Pediatric Atopic Dermatitis: Practice Essentials, Pathophysiology, Etiology (original) (raw)
Practice Essentials
Atopic dermatitis (AD) is a chronically relapsing skin disorder with an immunologic basis. [1] The clinical presentation varies from mild to severe. In the worst cases, atopic dermatitis may interfere with normal growth and development. Treatment consists of adequate skin hydration, avoidance of allergenic precipitants, topical anti-inflammatory medications, systemic antihistamines, and antibiotic coverage of secondary infections.
Although often used interchangeably, the terms eczema and atopic dermatitis are not equivalent. Eczema is a reaction pattern with various causes and the most common pediatric cause is atopic dermatitis. Other causes of eczematous dermatitis include allergic contact dermatitis, irritant contact dermatitis, seborrheic dermatitis, nummular eczema, dyshidrotic eczema, asteatotic eczema, and lichen simplex chronicus. Eczematous reactions can be classified as acute, subacute, or chronic, depending on historical and physical characteristics.
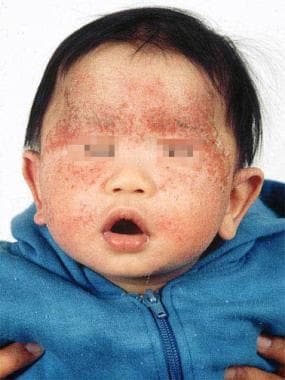
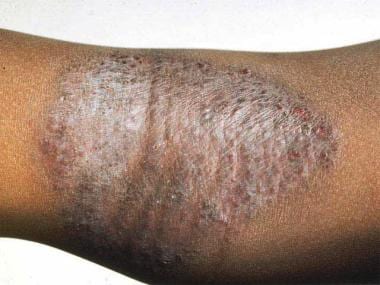
The images below depict patients with atopic dermatitis.
Typical atopic dermatitis on the face of an infant.
Flexural involvement in childhood atopic dermatitis.
Prognosis
Atopic dermatitis persists into adulthood in 20-40% of children with the condition. Many children outgrow severe atopic dermatitis and only experience itchy or inflamed skin if exposed to exogenous irritants as adults.
Signs and symptoms
See Physical Examination.
Diagnostics
No definitive laboratory tests are used to diagnose atopic dermatitis (AD). Elevated serum immunoglobulin E (IgE) levels and peripheral blood eosinophilia occur in most individuals with atopic dermatitis, and these findings may be useful in confirming the atopic status of suspected cases. The presence of serum IgE directed against the cell wall of S aureus is observed in hyper-IgE syndrome and atopic dermatitis.
Common infections that mimic or complicate atopic dermatitis can be tested for as follows: conduct a Tzanck smear for herpes simplex virus (HSV), a potassium hydroxide (KOH) preparation for dermatophytes, and a Gram stain for bacterial infections.
Histologic findings
Acute eczematous lesions show histologic markings of hyperkeratosis, parakeratosis, and acanthosis with a decreased or absent granular cell layer.
Important features in histologic diagnosis include spongiosis (accumulation of fluid in the intercellular and intracellular areas) and exocytosis (infiltration of leukocytes through the epidermis).
Chronic eczematous lesions display hyperkeratosis with areas of parakeratosis and papillomatosis (upward proliferation of dermal papillae).
Also see Other Tests.
Management
See Treatment and Medication.

Pathophysiology
Clinically unaffected skin in patients with atopic dermatitis has increased numbers of T-helper type 2 (Th2) cells compared with skin in patients without atopic dermatitis. Increased levels of interleukin (IL)-4 and IL-13 (Th2 cytokines) are seen in acute atopic dermatitis skin lesions, whereas chronic atopic dermatitis lesions show increased expression of IL-5 (Th2 cytokine) and IL-12 and interferon (IFN)-γ (Th1 cytokines). Chronic atopic dermatitis lesions also exhibit greater eosinophil infiltration compared with skin in patients without atopic dermatitis.
IL-4 enhances differentiation of T-helper cells along the Th2 pathway, and IL-13 acts as a chemoattractant for Th2 cells to infiltrate atopic dermatitis lesions. IL-13 may also directly induce IL-5 expression and eosinophil infiltration, thereby facilitating the transition from acute lesions into chronic lesions. [2]
In addition, patients with atopic dermatitis appear to have significantly decreased levels of skin barrier molecules compared with normal controls. Ceramide lipids in the stratum corneum, which are responsible for water retention and permeability functions, and skin barrier proteins such as filaggrin are expressed at significantly lower levels in the skin of patients with atopic dermatitis compared with the skin of patients without atopic dermatitis. [2, 3]
Significant evidence favors the hygiene hypothesis for the development of atopic dermatitis. An inverse relationship is recognized between helminth infections and atopic dermatitis but no other pathogens. [4] In addition, early day care, endotoxin, unpasteurized farm milk, and animal exposure appear to be beneficial, likely because of a general increase in exposure to nonpathogenic microbes.
Positive association was shown between maternal exposure to increased concentrations of particulate matter and atopic dermatitis. [5]

Etiology
The etiology of atopic dermatitis appears to be linked both to genetic causes and to environmental agents.
The prevalence of atopic dermatitis in children with one affected parent is 60% and rises to nearly 80% for children of two affected parents. Additionally, nearly 40% of patients with newly diagnosed cases report a positive family history for atopic dermatitis in at least one first-degree relative. Children of parents with atopic dermatitis have an increased risk of developing atopic dermatitis by age 3 years. [6] Much higher concordance rates for atopic dermatitis are observed in monozygotic twins (77%) than in dizygotic twins (15%). [2]
Evidence has demonstrated a strong genetic predisposition towards the development of atopic dermatitis in patients with loss-of-function mutations in the gene that encodes the epidermal structural protein filaggrin (FLG). Filaggrin deficiency causes a significant defect in the normal epidermal barrier that allows for enhanced allergen absorption through the skin, resulting in a higher incidence of dermatitis. FLG gene mutations have been associated with a more severe atopic dermatitis phenotype, earlier onset of atopic dermatitis, increased levels of systemic allergen sensitivity, and a higher proportion of patients with atopic dermatitis who eventually develop asthma. [7]
In addition, the specific loss-of-function null mutation R501x in the filaggrin gene appears to confer a higher risk of developing eczema herpeticum, which is a rare but serious complication that requires treatment with antiviral medications. [8]
Prenatal risk factors for atopic dermatitis are under investigation. Term infants of mothers who had gestational diabetes during pregnancy had an almost 8-fold increase in the prevalence of atopic dermatitis by age 6 years. Interestingly, this relationship did not occur in preterm infants of mothers with gestational diabetes. The reasons for this discrepancy are yet to be determined. [9]
A retrospective study of 414 children and adolescents with atopic dermatitis suggested that prolonged obesity in early childhood may be a risk factor for atopic dermatitis; this advocated the concept that weight loss may facilitate prevention and treatment of childhood atopic dermatitis. [10]
Environmental allergens repeatedly have been shown to trigger exacerbations of atopic dermatitis in susceptible individuals. Contact irritants, climate, sweating, aeroallergens, microbial organisms, and stress/psyche commonly trigger exacerbations.
Contact irritants (eg, soaps, solvents, wool clothing, mechanical irritants, detergents, preservatives, perfumes) compromise the integument, creating inflammation, irritation, and a portal of entry for further environmental insult. These surface irritants, along with the macerative effects of sweating and the drying effects of low humidity, lower the pruritic threshold. A vicious cycle of itching and scratching ensues, in which added cutaneous damage caused by scratching further lowers the pruritic threshold and subsequently causes increased itching.
Aeroallergens (eg, house dust mite, molds, pollen, dander) induce peripheral eosinophilia and elevate serum IgE levels. These early effects lead to increased histamine release from IgE-activated mast cells and elevated activity of the T-helper cell–mediated immune system. The increased release of vascular mediators (eg, bradykinin, histamine, slow-reacting substance of anaphylaxis [SRS-A]) induces vasodilation, edema, and urticaria, which in turn stimulate pruritus and inflammatory cutaneous changes.
Microbial agents (eg, S aureus, Pityrosporum yeasts, Candida organisms, Trichophyton dermatophytes) act in two different ways to promote the flares of atopic dermatitis. The microorganisms directly invade the skin, creating local injury and inflammation, and they induce a systemic allergic response to specific antigens, causing a rise in serum IgE and enhanced activity of the immune system. Anti-IgE drugs diminish pediatric atopic dermatitis severity. [11]
Nearly all patients with atopic dermatitis are colonized by S aureus on lesional skin. More than half of patients with atopic dermatitis are colonized by S aureus strains capable of producing superantigens. These patients can develop superantigen-specific IgE antibodies that activate inflammatory cells in the skin. Staphylococcal enterotoxin B is a superantigen known to upregulate IL-31 expression in skin. IL-31 has been shown to induce pruritus and skin lesions resembling atopic dermatitis in mice. [2] In addition, methicillin-resistant S aureus strains with reduced susceptibility to vancomycin are increasing worldwide and have been documented in atopic children. [12]
Specific IgE levels to Malassezia furfur have been correlated with atopic dermatitis severity in a subgroup of patients. These Malassezia -specific IgE antibodies have been shown to crossreact with autoantigens in atopic dermatitis skin. [2]
Food allergy is implicated as a cause in one third to one half of children with atopic dermatitis. Food allergens may be the initial trigger for IgE autoreactivity to epithelial autoantigens in young children with atopic dermatitis. [2] The most common food allergens in children are egg, soy, milk, wheat, fish, shellfish, and peanut, which together account for 90% of food-induced cases of atopic dermatitis in double-blind, placebo-controlled food challenges. Fortunately, many clinically significant food allergies self-resolve within the first 5 years of life, eliminating the need for long-term restrictive diets.
Stress may trigger atopic dermatitis at the sites of activated cutaneous nerve endings, possibly by the actions of substance P, vasoactive intestinal peptide (VIP), or via the adenyl cyclase–cyclic adenosine monophosphate (cAMP) system.
Atopic dermatitis is the result of a complex relationship between genetic predisposition and environmental exposures, including climate. [13] Atopic dermatitis prevalence was significantly lower with highest-quartile mean annual relative humidity, and with 2 other factors associated with increased UV exposure.

Epidemiology
Frequency
United States
Atopic dermatitis occurs in approximately 10-20% of children and 2% of adults. [2] Children with concurrent asthma or hayfever have a 30-50% incidence of developing atopic dermatitis.
International
Prevalence rates for atopic dermatitis in children over a 1-year period ranged from around 2% in Iran and China to about 20% in Australasia, England, and Scandinavia. [14] Interestingly, populations that migrate from areas of low prevalence to areas of higher prevalence have shown an increased incidence of atopic dermatitis, bolstering the idea of strong environmental influences in the development of atopic dermatitis.
Race, sex, and age-related information
No clear racial predilections have been identified.
Males and females are affected with equal incidence and severity.
Atopic dermatitis may occur in people of any age but often starts in infants aged 2-6 months. Ninety percent of patients with atopic dermatitis experience the onset of disease prior to age 5 years. [15] Seventy-five percent of individuals experience marked improvement in the severity of their atopic dermatitis by age 14 years; however, the remaining 25% continue to have significant relapses during their adult life. A 2009 study concluded that the prevalence of atopic dermatitis in children younger than 2 years was 18.6%. [16]

- Turner JD, Schwartz RA. Atopic dermatitis. A clinical challenge. Acta Dermatovenerol Alp Panonica Adriat. 2006 Jun. 15(2):59-68. [QxMD MEDLINE Link].
- Ong PY, Leung DY. Immune dysregulation in atopic dermatitis. Curr Allergy Asthma Rep. 2006 Sep. 6(5):384-9. [QxMD MEDLINE Link].
- Oranje AP, Devillers AC, Kunz B, et al. Treatment of patients with atopic dermatitis using wet-wrap dressings with diluted steroids and/or emollients. An expert panel's opinion and review of the literature. J Eur Acad Dermatol Venereol. 2006 Nov. 20(10):1277-86. [QxMD MEDLINE Link].
- Flohr C, Yeo L. Atopic dermatitis and the hygiene hypothesis revisited. Curr Probl Dermatol. 2011. 41:1-34. [QxMD MEDLINE Link].
- Stelmach I, Bobrowska-Korzeniowska M, Smejda K, Majak P, Jerzynska J, Stelmach W, et al. Risk factors for the development of atopic dermatitis and early wheeze. Allergy Asthma Proc. 2014 Sep. 35(5):382-389. [QxMD MEDLINE Link].
- Bisgaard H, Halkjaer LB, Hinge R, et al. Risk analysis of early childhood eczema. J Allergy Clin Immunol. 2009 Jun. 123(6):1355-60.e5. [QxMD MEDLINE Link].
- Leung DY. Our evolving understanding of the functional role of filaggrin in atopic dermatitis. J Allergy Clin Immunol. 2009 Sep. 124(3):494-5. [QxMD MEDLINE Link].
- Gao PS, Rafaels NM, Hand T, et al. Filaggrin mutations that confer risk of atopic dermatitis confer greater risk for eczema herpeticum. J Allergy Clin Immunol. 2009 Sep. 124(3):507-13, 513.e1-7. [QxMD MEDLINE Link].
- Kumar R, Ouyang F, Story RE, et al. Gestational diabetes, atopic dermatitis, and allergen sensitization in early childhood. J Allergy Clin Immunol. 2009 Nov. 124(5):1031-8.e1-4. [QxMD MEDLINE Link].
- Silverberg JI, Kleiman E, Lev-Tov H, et al. Association between obesity and atopic dermatitis in childhood: A case-control study. J Allergy Clin Immunol. 2011 May. 127(5):1180-1186.e1. [QxMD MEDLINE Link].
- Slomski A. Anti-IgE Medication Lessens Pediatric Atopic Dermatitis Severity. JAMA. 2020 Feb 25. 323 (8):701. [QxMD MEDLINE Link].
- Alzolibani AA, Al Robaee AA, Al Shobaili HA, Bilal JA, Issa Ahmad M, Bin Saif G. Documentation of vancomycin-resistant Staphylococcus aureus (VRSA) among children with atopic dermatitis in the Qassim region, Saudi Arabia. Acta Dermatovenerol Alp Panonica Adriat. 2012 Sep. 21(3):51-3. [QxMD MEDLINE Link].
- Silverberg JI, Hanifin J, Simpson EL. Climatic factors are associated with childhood eczema prevalence in US. J Invest Dermatol. 2013 Jan 18. [QxMD MEDLINE Link].
- Williams H, Stewart A, von Mutius E, Cookson W, Anderson HR,. Is eczema really on the increase worldwide?. J Allergy Clin Immunol. 2008 Apr. 121(4):947-54.e15. [QxMD MEDLINE Link].
- Ong PY, Boguniewicz M. Atopic dermatitis. Prim Care. 2008 Mar. 35(1):105-17, vii. [QxMD MEDLINE Link].
- Kvenshagen B, Jacobsen M, Halvorsen R. Atopic dermatitis in premature and term children. Arch Dis Child. 2009 Mar. 94(3):202-5. [QxMD MEDLINE Link].
- Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venreol. 1980. 92:44-7.
- Mrabet-Dahbi S, Maurer M. Innate immunity in atopic dermatitis. Curr Probl Dermatol. 2011. 41:104-11. [QxMD MEDLINE Link].
- Lee R, Schwartz RA. Pediatric molluscum contagiosum: reflections on the last challenging poxvirus infection, Part 2. Cutis. 2010 Dec. 86(6):287-92. [QxMD MEDLINE Link].
- Lee R, Schwartz RA. Pediatric molluscum contagiosum: reflections on the last challenging poxvirus infection, Part 1. Cutis. 2010 Nov. 86(5):230-6. [QxMD MEDLINE Link].
- Glazenburg EJ, Mulder PG, Oranje AP. A statistical model to predict the reduction of lichenification in atopic dermatitis. Acta Derm Venereol. 2015 Mar. 95 (3):294-7. [QxMD MEDLINE Link].
- Chopra R, Vakharia PP, Sacotte R, Patel N, Immaneni S, White T, et al. Severity strata for Eczema Area and Severity Index (EASI), modified EASI, Scoring Atopic Dermatitis (SCORAD), objective SCORAD, Atopic Dermatitis Severity Index and body surface area in adolescents and adults with atopic dermatitis. Br J Dermatol. 2017 Nov. 177 (5):1316-1321. [QxMD MEDLINE Link].
- Schram ME, Spuls PI, Leeflang MM, Lindeboom R, Bos JD, Schmitt J. EASI, (objective) SCORAD and POEM for atopic eczema: responsiveness and minimal clinically important difference. Allergy. 2012 Jan. 67 (1):99-106. [QxMD MEDLINE Link].
- Silverberg JI, Gelfand JM, Margolis DJ, Fonacier L, Boguniewicz M, Schwartz LB, et al. Severity strata for POEM, PO-SCORAD, and DLQI in US adults with atopic dermatitis. Ann Allergy Asthma Immunol. 2018 Oct. 121 (4):464-468.e3. [QxMD MEDLINE Link].
- Oranje AP. Practical Issues on Interpretation of Scoring Atopic Dermatitis: SCORAD Index, Objective SCORAD, Patient-Oriented SCORAD and Three-Item Severity Score. Curr Probl Dermatol. 2011. 41:149-55. [QxMD MEDLINE Link].
- van Oosterhout M, Janmohamed SR, Spierings M, Hiddinga J, de Waard-van der Spek FB, Oranje AP. Correlation between Objective SCORAD and Three-Item Severity Score used by physicians and Objective PO-SCORAD used by parents/patients in children with atopic dermatitis. Dermatology. 2015. 230 (2):105-12. [QxMD MEDLINE Link].
- Perrett KP, Peters RL. Emollients for prevention of atopic dermatitis in infancy. Lancet. 2020 Feb 19. [QxMD MEDLINE Link].
- Chamlin SL, Kao J, Frieden IJ, et al. Ceramide-dominant barrier repair lipids alleviate childhood atopic dermatitis: changes in barrier function provide a sensitive indicator of disease activity. J Am Acad Dermatol. 2002 Aug. 47(2):198-208. [QxMD MEDLINE Link].
- Zirwas MJ, Barkovic S. Anti-Pruritic Efficacy of Itch Relief Lotion and Cream in Patients With Atopic History: Comparison With Hydrocortisone Cream. J Drugs Dermatol. 2017 Mar 1. 16 (3):243-247. [QxMD MEDLINE Link].
- Leloup P, Stalder JF, Barbarot S. Outpatient Home-based Wet Wrap Dressings with Topical Steroids with Children with Severe Recalcitrant Atopic Dermatitis: A Feasibility Pilot Study. Pediatr Dermatol. 2015 Apr 22. [QxMD MEDLINE Link].
- Paller AS, Tom WL, Lebwohl MG, Blumenthal RL, Boguniewicz M, Call RS, et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J Am Acad Dermatol. 2016 Sep. 75 (3):494-503.e4. [QxMD MEDLINE Link]. [Full Text].
- Eucrisa (crisaborole) [package insert]. Collegeville, Pa: Anacor Pharmaceuticals, Inc. March 2020. Available at [Full Text].
- Eichenfield L, Boguniewicz M, Simpson E, et al. Ann Allergy Asthma Immunol. Once-Daily Roflumilast Cream 0.15% for Atopic “Dermatitis: Pooled Results: From INTEGUMENT-1/2 Phase 3 Trials. 2023 Nov 131(5; suppl 1):S91. [Full Text].
- Beck LA, Thaçi D, Hamilton JD, Graham NM, Bieber T, Rocklin R, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014 Jul 10. 371 (2):130-9. [QxMD MEDLINE Link].
- Thaçi D, Simpson EL, Beck LA, Bieber T, Blauvelt A, Papp K, et al. Efficacy and safety of dupilumab in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical treatments: a randomised, placebo-controlled, dose-ranging phase 2b trial. Lancet. 2016 Jan 2. 387 (10013):40-52. [QxMD MEDLINE Link].
- Simpson EL, Bieber T, Guttman-Yassky E, Beck LA, Blauvelt A, Cork MJ, et al. Two Phase 3 Trials of Dupilumab versus Placebo in Atopic Dermatitis. N Engl J Med. 2016 Dec 15. 375 (24):2335-2348. [QxMD MEDLINE Link].
- Frellick M. Dupilumab Effective in Teens With Moderate to Severe Eczema. Medscape Medical News. Available at https://www.medscape.com/viewarticle/902207. September 19, 2018; Accessed: April 2, 2020.
- Paller AS, Siegfried E, Gooderham M, Beck LA, Boguniewica M, Sher L, et al. Dupilumab significantly improves atopic dermatitis in children aged 6 to 12 years: Results from phase 3 trial (LIBERTY AD PEDS) (abstract 215). Presented at Revolutionizing Atopic Dermatitis 2020 virtual meeting. April 5, 2020. [Full Text].
- Paller AS, Flohr C, Cork M, Bewley A, Blauvelt A, Hong HC, et al. Efficacy and Safety of Tralokinumab in Adolescents With Moderate to Severe Atopic Dermatitis: The Phase 3 ECZTRA 6 Randomized Clinical Trial. JAMA Dermatol. 2023 Jun 1. 159 (6):596-605. [QxMD MEDLINE Link]. [Full Text].
- Schwartz DM, Bonelli M, Gadina M, O'Shea JJ. Type I/II cytokines, JAKs, and new strategies for treating autoimmune diseases. Nat Rev Rheumatol. 2016 Jan. 12 (1):25-36. [QxMD MEDLINE Link]. [Full Text].
- Oetjen LK, Mack MR, Feng J, Whelan TM, Niu H, Guo CJ, et al. Sensory Neurons Co-opt Classical Immune Signaling Pathways to Mediate Chronic Itch. Cell. 2017 Sep 21. 171 (1):217-228.e13. [QxMD MEDLINE Link]. [Full Text].
- Papp K, Szepietowski JC, Kircik L, Toth D, Eichenfield LF, Leung DYM, et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: Results from 2 phase 3, randomized, double-blind studies. J Am Acad Dermatol. 2021 Oct. 85 (4):863-872. [QxMD MEDLINE Link]. [Full Text].
- Guttman-Yassky E, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021 Jun 5. 397 (10290):2151-2168. [QxMD MEDLINE Link].
- Reich K, Teixeira HD, de Bruin-Weller M, Bieber T, Soong W, Kabashima K, et al. Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis (AD Up): results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2021 Jun 5. 397 (10290):2169-2181. [QxMD MEDLINE Link].
- Simpson EL, Sinclair R, Forman S, Wollenberg A, Aschoff R, Cork M, et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. 2020 Jul 25. 396 (10246):255-266. [QxMD MEDLINE Link]. [Full Text].
- Eichenfield LF, Flohr C, Sidbury R, Siegfried E, Szalai Z, Galus R, et al. Efficacy and Safety of Abrocitinib in Combination With Topical Therapy in Adolescents With Moderate-to-Severe Atopic Dermatitis: The JADE TEEN Randomized Clinical Trial. JAMA Dermatol. 2021 Oct 1. 157 (10):1165-1173. [QxMD MEDLINE Link]. [Full Text].
- Novak N. Allergen specific immunotherapy for atopic dermatitis. Curr Opin Allergy Clin Immunol. 2007 Dec. 7(6):542-46. [QxMD MEDLINE Link].
- Huang C, Tang J. Sublingual immunotherapy with Dermatophagoides farinae drops for pediatric atopic dermatitis. Int J Dermatol. 2022 Feb. 61 (2):246-251. [QxMD MEDLINE Link].
- [Guideline] Greer FR, Sicherer SH, Burks AW. Effects of early nutritional interventions on the development of atopic disease in infants and children: the role of maternal dietary restriction, breastfeeding, timing of introduction of complementary foods, and hydrolyzed formulas. Pediatrics. 2008 Jan. 121(1):183-91. [QxMD MEDLINE Link].
- Yang YW, Tsai CL, Lu CY. Exclusive breastfeeding and incident atopic dermatitis in childhood: a systematic review and meta-analysis of prospective cohort studies. Br J Dermatol. 2009 Aug. 161(2):373-83. [QxMD MEDLINE Link].
- Miyake Y, Tanaka K, Sasaki S, et al. Breastfeeding and atopic eczema in Japanese infants: The Osaka Maternal and Child Health Study. Pediatric Allergy & Immunology. May 2009. 20:234-241. [QxMD MEDLINE Link].
- Jin YY, Cao RM, Chen J, Kaku Y, Wu J, Cheng Y, et al. Partially hydrolyzed cow's milk formula has a therapeutic effect on the infants with mild to moderate atopic dermatitis: a randomized, double-blind study. Pediatr Allergy Immunol. 2011 May 4. [QxMD MEDLINE Link].
- Arellano FM, Arana A, Wentworth CE, et al. Lymphoma among patients with atopic dermatitis and/or treated with topical immunosuppressants in the United Kingdom. J Allergy Clin Immunol. 2009 May. 123(5):1111-6, 116.e1-13. [QxMD MEDLINE Link].
- Chang YS, Chou YT, Lee JH, Lee PL, Dai YS, Sun C, et al. Atopic dermatitis, melatonin, and sleep disturbance. Pediatrics. 2014 Aug. 134(2):e397-405. [QxMD MEDLINE Link].
- Xerfan EMS, Tomimori J, Andersen ML, Tufik S, Facina AS. Sleep disturbance and atopic dermatitis: A bidirectional relationship?. Med Hypotheses. 2020 Feb 18. 140:109637. [QxMD MEDLINE Link].
- Capozza K, Gadd H, Kelley K, Russell S, Shi V, Schwartz A. Insights From Caregivers on the Impact of Pediatric Atopic Dermatitis on Families: "I'm Tired, Overwhelmed, and Feel Like I'm Failing as a Mother". Dermatitis. 2020 Feb 21. [QxMD MEDLINE Link].
- Boguniewicz M. Topical treatment of atopic dermatitis. Immunol Allergy Clin North Am. 2004 Nov. 24(4):631-44, vi-vii. [QxMD MEDLINE Link].
- Lee J, Seto D, Bielory L. Meta-analysis of clinical trials of probiotics for prevention and treatment of pediatric atopic dermatitis. J Allergy Clin Immunol. 2008 Jan. 121(1):116-121.e11. [QxMD MEDLINE Link].
- Epstein TG, Bernstein DI, Levin L, Khurana Hershey GK, Ryan PH, Reponen T, et al. Opposing effects of cat and dog ownership and allergic sensitization on eczema in an atopic birth cohort. J Pediatr. 2011 Feb. 158(2):265-71.e1-5. [QxMD MEDLINE Link].
- Thyssen JP, Godoy-Gijon E, Elias PM. Ichthyosis vulgaris - the filaggrin mutation disease. Br J Dermatol. 2013 Jan 10. [QxMD MEDLINE Link].
- Blattner CM, Murase JE. A practice gap in pediatric dermatology: does breast-feeding prevent the development of infantile atopic dermatitis?. J Am Acad Dermatol. 2014 Aug. 71(2):405-6. [QxMD MEDLINE Link].
- Thyssen JP, Vestergaard C, Barbarot S, et al. European Task Force on Atopic Dermatitis: position on vaccination of adult patients with atopic dermatitis against COVID-19 (SARS-CoV-2) being treated with systemic medication and biologics. J Eur Acad Dermatol Venereol. 2021 Feb 15. [QxMD MEDLINE Link].
- Broeders JA, Ahmed Ali U, Fischer G. Systematic review and meta-analysis of randomized clinical trials (RCTs) comparing topical calcineurin inhibitors with topical corticosteroids for atopic dermatitis: A 15-year experience. J Am Acad Dermatol. 2016 May 11. [QxMD MEDLINE Link].
- Tiplica GS, Boralevi F, Konno P, Malinauskiene L, Kaszuba A, Laurens C, et al. The regular use of an emollient improves symptoms of atopic dermatitis in children: a randomized controlled study. J Eur Acad Dermatol Venereol. 2018 Feb 8. [QxMD MEDLINE Link].
- Janmohamed SR, Oranje AP, Devillers AC, Rizopoulos D, van Praag MC, Van Gysel D, et al. The proactive wet-wrap method with diluted corticosteroids versus emollients in children with atopic dermatitis: a prospective, randomized, double-blind, placebo-controlled trial. J Am Acad Dermatol. 2014 Jun. 70(6):1076-82. [QxMD MEDLINE Link].
- Draelos ZD, Stein Gold LF, Murrell DF, Hughes MH, Zane LT. Post Hoc Analyses of the Effect of Crisaborole Topical Ointment, 2% on Atopic Dermatitis: Associated Pruritus from Phase 1 and 2 Clinical Studies. J Drugs Dermatol. 2016 Feb 1. 15 (2):172-6. [QxMD MEDLINE Link].
- Paghdal KV, Schwartz RA. Topical tar: back to the future. J Am Acad Dermatol. 2009 Aug. 61(2):294-302. [QxMD MEDLINE Link].
- Luger T, Boguniewicz M, Carr W, et al. Pimecrolimus in atopic dermatitis: Consensus on safety and the need to allow use in infants. Pediatr Allergy Immunol. 2015 Jun. 26 (4):306-15. [QxMD MEDLINE Link].
- Leung DY, Hanifin JM, Pariser DM, et al. Effects of pimecrolimus cream 1% in the treatment of patients with atopic dermatitis who demonstrate a clinical insensitivity to topical corticosteroids: a randomized, multicentre vehicle-controlled trial. Br J Dermatol. 2009 Aug. 161(2):435-43. [QxMD MEDLINE Link].
- Doss N, Reitamo S, Dubertret L, et al. Superiority of tacrolimus 0.1% ointment compared with fluticasone 0.005% in adults with moderate to severe atopic dermatitis of the face: results from a randomized, double-blind trial. Br J Dermatol. 2009 Aug. 161(2):427-34. [QxMD MEDLINE Link].
- Remitz A, Reitamo S. Long-term safety of tacrolimus ointment in atopic dermatitis. Expert Opin Drug Saf. 2009 Jul. 8(4):501-6. [QxMD MEDLINE Link].
- Jensen JM, Pfeiffer S, Witt M, et al. Different effects of pimecrolimus and betamethasone on the skin barrier in patients with atopic dermatitis. J Allergy Clin Immunol. 2009 May. 123(5):1124-33. [QxMD MEDLINE Link].
- [Guideline] Patel TS, Greer SC, Skinner RB Jr. Cancer concerns with topical immunomodulators in atopic dermatitis: overview of data and recommendations to clinicians. Am J Clin Dermatol. 2007. 8(4):189-94. [QxMD MEDLINE Link].
- Ring J, Mohrenschlager M, Henkel V. The US FDA 'black box' warning for topical calcineurin inhibitors: an ongoing controversy. Drug Saf. 2008. 31(3):185-98. [QxMD MEDLINE Link].
- Kraft M, Worm M. Dupilumab in the treatment of moderate-to-severe atopic dermatitis. Expert Rev Clin Immunol. 2017 Apr. 13 (4):301-310. [QxMD MEDLINE Link].
- Ferreira S, Torres T. Dupilumab for the Treatment of Atopic Dermatitis. Actas Dermosifiliogr. 2018 Feb 5. [QxMD MEDLINE Link].
- Simon D, Hosli S, Kostylina G, Yawalkar N, Simon HU. Anti-CD20 (rituximab) treatment improves atopic eczema. J Allergy Clin Immunol. 2008 Jan. 121(1):122-8. [QxMD MEDLINE Link].
- Bukutu C, Deol J, Shamseer L, Vohra S. Complementary, holistic, and integrative medicine: atopic dermatitis. Pediatr Rev. 2007 Dec. 28(12):e87-94. [QxMD MEDLINE Link].
- Deo M, Yung A, Hill S, Rademaker M. Methotrexate for treatment of atopic dermatitis in children and adolescents. Int J Dermatol. 2014 Aug. 53(8):1037-41. [QxMD MEDLINE Link].
- Bieber T. Atopic dermatitis: an expanding therapeutic pipeline for a complex disease. Nat Rev Drug Discov. 2022 Jan. 21 (1):21-40. [QxMD MEDLINE Link]. [Full Text].
- Simpson EL, Flohr C, Eichenfield LF, Bieber T, Sofen H, Taïeb A, et al. Efficacy and safety of lebrikizumab (an anti-IL-13 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical corticosteroids: A randomized, placebo-controlled phase II trial (TREBLE). J Am Acad Dermatol. 2018 Jan 15. [QxMD MEDLINE Link].
Author
Robert A Schwartz, MD, MPH Professor and Head of Dermatology, Professor of Pathology, Professor of Pediatrics, Professor of Medicine, Rutgers New Jersey Medical School
Robert A Schwartz, MD, MPH is a member of the following medical societies: Alpha Omega Alpha, American Academy of Dermatology, New York Academy of Medicine, Royal College of Physicians of Edinburgh, Sigma Xi, The Scientific Research Honor Society
Disclosure: Nothing to disclose.
Specialty Editor Board
Mary L Windle, PharmD Adjunct Associate Professor, University of Nebraska Medical Center College of Pharmacy; Editor-in-Chief, Medscape Drug Reference
Disclosure: Nothing to disclose.
Robert A Schwartz, MD, MPH Professor and Head of Dermatology, Professor of Pathology, Professor of Pediatrics, Professor of Medicine, Rutgers New Jersey Medical School
Robert A Schwartz, MD, MPH is a member of the following medical societies: Alpha Omega Alpha, American Academy of Dermatology, New York Academy of Medicine, Royal College of Physicians of Edinburgh, Sigma Xi, The Scientific Research Honor Society
Disclosure: Nothing to disclose.
Chief Editor
Dirk M Elston, MD Professor and Chairman, Department of Dermatology and Dermatologic Surgery, Medical University of South Carolina College of Medicine
Dirk M Elston, MD is a member of the following medical societies: American Academy of Dermatology
Disclosure: Nothing to disclose.
Additional Contributors
Kevin P Connelly, DO Clinical Assistant Professor, Department of Pediatrics, Division of General Pediatrics and Emergency Care, Virginia Commonwealth University School of Medicine; Medical Director, Paws for Health Pet Visitation Program of the Richmond SPCA; Pediatric Emergency Physician, Emergency Consultants Inc, Chippenham Medical Center
Kevin P Connelly, DO is a member of the following medical societies: American Academy of Pediatrics, American College of Osteopathic Pediatricians, American Osteopathic Association
Disclosure: Nothing to disclose.
Acknowledgements
The authors and editors of Medscape Reference gratefully acknowledge the contributions of previous author, Caroline C Spagnola, MD, to the development and writing of this article.