Emerging concepts for immune checkpoint blockade-based combination therapies (original) (raw)
. Author manuscript; available in PMC: 2019 Apr 9.
Abstract
Checkpoint blockade has formally demonstrated that reactivating anti-tumor immune responses can regress tumors. However, this only occurs in a fraction of patients. Incorporating these therapies in more powerful combinations is thus a logical next step. Here, we review functional roles of immune checkpoints and molecular determinants of checkpoint-blockade clinical activity. Limited-size T-cell-infiltrated tumors, differing substantially from “self”, generally respond to checkpoint blockade. Therefore, we propose that reducing tumor burden and increasing tumor immunogenicity are key factors to improve immunotherapy. Lastly, we outline criteria to select proper immunotherapy combination partners and highlight the importance of activity biomarkers for timely treatment optimization.
Introduction
The capability of the immune system to recognize and eliminate incipient tumor cells, and thus surveying against tumor development, was recognized more than a century ago by Paul Ehrlich (Ehrlich et al., 1957) and studied in further detail by Lewis Thomas and MacFarlane Burnet (Burnet, 1957; Lawrence, 1959). Given the physiologic tumor suppressive function of a healthy immune system, a cancer diagnosis may also be considered a diagnosis of a dysfunctional immune system. The evolution of immune-tumor interactions – from tumor protective to tumor-promoting – during cancer progression has been conceptualized in the elegant theory of immunoediting by Robert Schreiber, whereby the immune system, which initially controls and eliminates malignant cells, unavoidably exerts a selective pressure favoring the outgrowth of poorly immunogenic clones that can escape immune recognition (Dunn et al., 2004). Approaches to reinvigorate anti-tumor immune functions and improve the capability of the immune system to recognize malignant cells have thus been devised with the attempt to eradicate cancer (cancer immunotherapy). Tumor remissions achieved by William Coley's strategy to inject tumors with a mixture of bacteria with the aim to reactivate anti-cancer immune functions constituted the first evidence that tumor immune evasion may be a reversible process (Coley, 1891).
Cancer immunotherapy has been profoundly inspired by immune studies in two major fields: infectious diseases and allogeneic bone marrow transplantation (BMT). These studies provided evidence, respectively, that the human immune system can be “trained” to recognize and clear pathogens for the development of specific immunity and that an allogeneic immune system can induce anti-tumor immune responses and clinical remissions in a different host (namely graft versus leukemia effects) (Barnes et al., 1956; Horowitz et al., 1990). Both these observations clearly indicated that immunity can be successfully established against “targets” of different origins (different organisms or individuals). The recognition that tumor cells constitute an altered form of “self” led thus to the use of tumor antigenic material as a cancer vaccine strategy to favor tumor-specific T-cell responses and disease eradication. However, despite sporadic activity in subsets of patients with certain malignancies, cancer vaccines have largely been unsuccessful in making a significant impact in late-phase clinical trials (Butts et al., 2014; Rosenberg et al., 2004) (Vansteenkiste J.F. et al., Annals of Oncology 25 (suppl_4): iv409, 2014 doi:10.1093/annonc/mdu347.1). Subsequent development of an alternative approach to elicit anti-tumor immune responses by inactivating inhibitory immune receptors (immune checkpoints)(Leach et al., 1996; Okazaki et al., 2013) has recently allowed for the demonstration that immunotherapy can durably control advanced cancer. Disinhibition of pre-existing immune responses by blocking the immune checkpoint CTLA-4 (cytotoxic T-lymphocyte-associated protein 4) and/or PD-1 (programmed cell death protein-1) to broadly facilitate immune activation significantly extends survival of advanced cancer patients. The successes of immune checkpoint blockade, initially obtained in patients with advanced melanoma(Hodi et al., 2010; Robert et al., 2011), have rapidly extended to patients with other types of cancer(Brahmer et al., 2012; Garon et al., 2015; Le et al., 2017; Le et al., 2015; Topalian et al., 2012). To date, immune checkpoint blockade therapy is part of standard of care for patients with advanced melanoma, non-small cell lung cancer (NSCLC, squamous and non-squamous carcinoma), Merkel cell carcinoma, head and neck squamous cell carcinoma, urothelial and kidney cancers, microsatellite instability (MSI)-high cancers (such as MSI-high colorectal cancer), refractory Hodgkin lymphoma, hepatocellular carcinoma and gastric cancer, and is intensively being investigated in clinical trials for the treatment of additional malignant diseases. These positive results, in addition to reinvigorating enthusiasm and interest in cancer immunotherapy, underscored the not-so-obvious biological information that, in a substantial fraction of cancer patients, the immune system can still recognize tumor cells if sufficient co-stimulatory signals are properly delivered. Reducing immune suppression by blocking immune checkpoints may thus provide sufficient immune stimulation to trigger therapeutic anti-tumor immunity. However, the clinical experience accumulated thus far with immune checkpoint blockade has also clearly shown that primary tumor refractoriness and acquired tumor resistance to these agents are common factors that prevent the achievement of a clinical benefit in the majority of the cases(Sharma et al., 2017). In addition, high-grade immune-related adverse events, in particular with dual CTLA-4 and PD-1 blockade, are to be considered when clinical decisions are being made. In this article, we discuss the steps toward the development of more effective immunotherapy programs for more cancer patients. Specifically, we review the immunologic and clinical information achieved with the use of checkpoint blockade as a guide to incorporate this approach into more successful therapeutic combinations based on two major strategies: reduction of tumor burden (direct anti-tumor effects) and increase of tumor immunogenicity (indirect immune-mediated anti-tumor effects).
Lesson learned from immune checkpoint blockade therapy
Antibodies (Abs) blocking the immune checkpoints CTLA-4 and PD-1/PD-L1 have been a quite successful form of cancer immunotherapy. In the past 10 years, many patients with different types of cancer have been treated with immune checkpoint blockade with varying degrees of success, with melanoma and Hodgkin lymphoma patients experiencing the greatest benefit, in terms of frequency of objective responses. CTLA-4 and PD-1 pathway blockade have significantly impacted the survival of metastatic melanoma patients, producing durable responses in about 20-40% of the patients when used as monotherapies and in up to 60% of the when combined(Hodi et al., 2010; Larkin et al., 2015; Robert et al., 2015a; Robert et al., 2015b; Weber et al., 2015; Wolchok et al., 2017). PD-1 blockade has also achieved impressive clinical results in advanced NSCLC patients, where it is now being investigated in combination with CTLA-4 blockade(Hellmann et al., 2016).
Clinical activity of checkpoint blockade has been shown to correlate with 3 major parameters:
- The number of tumor nonsynonymous/frameshift somatic mutations, presumably through production of ‘neoantigens’ – mutant non-self and as such more immunogenic proteins – in particular when they are shared by multiple tumor clones (arising from “truncal” mutations)(Carbone et al., 2017; Gubin et al., 2014; McGranahan et al., 2016; Rizvi et al., 2015; Snyder et al., 2014; Turajlic et al., 2017; van Rooij et al., 2013)(Gandara D. et al., Annals of Oncology (2017) 28 (suppl_5): v460-v496. 10.1093/annonc/mdx380; Rizvi N. et al., WCLC 2017, OA 07.03a ID11063);
- Tumor expression of the PD-1 ligand (PD-L1), with high PD-L1 levels in tumor cells or tumor-infiltrating immune cells being associated with an increased incidence of clinical responses to PD-1/PD-L1 blockade(Herbst et al., 2014; Topalian et al., 2012; Wang et al., 2017);
- Frequency of circulating Ki67-expressing CD8+ T cells relative to tumor burden, with patients having limited disease before treatment being more likely to achieve a clinical benefit if ki67+CD8+ T cells increase upon PD-1 blockade(Huang et al., 2017).
These results imply that immunogenic tumors in patients with lesser burdens of disease, differing substantially from “self” (based on neoantigen load) and interacting with effector T cells (based on PD-L1 expression), are more likely to respond to checkpoint blockade (ki67+CD8+ T-cell responses leading to tumor elimination). Therefore, strategies that are able to increase tumor immunogenicity, favor intra-tumor T-cell trafficking and reduce tumor burden should theoretically be prioritized for combination with checkpoint blockade to maximize the probability of a therapeutic success.
In parallel to the exponential increase in the number clinical trials with immune checkpoint blocking Abs, preclinical studies of the mechanisms of therapeutic activity of these agents have also increased. We are thus accumulating clinical and molecular data from the first immunotherapy approach to show consistent clinical benefit in patients with advanced cancer. Deepening the analysis of these results is thus a logical next step toward the development of more effective combination immunotherapy programs. A complete characterization of the molecular mechanisms underlying checkpoint blockade activity and resistance is now fundamental for the design of the next more powerful immunotherapies and immunotherapy-based combinations.
Molecular basis for distinct mechanisms of action of CTLA-4 and PD-1 blockade
Combined CTLA-4 and PD-1 blockade generally shows superior anti-tumor activity compared with blockade of each individual checkpoint, and refractoriness to anti-CTLA-4 does not portend an inferior outcome to anti-PD-1(Hellmann et al., 2016; Wolchok et al., 2017). These clinical results reflect distinct immune regulatory roles for the CTLA-4 and PD-1 pathways, which are in fact non-redundant, as indicated by the distinct autoimmune phenotypes in mice lacking the CTLA-4 or the PD-1 gene(Nishimura et al., 1999; Nishimura et al., 2001; Salomon and Bluestone, 2001; Wang et al., 2005). Understanding the molecular basis of the different immunologic roles of CTLA-4 and PD-1 is important to optimize their (co)-targeting. Four major mechanisms explaining the distinct involvement of CTLA-4 and PD-1 during immune responses have been defined: expression kinetic, expression pattern of respective ligands, engagement of specific protein phosphatases and metabolic regulation. While CTLA-4 is generally up-regulated on T cells upon proper engagement of T-cell receptor (TCR) together with the co-stimulatory receptor CD28(Walunas et al., 1994), PD-1 is induced upon sustained antigen stimulation and/or reduced CD4+ T-cell help, and as such is considered a hallmark of “exhausted” T cells(Wherry, 2011). In addition, the ligands of CTLA-4, B7-1 and B7-2, are specifically expressed on professional antigen presenting cells (APCs), predominantly in secondary lymphoid organs to deliver important signals for T-cell priming.
PD-1 ligands (PD-L1 and PD-L2), besides being expressed by myeloid and activated T cells, can also be found in peripheral tissues, where they are specifically and potently up-regulated as a defense mechanism to dampen T-cell effector function during tissue insult, such as for example upon exposure to inflammatory cytokines (i.e. IFN-γ) or hypoxia through HIF-1α-dependent mechanisms(Keir et al., 2008; Yamazaki et al., 2002). These temporal and spatial differences of CTLA-4 and PD-1 pathways (due to receptor expression kinetics and ligand expression pattern) suggest that CTLA-4 preferentially controls early stages of T-cell activation and PD-1 mainly limits T-cell effector functions in peripheral tissue(Fife and Bluestone, 2008) (Figure 1), although exceptions may occur. In fact, regulatory T cells (Tregs) constitutively express CTLA-4 and up-regulate this molecule in sites of inflammation as an immune-inhibitory feedback mechanism(Wing et al., 2008) (Figure 1). In addition, PD-L1 can be expressed by immune cells in secondary lymphoid organs, thus contributing to the control of excessive T-cell activation even in this anatomic location(Yamazaki et al., 2002). However, in tumor bearing hosts, PD-1 and PD-L1 are generally expressed at significantly higher levels within the tumor compared to secondary lymphoid organ and reinvigoration of intra-tumor PD-1+ T cells with PD-1 pathways blockade seems to be crucial for the anti-tumor activity(Kamphorst et al., 2017), pointing to the tumor mass as the preferential site of PD-1/PD-L1 targeting in this setting (Figure 1).
Figure 1. Differences between CTLA-4 and PD-1 immune inhibitory pathways.
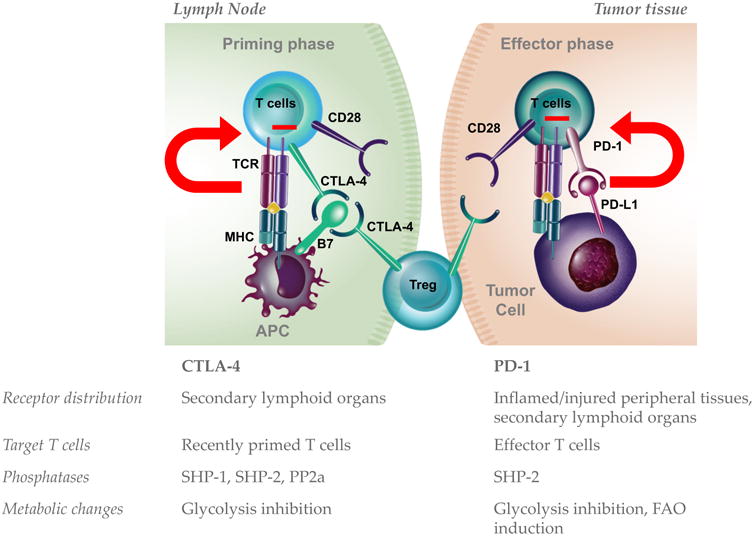
Schematic representation of CTLA-4 and PD-1 molecular functions. Different outcomes of these molecular pathways in tumor-bearing hosts may be mechanistically explained at least at 4 levels: expression pattern and localization, expression kinetic during T-cell activation, partner phosphatases in the downstream signaling pathway, and metabolic effects. TCR, T-cell receptor; MHC, major histocompatibility class molecules; FAO, fatty acid oxidation; Treg, regulatory T cell.
The inhibitory effects of PD-1 and CTLA-4 may also be mediated by slightly distinct mechanisms controlling the TCR downstream signaling cascade. CTLA-4 competes with higher affinity with the master co-stimulatory receptor CD28 for binding to the same ligands B7-1 and B7-2. As CD28 co-stimulation provides key survival, activation and proliferation signals through the up-regulation of anti-apoptotic molecules (Bcl-XL), T-cell growth factors (IL-2)(Lenschow et al., 1996) and activation of anabolic pathways (phosphatidylinositol 3-kinase (PI3K)/AKT)(Pages et al., 1994; Truitt et al., 1994), the effect of CTLA-4 to displace CD28 co-stimulation is to prevent T-cell activation. Mechanistically, CTLA-4 engagement induces its cytoplasmic tail to recruit the Src homology 2 (SH2) domain-containing tyrosine phosphatases, SHP-1 and SHP-2, and PP2a to the site of the immunologic synapse to switch off the TCR downstream phosphorylation cascade (Figure 2).
Figure 2. T-cell molecular pathways regulated by CTLA-4 and PD-1.
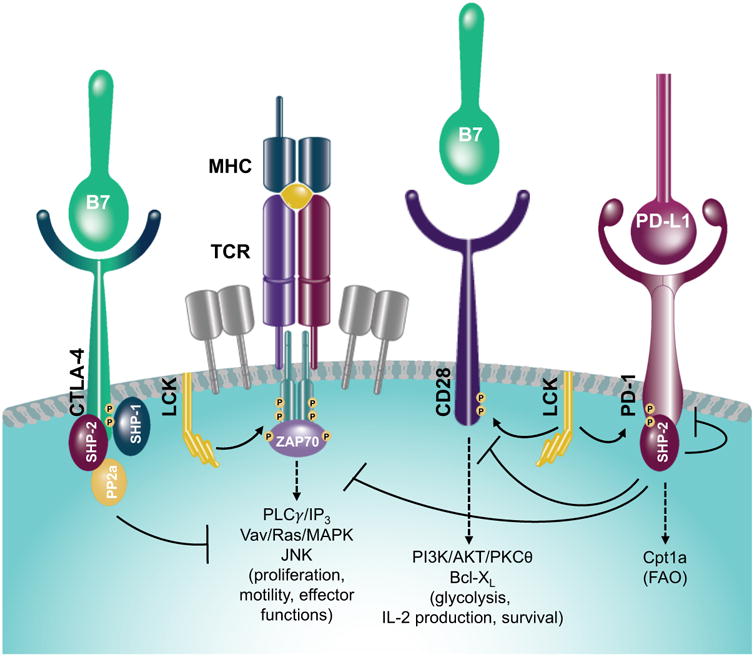
Upon TCR and CD28 engagement, CTLA-4 is rapidly up-regulated and binds with high affinity to B7 molecules. This leads to the CTLA-4 cytoplasmic tail recruitment of tyrosine phosphatases (SHP-1, SHP-2 and PP2a) that switch off TCR/CD28 downstream signaling pathways, otherwise promoting cell proliferation, differentiation, motility, acquisition of effector functions and glycolysis. On the other hand, PD-1 engagement induces LCK to phosphorylate its cytoplasmic tail with the consequent recruitment of tyrosine phosphatases (in particular SHP-2), which, besides down-regulating TCR/CD28 activation, reduces phosphorylation of PD-1 itself. PD-1 downstream signaling pathway also leads to Cpt1a up-regulation and promotes fatty acid oxidation (FAO).
In contrast to the CTLA-4-CD28 competition model, PD-1 engagement by PD-L1 and PD-L2 delivers direct T-cell inhibitory signals. Lck phosphorylates the PD-1 cytoplasmic tail on two tyrosines that function as high affinity docking sites specifically for SHP-2. Importantly, as a safeguard mechanism, activation of SHP-2 rapidly dephosphorylates PD-1, and continuous Lck signaling is required to maintain activity of the PD-1 signaling pathway(Hui et al., 2017), highlighting the potential high sensitivity of this system to disinhibition (Figure 2). Through this pathway, PD-1 actively decreases phosphorylation of effector signaling kinases in TCR and co-stimulatory pathways, including ERK, Vav, PLCγ and PI3K and inhibits the induction of the pro-survival factor Bcl-XL(Bennett et al., 2003; Parry et al., 2005; Yokosuka et al., 2012). Despite these molecular differences, PD-1 similar to CTLA-4 has been recently shown to directly dephosphorylate CD28 in silico(Hui et al., 2017). Therefore, through different molecular mechanisms and potency, and at different stages of immune activation, CTLA-4 and PD-1 can ultimately control CD28 co-stimulatory signals for respectively T-cell activation and effector function (Figure 2). Furthermore, by dampening this axis, CTLA-4 and PD-1 also interfere with T-cell metabolic fitness. The CD28 signaling pathway is extremely important to establish the T-cell metabolic shift toward glycolysis, which is needed for T-cell maturation, proliferation and effector function(Frauwirth et al., 2002; Jacobs et al., 2008; MacIver et al., 2013). By interfering with CD28 signal transduction, both CTLA-4 and PD-1 can thus limit T-cell glycolysis (Figure 2). However, PD-1, in contrast to CTLA-4, concurrently promotes fatty acid β-oxidation (FAO) by inducing the FAO rate-limiting enzyme carnitine palmitoyl transferase (Cpt1a)(Patsoukis et al., 2015) (Figure 2). Persistence and longevity of exhausted PD-1+ T cells may thus be enabled by the FAO metabolic shift promoted by PD-1 ligation. Importantly, the effect of PD-1 signaling to induce FAO is dependent on concurrent inhibition of PI3K/AKT and MEK/ERK pathways, which control glucose metabolism(Patsoukis et al., 2015). Under the influence of CTLA-4 signals, instead, T cells maintain the quiescent metabolic profile of unstimulated cells(Patsoukis et al., 2015). These findings highlight distinct roles of CTLA-4 and PD-1 even in the regulation of T-cell metabolism and indicate that CTLA-4 can generally function as an early immune checkpoint, controlling T-cell activation and induction of the consequently required metabolic switch, and PD-1 as a late immune checkpoint, which keeps previously activated antigen-specific T cells functionally and metabolically on hold. Careful consideration of the molecular and metabolic differences of CTLA-4 and PD-1 signaling (Figure 1 and 2) is now key to optimize the design of successful combinatorial immunotherapeutic anti-cancer approaches based on CTLA-4 and/or PD-1 blockade.
Implication of CTLA-4 and PD-1 molecular profiles for cancer immunotherapy with checkpoint blockade
CTLA-4 and PD-1 are the two most studied examples of the many negative regulators of immune responses(Chen and Flies, 2013). CTLA-4 is unique in its preponderant role in controlling T-cell priming and PD-L1 /PD-L2 up-regulation is a well-conserved tissue defense program to block excessive damage by activated PD-1+ T cells. Understanding how to properly modulate these two pathways may provide important information to guide the development of new therapies based on modulation of these and additional immune receptors operating in these two phases of the immune response. Preclinical studies have shown that CTLA-4 blockade cannot reinvigorate exhausted T cells and that PD-1 pathway blockade cannot unleash anti-tumor T-cell responses if fully primed and committed tumor-specific T cells are not present (Fife et al., 2006). The incremental effects of combination CTLA-4 and PD-1 blockade may thus rely on the ability of anti-CTLA-4 to channel anti-tumor T-cell responses for PD-1/PD-L1 inhibition, thus resulting in a more potent anti-tumor response. However, this paradigm holds true only if immunogenic tumor antigens are expressed and properly presented. As an example, in preclinical tolerance models, CTLA-4 blockade was not able to break tolerance unless it was administered together with high-dose target antigen in complete Freund's adjuvant to stimulate T-cell priming(Eagar et al., 2002). This may explain why anti-CTLA-4 more likely achieve a long clinical benefit in cancer patients with high tumor mutational burden and neoantigen load(Snyder et al., 2014). In addition, favoring the priming phase with anti-CTLA-4 monotherapy is generally less effective than unleashing pre-existing T-cell responses with PD-1/PD-L1 blockade(Wolchok et al., 2017). This indicates that using PD-1 pathway blockade to take advantage of already activated tumor-specific T cells may provide an initial level of protection that can be eventually potentiated by additional waves of anti-tumor T-cell responses covering multiple tumor specificities. In this regard, promoting tumor-antigen exposure/presentation with cancer vaccines or cytotoxic therapies, which induce the release of tumor antigens in vivo, in combination with checkpoint blockade may be a rational approach for improving anti-tumor effects of immunotherapy, as discussed further below (Figure 3).
Figure 3. Strategy to interfere with tumor fitness for potentiating cancer immunotherapy.
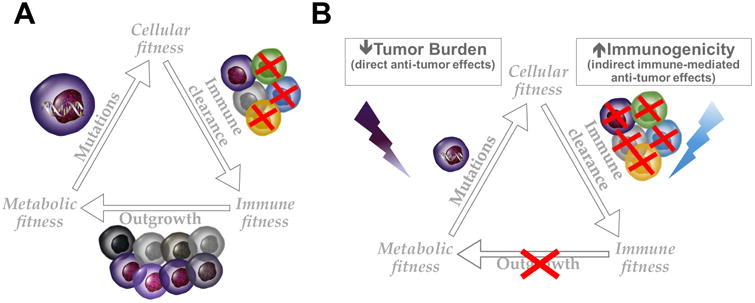
(A) A growing tumor is the result of multiple levels of adaptation in a relatively unfavorable environment. In order to develop and spread, malignant cells need to acquire survival/proliferation advantage, which is typically conferred by mutations in oncogenes/tumor-suppressor genes (cellular fitness), evade immune surveillance (immune fitness) and gain preferential access to nutrient (metabolic fitness). (B) Potentiating immune control of tumor growth, for example with immune checkpoint blockade, has shown efficacy in a limited number of patients. Therapeutic anti-tumor immune responses require that a sufficient amount of activated tumor-specific killer T cells are mobilized toward the tumor. As immune effector cells and tumor cells rely on the same glycolytic metabolism, immune cells need to gain metabolic advantage to sufficiently expand and efficiently eradicate the tumor. To this end, the use of anti-neoplastic agents to reduce tumor burden by directly interfering with tumor growth may favorably combine with immunotherapy, in particular if these anti-cancer treatments have positive immune modulatory effects. At the same time, improving tumor immunogenicity may facilitate immune recognition and clearance of malignant cells.
It is now clear that metabolic competition in the tumor microenvironment affects T-cell function (Figure 3A). By favoring CD28 signaling and in turn T-cell glycolytic capacity(Frauwirth et al., 2002; Parry et al., 2005), both CTLA-4 and PD-1 blockade can promote T-cell metabolic fitness. In support of this hypothesis, preclinical studies in mice have shown that blockade of either CTLA-4, PD-1 or PD-L1 increases glucose availability in the tumor microenvironment and restores the glycolytic capacity of tumor-infiltrating T cells (TILs) in association with the re-activation of the mTOR pathway and immune effector function (IFN-γ production)(Chang et al., 2015). Releasing tumor-specific T-cell responses with checkpoint blockade can lead to initial tumor shrinkage, which may per se shift the microenvironmental metabolic competition in favor of TILs and triggers a chain reaction, with further tumor antigen release and proper T-cell functional and metabolic activation, toward durable and dynamic tumor immunity. Expression of PD-L1 by tumor cells, besides protecting from a T-cell attack, also plays a direct role in promoting tumor glycolysis via AKT/mTOR signaling(Chang et al., 2015). Blocking this pathway may thus result in a dual synergistic anti-tumor effect: disinhibition of tumor-specific T-cell effector function and reduction in tumor glycolysis, with the consequent increase in glucose availability for further T-cell activation in the tumor microenvironment. Helping checkpoint-blocked T cells to gain a metabolic advantage over cancer cells by slowing tumor metabolism down and/or reducing tumor burden may be a logical approach to increase the occurrence of immune-mediated rejection of malignant diseases (Figure 3B).
Rational immunotherapy-based combination approaches
The recent renewed interest in cancer immunotherapy has necessitated a deeper understanding of the mechanisms underlying the anti-tumor activity of immunotherapy. T-cell activation and acquisition of cytotoxic function are complex biologic process tightly regulated by several fail-safe mechanisms to avoid unwanted and dangerous T-cell reactions. The list of immune checkpoints and co-stimulatory receptors that modulate immune responses is continuously growing(Chen and Flies, 2013). Each of these molecules has a complex mechanism of action, with many of them competing with others for the same ligands to deliver stimulatory or inhibitory signals (i.e. CD28 and CTLA-4, binding to the same B7 ligands(Fife and Bluestone, 2008); LIGHT, BTLA and CD160 binding to the same ligand HVEM(Cai and Freeman, 2009); CD226, TIGIT and CD96 binding to the same ligand CD155(Dougall et al., 2017)), and/or being expressed on multiple cell types with different functions (i.e. activated T cells and Tregs). Moreover, co-inhibitory receptors are rapidly up-regulated on activated T cells along with co-stimulatory molecules and both can be markers of T-cell activation and antigen experience. The presence of “immune brakes” on activated T cells ensures that they mount an effective cytotoxic response only when antigen stimulation is perceived with sufficient strength/affinity and in the context of appropriate pro-inflammatory signals. In addition, upon clearance of non-self antigens, similar immune-inhibitory mechanisms are used to contract the immune response and re-establish the immune homeostasis. Given the complexity of immune activation and the physiologic homeostatic mechanisms controlling immune responses, combinatorial approaches are likely needed to efficiently mobilize the immune system against cancer. The observed efficacy of checkpoint blockade may stem from the capability of these therapies to simultaneously affect multiple immune and metabolic pathways, as described above. On the other hand, lack of response to checkpoint blockade may not necessarily indicate a complete failure of treatment, and may rather suggest that the minimal requirements for mounting a full-fledged anti-tumor immune response are not met. Understanding the nature of these requirements is now key to improve the cure rate of patients treated with immunotherapy. By studying patients successfully treated with checkpoint blockade, we have learned that tumor burden (probably due to the proportional immune metabolic competition and immune suppression), tumor immunogenicity (i.e. tumor neoantigen load as a measure of tumor “non-selfness” and functionality of tumor-antigen presentation machinery) and the degree of immune suppression in the microenvironment are important factors determining the likelihood of a clinical response (Figure 3). Therefore, containing tumor spread and improving tumor-antigen presentation and immune recognition and further reducing immunosuppression may be all strategies that can positively combine with checkpoint blockade (Figure 3B).
Reducing tumor burden
The effect of cytotoxic agents to shrink the tumor mass may also positively impact anti-tumor immune activation through two major mechanisms: reduction of tumor-mediated immune suppression and release of tumor-associated (neo)antigens. Moreover, certain cytotoxic drugs have been shown to exert additional and more specific immunomodulatory functions (enhancement of tumor antigen presentation, activation of anti-tumor immunity and/or inhibition of immune suppressive mechanisms) and should thus be favored as partners for combination with immunotherapy. After surgery, chemotherapy, radiation and molecular targeted therapies are the most exploited modalities to reduce tumor mass (Figure 4). While exposure to radiation can be restricted to the tumor site, chemotherapy is a systemic therapy with consequent immune-toxic lympho-/myelo-ablative side effects. However, over the past ten years, it has become clear that the ways chemotherapeutic agents drive tumor cell death may differ in the ability to provide immunogenic signals. Certain chemotherapies induce dying tumor cells to expose immunogenic molecular chaperones on the cell-surface (calreticulin) and release soluble immunogenic mediators (such as HMGB1) that attract and activate APCs for efficient T-cell cross-presentation of tumor antigens(Apetoh et al., 2007; Obeid et al., 2007). The effect of chemotherapy to directly kill the tumor in an immunogenic manner may lead to in vivo vaccination, which can be invigorated by immunotherapy with checkpoint blockade (Figure 4). In agreement with this hypothesis, oxaliplatin, cyclophosphamide, doxorubicin and paclitaxel, which have all demonstrated these immunogenic features, have been shown to potentiate activity of checkpoint blockade in multiple preclinical mouse tumor models(Lu et al., 2014; Pfirschke et al., 2016). Ongoing clinical trials are testing chemotherapy in combination with PD-1 pathway blockade in patients with lung adenocarcinoma (NCT02366143; NCT02763579; NCT02574078) and the FDA has recently approved the use of pembrolizumab along with chemotherapy in the first-line treatment setting for NSCLC.
Figure 4. Improving checkpoint blockade activity in rational therapeutic combinations.
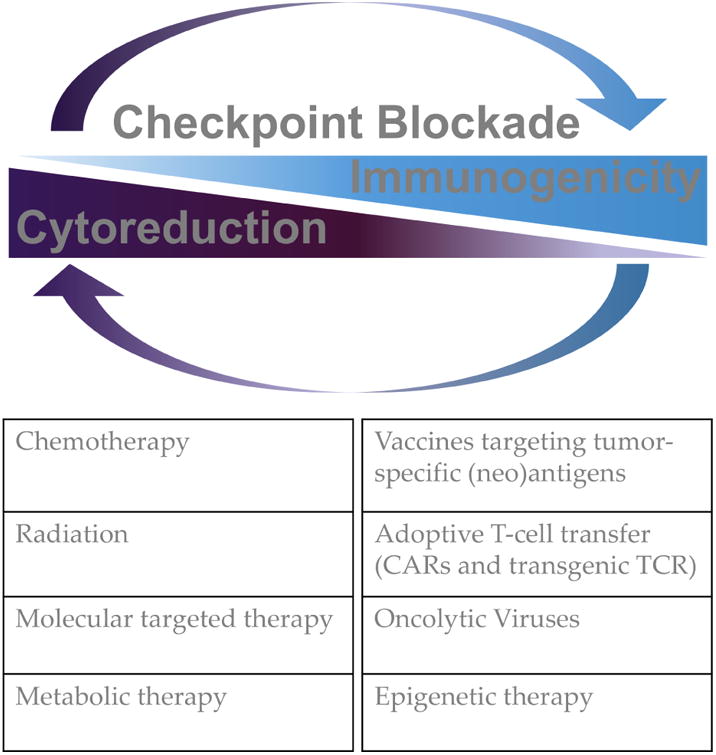
Examples of available anti-neoplastic agents and immunotherapies to exploit in combination with checkpoint blockade based on the rationale discussed in the article.
Radiation therapy has also been shown to cause immunogenic tumor cell death(Apetoh et al., 2007; Obeid et al., 2007). In addition, it enhances APC activation and presentation, via the type-I interferon response pathway(Deng et al., 2014b), intra-tumor T-cell trafficking by promoting pro-inflammatory chemokine production(Lugade et al., 2008; Matsumura et al., 2008) and tumor vascular normalization(Klug et al., 2013), and immune cytotoxic functions by up-regulating T-cell ligands for tumor cell recognition and killing (MHC-I, NKG2D-L and FAS-L)(Chakraborty et al., 2003; Reits et al., 2006). This biology contributes to the possibility of regression of distant metastasis upon local irradiation of a target lesion via the induction of systemic anti-tumor immune activation, a phenomenon termed the “abscopal effect”. When modeled in mice, the abscopal effect of radiation was found to be lost in animals lacking a functional adaptive immune system, thus mechanistically linking it to anti-tumor immunity(Demaria et al., 2004). In response to the direct cytotoxic and inflammatory damage, radiation can also induce signals for limiting immune activation. By favoring tumor up-regulation of CSF-1 and production of reactive oxygen species (ROS), radiation therapy can promote tumor infiltration by myeloid derived suppressor cells (MDSCs)(Xu et al., 2013) and activation of the immune suppressive factor TGFβ respectively(Barcellos-Hoff et al., 1994; Vanpouille-Box et al., 2015). Furthermore, as a consequence of T-cell infiltration and activation after radiation, tumor cells up-regulate PD-L1(Dovedi et al., 2014). In addition, in light of the intrinsic radiation resistance of Tregs, these immunosuppressive cells can persist in the tumor microenvironment at a relative high frequency after radiation(Schaue et al., 2012). Exploiting the anti-tumor stimulatory T-cell function of radiation therapy in combinatorial approaches to overcome these immunosuppressive mechanisms has been studied to increase the likelihood of achieving therapeutic anti-tumor responses. Clinical trials combining radiation therapy with dendritic cell (DC) stimulatory factors (TLR agonist or growth factors, such as GM-CSF) have provided the proof-of-concept that radiation can potentiate the effects of immunotherapy(Brody et al., 2010; Golden et al., 2015). These results have led to an evaluation of radiation therapy in combination with checkpoint blockade (Figure 4). Preclinical evidence in different mouse tumor models indicates significant therapeutic improvements when radiation is combined with CTLA-4 and/or PD-1/PD-L1 blockade depending on the model(Demaria et al., 2005; Deng et al., 2014a; Dewan et al., 2009; Dovedi et al., 2014; Twyman-Saint Victor et al., 2015). In addition, retrospective analyses of patients receiving radiation during anti-CTLA-4 therapy have reported induction of abscopal effects(Golden et al., 2013; Grimaldi et al., 2014; Hiniker et al., 2012; Postow et al., 2012); however, the clinical benefit of the combination still needs to be confirmed in prospective clinical trials. There is also growing interest to evaluate the possibility of using radiation therapy to overcome resistance to PD-1 pathway blockade in cancer patients. In order to optimize the efficacy of radiation in combination with checkpoint blockade, several variables in radiation therapy need to be systematically assessed to maximize the immune stimulating effects, including dose range, schedule, treatment sequence for combination, choice of the target lesion (anatomic location with respect to immune cells accessibility, size), susceptibility of different tumor types and tumor heterogeneity.
To reduce tumor burden, cancers “addicted” to specific oncogenes can be treated with molecular targeted therapies that inhibit these pathways (Figure 4). Recognition of a mutant, constitutively active BRAF isoform (mainly the V600E substitution) in about 50% of cutaneous melanomas led to the development of specific RAF pathway antagonists, including the BRAF inhibitors vemurafenib and dabrafenib and the MEK inhibitors trametinib and cobimetinib, which are now part of the treatment armamentarium of metastatic melanoma(Chapman et al., 2011; Hauschild et al., 2012; Larkin et al., 2014; Long et al., 2015). Due to the parallel introduction in the therapeutic algorithm for advanced melanoma of CTLA-4 blockade(Hodi et al., 2010) and soon after of PD-1/PD-L1 blockade(Hamid et al., 2013; Larkin et al., 2015; Topalian et al., 2012), studying the possibility to integrate BRAF pathway inhibition and immune checkpoint blockade in a combinatorial approach was thus a logical step (Figure 4). The immune modulatory activity of BRAF/MEK inhibitors thus started to be appreciated, providing the rationale for assessing immune effects of molecular targeted therapies available for other diseases potentially susceptible to immunotherapy, such as lung cancer. Both BRAF and MEK inhibitors have been shown to up-regulate tumor-associated antigens, thus favoring intra-tumor T-cell homing and pro-inflammatory cytokine production(Boni et al., 2010; Brea et al., 2016; Callahan et al., 2014; Frederick et al., 2013; Hu-Lieskovan et al., 2014). In addition, while MEK inhibition attenuates terminal T-cell differentiation(Ebert et al., 2016), BRAF inhibitors can activate the MAPK pathway in T cells, which carry wild-type RAF(Callahan et al., 2014). Interestingly, BRAF inhibition can also promote Treg and MDSC accumulation in the tumor microenvironment, whereas MEK inhibition counteracts this effect, thus supporting the combination of dual BRAF/MEK inhibition with immunotherapy in BRAF mutant melanomas (Hu-Lieskovan et al., 2015; Ott et al., 2013). Initial clinical investigation of BRAF inhibitors in combination with anti-CTLA-4 has raised some concern because of substantial liver toxicity, which was attributed to the paradoxical T-cell activation induced by RAF inhibition(Callahan et al., 2014). On the other hand, MEK inhibition in combination with PD-L1 blockade has shown initial signs of activity and a favorable safety profile in colorectal cancer patients (NCT01988896)(Bendell et al., 2016). Phase II/III studies are currently investigating the optimal sequencing of BRAF/MEK inhibition and checkpoint blockade. In addition, triple concurrent blockade of BRAF, MEK and PD-L1 has started to be tested in patients with advanced melanoma (Hwu et al., 2016; Ribas et al., 2015).
Although less prevalent than KRAS genetic alterations, EGFR mutations and ALK translocations constitute additional targetable oncogenic drivers in NSCLC. Interestingly, hyper-activation of EGFR or ALK is associated with increased expression levels of PD-L1(Akbay et al., 2013; D'Incecco et al., 2015; Ota et al., 2015), providing a rationale for combining EGFR/ALK inhibitors with PD-1 pathway blockade. However, retrospective analyses of patients having NSCLC harboring EGFR mutations or ALK translocations enrolled in clinical trials with PD-1/PD-L1 blockade have not shown improved activity over standard chemotherapy(Gainor et al., 2016; Lee et al., 2017). Tumors with these genetic alterations tend to have limited mutational burden(Spigel DR et al., J Clin Oncol 2016; 34(Suppl.): abstract 9017) and are characterized by a highly immunosuppressive microenvironment (Streicher K. et al., J Clin Oncol 2017; 35(Suppl.): abstract 11505), thus potentially explaining the limited efficacy of immunotherapy. Further clinical results of PD-1 or CTLA-4 blockade in combination with EGFR or ALK inhibition will be extremely important to guide the use of immunotherapy in lung cancer patients harboring mutations in these oncogenic drivers.
Another signaling cascade altered in many types of cancers, whose inhibition may found application in combination with immunotherapy is the Wnt/β-catenin pathway. Activation of this pathway correlates with lack of T-cell infiltration in human melanoma(Spranger et al., 2015). Interestingly, transcriptional inhibition of the chemokine CCL4 by the Wnt/β-catenin/ATF3 axis was reported as the mechanism underlying reduced intra-tumor infiltration of DCs and T cells(Spranger et al., 2015). Despite the challenges of blocking Wnt/β-catenin signaling – due to the complex network of interactions with other pathways and its role in normal tissue stem cell homeostasis – some Wnt inhibitors are already in clinical development, and the possibility to test their combination with checkpoint blockade may be soon a reality.
As a consequence of oncogene inhibition and tumor shrinkage, tumor metabolic activity is also down-regulated, thus generating a more favorable microenvironment with increased nutrient availability for immune cells (Figure 3). In light of the crucial metabolic competition between tumor and immune cells (Figure 3), combining therapies that directly dampen tumor metabolism with immunotherapy is now an attractive option (Figure 4). Even though activated immune cells and malignant cells use similar metabolic pathways to proliferate, initial evidence suggests that tumor cells are more “addicted” than immune cells to glucose consumption and glycolysis, and determining a therapeutic window where immune functions are not compromised by metabolic inhibition may be possible. Careful preclinical studies of dose regimens and schedules of metabolic inhibitors based on specific immune readouts in immunocompetent animals will be crucial for further clinical development of this approach. For example, clinically approved mTOR inhibitors (everolimus, temsirolimus and other rapalogues) can be used to limit tumor glycolysis. However, these compounds have also potent immunosuppressive functions(Araki et al., 2011), in part due to their capability of selectively expanding Tregs, whose metabolism does not rely on glycolysis(Delgoffe et al., 2009). To uncover the potential therapeutic advantage of mTOR inhibition in combination with immunotherapy, extensive evaluation of doses, timing and sequencing of these agents is thus required. The recent observation in elderly volunteers that low-dose everolimus before influenza vaccination enhances vaccine-specific immunity and reduces circulating PD-1-expressing, potentially exhausted, T cells supports the hypothesis that, in specific regimens, mTOR inhibitors may positively modulate immune functions(Mannick et al., 2014).
As an additional strategy to target tumor glucose metabolism, the use of anti-diabetic drugs metformin and phenformin has shown promising anti-tumor activity in preclinical studies(Kim et al., 2017; Pollak, 2013; Yuan et al., 2013). By decreasing blood glucose, insulin levels and mitochondrial ATP production, these agents can drive cancer cells into a metabolic crisis(Hardie, 2013). Interestingly, the same effects do not necessarily block T-cell functions. T cells can more easily adapt to alternative catabolic metabolism – a feature associated with T-cell longevity and memory development(Pearce et al., 2009). As a result, treatment with metformin has shown to limit T-cell apoptosis and exhaustion in favor to antigen-specific T-cell function and TIL expansion(Eikawa et al., 2015). In addition, recent studies in BRAF/ PTEN-mutant melanoma models have described the capability of phenformin, but not metformin, to decrease peripheral and intratumoral development of granulocytic MDSCs in association with therapeutic effects(Kim et al., 2017). Phenformin is more lipophilic than metformin and as such can easily access different types of cells independently of the expression of specific organic cation transporters (OCTs). OCTs have a very limited pattern of expression (mainly liver and kidney), and can be up-regulated in malignant cells(Emami Riedmaier et al., 2013). Therefore, metformin is less toxic but also less potent than phenformin as an anti-neoplastic agent. Assessing the relative expression of OCTs in tumor tissue and immune cells at different stages of activation will be important for the appropriate selection of the agent to use in each case. Building upon the successful pre-clinical results of phenformin against BRAF-mutant melanoma(Kim et al., 2017; Yuan et al., 2013), a phase I clinical trial of phenformin in combination with the BRAF inhibitor dabrafenib and the MEK inhibitor trametinib has recently started (NCT03026517). This study will provide key information for further development of phenformin in immunotherapy-based combination anti-cancer treatments (Figure 4).
As a consequence of accelerated anaerobic glycolytic metabolism, cancer cells need to extrude a high amount of lactate to avoid toxic cytoplasmic acidification. Interestingly, depending on the level of hypoxia in the tumor microenvironment, malignant cells adapt so that they produce and consume lactate in a symbiotic relationship. In highly hypoxic tumor regions, cells typically consume glucose and release lactate, which can be used by more oxidative cells in less hypoxic regions of the tumor(Sonveaux et al., 2008). Lactate transport relies on the expression of monocarboxylic acid transporters (MCT1-4), which regulate lactate import and export based on intracellular/extracellular lactate concentration, pH and presence of other MCT substrates. This symbiotic lactate-based interaction within the tumor thus prevents activated immune cells from eliminating their own glycolysis bio-products. As a consequence, immune effector functions are inhibited and tolerogenic polarization of macrophages and DCs favored(Brand et al., 2016; Fischer et al., 2007; Gottfried et al., 2006; Husain et al., 2013; Mendler et al., 2012; Nasi et al., 2013). Based on level of MCT up-regulation in cancer cells, MCT can be targeted to preferentially affect lactate transport in tumor cells and restore glucose metabolism in immune cells(Doherty and Cleveland, 2013; Hong et al., 2016). We have recently shown that selective MCT1/4 inhibition can re-invigorate effector functions of T cells co-cultured with the highly glycolytic 4T1 breast cancer model(Zappasodi et al., 2016). This is in agreement with previous findings indicating that effects of lactate on T-cell function are reversible(Fischer et al., 2007). Given that tumor lactate metabolism is emerging as a negative prognostic factor in melanoma patients treated with checkpoint blockade(Diem et al., 2016), pharmacologic inhibition of this metabolic pathway preferentially in tumor cells may be a rational strategy to enhance anti-tumor activity of immunotherapy (Figure 4). AZD3965 is an MCT1 inhibitor entering the clinical evaluation in cancer patients(Polanski et al., 2014). Results from this phase I trial will provide important information on the possibility to develop this therapy further (NCT01791595). Toward this direction, it is very likely that multiple key players in tumor metabolism need to be concurrently targeted to avoid further tumor metabolic adaptation and escape.
Increasing tumor immunogenicity
Tumor immunogenicity – the capability of a tumor to be recognized by the immune system – may be limited if amount and/or presentation of tumor (neo)antigens is inadequate and tumor antigen presentation machinery dysfunctional. We and others have reported that tumor-derived non-synonymous and frameshift mutation burden and neoantigen load are associated with long-term survival in immunotherapy-naïve cancer patients(Balachandran et al., 2017; Brown et al., 2014; Turajlic et al., 2017) and can predispose to the clinical efficacy of immune checkpoint blockade therapy(Carbone et al., 2017; Rizvi et al., 2015; Snyder et al., 2014; Turajlic et al., 2017)(Gandara D. et al., Annals of Oncology (2017) 28 (suppl_5): v460-v496. 10.1093/annonc/mdx380; Rizvi N. et al., WCLC 2017, OA 07.03a ID11063). These observations are based on retrospective studies in patients with different tumor types (mainly melanoma, NSCLC and renal carcinoma) showing that non-synonymous mutational load, frameshift mutation (insertion/deletion) burden, and/or tumor neoantigens correlate with the clinical outcome of CTLA-4 and PD-1 blockade(McGranahan et al., 2016; Rizvi et al., 2015; Snyder et al., 2014; Turajlic et al., 2017; Van Allen et al., 2015). The hypothesis being that acquisition of mutations during tumor development may generate neoantigens, which, being distinct from self-proteins, can elicit specific T-cell responses that are potentiated in the context of reduced immune suppression with checkpoint blockade therapy. Blockade of immune checkpoints reduces the threshold of immune activation, and if “non-self” antigens are sufficiently presented, T cells may more easily mount an attack against the tumor. However, the quantity of tumor neoantigens is not a perfect predictor of clinical outcome to checkpoint blockade. There are cases with high tumor mutational and neoantigen load that do not respond. In addition, the quality of neoantigens, in terms of clonal representation in a tumor(McGranahan et al., 2016; Turajlic et al., 2017) and immunogenicity(Luksza et al., 2017), are emerging as important determinants of response to checkpoint blockade.
In preclinical animal studies, immune checkpoint blockade has proven insufficient to break tolerance, unless co-administered with vaccines(Curran et al., 2010; Eagar et al., 2002). Supplying the required amount of immunogenic neoantigens in a vaccine in the context of checkpoint blockade may thus favor the induction of the desired T-cell responses for tumor eradication (Figure 4). Pulsing the immune system with tumor neoantigens may also reduce the risk of autoimmunity associated with checkpoint blockade, as immune activation promoted by checkpoint blockade will be more likely directed to the vaccine neoantigens. Two recent clinical studies in melanoma patients have shown feasibility and safety of peptide- and RNA-based vaccines to vehicle multiple tumor neoantigens predicted to be immunogenic in silico (Ott et al., 2017; Sahin et al., 2017). In both cases, neaontigen-specific T-cell responses were enhanced(Ott et al., 2017; Sahin et al., 2017). In addition, patients whose disease progressed could achieve complete response after subsequent treatment with PD-1 pathway blockade, indicating the potential therapeutic advantage of combining tumor-neoantigen vaccines with checkpoint blockade(Ott et al., 2017; Sahin et al., 2017). Integration of conventional algorithms for tumor neoepitope prediction – based on MHC binding and likelihood of MHC-peptide complex to be recognized by TCR – with tumor clonality (truncal or branch mutations) and neoepitope immunogenicity – based on sufficient divergence from self (i.e. when the wild type peptide counterpart is not a strong binder)(Ghorani et al., 2018) and/or resemblance to infectious-disease-associated antigens(Luksza et al., 2017) – is one approach for optimizing selection of neoantigens for vaccines. Recent reports have shown for example the potential increased immunogenicity of frameshift mutations, which are caused by genetic insertions/deletions(Giannakis et al.; Turajlic et al., 2017). Continuing to improve tumor-neoantigen prediction algorithms for a faithful and precise identification of tumor neoantigens is a key step for the development of more effective therapeutic cancer vaccines (Figure 4). In light of the heterogeneity of tumor lesions in a metastatic setting(Jimenez-Sanchez et al., 2017) and the difficulties associated with sampling multiple lesions for neoantigen prediction, it will be also important to systematically assess feasibility and reliability of predicting immunogenic “rejection” neoantigens from circulating tumor cell-free DNA. As the majority of clonal tumor mutations are found in circulating tumor cell-free DNA(Adalsteinsson et al., 2017), this material may offer a natural filter for the detection of relevant targets for immunotherapy.
To ensure tumor immune recognition, T cells with the desired specificity may be engineered and properly activated and expanded ex vivo and then re-infused in adequate numbers into cancer patients, a technique known as adoptive T-cell therapy (ACT). T-cell tumor specificity may be conferred by either insertion of tumor-antigen specific transgenic TCRs or chimeric antigen receptors (CARs), which couple the extracellular Ab portion for epitope recognition with the TCR cytoplasmic machinery for transduction of T-cell activation signals. CAR T cells use the Ab modality to recognize epitopes of surface antigens; whereas, TCR-transgenic T cells rely on patient-specific HLA-presentation of peptides deriving from any protein independent of the cellular localization. CAR T cells targeting the B-cell surface antigen CD19 have demonstrated frequent durable remissions in patients with refractory and relapsed B-cell malignancies(Garfall et al., 2015; Kochenderfer et al., 2015; Lee et al., 2015; Porter et al., 2011), and a similar strategy is currently being exploited to target antigens expressed by epithelial solid tumors. T cells engineered to express TCRs specific for the melanoma antigens MART-1 and gp100 and cancer-testis tumor-associated antigens MAGE-A3 and NY-ESO-1 have shown clinical responses in patients with solid and hematologic cancers, including metastatic melanoma, advanced multiple myeloma and synovial cell sarcoma(Morgan et al., 2013; Morgan et al., 2006; Rapoport et al., 2015; Robbins et al., 2015). As efficacy of checkpoint blockade relies on the presence of tumor-specific T cells, combining checkpoint blockade with adoptive transfer of tumor-specific T cells may increase the rate of therapeutic responses (Figure 4). This strategy is currently being investigated by either engineering TCR-transgenic T cells or CAR T cells to concurrently produce CTLA-4 and/or PD-1 blocking antibodies, or to express extracellular domains of CTLA-4 or PD-1 fused with the cytoplasmic tail of CD28 – for the transduction of positive stimuli upon engagement of these chimeric molecules – or “dominant negative” PD-1 isoforms, or by knocking out the PD-1 gene. Preclinical results from these studies have been encouraging(Ankri et al., 2013; Cherkassky et al., 2016; John et al., 2013; Moon et al., 2014; Shin et al., 2012). Adoptive transfer of ex vivo expanded tumor-antigen specific cytotoxic T cells has also started to be investigated in combination with standard systemic CTLA-4 blockade, with initial promising clinical results(Chapuis et al., 2016a; Chapuis et al., 2016b). However, potentiating adoptive T-cell transfer with checkpoint blockade therapy in the setting of solid cancers may raise concerns when the target antigen is expressed in normal tissues, because it can expose patients to the risk of severe life-threatening autoimmune side effects.
Supplementation of immunogenic tumor antigens (with vaccines) or tumor-specific T cells (with ACT) both rely on the capability of tumor cells to present/express the target/cognate antigen. Tumors with altered antigen presentation machinery, HLA/β-2 microglobulin down-regulation or loss of antigen expression may thus be refractory to these immune interventions. To overcome these limitations and efficiently and broadly activate the immune system against poorly immunogenic malignancies, means to promote local immune destruction and immune activation are being explored. Intratumoral injection of oncolytic viruses has the potential to directly kill malignant cells, thus directly decreasing tumor burden, and to concurrently induce the release of danger signals (PAMPs and DAMPs) and activation of the type I IFN anti-viral response for the engagement of both innate and adaptive immune responses in a cascade reaction leading to immune-mediated tumor eradiation(Kaufman et al., 2015). Viral antigens expressed in infected tumor cells may help mobilize the immune system toward the tumor site. In addition, tumor (neo)antigens released by dying and/or killed malignant cells in the context of an active viral infection may elicit multiple anti-tumor immune responses targeting cancer cells independently of the presence of the virus. Through the development of systemic anti-tumor immunity, therapeutic responses may thus occur in both locally injected and distant tumors. Talimogene laherparepvec (T-VEC; Amgen) – a modified herpes simplex virus type 1 (HSV-1), encoding granulocyte–macrophage colony-stimulating factor (GM-CSF) for chemo-attraction and maturation of DCs – has been recently approved for the treatment of advanced melanoma(Andtbacka et al., 2015) and is currently under investigation in combination with checkpoint blockade with initial promising results(Chesney et al., 2017; Ribas et al., 2017) (Figure 4). Due to safety concerns, oncolytic viruses have been generally delivered by intratumor administration, and this may restrict the use of this strategy to the treatment of superficial and accessible tumors, although innovative formulations and the use of radiographic image-guided delivery may help to overcome these challenges.
Epigenetic drugs constitute an alternative approach to re-establish tumor immunogenicity without the need of physically delivering antigenic material into an accessible tumor. These agents include inhibitors of DNA methyltransferases (DNMTs) and histone deacetylases (HDACs). Originally developed with the aim to reverse the abnormal epigenome of cancer cells, which use DNMTs and HDACs to modulate expression of oncogenes and tumor suppressor genes(Baylin and Jones, 2011; Shen and Laird, 2013), these agents have more recently demonstrated broad immunomodulatory function. It is becoming clear that epigenetic silencing of immune-related genes is a major mechanism of tumor immune escape. Inhibition of DNMTs, and to lesser extent HDACs, induces the re-expression of immunogenic tumor-associated antigens, such as cancer testis antigens (NY-ESO-1, MAGE-A1)(James et al., 2006; Karpf, 2006; Odunsi et al., 2014) as well as up-regulation of genes involved in antigen processing and presentation (β-2 microglobulin, HLA molecules, TAP1)(Li et al., 2014; Wrangle et al., 2013). Recent reports have demonstrated that re-activation of the viral defense pathway via demethylation and de-repression of endogeneous retroviral transcripts and other interferon-response transcription factors (such as IRF7) is one of the key mechanisms underlying increased immunogenicity of cancer cells after inhibition of DNMTs(Chiappinelli et al., 2015; Roulois et al., 2015). Activation of viral/interferon response genes by demethylating agents is also at the basis of their capability to induce PD-L1 up-regulation on cancer cells(Li et al., 2014; Wrangle et al., 2013). Class I HDAC inhibitors have similarly shown to enhance PD-L1 and PD-L2 expression in melanoma by directly favoring histone acetylation of PD-1 ligand genes. Interestingly, selective HDAC6 inhibition interferes with the IL6-STAT3 pathway and can instead down-regulate immune inhibitory ligands, indicating that, depending on the class of targeted HDACs, distinct immune modulatory effects can be achieved(M et al., 2016). Epigenetic modulation may also affect immune cells. Transition toward T-cell dysfunction and exhaustion during chronic antigen stimulation (such as chronic infection and cancer) is regulated by massive chromatin remodeling, including acquisition of de novo DNA methylation in T-cell functional gene loci and regulatory regions (IFNG, TCF7, TBX21, CCR7, EOMES). Therefore, promoting demethylation may favor reversal of T-cell exhaustion(Ghoneim et al., 2017; Pauken et al., 2016; Philip et al., 2017). Pan-HDAC inhibitors instead have been shown to counteract FAS-L-mediated activation-induced cell death (AICD) of tumor-infiltrating T cells(Cao et al., 2015). AICD of effector T cells is emerging as a key factor preventing maximal anti-tumor effects in immunotherapy-based combination programs(Messenheimer et al., 2017; Shrimali et al., 2017), and pan-HDAC inhibitors may thus find rational application in this setting. DNMT and HDAC inhibitors can also functionally affect MDSCs(Kim et al., 2014), thus highlighting the potential to sensitize to immunotherapy through multiple mechanisms (Figure 4). Accordingly, pre-clinical studies have shown enhanced anti-tumor efficacy of immune checkpoint blockade in combination with demethylating agents or HDAC inhibitors in otherwise immune refractory solid cancer models(Cao et al., 2015; Chiappinelli et al., 2015; Kim et al., 2014; Wang et al., 2015; Woods et al., 2015). Building upon these observations, combinations of checkpoint blockade and DNMT or HDAC inhibitors have started to be investigated in patients with advanced cancer(Mazzone et al., 2017). Understanding the optimal schedules of DNMT and HDAC inhibitors (sequential vs. concurrent, continuous vs. intermittent) in combination with systemic checkpoint blockade therapy is an important point to address in these studies.
Strategy prioritization
Many cancer treatments have demonstrated immune modulating effects and promising results in combination with immunotherapy in preclinical studies. Defining criteria for prioritizing effective combinations for clinical development is a current challenge. In addition to rational treatment selection, identifying the patient population(s) that would more likely benefit from such immunotherapeutic programs is also crucial. Based on experience accumulated thus far, reducing tumor burden and enhancing tumor immunogenicity are two necessary steps to improve efficacy of immunotherapy (Figure 3). As available anti-neoplastic and immunotherapeutic treatments have different levels of potency, combining therapies with adequate activity to affect the tumor and reinvigorate the immune system based on the extent of tumor invasiveness and immune dysfunction in each case would be a logical approach (Figure 5).
Figure 5. Selection and optimization of immunotherapy-based combinations.
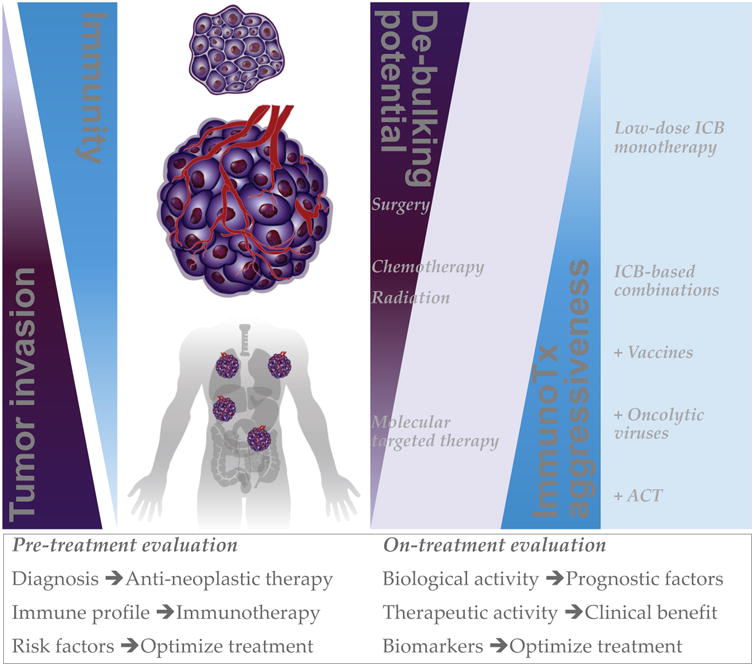
Proposed strategy to improve combination of anti-neoplastic and immunotherapeutic treatments based on their reciprocal levels of activity against tumors of different stage and immunogenicity. The extent of tumor invasion is expected to inversely correlate with immunogenicity and/or immune functions. De-bulking potential of standard anti-neoplastic treatments is generally stronger against limited diseases than disseminated tumors. Given that reducing tumor burden may facilitate long-lasting protective immunity, it is logical to combine therapies with the maximal de-bulking potential in each case with the immunotherapy (ImmunoTx) that can best complement such activity toward sustained anti-tumor responses. This approach would also ensure that immunotherapy is appropriately dosed to limit the occurrence of immune-related adverse events. The possibility to monitor the biological and therapeutic performance of the selected combination would allow for timely and patient-specific treatment optimization. ICB, immune checkpoint blockade.
Type, stage, aggressiveness and molecular profile of the disease can guide the choice of the most appropriate tumor-de-bulking/anti-neoplastic therapy to employ (Figure 5). Based on the anticipated therapeutic outcome of this treatment, it is possible to identify appropriate candidate partner immunotherapeutic options that are expected to best complement such activity toward complete disease control. In this context, following the approach of incorporating immunotherapy into standard cancer treatment programs may be prioritized to facilitate clinical development. Appropriate immunotherapy selection is challenging, as it requires sophisticated analyses of robust biomarkers to measure tumor immunogenicity and/or degree of immune dysfunction. Tumor non-synonymous/frameshift mutation load, neoantigen burden and presentation, and immune infiltration are interconnected features that can contribute to the definition of tumor immunogenicity and have all been found associated with the clinical outcome of immunotherapy. Human cancer types differ in the number of somatic non-synonymous mutations(Alexandrov et al., 2013), and immune checkpoint blockade therapy is generally more efficacious against tumors characterized by high mutation burden, such as melanoma(Snyder et al., 2014), NSCLC(Hellmann et al., 2016) and MSI-high colorectal carcinoma(Le et al., 2017; Le et al., 2015). As tumor (neo)antigens become real targets for T-cell responses only after being processed and presented on the cell surface as peptides bound to HLA, alterations in these processes also play a key role in dictating the level of immunogenicity of a tumor(Campoli and Ferrone, 2008; Tran et al., 2016). The capability of a tumor to correctly present immunogenic antigens has also been linked to the tumor's capacity to attract immune cells. Quantitation and distribution of immune cells within a tumor have been proposed as broad measurements of tumor immunogenicity, with 3 typical scenarios being recognized: inflamed tumors (“hot”, immune infiltration), immune excluded tumors (presence of T cells at the tumor margins but not in the tumor core) and immune desert tumors (“cold”, no immune infiltration)(Lanitis et al., 2017). Availability of specific gene signatures for individual immune cell subsets now makes it possible to dissect the tumor immune infiltrate using tumor transcriptomic data in a relatively straightforward manner(Bindea et al., 2013; Senbabaoglu et al., 2016). Tumor immunogenicity not only relies on signals delivered by malignant cells to the immune system but also on the ability of the tumor to respond to immune signals. One of the major mediators of such crosstalk is IFN-γ, which exerts both direct and indirect/immune-mediated anti-tumor effects by respectively limiting tumor cell survival and enhancing tumor antigen presentation(Platanias, 2005). Tumor cells can escape this mechanism by acquiring mutations or epigenetic silencing of molecules involved in IFN-γ-response pathway (e.g. JAK1/2 and STAT1). Characterizing tumor somatic non-synonymous/frameshift mutations, HLA expression, immune infiltration and IFN-γ responsiveness is thus an important step in beginning to assess patients' likelihood of response to immunotherapy and guide the choice of appropriate immune interventions. Tumors with broad mutation burden, intact HLA presentation capability and a functional IFN-γ-response pathway are expected to easily recruit T cells and respond to immunotherapy(Ayers et al., 2017; Gao et al., 2016; Le et al., 2015; Rizvi et al., 2015; Snyder et al., 2014; Zaretsky et al., 2016). Poorly immunogenic tumors, based on limited expression of neoantigens, genes involved in antigen-presentation machinery or IFN-γ-response pathway, will likely require more specific interventions to re-establish immune recognition and effector functions, including administration of oncolytic viruses and epigenetic therapies (Figure 5). Furthermore, in the case of advanced and metastatic diseases, for which conventional therapies may have limited de-bulking potential, more potent immunotherapeutic treatments may be necessary. If tumor-associated antigens are known, transfer of specific T cells may be considered to achieve rapid anti-tumor effects (Figure 5). In case of stable disease, enhancing endogenous anti-tumor immune responses and long-lasting immunologic memory may be attempted using vaccines targeting tumor-specific (neo)antigens, or, if tumor antigens are not known, through the administration of radiation/immunogenic chemotherapy supplemented with adjuvants (TLR agonists) to induce an in situ vaccination event.
Following the hypothesis that restoring immune control of tumor growth may facilitate clinical efficacy of immune checkpoint blockade, combining anti-neoplastic agents and immunotherapy to re-establish “cancer-immune equilibrium”(Dunn et al., 2004) has the potential to predispose more patients to benefit from checkpoint blockade therapy. With the aim of making checkpoint blockade more effective across different cancer types and stages – eventually in multivalent combinatorial programs (Figure 5) – it is important to define biomarkers of activity that can guide the selection of efficacious and safe dose regimens in each case and generate a so call personalized immune therapy approach. Although immune responses do not always correlate with the achievement of a therapeutic effect, immunotherapy cannot generate tumor regression without inducing immune responses. Accumulating evidence is beginning to show that on-treatment peripheral blood biomarkers may be valuable to assess biologic activity of immune modulating agents(Zappasodi et al., SITC Annual Meeting 2017, abstract P391)(Huang et al., 2017; Zappasodi et al., 2017). As an example, T-cell functional re-invigoration based on Ki67 up-regulation has been identified as a pharmacodynamic biomarker of checkpoint blockade, and this effect can be easily detected in peripheral blood(Blackburn et al., 2009; Huang et al., 2017; Twyman-Saint Victor et al., 2015). Moreover, we have recently observed that CTLA-4 and PD-1 blockade modulate peripheral non-conventional immunosuppressive CD4+ T cells in opposing directions, suggesting that optimizing combination treatment to avoid excessive increases in this cell population may favor a clinical benefit(Zappasodi et al., SITC Annual Meeting 2017, abstract P391). Monitoring biomarkers associated with immune-related adverse events is also very important for anticipating toxicity. It is becoming clear that activity and tolerability of immune checkpoint blockade vary depending on the tumor type, with melanoma patients, for example, tolerating higher doses of anti-CTLA-4-based combination therapy better than NSCLC and renal cell carcinoma patients(Hammers et al., 2017; Hellmann et al., 2016; Larkin et al., 2015; Postow et al., 2015; Wolchok et al., 2013). The definition of checkpoint blockade doses and schedules in these settings was achieved empirically, with the risk of exposing patients to high-grade immune-related adverse events(Hammers et al., 2017; Hellmann et al., 2016). Given the increasing interest to incorporate checkpoint blockade therapy into earlier lines of treatment, including neo-adjuvant and adjuvant settings, the possibility of timely assessment of biomarkers for immune activity during treatment would be invaluable to optimize regimens. During this exploratory phase of new immunotherapy-based combination treatments, systematic longitudinal analyses of sequential biologic specimens for biomarker discovery would thus be primordial.
Conclusions and perspectives
Over the last decade, cancer immunotherapy with checkpoint blockade has achieved impressive clinical results across different types of cancer, thus leading to renewed interest in the field. The intense investigation of molecular and immune effects of CTLA-4 and PD-1/PD-L1 pathways has clearly indicated that blockade of these checkpoints can act at multiple levels to restore anti-tumor immune functions, especially when the two pathways are concurrently targeted. The broad activity of checkpoint blockade may thus be attributed to a combination of immune events that lead to durable and dynamic tumor immunity. This, however, does not happen in all cases. Limited tumor immunogenicity, accelerated tumor growth and extensive tumor metabolic competition are emerging as major barriers to effective tumor eradication by a “re-invigorated” immune system (such as upon checkpoint blockade). Proper integration of immunotherapeutic and anti-neoplastic approaches may thus be key to overcome these limitations and improve cancer control. Genomic, transcriptomic and immune profiles of tumors from patients treated with checkpoint blockade are providing new insights into the molecular determinants of effective anti-cancer immune responses. Characterizing these factors as part of routine analyses for cancer patients will allow for improved disease classification and therapeutic decisions. The analysis of tumor-immune system interactions is reaching higher levels of resolution and computational/mathematical models are needed to project the outcome of these molecular complexities. Through this approach, cancer immunotherapy is expected to progressively evolve toward patient-tailored precision medicine combinatorial programs. In light of the dynamic nature of tumor-immune-system crosstalk, in particular during immunotherapeutic interventions, performing similar systematic analyses at baseline and during treatment will also be informative for the definition of robust biomarkers predicting therapeutic activity and/or toxicity.
Acknowledgments
We would like to thank the Swim Across America, Ludwig Institute for Cancer Research, the Parker Institute for Cancer Immunotherapy and the NIH/NCI Cancer Center Support Grant (P30 CA008748) for their support. R.Z. is the recipient of the Parker Institute for Cancer Immunotherapy scholar award.
Footnotes
Declaration of interests: The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adalsteinsson VA, Ha G, Freeman SS, Choudhury AD, Stover DG, Parsons HA, Gydush G, Reed SC, Rotem D, Rhoades J, et al. Scalable whole-exome sequencing of cell-free DNA reveals high concordance with metastatic tumors. Nat Commun. 2017;8:1324. doi: 10.1038/s41467-017-00965-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbay EA, Koyama S, Carretero J, Altabef A, Tchaicha JH, Christensen CL, Mikse OR, Cherniack AD, Beauchamp EM, Pugh TJ, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov. 2013;3:1355–1363. doi: 10.1158/2159-8290.CD-13-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SAJR, Behjati S, Biankin AV, Bignell GR, Bolli N, Borg A, Børresen-Dale AL, et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andtbacka RH, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J, Delman KA, Spitler LE, Puzanov I, Agarwala SS, et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. J Clin Oncol. 2015;33:2780–2788. doi: 10.1200/JCO.2014.58.3377. [DOI] [PubMed] [Google Scholar]
- Ankri C, Shamalov K, Horovitz-Fried M, Mauer S, Cohen CJ. Human T cells engineered to express a programmed death 1/28 costimulatory retargeting molecule display enhanced antitumor activity. J Immunol. 2013;191:4121–4129. doi: 10.4049/jimmunol.1203085. [DOI] [PubMed] [Google Scholar]
- Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- Araki K, Ellebedy AH, Ahmed R. TOR in the immune system. Curr Opin Cell Biol. 2011;23:707–715. doi: 10.1016/j.ceb.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayers M, Lunceford J, Nebozhyn M, Murphy E, Loboda A, Kaufman DR, Albright A, Cheng JD, Kang SP, Shankaran V, et al. IFN-gamma-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest. 2017;127:2930–2940. doi: 10.1172/JCI91190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balachandran VP, Luksza M, Zhao JN, Makarov V, Moral JA, Remark R, Herbst B, Askan G, Bhanot U, Senbabaoglu Y, et al. Identification of unique neoantigen qualities in long-term survivors of pancreatic cancer. Nature. 2017;551:512–516. doi: 10.1038/nature24462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcellos-Hoff MH, Derynck R, Tsang ML, Weatherbee JA. Transforming growth factor-beta activation in irradiated murine mammary gland. J Clin Invest. 1994;93:892–899. doi: 10.1172/JCI117045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes DWH, Corp MJ, Loutit JF, Neal FE. Treatment of Murine Leukaemia with X Rays and Homologous Bone Marrow. British Medical Journal. 1956;2:626–627. doi: 10.1136/bmj.2.4993.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylin SB, Jones PA. A decade of exploring the cancer epigenome - biological and translational implications. Nat Rev Cancer. 2011;11:726–734. doi: 10.1038/nrc3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendell JC, Kim TW, Goh BC, Wallin J, Oh DY, Han SW, Lee CB, Hellmann MD, Desai J, Lewin JH, et al. Clinical activity and safety of cobimetinib (cobi) and atezolizumab in colorectal cancer (CRC) Journal of Clinical Oncology. 2016;34:3502–3502. [Google Scholar]
- Bennett F, Luxenberg D, Ling V, Wang IM, Marquette K, Lowe D, Khan N, Veldman G, Jacobs KA, Valge-Archer VE, et al. Program death-1 engagement upon TCR activation has distinct effects on costimulation and cytokine-driven proliferation: attenuation of ICOS, IL-4, and IL-21, but not CD28, IL-7, and IL-15 responses. J Immunol. 2003;170:711–718. doi: 10.4049/jimmunol.170.2.711. [DOI] [PubMed] [Google Scholar]
- Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, Obenauf AC, Angell H, Fredriksen T, Lafontaine L, Berger A, et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity. 2013;39:782–795. doi: 10.1016/j.immuni.2013.10.003. [DOI] [PubMed] [Google Scholar]
- Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nature immunology. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boni A, Cogdill AP, Dang P, Udayakumar D, Njauw CN, Sloss CM, Ferrone CR, Flaherty KT, Lawrence DP, Fisher DE, et al. Selective BRAFV600E inhibition enhances T-cell recognition of melanoma without affecting lymphocyte function. Cancer Res. 2010;70:5213–5219. doi: 10.1158/0008-5472.CAN-10-0118. [DOI] [PubMed] [Google Scholar]
- Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand A, Singer K, Koehl GE, Kolitzus M, Schoenhammer G, Thiel A, Matos C, Bruss C, Klobuch S, Peter K, et al. LDHA-Associated Lactic Acid Production Blunts Tumor Immunosurveillance by T and NK Cells. Cell Metab. 2016;24:657–671. doi: 10.1016/j.cmet.2016.08.011. [DOI] [PubMed] [Google Scholar]
- Brea EJ, Oh CY, Manchado E, Budhu S, Gejman RS, Mo G, Mondello P, Han JE, Jarvis CA, Ulmert D, et al. Kinase Regulation of Human MHC Class I Molecule Expression on Cancer Cells. Cancer immunology research. 2016;4:936. doi: 10.1158/2326-6066.CIR-16-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody JD, Ai WZ, Czerwinski DK, Torchia JA, Levy M, Advani RH, Kim YH, Hoppe RT, Knox SJ, Shin LK, et al. In situ vaccination with a TLR9 agonist induces systemic lymphoma regression: a phase I/II study. J Clin Oncol. 2010;28:4324–4332. doi: 10.1200/JCO.2010.28.9793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SD, Warren RL, Gibb EA, Martin SD, Spinelli JJ, Nelson BH, Holt RA. Neo-antigens predicted by tumor genome meta-analysis correlate with increased patient survival. Genome Res. 2014;24:743–750. doi: 10.1101/gr.165985.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnet M. Cancer—A Biological Approach: I. The Processes Of Control. II. The Significance of Somatic Mutation. British Medical Journal. 1957;1:779–786. doi: 10.1136/bmj.1.5022.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butts C, Socinski MA, Mitchell PL, Thatcher N, Havel L, Krzakowski M, Nawrocki S, Ciuleanu TE, Bosquee L, Trigo JM, et al. Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non-small-cell lung cancer (START): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15:59–68. doi: 10.1016/S1470-2045(13)70510-2. [DOI] [PubMed] [Google Scholar]
- Cai G, Freeman GJ. The CD160, BTLA, LIGHT/HVEM pathway: a bidirectional switch regulating T-cell activation. Immunol Rev. 2009;229:244–258. doi: 10.1111/j.1600-065X.2009.00783.x. [DOI] [PubMed] [Google Scholar]
- Callahan MK, Masters G, Pratilas CA, Ariyan C, Katz J, Kitano S, Russell V, Gordon RA, Vyas S, Yuan J, et al. Paradoxical activation of T cells via augmented ERK signaling mediated by a RAF inhibitor. Cancer immunology research. 2014;2:70–79. doi: 10.1158/2326-6066.CIR-13-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campoli M, Ferrone S. HLA antigen changes in malignant cells: epigenetic mechanisms and biologic significance. Oncogene. 2008;27:5869–5885. doi: 10.1038/onc.2008.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao K, Wang G, Li W, Zhang L, Wang R, Huang Y, Du L, Jiang J, Wu C, He X, et al. Histone deacetylase inhibitors prevent activation-induced cell death and promote anti-tumor immunity. Oncogene. 2015;34:5960–5970. doi: 10.1038/onc.2015.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone DP, Reck M, Paz-Ares L, Creelan B, Horn L, Steins M, Felip E, van den Heuvel MM, Ciuleanu TE, Badin F, et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N Engl J Med. 2017;376:2415–2426. doi: 10.1056/NEJMoa1613493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty M, Abrams SI, Camphausen K, Liu K, Scott T, Coleman CN, Hodge JW. Irradiation of tumor cells up-regulates Fas and enhances CTL lytic activity and CTL adoptive immunotherapy. J Immunol. 2003;170:6338–6347. doi: 10.4049/jimmunol.170.12.6338. [DOI] [PubMed] [Google Scholar]
- Chang CH, Qiu J, O'Sullivan D, Buck MD, Noguchi T, Curtis JD, Chen Q, Gindin M, Gubin MM, van der Windt GJ, et al. Metabolic Competition in the Tumor Microenvironment Is a Driver of Cancer Progression. Cell. 2015;162:1229–1241. doi: 10.1016/j.cell.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapuis AG, Lee SM, Thompson JA, Roberts IM, Margolin KA, Bhatia S, Sloan HL, Lai I, Wagener F, Shibuya K, et al. Combined IL-21-primed polyclonal CTL plus CTLA4 blockade controls refractory metastatic melanoma in a patient. J Exp Med. 2016a;213:1133–1139. doi: 10.1084/jem.20152021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapuis AG, Roberts IM, Thompson JA, Margolin KA, Bhatia S, Lee SM, Sloan HL, Lai IP, Farrar EA, Wagener F, et al. T-Cell Therapy Using Interleukin-21-Primed Cytotoxic T-Cell Lymphocytes Combined With Cytotoxic T-Cell Lymphocyte Antigen-4 Blockade Results in Long-Term Cell Persistence and Durable Tumor Regression. J Clin Oncol. 2016b;34:3787–3795. doi: 10.1200/JCO.2015.65.5142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13:227–242. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherkassky L, Morello A, Villena-Vargas J, Feng Y, Dimitrov DS, Jones DR, Sadelain M, Adusumilli PS. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J Clin Invest. 2016;126:3130–3144. doi: 10.1172/JCI83092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesney J, Puzanov I, Collichio F, Singh P, Milhem MM, Glaspy J, Hamid O, Ross M, Friedlander P, Garbe C, et al. Randomized, Open-Label Phase II Study Evaluating the Efficacy and Safety of Talimogene Laherparepvec in Combination With Ipilimumab Versus Ipilimumab Alone in Patients With Advanced, Unresectable Melanoma. J Clin Oncol, JCO2017737379. 2017 doi: 10.1200/JCO.2017.73.7379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiappinelli KB, Strissel PL, Desrichard A, Li H, Henke C, Akman B, Hein A, Rote NS, Cope LM, Snyder A, et al. Inhibiting DNA Methylation Causes an Interferon Response in Cancer via dsRNA Including Endogenous Retroviruses. Cell. 2015;162:974–986. doi: 10.1016/j.cell.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coley WB. II. Contribution to the Knowledge of Sarcoma. Annals of Surgery. 1891;14:199–220. doi: 10.1097/00000658-189112000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A. 2010;107:4275–4280. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Incecco A, Andreozzi M, Ludovini V, Rossi E, Capodanno A, Landi L, Tibaldi C, Minuti G, Salvini J, Coppi E, et al. PD-1 and PD-L1 expression in molecularly selected non-small-cell lung cancer patients. Br J Cancer. 2015;112:95–102. doi: 10.1038/bjc.2014.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao B, Worley PF, Kozma SC, Powell JD. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–844. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaria S, Kawashima N, Yang AM, Devitt ML, Babb JS, Allison JP, Formenti SC. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res. 2005;11:728–734. [PubMed] [Google Scholar]
- Demaria S, Ng B, Devitt ML, Babb JS, Kawashima N, Liebes L, Formenti SC. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58:862–870. doi: 10.1016/j.ijrobp.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, Fu YX. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014a;124:687–695. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A, Li XD, Mauceri H, Beckett M, Darga T, et al. STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors. Immunity. 2014b;41:843–852. doi: 10.1016/j.immuni.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, Demaria S. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009;15:5379–5388. doi: 10.1158/1078-0432.CCR-09-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diem S, Kasenda B, Spain L, Martin-Liberal J, Marconcini R, Gore M, Larkin J. Serum lactate dehydrogenase as an early marker for outcome in patients treated with anti-PD-1 therapy in metastatic melanoma. Br J Cancer. 2016;114:256–261. doi: 10.1038/bjc.2015.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty JR, Cleveland JL. Targeting lactate metabolism for cancer therapeutics. J Clin Invest. 2013;123:3685–3692. doi: 10.1172/JCI69741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougall WC, Kurtulus S, Smyth MJ, Anderson AC. TIGIT and CD96: new checkpoint receptor targets for cancer immunotherapy. Immunol Rev. 2017;276:112–120. doi: 10.1111/imr.12518. [DOI] [PubMed] [Google Scholar]
- Dovedi SJ, Adlard AL, Lipowska-Bhalla G, McKenna C, Jones S, Cheadle EJ, Stratford IJ, Poon E, Morrow M, Stewart R, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014;74:5458–5468. doi: 10.1158/0008-5472.CAN-14-1258. [DOI] [PubMed] [Google Scholar]
- Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–360. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- Eagar TN, Karandikar NJ, Bluestone JA, Miller SD. The role of CTLA-4 in induction and maintenance of peripheral T cell tolerance. Eur J Immunol. 2002;32:972–981. doi: 10.1002/1521-4141(200204)32:4<972::AID-IMMU972>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Ebert PJR, Cheung J, Yang Y, McNamara E, Hong R, Moskalenko M, Gould SE, Maecker H, Irving BA, Kim JM, et al. MAP Kinase Inhibition Promotes T Cell and Anti-tumor Activity in Combination with PD-L1 Checkpoint Blockade. Immunity. 2016;44:609–621. doi: 10.1016/j.immuni.2016.01.024. [DOI] [PubMed] [Google Scholar]
- Ehrlich P, Himmelweit F, Marquadt M, Dale HH. The Collected Papers of Paul Ehrlich of Paul Ehrlich: Immunology and cancer research: Immunology and cancer research: Pergamon P 1957 [Google Scholar]
- Eikawa S, Nishida M, Mizukami S, Yamazaki C, Nakayama E, Udono H. Immune-mediated antitumor effect by type 2 diabetes drug, metformin. Proc Natl Acad Sci U S A. 2015;112:1809–1814. doi: 10.1073/pnas.1417636112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emami Riedmaier A, Fisel P, Nies AT, Schaeffeler E, Schwab M. Metformin and cancer: from the old medicine cabinet to pharmacological pitfalls and prospects. Trends Pharmacol Sci. 2013;34:126–135. doi: 10.1016/j.tips.2012.11.005. [DOI] [PubMed] [Google Scholar]
- Fife BT, Bluestone JA. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol Rev. 2008;224:166–182. doi: 10.1111/j.1600-065X.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- Fife BT, Guleria I, Gubbels Bupp M, Eagar TN, Tang Q, Bour-Jordan H, Yagita H, Azuma M, Sayegh MH, Bluestone JA. Insulin-induced remission in new-onset NOD mice is maintained by the PD-1-PD-L1 pathway. J Exp Med. 2006;203:2737–2747. doi: 10.1084/jem.20061577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer K, Hoffmann P, Voelkl S, Meidenbauer N, Ammer J, Edinger M, Gottfried E, Schwarz S, Rothe G, Hoves S, et al. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood. 2007;109:3812–3819. doi: 10.1182/blood-2006-07-035972. [DOI] [PubMed] [Google Scholar]
- Frauwirth KA, Riley JL, Harris MH, Parry RV, Rathmell JC, Plas DR, Elstrom RL, June CH, Thompson CB. The CD28 signaling pathway regulates glucose metabolism. Immunity. 2002;16:769–777. doi: 10.1016/s1074-7613(02)00323-0. [DOI] [PubMed] [Google Scholar]
- Frederick DT, Piris A, Cogdill AP, Cooper ZA, Lezcano C, Ferrone CR, Mitra D, Boni A, Newton LP, Liu C, et al. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clin Cancer Res. 2013;19:1225–1231. doi: 10.1158/1078-0432.CCR-12-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainor JF, Shaw AT, Sequist LV, Fu X, Azzoli CG, Piotrowska Z, Huynh TG, Zhao L, Fulton L, Schultz KR, et al. EGFR Mutations and ALK Rearrangements Are Associated with Low Response Rates to PD-1 Pathway Blockade in Non-Small Cell Lung Cancer: A Retrospective Analysis. Clin Cancer Res. 2016;22:4585–4593. doi: 10.1158/1078-0432.CCR-15-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Shi LZ, Zhao H, Chen J, Xiong L, He Q, Chen T, Roszik J, Bernatchez C, Woodman SE, et al. Loss of IFN-gamma Pathway Genes in Tumor Cells as a Mechanism of Resistance to Anti-CTLA-4 Therapy. Cell. 2016;167:397–404 e399. doi: 10.1016/j.cell.2016.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfall AL, Maus MV, Hwang WT, Lacey SF, Mahnke YD, Melenhorst JJ, Zheng Z, Vogl DT, Cohen AD, Weiss BM, et al. Chimeric Antigen Receptor T Cells against CD19 for Multiple Myeloma. N Engl J Med. 2015;373:1040–1047. doi: 10.1056/NEJMoa1504542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- Ghoneim HE, Fan Y, Moustaki A, Abdelsamed HA, Dash P, Dogra P, Carter R, Awad W, Neale G, Thomas PG, Youngblood B. De Novo Epigenetic Programs Inhibit PD-1 Blockade-Mediated T Cell Rejuvenation. Cell. 2017;170:142–157 e119. doi: 10.1016/j.cell.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghorani E, Rosenthal R, McGranahan N, Reading JL, Lynch M, Peggs KS, Swanton C, Quezada SA. Differential binding affinity of mutated peptides for MHC class I is a predictor of survival in advanced lung cancer and melanoma. Annals of Oncology. 2018;29:271–279. doi: 10.1093/annonc/mdx687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannakis M, Mu Xinmeng J, Shukla Sachet A, Qian Zhi R, Cohen O, Nishihara R, Bahl S, Cao Y, Amin-Mansour A, Yamauchi M, et al. Genomic Correlates of Immune-Cell Infiltrates in Colorectal Carcinoma. Cell Reports. 15:857–865. doi: 10.1016/j.celrep.2016.03.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden EB, Chhabra A, Chachoua A, Adams S, Donach M, Fenton-Kerimian M, Friedman K, Ponzo F, Babb JS, Goldberg J, et al. Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: a proof-of-principle trial. Lancet Oncol. 2015;16:795–803. doi: 10.1016/S1470-2045(15)00054-6. [DOI] [PubMed] [Google Scholar]
- Golden EB, Demaria S, Schiff PB, Chachoua A, Formenti SC. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer immunology research. 2013;1:365–372. doi: 10.1158/2326-6066.CIR-13-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried E, Kunz-Schughart LA, Ebner S, Mueller-Klieser W, Hoves S, Andreesen R, Mackensen A, Kreutz M. Tumor-derived lactic acid modulates dendritic cell activation and antigen expression. Blood. 2006;107:2013–2021. doi: 10.1182/blood-2005-05-1795. [DOI] [PubMed] [Google Scholar]
- Grimaldi AM, Simeone E, Giannarelli D, Muto P, Falivene S, Borzillo V, Giugliano FM, Sandomenico F, Petrillo A, Curvietto M, et al. Abscopal effects of radiotherapy on advanced melanoma patients who progressed after ipilimumab immunotherapy. Oncoimmunology. 2014;3:e28780. doi: 10.4161/onci.28780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubin MM, Zhang X, Schuster H, Caron E, Ward JP, Noguchi T, Ivanova Y, Hundal J, Arthur CD, Krebber WJ, et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515:577–581. doi: 10.1038/nature13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammers HJ, Plimack ER, Infante JR, Rini BI, McDermott DF, Lewis LD, Voss MH, Sharma P, Pal SK, Razak ARA, et al. Safety and Efficacy of Nivolumab in Combination With Ipilimumab in Metastatic Renal Cell Carcinoma: The CheckMate 016 Study. J Clin Oncol. 2017;35:3851–3858. doi: 10.1200/JCO.2016.72.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG. AMPK: a target for drugs and natural products with effects on both diabetes and cancer. Diabetes. 2013;62:2164–2172. doi: 10.2337/db13-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauschild A, Grob JJ, Demidov LV, Jouary T, Gutzmer R, Millward M, Rutkowski P, Blank CU, Miller WH, Jr, Kaempgen E, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380:358–365. doi: 10.1016/S0140-6736(12)60868-X. [DOI] [PubMed] [Google Scholar]
- Hellmann MD, Rizvi NA, Goldman JW, Gettinger SN, Borghaei H, Brahmer JR, Ready NE, Gerber DE, Chow LQ, Juergens RA, et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol. 2016 doi: 10.1016/S1470-2045(16)30624-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiniker SM, Chen DS, Reddy S, Chang DT, Jones JC, Mollick JA, Swetter SM, Knox SJ. A systemic complete response of metastatic melanoma to local radiation and immunotherapy. Transl Oncol. 2012;5:404–407. doi: 10.1593/tlo.12280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong CS, Graham NA, Gu W, Espindola Camacho C, Mah V, Maresh EL, Alavi M, Bagryanova L, Krotee PAL, Gardner BK, et al. MCT1 Modulates Cancer Cell Pyruvate Export and Growth of Tumors that Co-express MCT1 and MCT4. Cell Rep. 2016;14:1590–1601. doi: 10.1016/j.celrep.2016.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ, Rimm AA, Ringden O, Rozman C, Speck B. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75:555. [PubMed] [Google Scholar]
- Hu-Lieskovan S, Mok S, Homet Moreno B, Tsoi J, Robert L, Goedert L, Pinheiro EM, Koya RC, Graeber TG, Comin-Anduix B, Ribas A. Improved antitumor activity of immunotherapy with BRAF and MEK inhibitors in BRAF(V600E) melanoma. Sci Transl Med. 2015;7:279ra241. doi: 10.1126/scitranslmed.aaa4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu-Lieskovan S, Robert L, Homet Moreno B, Ribas A. Combining targeted therapy with immunotherapy in BRAF-mutant melanoma: promise and challenges. J Clin Oncol. 2014;32:2248–2254. doi: 10.1200/JCO.2013.52.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang AC, Postow MA, Orlowski RJ, Mick R, Bengsch B, Manne S, Xu W, Harmon S, Giles JR, Wenz B, et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature. 2017;545:60–65. doi: 10.1038/nature22079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui E, Cheung J, Zhu J, Su X, Taylor MJ, Wallweber HA, Sasmal DK, Huang J, Kim JM, Mellman I, Vale RD. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science. 2017;355:1428–1433. doi: 10.1126/science.aaf1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain Z, Huang Y, Seth P, Sukhatme VP. Tumor-derived lactate modifies antitumor immune response: effect on myeloid-derived suppressor cells and NK cells. J Immunol. 2013;191:1486–1495. doi: 10.4049/jimmunol.1202702. [DOI] [PubMed] [Google Scholar]
- Hwu P, Hamid O, Gonzalez R, Infante JR, Patel MR, Hodi FS, Lewis KD, Wallin J, Mwawasi G, Cha E, et al. Preliminary safety and clinical activity of atezolizumab combined with cobimetinib and vemurafenib in BRAF V600-mutant metastatic melanoma. Annals of Oncology. 2016;27:1109PD–1109PD. [Google Scholar]
- Jacobs SR, Herman CE, MacIver NJ, Wofford JA, Wieman HL, Hammen JJ, Rathmell JC. Glucose Uptake Is Limiting in T Cell Activation and Requires CD28-Mediated Akt-Dependent and Independent Pathways. The Journal of Immunology. 2008;180:4476. doi: 10.4049/jimmunol.180.7.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James SR, Link PA, Karpf AR. Epigenetic regulation of X-linked cancer/germline antigen genes by DNMT1 and DNMT3b. Oncogene. 2006;25:6975–6985. doi: 10.1038/sj.onc.1209678. [DOI] [PubMed] [Google Scholar]
- Jimenez-Sanchez A, Memon D, Pourpe S, Veeraraghavan H, Li Y, Vargas HA, Gill MB, Park KJ, Zivanovic O, Konner J, et al. Heterogeneous Tumor-Immune Microenvironments among Differentially Growing Metastases in an Ovarian Cancer Patient. Cell. 2017;170:927–938 e920. doi: 10.1016/j.cell.2017.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John LB, Devaud C, Duong CP, Yong CS, Beavis PA, Haynes NM, Chow MT, Smyth MJ, Kershaw MH, Darcy PK. Anti-PD-1 antibody therapy potently enhances the eradication of established tumors by gene-modified T cells. Clin Cancer Res. 2013;19:5636–5646. doi: 10.1158/1078-0432.CCR-13-0458. [DOI] [PubMed] [Google Scholar]
- Kamphorst AO, Wieland A, Nasti T, Yang S, Zhang R, Barber DL, Konieczny BT, Daugherty CZ, Koenig L, Yu K, et al. Rescue of exhausted CD8 T cells by PD-1-targeted therapies is CD28-dependent. Science. 2017;355:1423–1427. doi: 10.1126/science.aaf0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpf AR. A potential role for epigenetic modulatory drugs in the enhancement of cancer/germ-line antigen vaccine efficacy. Epigenetics. 2006;1:116–120. doi: 10.4161/epi.1.3.2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman HL, Kohlhapp FJ, Zloza A. Oncolytic viruses: a new class of immunotherapy drugs. Nat Rev Drug Discov. 2015;14:642–662. doi: 10.1038/nrd4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Skora AD, Li Z, Liu Q, Tam AJ, Blosser RL, Diaz LA, Jr, Papadopoulos N, Kinzler KW, Vogelstein B, Zhou S. Eradication of metastatic mouse cancers resistant to immune checkpoint blockade by suppression of myeloid-derived cells. Proc Natl Acad Sci U S A. 2014;111:11774–11779. doi: 10.1073/pnas.1410626111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Li M, Trousil S, Zhang Y, Pasca di Magliano M, Swanson KD, Zheng B. Phenformin Inhibits Myeloid-Derived Suppressor Cells and Enhances the Anti-Tumor Activity of PD-1 Blockade in Melanoma. J Invest Dermatol. 2017;137:1740–1748. doi: 10.1016/j.jid.2017.03.033. [DOI] [PubMed] [Google Scholar]
- Klug F, Prakash H, Huber PE, Seibel T, Bender N, Halama N, Pfirschke C, Voss RH, Timke C, Umansky L, et al. Low-dose irradiation programs macrophage differentiation to an iNOS(+)/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell. 2013;24:589–602. doi: 10.1016/j.ccr.2013.09.014. [DOI] [PubMed] [Google Scholar]
- Kochenderfer JN, Dudley ME, Kassim SH, Somerville RP, Carpenter RO, Stetler-Stevenson M, Yang JC, Phan GQ, Hughes MS, Sherry RM, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015;33:540–549. doi: 10.1200/JCO.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanitis E, Dangaj D, Irving M, Coukos G. Mechanisms regulating T-cell infiltration and activity in solid tumors. Ann Oncol. 2017;28:xii18–xii32. doi: 10.1093/annonc/mdx238. [DOI] [PubMed] [Google Scholar]
- Larkin J, Ascierto PA, Dreno B, Atkinson V, Liszkay G, Maio M, Mandala M, Demidov L, Stroyakovskiy D, Thomas L, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371:1867–1876. doi: 10.1056/NEJMoa1408868. [DOI] [PubMed] [Google Scholar]
- Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence HS. Cellular and humoral aspects of the hypersensitive states: a symposium held at the New York Academy of Medicine: PP.B. Hoeber 1959 [Google Scholar]
- Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, et al. Mismatch-repair deficiency predicts response of solid tumors to PD-1blockade. Science. 2017 doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- Lee CK, Man J, Lord S, Links M, Gebski V, Mok T, Yang JC. Checkpoint Inhibitors in Metastatic EGFR-Mutated Non-Small Cell Lung Cancer-A Meta-Analysis. J Thorac Oncol. 2017;12:403–407. doi: 10.1016/j.jtho.2016.10.007. [DOI] [PubMed] [Google Scholar]
- Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, Fry TJ, Orentas R, Sabatino M, Shah NN, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385:517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- Li H, Chiappinelli KB, Guzzetta AA, Easwaran H, Yen RW, Vatapalli R, Topper MJ, Luo J, Connolly RM, Azad NS, et al. Immune regulation by low doses of the DNA methyltransferase inhibitor 5-azacitidine in common human epithelial cancers. Oncotarget. 2014;5:587–598. doi: 10.18632/oncotarget.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long GV, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, Larkin J, Garbe C, Jouary T, Hauschild A, Grob JJ, et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicentre, double-blind, phase 3 randomised controlled trial. Lancet. 2015;386:444–451. doi: 10.1016/S0140-6736(15)60898-4. [DOI] [PubMed] [Google Scholar]
- Lu L, Xu X, Zhang B, Zhang R, Ji H, Wang X. Combined PD-1 blockade and GITR triggering induce a potent antitumor immunity in murine cancer models and synergizes with chemotherapeutic drugs. J Transl Med. 2014;12:36. doi: 10.1186/1479-5876-12-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugade AA, Sorensen EW, Gerber SA, Moran JP, Frelinger JG, Lord EM. Radiation-induced IFN-gamma production within the tumor microenvironment influences antitumor immunity. J Immunol. 2008;180:3132–3139. doi: 10.4049/jimmunol.180.5.3132. [DOI] [PubMed] [Google Scholar]
- Luksza M, Riaz N, Makarov V, Balachandran VP, Hellmann MD, Solovyov A, Rizvi NA, Merghoub T, Levine AJ, Chan TA, et al. A neoantigen fitness model predicts tumour response to checkpoint blockade immunotherapy. Nature. 2017;551:517–520. doi: 10.1038/nature24473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- M L, P PV, T K, M P, E S, J P, K VW, C L, F C, S D, et al. Essential role of HDAC6 in the regulation of PD-L1 in melanoma. Mol Oncol. 2016;10:735–750. doi: 10.1016/j.molonc.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIver NJ, Michalek RD, Rathmell JC. Metabolic regulation of T lymphocytes. Annu Rev Immunol. 2013;31:259–283. doi: 10.1146/annurev-immunol-032712-095956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannick JB, Del Giudice G, Lattanzi M, Valiante NM, Praestgaard J, Huang B, Lonetto MA, Maecker HT, Kovarik J, Carson S, et al. mTOR inhibition improves immune function in the elderly. Sci Transl Med. 2014;6:268ra179. doi: 10.1126/scitranslmed.3009892. [DOI] [PubMed] [Google Scholar]
- Matsumura S, Wang B, Kawashima N, Braunstein S, Badura M, Cameron TO, Babb JS, Schneider RJ, Formenti SC, Dustin ML, Demaria S. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J Immunol. 2008;181:3099–3107. doi: 10.4049/jimmunol.181.5.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzone R, Zwergel C, Mai A, Valente S. Epi-drugs in combination with immunotherapy: a new avenue to improve anticancer efficacy. Clin Epigenetics. 2017;9:59. doi: 10.1186/s13148-017-0358-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGranahan N, Furness AJ, Rosenthal R, Ramskov S, Lyngaa R, Saini SK, Jamal-Hanjani M, Wilson GA, Birkbak NJ, Hiley CT, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351:1463–1469. doi: 10.1126/science.aaf1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendler AN, Hu B, Prinz PU, Kreutz M, Gottfried E, Noessner E. Tumor lactic acidosis suppresses CTL function by inhibition of p38 and JNK/c-Jun activation. Int J Cancer. 2012;131:633–640. doi: 10.1002/ijc.26410. [DOI] [PubMed] [Google Scholar]
- Messenheimer DJ, Jensen SM, Afentoulis ME, Wegmann KW, Feng Z, Friedman DJ, Gough MJ, Urba WJ, Fox BA. Timing of PD-1 Blockade Is Critical to Effective Combination Immunotherapy with Anti-OX40. Clin Cancer Res. 2017;23:6165–6177. doi: 10.1158/1078-0432.CCR-16-2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon EK, Wang LC, Dolfi DV, Wilson CB, Ranganathan R, Sun J, Kapoor V, Scholler J, Pure E, Milone MC, et al. Multifactorial T-cell hypofunction that is reversible can limit the efficacy of chimeric antigen receptor-transduced human T cells in solid tumors. Clin Cancer Res. 2014;20:4262–4273. doi: 10.1158/1078-0432.CCR-13-2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan RA, Chinnasamy N, Abate-Daga D, Gros A, Robbins PF, Zheng Z, Dudley ME, Feldman SA, Yang JC, Sherry RM, et al. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J Immunother. 2013;36:133–151. doi: 10.1097/CJI.0b013e3182829903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, Royal RE, Topalian SL, Kammula US, Restifo NP, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasi A, Fekete T, Krishnamurthy A, Snowden S, Rajnavolgyi E, Catrina AI, Wheelock CE, Vivar N, Rethi B. Dendritic cell reprogramming by endogenously produced lactic acid. J Immunol. 2013;191:3090–3099. doi: 10.4049/jimmunol.1300772. [DOI] [PubMed] [Google Scholar]
- Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, Sasayama S, Mizoguchi A, Hiai H, Minato N, Honjo T. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291:319–322. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T, Casares N, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- Odunsi K, Matsuzaki J, James SR, Mhawech-Fauceglia P, Tsuji T, Miller A, Zhang W, Akers SN, Griffiths EA, Miliotto A, et al. Epigenetic potentiation of NY-ESO-1 vaccine therapy in human ovarian cancer. Cancer immunology research. 2014;2:37–49. doi: 10.1158/2326-6066.CIR-13-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki T, Chikuma S, Iwai Y, Fagarasan S, Honjo T. A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nature immunology. 2013;14:1212–1218. doi: 10.1038/ni.2762. [DOI] [PubMed] [Google Scholar]
- Ota K, Azuma K, Kawahara A, Hattori S, Iwama E, Tanizaki J, Harada T, Matsumoto K, Takayama K, Takamori S, et al. Induction of PD-L1 Expression by the EML4-ALK Oncoprotein and Downstream Signaling Pathways in Non-Small Cell Lung Cancer. Clin Cancer Res. 2015;21:4014–4021. doi: 10.1158/1078-0432.CCR-15-0016. [DOI] [PubMed] [Google Scholar]
- Ott PA, Henry T, Baranda SJ, Frleta D, Manches O, Bogunovic D, Bhardwaj N. Inhibition of both BRAF and MEK in BRAF(V600E) mutant melanoma restores compromised dendritic cell (DC) function while having differential direct effects on DC properties. Cancer Immunol Immunother. 2013;62:811–822. doi: 10.1007/s00262-012-1389-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott PA, Hu Z, Keskin DB, Shukla SA, Sun J, Bozym DJ, Zhang W, Luoma A, Giobbie-Hurder A, Peter L, et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. 2017;547:217–221. doi: 10.1038/nature22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pages F, Ragueneau M, Rottapel R, Truneh A, Nunes J, Imbert J, Olive D. Binding of phosphatidylinositol-3-OH kinase to CD28 is required for T-cell signalling. Nature. 1994;369:327–329. doi: 10.1038/369327a0. [DOI] [PubMed] [Google Scholar]
- Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, Linsley PS, Thompson CB, Riley JL. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsoukis N, Bardhan K, Chatterjee P, Sari D, Liu B, Bell LN, Karoly ED, Freeman GJ, Petkova V, Seth P, et al. PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation. Nat Commun. 2015;6:6692. doi: 10.1038/ncomms7692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauken KE, Sammons MA, Odorizzi PM, Manne S, Godec J, Khan O, Drake AM, Chen Z, Sen DR, Kurachi M, et al. Epigenetic stability of exhausted T cells limits durability of reinvigoration by PD-1 blockade. Science. 2016;354:1160–1165. doi: 10.1126/science.aaf2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, Jones RG, Choi Y. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfirschke C, Engblom C, Rickelt S, Cortez-Retamozo V, Garris C, Pucci F, Yamazaki T, Poirier-Colame V, Newton A, Redouane Y, et al. Immunogenic Chemotherapy Sensitizes Tumors to Checkpoint Blockade Therapy. Immunity. 2016;44:343–354. doi: 10.1016/j.immuni.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip M, Fairchild L, Sun L, Horste EL, Camara S, Shakiba M, Scott AC, Viale A, Lauer P, Merghoub T, et al. Chromatin states define tumour-specific T cell dysfunction and reprogramming. Nature. 2017;545:452–456. doi: 10.1038/nature22367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol. 2005;5:375–386. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- Polanski R, Hodgkinson CL, Fusi A, Nonaka D, Priest L, Kelly P, Trapani F, Bishop PW, White A, Critchlow SE, et al. Activity of the monocarboxylate transporter 1 inhibitor AZD3965 in small cell lung cancer. Clin Cancer Res. 2014;20:926–937. doi: 10.1158/1078-0432.CCR-13-2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak M. Potential applications for biguanides in oncology. J Clin Invest. 2013;123:3693–3700. doi: 10.1172/JCI67232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, Mu Z, Rasalan T, Adamow M, Ritter E, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366:925–931. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, Linette GP, Meyer N, Giguere JK, Agarwala SS, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006–2017. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport AP, Stadtmauer EA, Binder-Scholl GK, Goloubeva O, Vogl DT, Lacey SF, Badros AZ, Garfall A, Weiss B, Finklestein J, et al. NY-ESO-1-specific TCR-engineered T cells mediate sustained antigen-specific antitumor effects in myeloma. Nat Med. 2015;21:914–921. doi: 10.1038/nm.3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, Camphausen K, Luiten RM, de Ru AH, Neijssen J, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203:1259–1271. doi: 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas A, Butler M, Lutzky J, Lawrence DP, Robert C, Miller W, Linette GP, Ascierto PA, Kuzel T, Algazi AP, et al. Phase I study combining anti-PD-L1 (MEDI4736) with BRAF (dabrafenib) and/or MEK (trametinib) inhibitors in advanced melanoma. Journal of Clinical Oncology. 2015;33:3003–3003. [Google Scholar]
- Ribas A, Dummer R, Puzanov I, VanderWalde A, Andtbacka RHI, Michielin O, Olszanski AJ, Malvehy J, Cebon J, Fernandez E, et al. Oncolytic Virotherapy Promotes Intratumoral T Cell Infiltration and Improves Anti-PD-1 Immunotherapy. Cell. 2017;170:1109–1119 e1110. doi: 10.1016/j.cell.2017.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins PF, Kassim SH, Tran TL, Crystal JS, Morgan RA, Feldman SA, Yang JC, Dudley ME, Wunderlich JR, Sherry RM, et al. A pilot trial using lymphocytes genetically engineered with an NY-ESO-1-reactive T-cell receptor: long-term follow-up and correlates with response. Clin Cancer Res. 2015;21:1019–1027. doi: 10.1158/1078-0432.CCR-14-2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015a;372:320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015b;372:2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- Robert C, Thomas L, Bondarenko I, O'Day S, M DJ, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roulois D, Loo Yau H, Singhania R, Wang Y, Danesh A, Shen SY, Han H, Liang G, Jones PA, Pugh TJ, et al. DNA-Demethylating Agents Target Colorectal Cancer Cells by Inducing Viral Mimicry by Endogenous Transcripts. Cell. 2015;162:961–973. doi: 10.1016/j.cell.2015.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra D, Crombet T. CIMAvax-EGF: A New Therapeutic Vaccine for Advanced Non-Small Cell Lung Cancer Patients. Front Immunol. 2017;8:269. doi: 10.3389/fimmu.2017.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin U, Derhovanessian E, Miller M, Kloke BP, Simon P, Lower M, Bukur V, Tadmor AD, Luxemburger U, Schrors B, et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature. 2017;547:222–226. doi: 10.1038/nature23003. [DOI] [PubMed] [Google Scholar]
- Salomon B, Bluestone JA. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu Rev Immunol. 2001;19:225–252. doi: 10.1146/annurev.immunol.19.1.225. [DOI] [PubMed] [Google Scholar]
- Schaue D, Xie MW, Ratikan JA, McBride WH. Regulatory T cells in radiotherapeutic responses. Front Oncol. 2012;2:90. doi: 10.3389/fonc.2012.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senbabaoglu Y, Gejman RS, Winer AG, Liu M, Van Allen EM, de Velasco G, Miao D, Ostrovnaya I, Drill E, Luna A, et al. Tumor immune microenvironment characterization in clear cell renal cell carcinoma identifies prognostic and immunotherapeutically relevant messenger RNA signatures. Genome Biol. 2016;17:231. doi: 10.1186/s13059-016-1092-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell. 2017;168:707–723. doi: 10.1016/j.cell.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Laird PW. Interplay between the cancer genome and epigenome. Cell. 2013;153:38–55. doi: 10.1016/j.cell.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JH, Park HB, Oh YM, Lim DP, Lee JE, Seo HH, Lee SJ, Eom HS, Kim IH, Lee SH, Choi K. Positive conversion of negative signaling of CTLA4 potentiates antitumor efficacy of adoptive T-cell therapy in murine tumor models. Blood. 2012;119:5678–5687. doi: 10.1182/blood-2011-09-380519. [DOI] [PubMed] [Google Scholar]
- Shrimali RK, Ahmad S, Verma V, Zeng P, Ananth S, Gaur P, Gittelman RM, Yusko E, Sanders C, Robins H, et al. Concurrent PD-1 Blockade Negates the Effects of OX40 Agonist Antibody in Combination Immunotherapy through Inducing T-cell Apoptosis. Cancer immunology research. 2017;5:755–766. doi: 10.1158/2326-6066.CIR-17-0292. [DOI] [PubMed] [Google Scholar]
- Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371:2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonveaux P, Vegran F, Schroeder T, Wergin MC, Verrax J, Rabbani ZN, De Saedeleer CJ, Kennedy KM, Diepart C, Jordan BF, et al. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Invest. 2008;118:3930–3942. doi: 10.1172/JCI36843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature. 2015;523:231–235. doi: 10.1038/nature14404. [DOI] [PubMed] [Google Scholar]
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran E, Robbins PF, Lu YC, Prickett TD, Gartner JJ, Jia L, Pasetto A, Zheng Z, Ray S, Groh EM, et al. T-Cell Transfer Therapy Targeting Mutant KRAS in Cancer. N Engl J Med. 2016;375:2255–2262. doi: 10.1056/NEJMoa1609279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truitt KE, Hicks CM, Imboden JB. Stimulation of CD28 triggers an association between CD28 and phosphatidylinositol 3-kinase in Jurkat T cells. J Exp Med. 1994;179:1071–1076. doi: 10.1084/jem.179.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turajlic S, Litchfield K, Xu H, Rosenthal R, McGranahan N, Reading JL, Wong YNS, Rowan A, Kanu N, Al Bakir M, et al. Insertion-and-deletion-derived tumour-specific neoantigens and the immunogenic phenotype: a pan-cancer analysis. Lancet Oncol. 2017;18:1009–1021. doi: 10.1016/S1470-2045(17)30516-8. [DOI] [PubMed] [Google Scholar]
- Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, Benci JL, Xu B, Dada H, Odorizzi PM, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520:373–377. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Allen EM, Miao D, Schilling B, Shukla SA, Blank C, Zimmer L, Sucker A, Hillen U, Foppen MHG, Goldinger SM, et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science. 2015;350:207–211. doi: 10.1126/science.aad0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooij N, van Buuren MM, Philips D, Velds A, Toebes M, Heemskerk B, van Dijk LJA, Behjati S, Hilkmann H, el Atmioui D, et al. Tumor Exome Analysis Reveals Neoantigen-Specific T-Cell Reactivity in an Ipilimumab-Responsive Melanoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31 doi: 10.1200/JCO.2012.47.7521. 10.1200/JCO.2012.1247.7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanpouille-Box C, Diamond JM, Pilones KA, Zavadil J, Babb JS, Formenti SC, Barcellos-Hoff MH, Demaria S. TGFbeta Is a Master Regulator of Radiation Therapy-Induced Antitumor Immunity. Cancer Res. 2015;75:2232–2242. doi: 10.1158/0008-5472.CAN-14-3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, Thompson CB, Bluestone JA. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- Wang J, Yoshida T, Nakaki F, Hiai H, Okazaki T, Honjo T. Establishment of NOD-Pdcd1-/- mice as an efficient animal model of type I diabetes. Proc Natl Acad Sci U S A. 2005;102:11823–11828. doi: 10.1073/pnas.0505497102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Amoozgar Z, Huang J, Saleh MH, Xing D, Orsulic S, Goldberg MS. Decitabine Enhances Lymphocyte Migration and Function and Synergizes with CTLA-4 Blockade in a Murine Ovarian Cancer Model. Cancer immunology research. 2015;3:1030–1041. doi: 10.1158/2326-6066.CIR-15-0073. [DOI] [PubMed] [Google Scholar]
- Wang Q, Liu F, Liu L. Prognostic significance of PD-L1 in solid tumor: An updated meta-analysis. Medicine. 2017;96:e6369. doi: 10.1097/MD.0000000000006369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber JS, D'Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, Hoeller C, Khushalani NI, Miller WH, Jr, Lao CD, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375–384. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- Wherry EJ. T cell exhaustion. Nature immunology. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, Lao CD, Wagstaff J, Schadendorf D, Ferrucci PF, et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med. 2017;377:1345–1356. doi: 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods DM, Sodre AL, Villagra A, Sarnaik A, Sotomayor EM, Weber J. HDAC Inhibition Upregulates PD-1 Ligands in Melanoma and Augments Immunotherapy with PD-1 Blockade. Cancer immunology research. 2015;3:1375–1385. doi: 10.1158/2326-6066.CIR-15-0077-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrangle J, Wang W, Koch A, Easwaran H, Mohammad HP, Vendetti F, Vancriekinge W, Demeyer T, Du Z, Parsana P, et al. Alterations of immune response of Non-Small Cell Lung Cancer with Azacytidine. Oncotarget. 2013;4:2067–2079. doi: 10.18632/oncotarget.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Escamilla J, Mok S, David J, Priceman S, West B, Bollag G, McBride W, Wu L. CSF1R signaling blockade stanches tumor-infiltrating myeloid cells and improves the efficacy of radiotherapy in prostate cancer. Cancer Res. 2013;73:2782–2794. doi: 10.1158/0008-5472.CAN-12-3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki T, Akiba H, Iwai H, Matsuda H, Aoki M, Tanno Y, Shin T, Tsuchiya H, Pardoll DM, Okumura K, et al. Expression of programmed death 1 ligands by murine T cells and APC. J Immunol. 2002;169:5538–5545. doi: 10.4049/jimmunol.169.10.5538. [DOI] [PubMed] [Google Scholar]
- Yokosuka T, Takamatsu M, Kobayashi-Imanishi W, Hashimoto-Tane A, Azuma M, Saito T. Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. J Exp Med. 2012;209:1201–1217. doi: 10.1084/jem.20112741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan P, Ito K, Perez-Lorenzo R, Del Guzzo C, Lee JH, Shen CH, Bosenberg MW, McMahon M, Cantley LC, Zheng B. Phenformin enhances the therapeutic benefit of BRAF(V600E) inhibition in melanoma. Proc Natl Acad Sci U S A. 2013;110:18226–18231. doi: 10.1073/pnas.1317577110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zappasodi R, Ghosh A, Serganova I, Cohen I, Senbabaoglu Y, Shindo M, Mane MM, Leftin A, Ackerstaff E, Koutcher JA, et al. Abstract PR06: Overcoming intratumor T-cell exclusion by modulation of lactate metabolism to improve immune checkpoint therapies in aggressive breast cancer. Cancer immunology research. 2016;4:PR06. [Google Scholar]
- Zappasodi R, Li Y, Abu-Akeel M, Qi J, Wong P, Sirard C, Postow M, Schaer DA, Newman W, Koon H, et al. Abstract CT018: Intratumor and peripheral Treg modulation as a pharmacodynamic biomarker of the GITR agonist antibody TRX-518 in the first in-human trial. Cancer Res. 2017;77:CT018. [Google Scholar]
- Zaretsky JM, Garcia-Diaz A, Shin DS, Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, Torrejon DY, Abril-Rodriguez G, Sandoval S, Barthly L, et al. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. N Engl J Med. 2016;375:819–829. doi: 10.1056/NEJMoa1604958. [DOI] [PMC free article] [PubMed] [Google Scholar]