern is accompanied by additional evidence of substantial injury to the His-Purkinje system (eg, left anterior hemiblock, first-degree AV block). Patients who have undergone repair for tetralogy of Fallot and who have an right bundle branch block pattern with a markedly prolonged QRS duration (>180 ms) may be at increased risk for significant ventricular arrhythmias and sudden death.
Patients with right bundle branch block from other causes may have diverse natural histories depending on the underlying disease. The outcome may be benign in some forms of familial right bundle branch block, or sudden death may result if the right bundle branch block pattern on ECG is due to Brugada syndrome, arrhythmogenic right ventricular cardiomyopathy (ARVC), or Kearns-Sayre syndrome.

Etiology
Hereditary factors
Hereditary right bundle branch block (RBBB) was observed in four Lebanese families and has been mapped to chromosome 19. [2]
A subset of patients with Brugada syndrome have mutations in SCN5A, the gene that encodes for the voltage-gated cardiac sodium channel. [3, 4]
Risk factors
In children, most cases of right bundle branch block occur after intracardiac surgery, such as congenital heart surgery associated with repair of a ventricular septal defect (VSD) and tetralogy of Fallot, as well as cardiac transplantation. Right bundle branch block has also been described in patients undergoing cardiac catheterization, particularly transcatheter closure of perimembranous VSDs and catheter ablation for ventricular tachycardia.
Right bundle branch block has been associated with cardiomyopathy, myocarditis, congestive heart failure, right ventricular systolic dysfunction, [5] atrial septal defect (ASD), and Ebstein anomaly of the tricuspid valve.
A transient form of right bundle branch may be observed in patients with premature atrial contractions (Ashman phenomenon) or supraventricular tachycardia (rate dependent right bundle branch block). This occurs when an early impulse is conducted from the atrioventricular (AV) node to the His bundle while the right bundle branch is still refractory but the left bundle is not. Conduction down the right bundle branch is therefore delayed or blocked, resulting in a transient right bundle branch block pattern on the electrocardiogram (ECG).
Incomplete right bundle branch block has been described in children with perinatal exposure to human immunodeficiency virus (HIV) type 1. [6] In a more recent study that evaluated retrospective (2005-2014) Boston Children’s Hospital data from 32,127 ECGs depicting isolated right bundle branch conduction disturbances, the investigators noted that incomplete right bundle branch block is not uncommon in a healthy school age children, and that there was high inter-reader variability (its prevalence among readers ranged from 1% to 20%). [7] While there is an increased use of echocardiography to evaluate these children, there was no evidence for higher echocardiographic abnormalities in these children than those with normal ECGs. [7]
Associated syndromes
Duchenne muscular dystrophy is an X-linked myopathy characterized by early onset and rapid progression with muscular weakness and pseudohypertrophy seen in the second year of life. Cardiac findings include mitral valve prolapse, pulmonary flow murmur, and an S3 or S4 gallop. (See Muscular Dystrophy.)
Myotonic dystrophy is characterized by muscular dystrophy, myotonias, hypogonadism, frontal balding, and cataracts. Congenital muscular dystrophy manifests with neonatal hypotonia, paresis, and myotonia. The adult form of myotonic dystrophy is the most common muscular dystrophy seen in adults. ECG findings may include first-degree AV block, left anterior fascicular block, and intraventricular conduction delay. Patients may have arrhythmias, Stokes-Adams attacks, or both. (See Muscular Dystrophy.)
Kearns-Sayre Syndrome is a mitochondrial myopathy with the physical findings of ptosis, chronic progressive external ophthalmoplegia, and abnormal retinal pigmentation. Patients are at risk for heart block and sudden death. [8] Rarely, patients present with dilated cardiomyopathy and heart failure.
Brugada syndrome is a channelopathy mediated by the SCN5A gene. The right bundle branch block pattern seen in patients with this syndrome is not actually right bundle branch block but a function of the unusual repolarization abnormality. The ECG shows ST-segment elevation in leads V1-V3, and patients are at risk for sudden cardiac death. An illustrative example of transient right bundle branch block masking the ST-segment changes in Brugada syndrome has been published. [9] Cocaine consumption or the use of the antiarrhythmic drugs propafenone, ajmaline, flecainide, or procainamide may reveal ECG findings consistent with Brugada syndrome. [10]
Right bundle branch block may also disguise the characteristic QRS morphology associated with ventricular preexcitation (see Wolff-Parkinson-White Syndrome). In patients with known right bundle branch block (eg, tetralogy of Fallot) and Wolff-Parkinson-White syndrome with a left-sided accessory pathway, the presence of the pathway is often undetected. In patients with right bundle branch block and a right-sided accessory pathway, the QRS may appear relatively normal without the expected right bundle branch block pattern.
The presence of right bundle branch block influences the diagnostic criterion for patients with arrhythmogenic right ventricular cardiomyopathy (ARVC), a condition associated with T-wave inversions in leads V1-V3. [11]
Patients may have isolated right bundle branch block or right bundle branch block with a left anterior fascicular block.
Right bundle branch block has been associated with blunt chest trauma, polymyositis, and, more recently, post-coronavirus disease (COVID) multisystem inflammatory syndrome in children (MIS-C). [12]

Epidemiology
United States data
The most common cause of right bundle branch block (RBBB) in children is surgery associated with repair of an isolated ventricular septal defect (VSD) or another congenital heart disease that includes a VSD (eg, double-chambered right ventricle, atrioventricular [AV] canal, or tetralogy of Fallot). The incidence of right bundle branch block ranges from 25%-81% after repair of a VSD alone to 60%-100% after repair of tetralogy of Fallot. The variation of right bundle branch block after surgery is likely due to the proximity of the VSD to the His bundle and right bundle, as well as the surgical technique. For example, right bundle branch block is less common with transatrial repair or exclusion ventriculotomy repair of a VSD than with other procedures.
Age-related demographics
Surgical repair of tetralogy of Fallot, in addition to closing the VSD, is often associated with a transannular patch of the right ventricular outflow tract. This situation often results in clinically significant pulmonic valve insufficiency and progressive right ventricular dilatation as the patient ages. In addition, some patients have residual stenosis at various levels in the pulmonary outflow tract. By the time the patient is in his or her late teens or young adulthood, the right ventricle has been subjected to years of abnormal hemodynamics. As noted above, patients with right bundle branch block and a markedly prolonged QRS duration (>180 ms) may be at increased risk for ventricular tachycardia and sudden death.

Prognosis
The prognosis for patients with isolated right bundle branch block (RBBB) is excellent, because the clinical course of right bundle branch block is generally benign.
In other patients with right bundle branch block, such as those who have undergone heart transplantation or repair of congenital heart disease and those with Brugada syndrome, Kearns-Sayre syndrome, myocarditis, or cardiomyopathy, the prognosis depends on the underlying condition causing the right bundle branch block and the morphology of the systemic ventricle.
In pediatric patients after undergoing ventricular septal defect (VSD) patch closure or tetralogy of Fallot repair, the presence of right bundle branch block has been shown to cause some mechanical dyssynchrony, a tendency toward left ventricular dilatation, and affect cardiac magnetic resonance imaging assessment of right ventricular size and function. [13, 14, 15] Therefore, long-term follow-up of these patients has been suggested, although the clinical significance of these changes is likely limited. [16]
Vojtovic et al found that acute right ventricular resynchronization led to short-term improvement of hemodynamics in children early after repair of tetralogy of Fallot, and the effect was maximized when paced from the site of the latest right ventricular activation. [17] The researchers suggested right ventricular resynchronization has the potential to be a useful nonpharmacologic adjunct in the management of hemodynamically compromised children.
Morbidity/mortality
Surgically induced right bundle branch block is generally considered to have in no clinically significant acute hemodynamic consequences and has a benign course over the long term. However, the role of right bundle branch block in acute right ventricular dysfunction following tetralogy of Fallot repair is unknown since this is a routine postoperative finding. Patients who have undergone tetralogy of Fallot repair and have a QRS duration more than 180 milliseconds may have a long-term risk of ventricular arrhythmias and sudden death. [18] In rare cases, if right bundle branch block is associated with injury to the proximal His-Purkinje system (eg, left anterior hemiblock, first-degree atrioventricular [AV] block), it can progress to complete heart block and sudden death.
Patients with familial right bundle branch block may have a benign course, whereas those with right bundle branch block in association with Brugada syndrome, arrhythmogenic right ventricular cardiomyopathy (ARVC), or Kearns-Sayre syndrome are at risk for sudden death.
Complications
If right bundle branch block progresses to complete heart block, the patient may experience fatigue, exercise intolerance, dizziness, syncope, or sudden death. Some patients who have undergone tetralogy of Fallot repair and have a right bundle branch block pattern and a markedly prolonged QRS may be at increased risk for ventricular tachycardia or sudden death.

Patient Education
Patients, parents, and primary medical providers should be informed that right bundle branch block (RBBB) per se does not typically predict specific future problems or management concerns.
Patients with right bundle branch block are not specifically limited in their activities; however, associated conditions may influence activity restrictions.
For patient education resources, see the Heart Health Center, as well as Tetralogy of Fallot.

- Stein R, Nguyen P, Abella J, Olson H, Myers J, Froelicher V. Prevalence and prognostic significance of exercise-induced right bundle branch block. Am J Cardiol. 2010 Mar 1. 105(5):677-80. [QxMD MEDLINE Link].
- Stephan E, Chedid R, Loiselet J, Bouvagnet P. Clinical and molecular genetics of familial bundle branch block related to chromosome 19 [in French]. Arch Mal Coeur Vaiss. 1998 Dec. 91(12):1465-74. [QxMD MEDLINE Link].
- Deschenes I, Baroudi G, Berthet M, et al. Electrophysiological characterization of SCN5A mutations causing long QT (E1784K) and Brugada (R1512W and R1432G) syndromes. Cardiovasc Res. 2000 Apr. 46(1):55-65. [QxMD MEDLINE Link].
- Keller DI, Barrane FZ, Gouas L, et al. A novel nonsense mutation in the SCN5A gene leads to Brugada syndrome and a silent gene mutation carrier state. Can J Cardiol. 2005 Sep. 21(11):925-31. [QxMD MEDLINE Link].
- Adams JC, Nelson MR, Chandrasekaran K, Jahangir A, Srivathsan K. Novel ECG criteria for right ventricular systolic dysfunction in patients with right bundle branch block. Int J Cardiol. 2013 Aug 20. 167(4):1385-9. [QxMD MEDLINE Link]. [Full Text].
- Diogenes MS, Succi RC, Machado DM, et al. Cardiac longitudinal study of children perinatally exposed to human immunodeficiency virus type 1 [in Portuguese]. Arq Bras Cardiol. 2005 Oct. 85(4):233-40. [QxMD MEDLINE Link].
- Meziab O, Abrams DJ, Alexander ME, et al. Utility of incomplete right bundle branch block as an isolated ECG finding in children undergoing initial cardiac evaluation. Congenit Heart Dis. 2018 May. 13(3):419-27. [QxMD MEDLINE Link].
- van Beynum I, Morava E, Taher M, et al. Cardiac arrest in Kearns-Sayre syndrome. JIMD Rep. 2012. 2:7-10. [QxMD MEDLINE Link]. [Full Text].
- Perez-Riera AR, Barbosa Barros R, Daminello-Raimundo R, de Abreu LC, Nikus K. Transient high-degree right bundle branch block masking the type 1 Brugada ECG pattern associated with possible transient early repolarization syndrome. Ann Noninvasive Electrocardiol. 2020 Jan. 25(1):e12673. [QxMD MEDLINE Link]. [Full Text].
- Daga B, Minano A, de la Puerta I, et al. Electrocardiographic findings typical of Brugada syndrome unmasked by cocaine consumption [in Spanish]. Rev Esp Cardiol. 2005 Nov. 58(11):1355-7. [QxMD MEDLINE Link].
- Marcus FI, McKenna WJ, Sherrill D, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the Task Force Criteria. Eur Heart J. 2010 Apr. 31(7):806-14. [QxMD MEDLINE Link]. [Full Text].
- Choi NH, Fremed M, Starc T, et al. MIS-C and cardiac conduction abnormalities. Pediatrics. 2020 Dec. 146(6):[QxMD MEDLINE Link]. [Full Text].
- Veeram Reddy SR, Du W, Zilberman MV. Left ventricular mechanical synchrony and global systolic function in pediatric patients late after ventricular septal defect patch closure: a three-dimensional echocardiographic study. Congenit Heart Dis. 2009 Nov-Dec. 4(6):454-8. [QxMD MEDLINE Link].
- Hui W, Slorach C, Dragulescu A, Mertens L, Bijnens B, Friedberg MK. Mechanisms of right ventricular electromechanical dyssynchrony and mechanical inefficiency in children after repair of tetralogy of Fallot. Circ Cardiovasc Imaging. 2014 Jul. 7(4):610-8. [QxMD MEDLINE Link].
- Marterer R, Hongchun Z, Tschauner S, Koestenberger M, Sorantin E. Cardiac MRI assessment of right ventricular function: impact of right bundle branch block on the evaluation of cardiac performance parameters. Eur Radiol. 2015 Dec. 25(12):3528-35. [QxMD MEDLINE Link].
- Karadeniz C, Atalay S, Demir F, et al. Does surgically induced right bundle branch block really effect ventricular function in children after ventricular septal defect closure?. Pediatr Cardiol. 2015 Mar. 36(3):481-8. [QxMD MEDLINE Link].
- Vojtovic P, Kucera F, Kubus P, et al. Acute right ventricular resynchronization improves haemodynamics in children after surgical repair of tetralogy of Fallot. Europace. 2018 Feb 1. 20(2):323-8. [QxMD MEDLINE Link].
- Gatzoulis MA, Balaji S, Webber SA, et al. Risk factors for arrhythmia and sudden cardiac death late after repair of tetralogy of Fallot: a multicentre study. Lancet. 2000 Sep 16. 356(9234):975-81. [QxMD MEDLINE Link].
- [Guideline] Finnish Medical Society Duodecim. Myocardial infarction. In: EBM Guidelines. Evidence-Based Medicine [Internet]. Helsinki, Finland: Wiley Interscience. John Wiley & Sons; 2008 Apr 2. [Full Text].
- Heiberg J, Eckerstrom F, Rex CE, et al. Heart rate variability is impaired in adults after closure of ventricular septal defect in childhood: A novel finding associated with right bundle branch block. Int J Cardiol. 2019 Jan 1. 274:88-92. [QxMD MEDLINE Link].
- Jain R, Dalal D, Daly A, et al. Electrocardiographic features of arrhythmogenic right ventricular dysplasia. Circulation. 2009 Aug 11. 120(6):477-87. [QxMD MEDLINE Link]. [Full Text].
- Sillanmaki S, Aapro S, Lipponen JA, et al. Electrical and mechanical dyssynchrony in patients with right bundle branch block. J Nucl Cardiol. 2020 Apr. 27(2):621-30. [QxMD MEDLINE Link].
- Chevallier S, Forclaz A, Tenkorang J, et al. New electrocardiographic criteria for discriminating between Brugada types 2 and 3 patterns and incomplete right bundle branch block. J Am Coll Cardiol. 2011 Nov 22. 58(22):2290-8. [QxMD MEDLINE Link].
- Becker AE, Anderson RH. Morphology of the human atrioventricular junctional area. In: Wellens JHH, Lie KI, Janse MJ, eds. The Conduction System of the Heart. Chicago: Lea & Febiger; 1976. 263-71.
- Brugada J, Brugada R, Brugada P. Right bundle-branch block and ST-segment elevation in leads V1 through V3: a marker for sudden death in patients without demonstrable structural heart disease. Circulation. 1998 Feb 10. 97(5):457-60. [QxMD MEDLINE Link].
- Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome. A multicenter report. J Am Coll Cardiol. 1992 Nov 15. 20(6):1391-6. [QxMD MEDLINE Link].
- Brugada P, Brugada R, Brugada J. Sudden death in patients and relatives with the syndrome of right bundle branch block, ST segment elevation in the precordial leads V(1)to V(3)and sudden death. Eur Heart J. 2000 Feb. 21(4):321-6. [QxMD MEDLINE Link].
- Colvin EV. Cardiac embryology. In: Garson A, Bricker JT, Fisher DJ, Neish SR, eds. The Science and Practice of Pediatric Cardiology. 2nd ed. Baltimore, MD: Williams & Wilkins; 1998. 91-123.
- Dubin D. Rapid Interpretation of EKGs. 3rd ed. Tampa, FL: Cover; 1984. 137-42.
- Esscher E, Hardell LI, Michaelsson M. Familial, isolated, complete right bundle-branch block. Br Heart J. 1975 Jul. 37(7):745-7. [QxMD MEDLINE Link].
- Ewing L. Bundle-branch and fasicular blocks. In: Gillette PC, Garson A, eds. Pediatric Arrhythmias: Electrophysiology and Pacing. Philadelphia: WB Saunders Co; 1990. 319-21.
- Garson A. Interventricular conduction disturbances. The Electrocardiogram in Infants and Children: A Systematic Approach. Philadelphia, PA: Lea & Febiger; 1983. 119-42.
- Garson A. Electrocardiography. In: Garson A, Bricker JT, Fisher DJ, Neish SR, eds. The Science and Practice of Pediatric Cardiology. 2nd ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 1998. 736.
- Horowitz LN, Alexander JA, Edmunds LH Jr. Postoperative right bundle branch block: identification of three levels of block. Circulation. 1980 Aug. 62(2):319-28. [QxMD MEDLINE Link].
- Husson GS, Blackman MS, Rogers MC, et al. Familial congenital bundle branch system disease. Am J Cardiol. 1973 Sep 7. 32(3):365-9. [QxMD MEDLINE Link].
- James TN. The connecting pathways between the sinus node and A-V node and between the right and the left atrium in the human heart. Am Heart J. 1963 Oct. 66:498-508. [QxMD MEDLINE Link].
- Kehoe RF, Bauernfeind R, Tommaso C, et al. Cardiac conduction defects in polymyositis: electrophysiologic studies in four patients. Ann Intern Med. 1981 Jan. 94(1):41-3. [QxMD MEDLINE Link].
- Komajda M, Frank R, Vedel J, et al. Intracardiac conduction defects in dystrophia myotonica. Electrophysiological study of 12 cases. Br Heart J. 1980 Mar. 43(3):315-20. [QxMD MEDLINE Link].
- Krongrad E. Prognosis for patients with congenital heart disease and postoperative intraventricular conduction defects. Circulation. 1978 May. 57(5):867-70. [QxMD MEDLINE Link].
- Li TC, Hu DY, Bian H, et al. Effects of transcatheter closure of perimembranous ventricular septal defects: intermediate and long-term follow-up of 68 cases [in Chinese]. Zhonghua Yi Xue Za Zhi. 2005 Oct 26. 85(40):2846-9. [QxMD MEDLINE Link].
- Massing GK, James TN. Anatomical configuration of the His bundle and bundle branches in the human heart. Circulation. 1976 Apr. 53(4):609-21. [QxMD MEDLINE Link].
- Nery PB, Ha AC, Keren A, Birnie DH. Cardiac resynchronization therapy in patients with left ventricular systolic dysfunction and right bundle branch block: a systematic review. Heart Rhythm. 2011 Jul. 8(7):1083-7. [QxMD MEDLINE Link].
- Ogura Y, Kato J, Ogawa Y, et al. A case of alternating bundle branch block in combination with intra-Hisian block. Int Heart J. 2005 Jul. 46(4):737-44. [QxMD MEDLINE Link].
- Pickhoff AS. Electrophysiology; development and function of the cardiac conduction system. In: Allen HD, Gutgesell HP, Clark EB, Driscoll DJ, eds. Heart Disease in Infants, Children, and Adolescents: Including the Fetus and Young Adult. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2000. 414-24.
- Priori SG, Napolitano C, Gasparini M, et al. Clinical and genetic heterogeneity of right bundle branch block and ST- segment elevation syndrome: A prospective evaluation of 52 families. Circulation. 2000 Nov 14. 102(20):2509-15. [QxMD MEDLINE Link].
- Rossi L. Histopathology of Cardiac Arrhythmias. 2nd ed. Philadelphia, PA: Lea & Febiger; 1979. 1-75.
- Sanyal SK, Johnson WW. Cardiac conduction abnormalities in children with Duchenne's progressive muscular dystrophy: electrocardiographic features and morphologic correlates. Circulation. 1982 Oct. 66(4):853-63. [QxMD MEDLINE Link].
- Schaal SF, Seidensticker J, Goodman R, Wooley CF. Familial right bundle-branch block, left axis deviation, complete heart block, and early death. A heritable disorder of cardiac conduction. Ann Intern Med. 1973 Jul. 79(1):63-6. [QxMD MEDLINE Link].
- Scheinman MM, Goldschlager NF, Peters RW. Bundle branch block. Cardiovasc Clin. 1980. 11(1):57-80. [QxMD MEDLINE Link].
- Silka MJ. Bundle branch block. In: Garson A, Bricker JT, Fisher DJ, Neish SR, eds. The Science and Practice of Pediatric Cardiology. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 1998. 2033-6.
- Simonsen EE, Madsen EG. Four cases of right-sided bundle-branch block and one case of atrioventricular block in three generations of a family. Br Heart J. 1970 Jul. 32(4):501-4. [QxMD MEDLINE Link].
- Stephan E. Hereditary bundle branch system defect. A new genetic entity?. Am Heart J. 1979 Jun. 97(6):708-18. [QxMD MEDLINE Link].
- Stephan E. Hereditary bundle branch system defect: survey of a family with four affected generations. Am Heart J. 1978 Jan. 95(1):89-95. [QxMD MEDLINE Link].
- Sun XJ, Gao W, Zhou AQ, et al. Risk factors for arrhythmia early after transcatheter closure of perimembranous ventricular septal defects [in Chinese]. Zhonghua Er Ke Za Zhi. 2005 Oct. 43(10):767-71. [QxMD MEDLINE Link].
- Sung RJ, Tamer DM, Garcia OL, et al. Analysis of surgically-induced right bundle branch block pattern using intracardiac recording techniques. Circulation. 1976 Sep. 54(3):442-6. [QxMD MEDLINE Link].
- Tawara S. Das Reizleitungssystem des Saugetierkerzens. Jena: Gustav Fisher; 1906.
- Udink ten Cate FE, van Heerde M, Rammeloo LA, Hruda J. Transientelectrocardiographic abnormalities following blunt chest trauma in a child. Eur J Pediatr. November 2008. 167(11):1331-3.
- Van Praagh R, Van Praagh S. Morphologic anatomy. In: Fyler DC, ed. Nadas' Pediatric Cardiology. Philadelphia, PA: Hanley & Belfus; 1992. 23.
- Waller BF, Schlant RC. Anatomy of the heart. In: O'Rourke RA, Schlant RC, Roberts R, et al, eds. Hurst's The Heart. 8th ed. New York, NY: McGraw-Hill Medical; 1994. 99-102.
- Walsh, EP. Electrocardiography and introduction to electrophysiologic techniques. In: Fyler DC, ed. Nadas' Pediatric Cardiology. Philadelphia, PA: Hanley & Belfus; 1992. 134.
- Yasui H, Yoshitoshi M, Komori M, et al. Cardiodynamic effects of experimental right bundle branch block in canine hearts with normal and hypertrophied right ventricles. Am Heart J. 1985 Jan. 109(1):69-77. [QxMD MEDLINE Link].
- Ksiazczyk TM, Tomik A, Godlewski K, Werner B. Transient complete right bundle branch block following right heart catheterization in children - a report of two cases. Postepy Kardiol Interwencyjnej. 2016. 12(4):372-6. [QxMD MEDLINE Link].
- Thornton MD. Lower-extremity weakness in a teenager due to thyrotoxic periodic paralysis. J Emerg Med. 2017 Apr. 52(4):e133-e137. [QxMD MEDLINE Link].
- Gatzoulis MA, Till JA, Somerville J, Redington AN. Mechanoelectrical interaction in tetralogy of Fallot. QRS prolongation relates to right ventricular size and predicts malignant ventricular arrhythmias and sudden death. Circulation. 1995 Jul 15. 92(2):231-7. [QxMD MEDLINE Link]. [Full Text].
- Zhou SH, Liebman J, Dubin AM, et al. Using 12-lead ECG and synthesized VCG in detection of right ventricular hypertrophy with terminal right conduction delay versus partial right bundle branch block in the pediatric population. J Electrocardiol. 2001. 34 suppl:249-57. [QxMD MEDLINE Link].
Author
Glenn T Wetzel, MD, PhD Associate Professor of Pediatric Cardiology, John Hopkins University School of Medicine; Director, Pediatric Interventional Electrophysiology, John Hopkins Children's Center
Glenn T Wetzel, MD, PhD is a member of the following medical societies: Heart Rhythm Society
Disclosure: Nothing to disclose.
Coauthor(s)
Kenneth R Knecht, MD Assistant Professor of Cardiology, Department of Pediatrics, Arkansas Children's Hospital, University of Arkansas for Medical Sciences College of Medicine
Kenneth R Knecht, MD is a member of the following medical societies: American Academy of Pediatrics
Disclosure: Nothing to disclose.
Karine Guerrier, DO, MPH Assistant Professor, Division of Cardiology, Department of Pediatrics, University of Tennessee Health Science Center College of Medicine; Director, Cardiac Rhythm Device Management, Le Bonheur Heart Institute, Le Bonheur Children’s Hospital
Karine Guerrier, DO, MPH is a member of the following medical societies: American College of Cardiology, American Heart Association, Heart Rhythm Society, Pediatric and Congenital Electrophysiology Society
Disclosure: Nothing to disclose.
Specialty Editor Board
Mary L Windle, PharmD Adjunct Associate Professor, University of Nebraska Medical Center College of Pharmacy; Editor-in-Chief, Medscape Drug Reference
Disclosure: Nothing to disclose.
Alvin J Chin, MD Emeritus Professor of Pediatrics, University of Pennsylvania School of Medicine
Disclosure: Nothing to disclose.
Chief Editor
Syamasundar Rao Patnana, MD Professor of Pediatrics and Medicine, Division of Cardiology, Emeritus Chief of Pediatric Cardiology, University of Texas Medical School at Houston and Children's Memorial Hermann Hospital
Syamasundar Rao Patnana, MD is a member of the following medical societies: American Academy of Pediatrics, American Pediatric Society, American College of Cardiology, American Heart Association, Society for Cardiovascular Angiography and Interventions, Society for Pediatric Research
Disclosure: Nothing to disclose.
Acknowledgements
The authors and editors of Medscape Drugs & Diseases gratefully acknowledge the contributions of previous authors Christopher Mart, MD; Kerry Rosen, MD; and Christopher Zachary, MD, to the development and writing of this article.