Rosuvastatin for Sepsis-Associated Acute Respiratory Distress Syndrome (original) (raw)
. Author manuscript; available in PMC: 2014 Dec 5.
Published in final edited form as: N Engl J Med. 2014 May 18;370(23):2191–2200. doi: 10.1056/NEJMoa1401520
Abstract
BACKGROUND
In the acute respiratory distress syndrome (ARDS), inflammation in the lungs and other organs can cause life-threatening organ failure. Inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A reductase (statins) can modulate inflammatory responses. Previous observational studies suggested that statins improved clinical outcomes in patients with sepsis. We hypothesized that rosuvastatin therapy would improve clinical outcomes in critically ill patients with sepsis-associated ARDS.
METHODS
We conducted a multicenter trial in which patients with sepsis-associated ARDS were randomly assigned to receive either enteral rosuvastatin or placebo in a double-blind manner. The primary outcome was mortality before hospital discharge home or until study day 60 if the patient was still in a health care facility. Secondary outcomes included the number of ventilator-free days (days that patients were alive and breathing spontaneously) to day 28 and organ-failure–free days to day 14.
RESULTS
The study was stopped because of futility after 745 of an estimated 1000 patients had been enrolled. There was no significant difference between study groups in 60-day in-hospital mortality (28.5% with rosuvastatin and 24.9% with placebo, P = 0.21) or in mean (±SD) ventilator-free days (15.1±10.8 with rosuvastatin and 15.1±11.0 with placebo, P = 0.96). The groups were well matched with respect to demographic and key physiological variables. Rosuvastatin therapy, as compared with placebo, was associated with fewer days free of renal failure to day 14 (10.1±5.3 vs. 11.0±4.7, P = 0.01) and fewer days free of hepatic failure to day 14 (10.8±5.0 vs. 11.8±4.3, P = 0.003). Rosuvastatin was not associated with an increased incidence of serum creatine kinase levels that were more than 10 times the upper limit of the normal range.
CONCLUSIONS
Rosuvastatin therapy did not improve clinical outcomes in patients with sepsis-associated ARDS and may have contributed to hepatic and renal organ dysfunction.
Despite progress in supportive care strategies for the acute respiratory distress syndrome (ARDS), mortality remains high, especially among patients with sepsis.1 Inflammation leading to cellular damage and cellular death contributes to both pulmonary and nonpulmonary organ failure. Therapies that attenuate inflammation may improve outcomes in patients with ARDS.2 Inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A reductase, known collectively as statins, are commonly prescribed for hypercholesterolemia. However, statins also reduce inflammation and have been shown to prevent ARDS in murine models.3–13 Several (but not all) large observational studies, a small randomized, controlled trial, and meta-analyses before study initiation have suggested that patients with sepsis or other life-threatening inflammatory conditions who receive statins have improved clinical outcomes or intermediate outcomes (e.g., oxygenation and lung compliance).14–24 We designed a trial to test the hypothesis that rosuvastatin therapy would improve the clinical outcomes of critically ill patients with sepsis-associated ARDS.
METHODS
STUDY DESIGN AND OVERSIGHT
This clinical trial was approved by the institutional review board at each of the 44 enrolling hospitals in the National Heart, Lung, and Blood Institute ARDS Clinical Trials Network (see the Supplementary Appendix, available with the full text of this article at NEJM.org). Participants or legally authorized surrogates provided written informed consent. (The study protocol and statistical analysis plan are available at NEJM.org.)
The study was conceived in 2004 and revised in 2009 by the ARDS Clinical Trials Network steering committee. The protocol committee wrote the first draft of the manuscript. The coordinating center performed all the analyses. All the authors had full and independent access to all the data and vouch for the integrity, accuracy, and completeness of the data and analysis and the fidelity of the study to the protocol. AstraZeneca supplied the study drugs and the resources to measure blood levels of rosuvastatin. AstraZeneca had no role in the study design, study conduct, data analysis, or data interpretation. AstraZeneca reviewed the manuscript before submission for publication and offered nonbinding suggestions related to the correction of typographic errors.
STUDY PATIENTS
Patients were eligible for enrollment if they were receiving positive-pressure mechanical ventilation through an endotracheal tube, had a ratio of the partial pressure of arterial oxygen (Pao2) to the fraction of inspired oxygen (Fio2) of 300 or less, and had bilateral infiltrates on chest radiography that were consistent with pulmonary edema, without evidence of left atrial hypertension.25 These criteria had been met during a single 24-hour period. Additional inclusion criteria were a known or suspected infection and either of the following criteria for a systemic inflammatory response: a white-cell count of more than 12,000 per cubic millimeter or less than 4000 per cubic millimeter or a differential count with more than 10% band forms, or a core body temperature of more than 38°C or less than 36°C.26
Reasons for exclusion are listed in the Supplementary Appendix. Major exclusion criteria were the presence of ARDS for more than 48 hours; chronic conditions that could adversely affect survival, impair weaning from the ventilator, or compromise adherence to the protocol; serum levels of creatine kinase, aspartate aminotransferase, or alanine aminotransferase of more than five times the upper limit of the normal range; ingestion of a statin (on an inpatient or outpatient basis) in the 48 hours before randomization; and an inability to obtain consent.
RANDOMIZATION AND STUDY DRUGS
Patients were randomly assigned in permuted blocks of eight to receive either enteral rosuvastatin or placebo. A 40-mg loading dose of the study drug was administered within 4 hours after randomization. Subsequently, maintenance doses of 20 mg were administered daily at 10 a.m. (±4 hours). If administration of the study-drug dose was delayed by more than 12 hours, that dose was not given and a 40-mg loading dose was given the following day. For patients with a morning serum creatinine level of 2.8 mg per deciliter (250 μmol per liter) or more who were not receiving renal-replacement therapy, the daily maintenance dose was reduced to 10 mg. The study drug was administered until the third day after discharge from the intensive care unit (ICU), study day 28, hospital discharge, or death, whichever came first. The study drug was stopped for reasons of safety if the creatine kinase level exceeded 10 times the upper limit of the normal range or if the level of alanine aminotransferase or aspartate aminotransferase exceeded 8 times the upper limit of the normal range, because liver and muscle injury are reported risks of statins.
STUDY MEASUREMENTS AND PROCEDURES
Plasma samples for measurement of peak and trough rosuvastatin levels were collected on day 6 (±2 days) from the first 60 enrolled patients and, in response to a request from the data and safety monitoring board, from an additional 344 enrolled patients. Serum levels of alanine aminotransferase, aspartate aminotransferase, and creatine kinase were measured on specified study days, as described in the protocol.
Ventilator management and weaning followed a modified ARDS Clinical Trials Network lower-tidal-volume protocol.27 Fluid management followed a simplified version of the ARDS Network conservative approach.28 The study was amended after 225 patients were enrolled to permit enrollment of patients without a central venous catheter, and the fluid-management approach was modified to recommend neutral or negative fluid balance after resolution of shock (see the protocol).
OUTCOMES
The primary outcome was mortality before hospital discharge home or until study day 60 if the patient was still in a health care facility. Secondary outcomes included the number of days that patients were alive and breathing without ventilator assistance (ventilator-free days) to day 28, organ-failure–free days to day 14, and ICU-free days to day 28.29,30 We examined changes in the Pao2:Fio2 ratio and the oxygenation index during the first 7 days. Additional secondary outcomes are listed in the protocol.
In an a priori analysis, we examined outcomes in patients who were in shock at baseline. In a post hoc analysis, we examined outcomes in patients who had been prescribed statins before the development of ARDS but had not received a statin for at least 48 hours before randomization. This subgroup analysis was added before the completion of enrollment, on the basis of a randomized trial showing that continued statin therapy may be beneficial.31
STATISTICAL ANALYSIS
In-hospital mortality to day 60 was estimated with the use of the Kaplan–Meier statistic, with patients who were discharged home before day 60 considered to be alive at day 60. Study patients who were still in a health care facility on day 61 were considered to be alive for this analysis. One patient was lost to follow-up on day 24, and data for this patient were censored on that day. For 81 patients who were also enrolled in a randomized clinical trial of two nutritional strategies,32 the analysis was stratified according to the study-group assignments in that trial. Ventilator-free days, ICU-free days, and organ-failure–free days were analyzed by means of analysis of variance, with the use of treatment assignment and co-enrollment assignment when applicable. A maximum of 1000 patients were to be enrolled; this maximum would provide a 92% probability of rejection of the null hypothesis for the effect on mortality if the true between-group difference in mortality was 9 percentage points (a reduction from 27% to 18%). The probability of rejecting the null hypothesis for the effect on ventilator-free days was 92%, with the assumption of a difference of 2.25 days and a standard deviation of 10.5 days.
The data and safety monitoring board conducted an interim analysis after enrollment of the initial 105 patients and then after approximately every 250 patients enrolled. Sequential stopping rules for futility and efficacy, as well as additional details of the study methods, are described in the study protocol. Only the site pharmacist, pharmacokinetic assay laboratory, coleaders of the coordinating center, and data and safety monitoring board were aware of the study-group assignments.
RESULTS
BASELINE CHARACTERISTICS OF THE PATIENTS
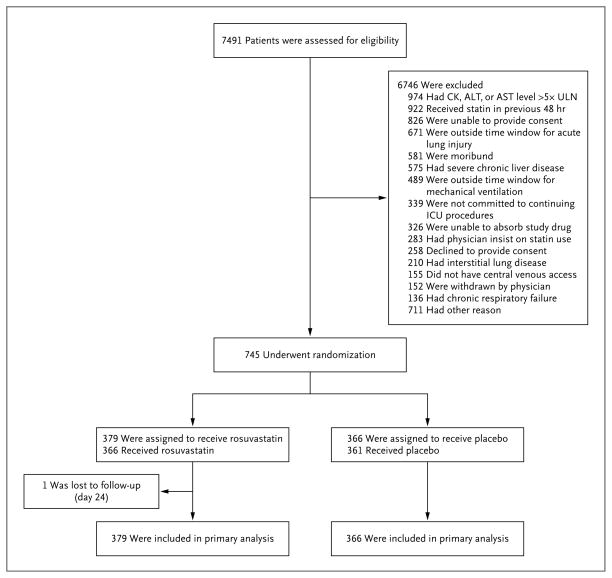
The first patient was enrolled on March 18, 2010, and the data and safety monitoring board stopped the study because of futility on September 30, 2013, after enrollment of 745 patients (Fig. 1, and Table S1 in the Supplementary Appendix). Follow-up was complete for all but 1 patient. Baseline demographic and clinical characteristics are shown in Table 1 (for more details, see Table S2 in the Supplementary Appendix). There were no significant differences between groups at baseline except for small differences in tidal volume and central venous pressure. Approximately 87% of patients underwent randomization within 48 hours after intubation, and all but 7 patients underwent randomization within 48 hours after meeting ARDS criteria.
Figure 1. Screening, Randomization, and Follow-up.
There may have been more than one reason for exclusion of a patient. The full exclusion criteria are listed in the study protocol, available at NEJM.org. One patient underwent randomization and was enrolled in the trial twice (once in each group). ALT denotes alanine aminotransferase, AST aspartate aminotransferase, CK creatine kinase, ICU intensive care unit, and ULN upper limit of the normal range.
Table 1.
Baseline Characteristics of All Patients and Those Who Had Used Statins Previously.*
| Characteristic | All Patients | Patients Who Had Used Statins Previously | ||
|---|---|---|---|---|
| Rosuvastatin (N = 379) | Placebo (N = 366) | Rosuvastatin (N = 54) | Placebo (N = 55) | |
| Age — yr | 54.2±17.1 | 54.1±15.6 | 64.3±14.6 | 62.7±12.5 |
| Female sex — no. (%) | 195 (51) | 185 (51) | 25 (46) | 27 (49) |
| White race — no./total no. (%)† | 301/365 (82) | 289/356 (81) | 49/53 (92) | 45/53 (85) |
| Hispanic or Latino ethnic group — no. (%)† | 46 (12) | 40 (11) | 4 (7) | 6 (11) |
| Medical ICU admission — no. (%) | 330 (87) | 317 (87) | 51 (94) | 52 (95) |
| Primary cause of lung injury — no./total no. (%) | ||||
| Pneumonia | 267/377 (71) | 260/365 (71) | 38/53 (72) | 37/55 (67) |
| Nonpulmonary infection | 72/377 (19) | 73/365 (20) | 9/53 (17) | 13/55 (24) |
| Aspiration | 26/377 (7) | 23/365 (6) | 4/53 (8) | 3/55 (5) |
| Other | 7/377 (2) | 4/365 (1) | 2/53 (4) | 2/55 (4) |
| Multiple transfusions | 3/377 (1) | 1/365 (0) | 0/53 | 0/55 |
| Trauma | 2/377 (1) | 4/365 (1) | 0/53 | 0/55 |
| Weight — kg | 88.4±29.9 | 86.7±30.8 | 93.2±23.9 | 91.6±29.6 |
| APACHE III score‡ | 92.1±28.4 | 94.8±27.9 | 94.6±24.2 | 94.4±25.8 |
| Shock at baseline — no. (%)§ | 173 (46) | 166 (45) | 29 (54) | 28 (51) |
| Prerandomization fluid balance — ml/24 hr | 2128±2635 | 2227±3136 | 1513±2286 | 2144±3418 |
| Arterial pressure — mm Hg | 76.5±13.5 | 77.0±13.9 | 77.1±16.1 | 77.5±12.7 |
| Central venous pressure — mm Hg | 12.3±4.9 | 11.2±4.8¶ | 12.0±5.2 | 12.1±5.0 |
| Tidal volume — ml/kg of predicted body weight | 6.6±1.1 | 6.8±1.3[| | ](#TFN6) | 7.0±1.6 |
| Minute ventilation — liters/min | 10.7±3.1 | 10.8±3.2 | 10.7±3.6 | 11.0±3.4 |
| Inspiratory plateau pressure — cm of water | 23.4±6.6 | 23.6±6.6 | 21.2±5.1 | 22.6±5.6 |
| PEEP — cm of water | 9.2±3.7 | 9.2±3.9 | 8.3±2.9 | 8.4±3.8 |
| Paco2 — mm Hg | 39.9±9.9 | 40.7±11.7 | 41.0±12.2 | 41.6±13.5 |
| Pao2:Fio2 — mm Hg | 170±71 | 170±67 | 179±63 | 178±78 |
| Pao2:Fio2 ≤200 mm Hg — no./total no. (%) | 267/371 (72) | 253/361 (70) | 32/52 (62) | 33/55 (60) |
| Creatinine — mg/dl** | 1.6±1.3 | 1.5±1.1 | 1.9±1.5 | 1.8±1.3 |
| Creatine kinase — U/liter | 224±417 | 214±396 | 207±288 | 196±224 |
| Alanine aminotransferase — U/liter | 37±37 | 36±34 | 35±26 | 38±35 |
| Aspartate aminotransferase — U/liter | 54±69 | 50±44 | 49±45 | 44±28 |
PHARMACOKINETIC FINDINGS
Patients received the study drug for a median of 9 days in the rosuvastatin group (interquartile range, 6 to 13) and 9 days in the placebo group (interquartile range, 6 to 14; P = 0.42). More patients in the rosuvastatin group than in the placebo group had dose reductions on some study days (Tables S3 and S4 in the Supplementary Appendix). Median peak and trough rosuvastatin levels were 7.3 ng per milliliter (interquartile range, 4.4 to 13.1; 171 patients) and 2.4 ng per milliliter (interquartile range, 1.0 to 4.5; 187 patients), respectively. The median half-life was calculated as 13.4 hours (interquartile range, 9.6 to 20.0). For details, see Table S5 in the Supplementary Appendix.
OUTCOMES
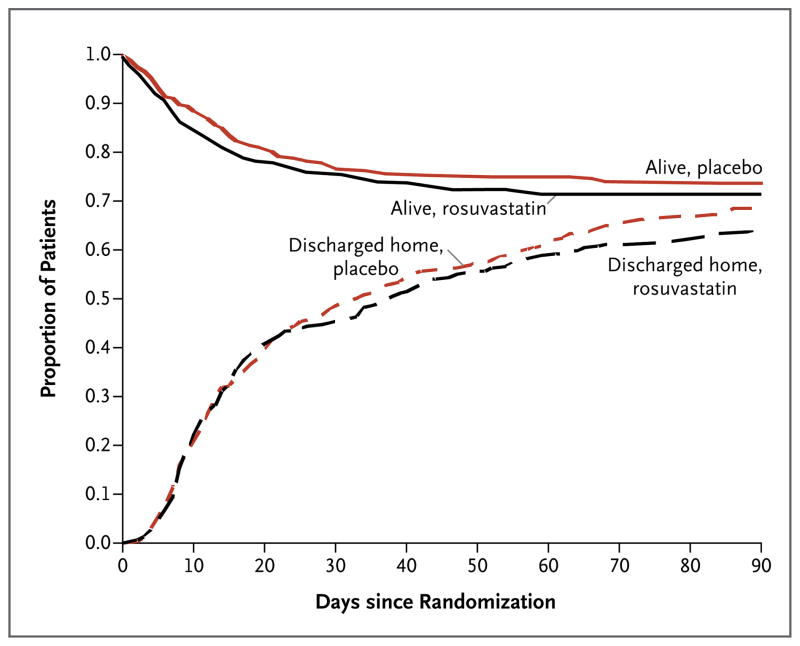
Mortality and the number of ventilator-free days did not differ significantly between the two groups (Table 2 and Fig. 2). The rosuvastatin group had fewer days free of hepatic or renal failure. There were no significant between-group differences in any of the other secondary outcomes (Table S6 in the Supplementary Appendix). During the study period, mean tidal volume, positive end-expiratory pressure, fluid balance, Pao2:Fio2 ratio, and oxygenation index did not differ significantly between the groups, nor were there any significant between-group differences in plateau pressure, static compliance of the respiratory system, minute ventilation, Paco2, or arterial pH (Tables S7 through S16 in the Supplementary Appendix).
Table 2.
Main Outcome Variables.*
| Variable | Rosuvastatin (N = 379) | Placebo (N = 366) | Difference (95% CI) | P Value |
|---|---|---|---|---|
| Death in health care facility to day 60 — no. (%)† | 108 (28.5) | 91 (24.9) | 4.0 (−2.3 to 10.2)‡ | 0.21 |
| Ventilator-free days to day 28 — no.§ | 15.1±10.8 | 15.1±11.0 | 0.0 (−1.6 to 1.5) | 0.96 |
| ICU-free days to day 28 — no. | 14.3±10.1 | 14.4±10.3 | −0.2 (−1.6 to 1.3) | 0.84 |
| Organ-failure–free days to day 14 — no.¶ | ||||
| Free of cardiovascular failure | 8.5±4.8 | 8.7±4.9 | −0.2 (−0.9 to 0.5) | 0.59 |
| Free of coagulation abnormality | 10.7±5.1 | 11.1±4.8 | −0.3 (−1.1 to 0.4) | 0.34 |
| Free of hepatic failure | 10.8±5.0 | 11.8±4.3 | −1.0 (−1.7 to −0.4) | 0.003 |
| Free of renal failure | 10.1±5.3 | 11.0±4.7 | −0.9 (−1.7 to −0.2) | 0.01 |
Figure 2. Probability of Survival and of Being Discharged Home during the First 90 Days after Randomization.
Data for the one patient in the rosuvastatin group who was lost to follow-up at day 24 were censored on that day.
Statin use in patients with sepsis has been associated with reduced C-reactive protein (CRP) levels.33 There were no significant differences between the rosuvastatin and placebo groups in the CRP level at baseline or during the study or (except on day 9) in the change in the CRP level from baseline (Tables S17 and S18 in the Supplementary Appendix). There was a significant but weak positive correlation between the CRP level and both the peak rosuvastatin level (r = 0.29, P<0.001) and the trough rosuvastatin level (r = 0.27, P<0.001) (Fig. S1 and S2 in the Supplementary Appendix).
PATIENTS IN SHOCK AT BASELINE
A total of 339 patients were in shock at baseline. There were no significant differences between the rosuvastatin and placebo groups in mortality (36% and 32%, respectively; 95% confidence interval [CI] for the difference, −4.0 to 14.0 percentage points; P = 0.39) or the number of ventilator-free days (12.2±10.8 and 13.2±11.2, respectively). There were no significant differences in mortality or the number of ventilator-free days according to the Pao2:Fio2 ratio and the presence or absence of shock at baseline (Table S19 in the Supplementary Appendix).
PATIENTS WHO HAD USED STATINS PREVIOUSLY
The baseline characteristics of the 109 patients who used statins before enrollment are shown in Table 1 (for details, see Table S2 in the Supplementary Appendix). There were no significant differences in baseline characteristics between those assigned to rosuvastatin and those assigned to placebo. Rosuvastatin therapy had no effect on mortality. There were 17 deaths among 54 prior statin users who received rosuvastatin (31%) and 11 deaths among 55 prior statin users who received placebo (20%) (95% CI for the difference, −4.8 to 27.8 percentage points; P = 0.14). The interaction between previous use of statins and randomization to rosuvastatin or placebo was not significant (P = 0.28). Similarly, there was no significant difference in the number of ventilator-free days with rosuvastatin as compared with placebo (14.7±11.0 days and 15.3±11.1 days, P = 0.80). The interaction between previous use of statins and randomized assignment to rosuvastatin or placebo was not significant (P = 0.78).
ADVERSE EVENTS
There were no significant differences between the rosuvastatin and placebo groups in the number of patients with creatine kinase levels exceeding 10 times the upper limit of the normal range (16 and 13 patients, respectively; P = 0.65) or in the number of patients with alanine aminotransferase levels exceeding 8 times the upper limit of the normal range (10 and 12 patients, respectively; P = 0.39). After 508 patients had been enrolled, monitoring of aspartate aminotransferase levels was added to the study protocol at the recommendation of the data and safety monitoring board because of 5 reported elevations in the rosuvastatin group. Among the final 237 patients enrolled, 11 additional adverse events of elevated aspartate aminotransferase levels were reported in the rosuvastatin group versus 2 events in the placebo group, bringing the total number of such events to 16 and 2 in the two groups, respectively (P<0.001). Of the 16 patients in the rosuvastatin group with elevated aspartate aminotransferase levels, 5 had creatine kinase levels exceeding 10 times the upper limit of the normal range, and 2 had alanine aminotransferase levels exceeding 8 times the upper limit of the normal range. Changes from baseline in the levels of creatine kinase, alanine aminotransferase, and aspartate aminotransferase were significantly less, on some study days, in the rosuvastatin group than in the placebo group, although the differences were small (Tables S20, S21, and S22 in the Supplementary Appendix). Three serious adverse events of hyperthermia occurred in the rosuvastatin group, leading to a protocol amendment.
DISCUSSION
In this large, multicenter, double-blind, randomized, placebo-controlled clinical trial involving patients with sepsis-associated ARDS, the administration of rosuvastatin did not reduce mortality or improve any of our prespecified secondary outcomes. Moreover, rosuvastatin had no beneficial effect on physiological or biochemical end points.
Rosuvastatin, as compared with placebo, was associated with fewer days free of renal or hepatic failure. These differences in organ-failure–free days were small, and their significance may be spurious owing to the number of secondary end points analyzed. However, we cannot rule out a detrimental effect of rosuvastatin. Aspartate aminotransferase levels were higher in the rosuvastatin group, and adverse events of elevated aspartate aminotransferase levels were more frequently reported in this group. Some patients with elevated aspartate aminotransferase levels had concomitant elevations in creatine kinase and alanine aminotransferase levels, findings that suggest that the source of aspartate aminotransferase was skeletal muscle or liver, respectively. There were no significant between-group differences in the number of patients with levels of alanine aminotransferase or creatine kinase above protocol-specified thresholds. Moreover, although levels of creatine kinase and alanine aminotransferase during the study period were significantly higher in the rosuvastatin group than in the placebo group, the differences between the groups were small and the clinical significance of these biochemical alterations is unclear. Additional studies, including long-term assessment of muscle function, are needed to determine whether these changes are clinically important.
The median peak plasma level of rosuvastatin, 7.3 ng per milliliter, was lower than our target range of 10 to 70 ng per milliliter, despite our selection of a moderate daily dose (20 mg [range approved by the Food and Drug Administration, 5 to 40]). Our target range was based on studies involving healthy persons.34,35 On the basis of potencies for the reduction of low-density lipoprotein cholesterol levels,36 statin doses used in other observational and interventional trials involving patients with sepsis or ARDS17,21,22,31,37–39 were lower than our maintenance dose. However, we do not know whether antiinflammatory effects correlate with relative potencies for lipid reduction.
The plasma levels of statins that are required to induce antiinflammatory effects are not known, and the lower-than-expected plasma levels of rosuvastatin in our study might have accounted for the lack of efficacy. However, given the signs of potential toxicity that we observed (e.g., elevated levels of aspartate aminotransferase and a decreased number of days free of hepatic or renal failure), it is unclear whether the use of higher doses would have been appropriate.
We selected rosuvastatin because it has potentially fewer drug interactions than other statins do.40 It is not known whether the more lipophilic statins with greater tissue penetration, such as atorvastatin and simvastatin, would have been effective. It is also not known whether all classes of statins have similar immunomodulatory effects. Finally, atorvastatin and simvastatin may have greater antibacterial effects than rosuvastatin.41
Observational studies have shown significantly reduced morbidity and mortality among patients with infection who were prescribed statins before hospitalization. In addition, several meta-analyses of these observational studies have suggested that mortality is reduced when statins are used in patients with infection, including those with pneumonia.23,24,42–44 Our results challenge these observations and are consistent with a recent meta-analysis of five randomized, controlled trials of statins in 650 patients with sepsis, which showed that neither atorvastatin nor simvastatin provided a benefit with respect to mortality.39 Similarly, in a recent randomized, controlled trial involving patients with ventilator-associated pneumonia, simvastatin did not reduce mortality or improve secondary outcomes.38 In a randomized, placebo-controlled trial involving 60 patients with ARDS, a high dose of simvastatin (80 mg) reduced nonpulmonary organ dysfunction at day 14, as compared with placebo,37 but there was no effect on mortality.
The finding in observational studies that previous statin use provides a benefit may reflect better access to health care among patients who use statins than among those who do not, with a shorter time to the initiation of antibiotic therapy at the onset of symptoms of infection in statin users. Delayed antibiotic therapy has been shown to correlate with increased mortality from septic shock.45,46 Another possible explanation is that statins have preventive effects when administered before infection that cannot be replicated with short-term administration of the drugs.
In our post hoc analysis of patients who had been prescribed statins before hospitalization and enrollment, randomized assignment to rosuvastatin, as compared with placebo, was not associated with improved outcomes. This finding contrasts with the results of a study involving patients with sepsis in which continued use of atorvastatin improved outcomes among those who had used statins previously.31 Our study differs both in the choice of statin and in our requirement for a 48-hour washout period for statins in order for patients to be eligible for enrollment. Our results suggest that reintroduction of statins after this interval is not beneficial.
In conclusion, our study showed that rosuvastatin therapy did not reduce in-hospital mortality or improve other important clinical outcomes in patients with sepsis-associated ARDS and that it may have had detrimental effects on kidney and hepatic function. These results, coupled with those of smaller randomized trials of other statins,39 do not provide support for initiating or continuing statin therapy for the treatment of sepsis-associated ARDS.
Supplementary Material
Supplement1
Acknowledgments
Funded by the National Heart, Lung, and Blood Institute and the Investigator-Sponsored Study Program of AstraZeneca; ClinicalTrials.gov number, NCT00979121.
Supported by a grant from the National Heart, Lung, and Blood Institute, National Institutes of Health (HHSN268200 536165C-536179C), and the Investigator-Sponsored Study Program of AstraZeneca.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–93. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 2.Raghavendran K, Pryhuber GS, Chess PR, Davidson BA, Knight PR, Notter RH. Pharmacotherapy of acute lung injury and acute respiratory distress syndrome. Curr Med Chem. 2008;15:1911–24. doi: 10.2174/092986708785132942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Almog Y. Statins, inflammation, and sepsis: hypothesis. Chest. 2003;124:740–3. doi: 10.1378/chest.124.2.740. [DOI] [PubMed] [Google Scholar]
- 4.Arnaud C, Braunersreuther V, Mach F. Toward immunomodulatory and anti-inflammatory properties of statins. Trends Cardiovasc Med. 2005;15:202–6. doi: 10.1016/j.tcm.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Arnaud C, Burger F, Steffens S, et al. Statins reduce interleukin-6-induced C-reactive protein in human hepatocytes: new evidence for direct antiinflammatory effects of statins. Arterioscler Thromb Vasc Biol. 2005;25:1231–6. doi: 10.1161/01.ATV.0000163840.63685.0c. [DOI] [PubMed] [Google Scholar]
- 6.Arnaud C, Veillard NR, Mach F. Cholesterol-independent effects of statins in inflammation, immunomodulation and atherosclerosis. Curr Drug Targets Cardiovasc Haematol Disord. 2005;5:127–34. doi: 10.2174/1568006043586198. [DOI] [PubMed] [Google Scholar]
- 7.Gao F, Linhartova L, Johnston AM, Thickett DR. Statins and sepsis. Br J Anaesth. 2008;100:288–98. doi: 10.1093/bja/aem406. [DOI] [PubMed] [Google Scholar]
- 8.Greenwood J, Mason JC. Statins and the vascular endothelial inflammatory response. Trends Immunol. 2007;28:88–98. doi: 10.1016/j.it.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kruger PS STATInS Investigators. Re: statins and sepsis. Br J Anaesth. 2008;100:862. doi: 10.1093/bja/aen114. [DOI] [PubMed] [Google Scholar]
- 10.Mach F. Statins as immunomodulatory agents. Circulation. 2004;109(Suppl 1):II-15–II-17. doi: 10.1161/01.CIR.0000129502.10459.fe. [DOI] [PubMed] [Google Scholar]
- 11.Terblanche M, Almog Y, Rosenson RS, Smith TS, Hackam DG. Statins: panacea for sepsis? Lancet Infect Dis. 2006;6:242–8. doi: 10.1016/S1473-3099(06)70439-X. [DOI] [PubMed] [Google Scholar]
- 12.Idem. Statins and sepsis: multiple modifications at multiple levels. Lancet Infect Dis. 2007;7:358–68. doi: 10.1016/S1473-3099(07)70111-1. [DOI] [PubMed] [Google Scholar]
- 13.Jacobson JR, Barnard JW, Grigoryev DN, Ma SF, Tuder RM, Garcia JG. Simvastatin attenuates vascular leak and inflammation in murine inflammatory lung injury. Am J Physiol Lung Cell Mol Physiol. 2005;288:L1026–L1032. doi: 10.1152/ajplung.00354.2004. [DOI] [PubMed] [Google Scholar]
- 14.Liappis AP, Kan VL, Rochester CG, Simon GL. The effect of statins on mortality in patients with bacteremia. Clin Infect Dis. 2001;33:1352–7. doi: 10.1086/323334. [DOI] [PubMed] [Google Scholar]
- 15.Almog Y, Shefer A, Novack V, et al. Prior statin therapy is associated with a decreased rate of severe sepsis. Circulation. 2004;110:880–5. doi: 10.1161/01.CIR.0000138932.17956.F1. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez R, De Pedro VJ, Artigas A. Statin therapy prior to ICU admission: protection against infection or a severity marker? Intensive Care Med. 2006;32:160–4. doi: 10.1007/s00134-005-2743-9. [DOI] [PubMed] [Google Scholar]
- 17.Kruger P, Fitzsimmons K, Cook D, Jones M, Nimmo G. Statin therapy is associated with fewer deaths in patients with bacteraemia. Intensive Care Med. 2006;32:75–9. doi: 10.1007/s00134-005-2859-y. [DOI] [PubMed] [Google Scholar]
- 18.Thomsen RW, Hundborg HH, Johnsen SP, et al. Statin use and mortality within 180 days after bacteremia: a population-based cohort study. Crit Care Med. 2006;34:1080–6. doi: 10.1097/01.CCM.0000207345.92928.E4. [DOI] [PubMed] [Google Scholar]
- 19.Almog Y, Novack V, Eisinger M, Porath A, Novack L, Gilutz H. The effect of statin therapy on infection-related mortality in patients with atherosclerotic diseases. Crit Care Med. 2007;35:372–8. doi: 10.1097/01.CCM.0000253397.42079.D5. [DOI] [PubMed] [Google Scholar]
- 20.Falagas ME, Makris GC, Matthaiou DK, Rafailidis PI. Statins for infection and sepsis: a systematic review of the clinical evidence. J Antimicrob Chemother. 2008;61:774–85. doi: 10.1093/jac/dkn019. [DOI] [PubMed] [Google Scholar]
- 21.Novack V, Eisinger M, Frenkel A, et al. The effects of statin therapy on inflammatory cytokines in patients with bacterial infections: a randomized double-blind placebo controlled clinical trial. Intensive Care Med. 2009;35:1255–60. doi: 10.1007/s00134-009-1429-0. [DOI] [PubMed] [Google Scholar]
- 22.Kor DJ, Iscimen R, Yilmaz M, Brown MJ, Brown DR, Gajic O. Statin administration did not influence the progression of lung injury or associated organ failures in a cohort of patients with acute lung injury. Intensive Care Med. 2009;35:1039–46. doi: 10.1007/s00134-009-1421-8. [DOI] [PubMed] [Google Scholar]
- 23.Kopterides P, Falagas ME. Statins for sepsis: a critical and updated review. Clin Microbiol Infect. 2009;15:325–34. doi: 10.1111/j.1469-0691.2009.02750.x. [DOI] [PubMed] [Google Scholar]
- 24.Tleyjeh IM, Kashour T, Hakim FA, et al. Statins for the prevention and treatment of infections: a systematic review and meta-analysis. Arch Intern Med. 2009;169:1658–67. doi: 10.1001/archinternmed.2009.286. Erratum, Arch Intern Med 2010;170:42. [DOI] [PubMed] [Google Scholar]
- 25.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS: definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–24. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 26.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. 1992;101:1644–55. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 27.The National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med. 2006;354:2213–24. doi: 10.1056/NEJMoa061895. [DOI] [PubMed] [Google Scholar]
- 28.Calfee CS, Matthay MA. Nonventilatory treatments for acute lung injury and ARDS. Chest. 2007;131:913–20. doi: 10.1378/chest.06-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–8. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 30.Bernard GR, Doig G, Hudson LD, et al. Quantification of organ failure for clinical trials and clinical practice. Am J Respir Crit Care Med. 1995;151(Suppl):A323. abstract. [Google Scholar]
- 31.Kruger P, Bailey M, Bellomo R, et al. A multicenter randomized trial of atorvastatin therapy in intensive care patients with severe sepsis. Am J Respir Crit Care Med. 2013;187:743–50. doi: 10.1164/rccm.201209-1718OC. [DOI] [PubMed] [Google Scholar]
- 32.National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Rice TW, Wheeler AP, et al. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA. 2012;307:795–803. doi: 10.1001/jama.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chalmers JD, Singanayagam A, Murray MP, et al. Prior statin use is associated with improved outcomes in community-acquired pneumonia. Am J Med. 2008;121(11):1002.e1–1007.e1. doi: 10.1016/j.amjmed.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 34.Lee E, Ryan S, Birmingham B, et al. Rosuvastatin pharmacokinetics and pharmacogenetics in white and Asian subjects residing in the same environment. Clin Pharmacol Ther. 2005;78:330–41. doi: 10.1016/j.clpt.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 35.Pasanen MK, Fredrikson H, Neuvonen PJ, Niemi M. Different effects of SLCO1B1 polymorphism on the pharmacokinetics of atorvastatin and rosuvastatin. Clin Pharmacol Ther. 2007;82:726–33. doi: 10.1038/sj.clpt.6100220. [DOI] [PubMed] [Google Scholar]
- 36.Smith ME, Lee NJ, Haney E, Carson S. Update 2. Portland: Oregon Health & Sciences University; Mar, 2004. Drug class review: HMG-CoA reductase inhibitors (statins) ( https://www.ncbi.nlm.nih.gov/books/NBK47273) [PubMed] [Google Scholar]
- 37.Craig TR, Duffy MJ, Shyamsundar M, et al. A randomized clinical trial of hydroxymethylglutaryl-coenzyme a reductase inhibition for acute lung injury (the HARP Study) Am J Respir Crit Care Med. 2011;183:620–6. doi: 10.1164/rccm.201003-0423OC. [DOI] [PubMed] [Google Scholar]
- 38.Papazian L, Roch A, Charles PE, et al. Effect of statin therapy on mortality in patients with ventilator-associated pneumonia: a randomized clinical trial. JAMA. 2013;310:1692–700. doi: 10.1001/jama.2013.280031. [DOI] [PubMed] [Google Scholar]
- 39.Pasin L, Landoni G, Castro ML, et al. The effect of statins on mortality in septic patients: a meta-analysis of randomized controlled trials. PLoS One. 2013;8(12):e82775. doi: 10.1371/journal.pone.0082775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shitara Y, Sugiyama Y. Pharmacokinetic and pharmacodynamic alterations of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors: drug-drug interactions and interindividual differences in transporter and metabolic enzyme functions. Pharmacol Ther. 2006;112:71–105. doi: 10.1016/j.pharmthera.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 41.Masadeh M, Mhaidat N, Alzoubi K, Al-Azzam S, Alnasser Z. Antibacterial activity of statins: a comparative study of atorvastatin, simvastatin, and rosuvastatin. Ann Clin Microbiol Antimicrob. 2012;11:13. doi: 10.1186/1476-0711-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Janda S, Young A, Fitzgerald JM, Etminan M, Swiston J. The effect of statins on mortality from severe infections and sepsis: a systematic review and meta-analysis. J Crit Care. 2010;25(4):656.e7–656.e22. doi: 10.1016/j.jcrc.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 43.Khan AR, Riaz M, Bin Abdulhak AA, et al. The role of statins in prevention and treatment of community acquired pneumonia: a systematic review and metaanalysis. PLoS One. 2013;8(1):e52929. doi: 10.1371/journal.pone.0052929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma Y, Wen X, Peng J, Lu Y, Guo Z, Lu J. Systematic review and meta-analysis on the association between outpatient statins use and infectious disease-related mortality. PLoS One. 2012;7(12):e51548. doi: 10.1371/journal.pone.0051548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gaieski DF, Mikkelsen ME, Band RA, et al. Impact of time to antibiotics on survival in patients with severe sepsis or septic shock in whom early goal-directed therapy was initiated in the emergency department. Crit Care Med. 2010;38:1045–53. doi: 10.1097/CCM.0b013e3181cc4824. [DOI] [PubMed] [Google Scholar]
- 46.Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34:1589–96. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement1