Lung cancer and interstitial lung disease: a literature review (original) (raw)
Abstract
The association between lung cancer (LC) and interstitial lung disease (ILD) can be explained by the shared risk factors like smoking and physiopathology of fibrogenesis and cancerogenesis. The relative LC risk is shown to be 3.5- to 7.3-times higher in ILD, with LC occurrence estimated at 10–20% in ILD, with >15% of ILD patients likely to die from LC. ILD incidence upon LC diagnosis varied from 2.4–10.9%. Primary radiological presentations consist of peripheral lesions, mostly in the inferior pulmonary lobes, either close to or within the ILD areas. There is a trend towards inverted proportion of adenocarcinomas and squamous-cell carcinomas, with EGFR mutations very rarely found. ILD negatively impacted LC prognosis, with surgery associated with increased morbidity-mortality, particularly due to acute exacerbation (AE) of ILD. Limited resection reduced this risk, whilst increasing that of cancer mortality. Studies on radiotherapy that can induce AE-ILD are scarce. Chemotherapy was associated with similar response rates to those in LC patients without ILD, yet worse survival. This difference may be accounted for by ILD patients’ poorer health and higher risk of drug-induced pneumonitis. Further studies are warranted to better understand cancer physiopathology within the fibrotic areas, along with the therapeutic strategies required.
Keywords: Lung cancer (LC), pulmonary fibrosis, interstital lung disease (ILD)
Introduction
The first literature reviews on the association between lung cancer (LC) and interstitial lung disease (ILD) date back to over 10 years. Primarily focused on epidemiology (1,2), these reviews reported increased LC risk in ILD. Published in 2017, the last review focused on LC associated with idiopathic pulmonary fibrosis (IPF) (3). Our current review sought to further enhance our understanding of LC-ILD, considering recently published data.
Sharing common risk factors with LC like smoking or exposure to chemicals (4,5), IPF must be distinguished from other ILDs. Like carcinogenesis, IPF physiopathology is primarily based on epithelial damage, repair abnormalities, and epithelial mesenchymal transition, whereas for the other ILD subtypes, inflammation and immunosuppression are paramount features. These last displays a better prognosis that IPF.
The review has several limitations we wish to highlight upfront. Most data were derived from retrospective Asian cohorts, with ILD diagnoses based on surgical samples or retrospective pre-operative CTs. While nearly all studies focused on IPF, only few concerned all ILDs.
Methods
Literature review
A systematic Medline search through PubMed was performed from inception to January 2018 for published reports restricted to human subjects and English or French language. Bibliographies from selected articles were screened for relevant publications.
Selection of studies
The inclusion criteria were: (I) ILD or IPF; (II) LC. Studies reporting ILD secondary to LC therapy or LC secondary to ILD therapy were excluded.
ILD classification
The commonly-affected area in ILD is the pulmonary interstitium. Pulmonary damage weakens alveolar wall integrity, causing epithelial cell lesions, inflammation, and myofibroblast infiltration, with extracellular matrix accumulation. This results in diminished pulmonary compliance and altered gas exchanges, with eventually pulmonary insufficiency.
In the early 2000’s, a new ILD classification was proposed, recently updated (6). IPF is the most serious ILD, occurring in male smokers aged ≥60, with a median survival of 2–3 years. Other ILDs essentially involve inflammatory processes, with better prognosis.
Common features of ILD and LC
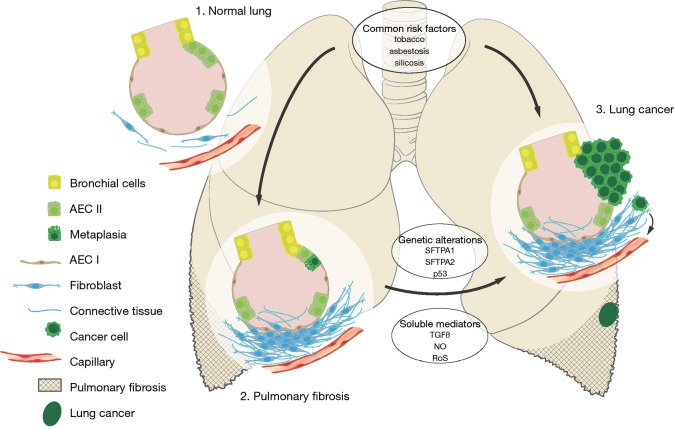
The two leading hypotheses regarding LC-ILD are the shared risk factors and common physiopathology (Figure 1).
Figure 1.
Pathobiological paradigm for LC in ILD. Evidence suggests that the pathobiology of LC in ILD involves both common risk factors (e.g., tobacco and asbestos) and common pathophysiology (e.g., cellular abnormalities). In ILD, a combination of genetic, environmental, and behavioral factors are believed to contribute to the development of a fibrotic phenotype characterized by extracellular matrix deposition, parenchymal scar formation, and lung remodeling. LC in ILD is most likely favored by genetic alterations (e.g., SFTPA, p53) and soluble mediators’ secretions (TGFβ, NO, RoS). TGFβ, transforming growth factor β; NO, nitric oxygen; RoS, reactive oxygen species; LC, lung cancer; ILD, interstitial lung disease.
Common risk factors
As smoking and exposure to occupational hazards are common risk factors for LC and ILD especially IPF, both may account for their concomitant occurrence (4,5). However, smoking is not always preponderant, since LC risk in scleroderma patients is no higher in smokers than non-smokers (7).
Common pathophysiology
The pathophysiology of ILDs, particularly IPF, resembles that of cancer development, including epithelial cell anomalies ranging from metaplasia to carcinomatous transformation, cellular bioenergetics, soluble mediator release, gene alterations, and aging with telomere attrition and aberrant recapitulation of developmental pathways.
Cellular abnormalities and cell-to-cell interactions
Epithelial layer abnomalities like hyperplasia of pulmonary cells, cuboidal cells, and mucous cells, along with epithelial metaplasia within fibrotic regions, were demonstrated in IPF (8). Epithelial metaplasia appears linked to cancerogenesis, with transition zones from metaplasia to invasive cancer located close to fibrosis areas (9,10). Histone deacetylase enzyme (HDAC) overexpression was recently demonstrated in aberrant bronchial cells populating fibrotic regions, with HDACs exhibiting anti-apoptotic activity via p53 inhibition or cMyc stimulation observed in cancer cells (11).
The metabolic perturbation in cancer cells is known as the Warburg effect, meaning that glycolysis is preferred over oxidative phosphorylation, even in the presence of oxygen. The Warburg effect has also been observed upon myofibroblast differentiation, a distinct physiopathological step in pulmonary fibrosis (12).
In LC, interactions between stroma and tumor influence tumor progression and metastatic diffusion. Peritumoral stroma shares common features with pulmonary fibrosis, and several authors have reported that this stroma facilitates mesenchymatous phenotype development in tumor cells, along with their invasive capability (13-15). Inflammatory cells are involved in carcinogenesis in smokers via oxygen-free radical production (16), with decreased tumor immune surveillance. A diminished T lymphocyte response was demonstrated in chronic obstructive pulmonary disease, contributing to enhanced LC risk (17).
Soluble mediators and gene alterations
The carcinomatous transformation of epithelial cells during fibrogenesis is likely facilitated by cytokine or nitrous derivative secretion (18,19) into the microenvironment, enhancing acquired genetic anomalies like p53 mutations.
Tumor growth factor beta (TGFβ) appears involved in fibrogenesis. In normal conditions, TGFβ exerts an anti-proliferative action on epithelial cells and stimulates the Wnt/β-catenin signalization pathway, transforming fibroblasts into myofibroblasts (20,21). Using cDNA arrays, a study revealed Smad4 expression, involved in TGFβ signaling, to be diminished in LC patients with concomitant IPF compared to those without, promoting diminished growth inhibitory response to TGFβ (22). TGFβ decreased cancer immune surveillance, inhibiting effector T lymphocytes while stimulating regulator T lymphocytes. In a tumor xenograft transplant model, TGFβ instillation increased regulator T lymphocyte levels, with significant tumor growth, increased lung metastasis, and decreased overall survival (OS). Naringenin, an antifibrotic agent, reduced TGFβ pro-tumoral activity in animals (23).
TGFβ’s role regarding LC-ILD association was evoked while investigating families with both conditions. Mutations in genes encoding surfactant proteins A2 (SFTPA2) that induce TGFβ secretion promoted pulmonary fibrosis, with associations found between cancer and ILD and SFTPA2 mutations (24,25). One study reported a family with 10 ILD patients, three exhibiting concomitant LC, while three others displayed LC without ILD, all patients exhibiting SFTPA2 mutations (25). Another study described a family including 10 patients exhibiting ILD and three others LC, with six of the patients exhibiting SFTPA1 mutations (26).
Nitric oxygen (NO) produced in stressed epithelial cells caused guanine nitrification in cellular DNA. NO overexpression, along with guanine nitrification, was more common in pulmonary tissues from IPF and squamous-cell carcinoma patients versus healthy subjects, regardless of smoking status (27).
Oncogene and tumor-suppressor gene mutations induce genomic instability within epithelial cells. The p53 gene is the most-frequently mutated gene in LC. Kawasaki et al., analyzing p53 gene mutations and expression in tumoral tissues from 19 LC-IPF patients, detected p53 mutations in cancer cells and squamous metaplasia cells in 57% and 26% of patients, respectively (8). Another study focused on expired air and blood samples from IPF patients revealed genetic alterations within tumor suppressor genes (28). Searching for common anomalies between IPF and LC in the AKT/MAPK and TLR signaling pathways proved inconclusive (29).
Most genetic hallmarks that characterize the ageing process were identified in both IPF and LC like abnormal telomere shortening, aberrant recapitulation of developmental pathways, and cellular senescence (30-32).
Contrasting LC risk between IPF and other ILDs
Increased LC risk in ILD
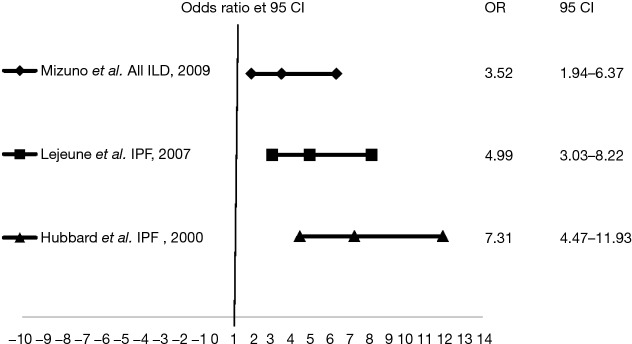
Increased LC risk in ILD was assessed in three studies (Figure 2). A Japanese case-control study comparing LC patients to healthy subjects participating in a CT screening program found the proportion of smokers to be higher in the LC group (88.5% vs. 45%) and LC risk to be 3.5 times superior in patients with fibrotic lesions, regardless of smoking status (33). Two IPF studies reported 4.99 and 7.3 times higher LC risk with this disease, respectively (34,35).
Figure 2.
LC risk in ILD patients (see text). LC, lung cancer; ILD, interstitial lung disease.
LC diagnosis is usually established in male smokers in their sixties (9,36,37). Concerning IPF patients, multivariate analysis demonstrated age >60 years, male gender, and tobacco smoking to be associated with relative LC risks of 2.41, 11.04, and 2.71, respectively (38). Two series found a significantly higher proportion of males with LC-IPF versus LC patients without ILD (92% vs. 62%; 94% vs. 74%, respectively) (39-41).
LC incidence and prevalence in IPF and other ILDs
Idiopathic ILD
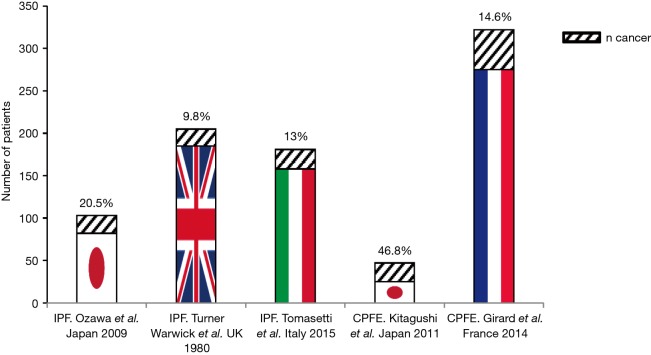
Figure 3 lists published series reporting on LC frequency in IPF, combining pulmonary fibrosis and emphysema (CPFE). The first, a retrospective Japanese study involving 103 IPF patients, reported 20.5% of patients having developed LC over a mean 52±44.8 months follow-up (36). In the second involving 205 cryptogenic fibrosing alveolitis (CFA) patients, LC frequency was 9.8% over a 4±21-year follow-up (42). An Italian cohort involving 181 IPF patients reported a 13% LC frequency (n=23) (37).
Figure 3.
Cancer rates in IPF and CPFE patients (see text). IPF, idiopathic pulmonary fibrosis; CPFE, combining pulmonary fibrosis and emphysema.
Analyzing CPFE populations, LC incidence was 46.8% (22/47) over a 3-year follow-up in a Japanese series and 14.6% (47/322) over a 10-year follow-up in the French GERM“O”P (Groupe d‘Etude et de Recherche des Maladies Orphelines Pulmonaires) registry (43,44). Data on other idiopathic ILDs like non-specific interstitial pneumonia (NSIP), desquamative interstitial pneumonia (DIP), respiratory bronchiolitis-associated interstitial lung disease (RB-ILD), and lymphocytic interstitial pneumonia (LIP) are quasi-inexistent.
Other ILDs
Data concerning LC risk in other ILDs are difficult to interpret, as certain ILDs are strongly associated with smoking or exposure to carcinogens. Moreover, myositis and dermatomyositis can manifest as a paraneoplastic syndrome complicating LC.
Connective tissue disease (CTD)-associated ILD
A retrospective study reported a 5.5% (7/127) LC incidence in CTD-related ILDs over a median 67.4-month follow-up, with 1-, 3-, and 5-year cumulative incidences of 0, 1.8%, and 2.9%. In univariate analysis, heavy smoking and emphysema on CT were LC risk factors (45).
In cohort studies, scleroderma was proven an LC risk factor, with LC being the most common scleroderma-associated cancer with an incidence of 4.9% in Sweden versus 5.7% in Japan and 5.9% in Australia (46-48).
A meta-analysis assessing cancer incidence in RA revealed increased cancer risk with a standardized incidence ratio (SIR) of 1.63 (49). Likewise, several cohort studies demonstrated an increased LC risk in systemic lupus erythematosus with an SIR of 1.37–3.1 (50). In these two CTD, no link was reported between LC and ILD.
Idiopathic inflammatory myopathies like polymyositis (PM) and dermatomyositis (DM) can manifest as paraneoplastic syndromes. ILD appears to diminish the LC cancer risk, especially among Asians (51-53).
Silicosis and asbestosis
In a meta-analysis, silicosis was an LC risk factor with a 2.37 RR, in contrast to silica exposure without silicosis (54). Smoking markedly increased LC risk in silicosis patients, with a 4.47 RR.
Concerning asbestos exposure, most patients who develop LC in this setting are also smokers. The association between asbestos exposure and LC proves difficult, with roughly 33% of such cancers presumably caused by double exposure (55). Asbestosis, an ILD secondary to asbestos fiber inhalation, increases LC risk (55,56).
Hypersensitivity pneumonia (HP)
HP refers to ILDs secondary to organic antigen exposure, the best known being bird fancier’s or farmer’s lung. A Japanese study identified 11 cancer cases among 104 (10.4%) HP patients, most exhibiting bird fancier’s lung (n=93), and all involving smokers. Seven patients underwent lung biopsy, revealing a pattern of usual interstitial pneumonia (UIP) similar to IPF (57).
Sarcoidosis
Data on sarcoidosis are controversial. In a meta-analysis, sarcoidosis was initially not associated with increased LC risk, though an increased risk was observed at 5 years post-diagnosis, followed by risk decrease, and ending with a diminished risk at 10 years (58).
Langerhans cell granulomatosis
Langerhans cell granulomatosis is strongly associated with smoking. Two series reported links between LC and Langerhans cell granulomatosis, suggesting increased LC risk (59,60).
LC mortality in IPF
A paper reviewed the published series on LC-related mortality in IPF, reporting a mean mortality of 17.3% (61). In a 1990–1992 UK cohort survey, IPF diagnosis was initially established in 588 patients; of these, 488 died, 46 due to LC (9.4%). A more recent German study revealed that of 272 IPF-diagnosed patients, 171 died, 13 (7.3%) from LC (62).
LC in ILD or ILD in LC?
Another means of investigating LC-ILD is to assess ILD frequency in LC populations (Table S1). Most series pertaining to IPF reported a frequency of 2.4–10.9% (10,38,41,63-70), with only three from Japan exhibiting higher rates of 16.8% and 24.3% (71,72). In the first study, IPF diagnosis was based on histological analysis (71), potentially including infra-radiological IPFs, which may explain the differences.
Table S1. Frequency of interstitial lung diseases in lung cancers.
| Series | Country | Recruitment | n cancers | n ILDs | % of ILD | ILD type |
|---|---|---|---|---|---|---|
| Park et al. 2001 | Korea | All stages | 2,660 | 63 | 2.4 | IPF |
| Khan et al. 2015 | Ireland | All stages | 637 | 34 | 5.3 | IPF |
| Kawasaki et al. 2002 | Japan | Surgical series | 711 | 53 | 5.3 | IPF |
| Chida et al. 2008 | Japan | Surgical series | 834 | 91 | 10.9 | IPF |
| Watanabee et al. 2008 | Japan | Surgical series | 858 | 56 | 6.5 | IPF |
| Saito et al. 2011 | Japan | Surgical series | 350 | 28 | 8 | IPF |
| Goto et al. 2014 | Japan | Surgical series | 387 | 65 | 16.8 | IPF |
| Kumar et al. 2003 | England | Surgical series | 951 | 22 | 2.3 | 16 IPF, 6 NSIP |
| Chiyo et al. 2003 | Japan | Surgical series | 931 | 36 | 3.9 | 26 IPF, 10? |
| Voltolini et al. 2013 | Italy | Surgical series | 775 | 37 | 4.8 | 11 IPF, 26? |
| Kanaji et al. 2016 | Japan | Stage IIIB, IV | 218 | 53 | 24.3 | 34 IPF, 19? |
| Usui et al. 2011 | Japan | All stages | 1,148 | 102 | 8.9 | CPFE |
| Hata et al. 2016 | Japan | Surgical series | 1,264 | 104 | 8.2 | 77 IPF, 10 CTD, 2 NSIP, 1 OP, 2 smoker ILD, 12? |
| Tao et al. 2017 | Japan | Surgical series | 257 | 60 | 23.3 | 25 UIP, 35 non UIP* |
| Sekihara et al. 2017 | Japan | Surgical series | 2054 | 106 | 5 | 79 UIP, 27 non UIP* |
| Personal data, 2016 | France | All stages | 907 | 48 | 5.3 | ILD |
Clinical presentation
Cancer detection mode and stage at diagnosis
LC is mostly diagnosed after ILD, with numerous LCs fortuitously detected upon CT ILD-surveillance (73). So far, 4,672 patients concomitantly exhibiting non-small cell lung cancer (NSCLC) were reported, with stage I, II, III, and IV in 42.7%, 14.9%, 22.5%, and 13.3% of cases, respectively. A recent surgery series comparing 104 patients with ILD-LC to 1,160 LC-alone patients revealed a higher proportion of the former exhibiting pleural invasion (69). Concerning small-cell LC, 209 cases were reported, 39.7% of whom were localized.
CT-scan presentation and PET-scan requirement
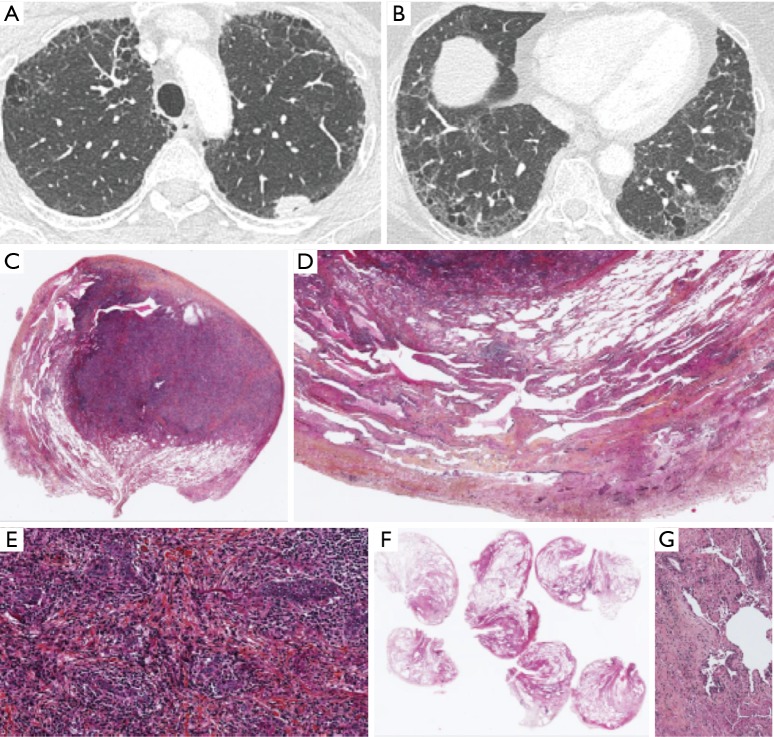
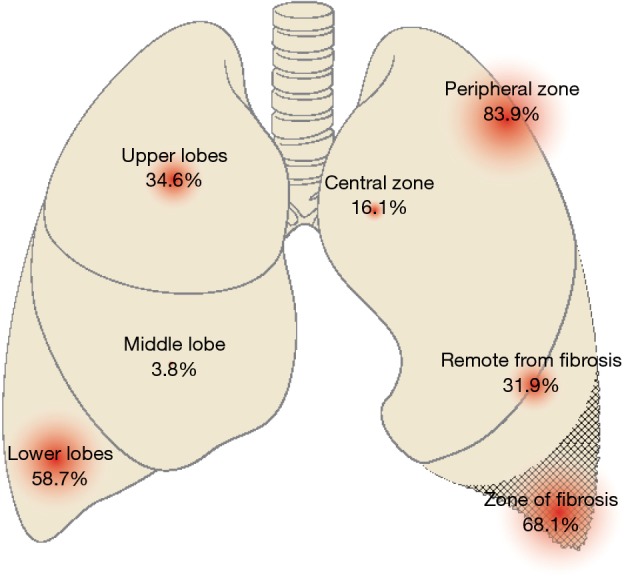
In ILD, LCs mostly manifests as peripheral tumors (83.9% of 503 patients) developing within/near fibrotic areas (68.1% of 474), in the inferior lung lobes (58.7% of 2,877) (Figures 4,S1,S2). Their limits are distinct, with spiculated contours and possibly an air bronchogram (73,74).
Figure 4.
CT scan presentation of LC associated with ILD (see text). LC, lung cancer; ILD, interstitial lung disease; CT, computed tomography.
Figure S1.
(A) CT scan of a 34-year-old man with ILD in a context of scleroderma in 2015; (B) CT scan of the same patient in 2017 showing a new right lower lobe opacity; (C) presence of a tumoural nodule of adenocarcinoma in a case of pulmonary involvement by scleroderma. ×1, HES; (D) adenocarcinoma is present in the right of the field and non tumoural lung parenchyma on the left, with NSIP. ×10, HES; (E) lung parenchyma with NSIP. The witness of the parietal wall is increased and there is inflammatory cells and alveolar hemorrhage. ×20, HES; (F) invasive acinous adenocarcinoma with inflammatory cells. ×20, HES. NSIP, non-specific interstitial pneumonia; ILD, interstitial lung disease; CT, computed tomography.
Figure S2.
(A) CT scan of a 52-year-old tobacco smoker woman with a left upper lobe nodule. Presence of subpleural reticulation and paraseptal emphysema; (B) CT scan of the lower lobes showing a predominance of peripheral and basal predominance of reticular abnormalities with emphysema. The radiological pattern is possible UIP; (C) lymphoepithelial carcinoma in a case of UIP. ×1, HES; (D) lung parenchyma surrounding the nodule with peripheral subpleural heterogeneous fibrosis. ×10, HES; (E) lymphoepithelial carcinoma with carcinomatous islands associated with fibrosis and a lot of inflammatory cells. ×20, HES; (F) heterogeneous fibrosis with subpleural and peripheral predominance; (G) fibroblastic focus. CT, computed tomography; UIP, usual interstitial pneumonia.
In a series of 66 peripheral T1N0 cancers, 64% occurred in the inferior lobes, 53% at the fibrosis/healthy tissue interface, and 31.8% in fibrotic areas. Most tumors consisted of solid, round or oval lesions, with a median doubling time of 77 days (75). In another study (76), tumors were more commonly found in areas with more fibrosis (12/16 patients). A South Korean study reported contrasting results involving 63 LC-IPF patients. While peripheral (56%), most lesions predominantly affected the superior lobes (51%), far from fibrotic areas (63%). One possible explanation was that 19% of patients exhibited small-cell LCs, more centrally located (38).
Assessing tumor size on CT proved difficult. In a study involving 1,357 surgical patients, 136 exhibiting ILD, tumor size was underestimated in 10.3% of ILD patients versus 3.2% of controls (77).
Mediastinal analysis of LC-ILD is more difficult, owing to reactively-increased mediastinal lymph node sizes in 55–93% ILD cases (78). CT specificity for mediastinal staging is diminished in IPF (47% vs. 84%). PET revealed similar sensitivity and specificity rates in patients with or without ILD (78). Comparing LC-IPF patients to those without ILD, the authors demonstrated that PET versus CT alone increased diagnostic accuracy from 14% to 33%.
Histology and molecular analysis
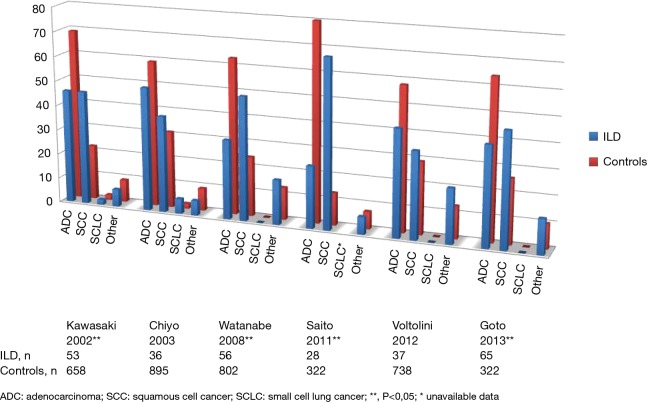
Obtaining histological cancer diagnosis can prove challenging owing to patient frailty. In the GERM“O”P study, histological diagnosis was impossible in 9 (19%) patients (43). Thirteen series, including six surgical ones, assessed LC histology distribution in ILD cases versus ILD-free controls. Seven revealed statistically different histological distribution in ILD from controls, with less adenocarcinoma (ADC) (24–40% vs. 42–80%) and more squamous-cell carcinoma (SCC) (29–68% vs. 13–26%) cases, the other six revealing similar distributions (9,38,41,64,65,67,68,72,79-83) (Figures S3,S4).
Figure S3.
Histological analysis of LC-ILD vs. LC: surgical series. LC, lung cancer; ILD, interstitial lung disease.
Figure S4.
Histological analysis of LC-ILD vs. LC: all stages. LC, lung cancer; ILD, interstitial lung disease.
Synchronous cancers were more frequently described in IPF patients versus ILD-free in two series, one based on autopsy data (17% vs. 5%; 21.9% vs. 3.4%) (9,41). A retrospective Japanese study reported higher frequency of combined small-cell LC with NSCLC (7/22; 33%) versus single cancers (17/119; 14%) (79).
Activating mutations in epidermal growth factor receptor (EGFR) gene were rarely detected in LC-ILD, owing to high frequencies of smokers and SCC histology (Table S2). Five Japanese series compared EGFR gene mutation frequencies in NSCLC patients with and without ILD. EGFR mutations were detected in 0–5.8% of tumors in ILD patients versus 24.3–47% in those without (72,84-87). A multivariate analysis revealed EGFR mutations to be independently associated with absent ILD (86). In another study involving ADC patients, those with histologically-confirmed ILD were more commonly Kras-mutation carriers (87). Guyard et al. reported NGS performed using an ampliseq panel to analyze hotspots and targeted regions in 22 cancer-associated genes in 27 samples from patients with LC-ILD (88). They authors found several alterations in MET, FGFR3, ERBB4, DDR2, EGFR, BRAF, and PI3KCA genes in 25 samples. There were neither EGFR mutations nor ALK or ROS rearrangements found. Surprinsingly, only one tumor was considered Kras-mutated, whereas PDL1 expression (≥1%) was reported in 10 cases (61%), with only one tumor >50% PD-L1 expression.
Table S2. EGFR mutations.
| Author | n | Stage | ADC % | Smoker % | ILD % | ILD+ | EGFR % | ||
|---|---|---|---|---|---|---|---|---|---|
| ADC % | Smoker % | ILD | No ILD | ||||||
| Fujimoto et al. 2013 | 555 | I–IV | 100 | 52 | 6 | 100 | 87 | 3 | 47 |
| Usui et al. 2011 | 198 | I–IV | 85.4 | 62.6 | 8.5 | nd | nd | 5.8 | 28.7 |
| Sekine et al. 2013 | 88 | I–IV | 91 | 100 | 11.3 | nd | 100 | 0 | 24.3 |
| Kanaji et al. 2016 | 218 | IIIB–IV | 69 | 73 | 24.3 | 40 | 96 | 2 | 32 |
| Masai et al., JTO 2016 | 2,309 | I–IV | 100 | 46.4 | 1.9 | 100 | 90.9 | 2.3 | 45.6 |
Lung cancer diagnostic approach in ILD
Due to the frailty of ILD patients with increased LC risk, the optimal LC diagnostic approach must be carefully assessed. In a recent letter, Tzouvelekis et al. proposed an algorithm, with HRCT to be performed once a year in all IPF patients for LC screening purposes (89). Yet, the benefit/risk of this approach has never been evaluated in IPF patients, with 90% of positive CTs eventually considered false-positive in the National Lung Screening Trial. For nodules with a diameter ≥8 mm, PET-CT scan appears highly recommended. If FDG uptake is indicative of tumor lesions, the authors suggested proceeding first to minimally-invasive diagnostics, including transthoracic needle biopsy (TTNB) for peripheral lesions or endobronchial ultrasound-guided trans-bronchial needle biopsy in the presence of pathological lymph nodes. Concerning the risk of pneumothorax, radial probe endobronchial ultrasound-guided transbronchial biopsy should be employed instead of TTNB in specific settings, such as emphysema and larger lesions with a bronchial sign within (90).
The role of liquid biopsy as non-invasive diagnostic tool for LC-ILD must still be defined, considering the technique’s specificity <60% for a pre-specified alteration, namely EGFR mutation in advanced NSCLC, being even lower in early-stage disease. Moreover, the molecular alterations to be tested probably differ from NSCLC cases without IPF (see above) (88).
Treating LC while reducing the risk of ILD exacerbation
Treating LC in ILD raises the issue as to which standards of care should be applied for these elderly patients with diminished respiratory reserve. In the GERM“O”P registry, 20/47 (43%) LC patients with combined pulmonary fibrosis and emphysema (CPFE) did not receive the standards of care, 8 (17%) on account of CPFE (43), while in the Miyazaki series involving stage I–IIIA patients, 67.6% ILDs (n=34) underwent surgery vs. 82.6% ILD-free (n=1,089) (40). In another study, 10/34 (29%) LC patients with IPF received only best supportive care (10).
Operable NSCLC
Tables S3,S4 display published series on operated LC in association with IPF or other ILDs, respectively.
Table S3. Surgical series on idiopathic pulmonary fibrosis.
| Citations | n* | Stage | ILD type | Surgery (pneumonectomy/lobectomy/< lobe) | Survival | AE |
|---|---|---|---|---|---|---|
| Kawasaki, J Surg Oncol 2002 | 53/711 | I, n=21; II, n=12; III, n=14; IV, n=6; | IPF | 0/52/1 | 5-year survival; 43% vs. 64.2% | ND |
| Fujimoto, Ann Thor Surg 2003 | 21 | I–II, n=14; III–IV, n=7 | IPF | 0/17/4 | 2-year survival; all stages: 52%; N0 and N1: 58%; N2: 25% | ? |
| Watanabee, J Thorac Cardiovasc Surg 2008 | 56/858 | I, n=28; II, n=5; III, n=14; IV, n=3; ND, n=6; | IPF | 0/44φ/12 | 5-year survival; stage I: 61.6% vs. 83%; stage II: 40% vs. 72.5%; stage III: 31.8% vs. 38%; stage IV: 0% vs. 15.4% | 7.1% |
| Saito, Ann Thor Surg 2011 | 28/350 | IA | IPF | 0/23/5 | 5-year survival; 54.2% vs. 88.3% | 10.7% |
| Suzuki, Surg Today 2011:914 | 28 | I–II, n=14; III–IV, n=14 | IPF | ND | 3-year survival; with AE: 42.5%; without AE: 55.2% | 32% |
| Mizuno, Eur J Cardio Thor Surg 2012 | 52 | I, n=32; II, n=13; III, n=17; | IPF | 0/47δ/5 | ND | 13.5% |
| Song, Korean J Patho 2014 | 43 | I, n=19; II, n=12;III, n=11; ND, n=1 | IPF | 3/37/3 | 1-year survival; 60% | ND |
| Goto, Int J Clin Oncol 2014 | 65/387 | IA, n=11; IB, n=17; IIB, n=10; IIIA, n=16; IIIB, n=8; IV, n=3 | IPF | 3/53/3 | ND | 6.2% |
| Lee, Resp Med 2014 | 33/66¶ | I, n=23; II, n=8; IIIA, n=2 | IPF | ?/?β/11 | 5-year survival; 37.5% vs. 72.5% | ND |
| Iwata, Surg Today 2014 | 28 | ND | IPF | 0/19/9 | 5-year survival; 62.8% | 25% |
Table S4. Surgical series on various interstitial lung diseases.
| Citations | n* | Stage | ILD type | Surgery (pneumonectomy/lobectomy/< lobe) | Survival | AE |
|---|---|---|---|---|---|---|
| Kumar, Gen Thor Cardio Surg 2003 | 22/951 | ND | Histology: UIP, n=16NSIP, n=6 (including 3 CTD¥) | 6/17/1 | 3-year survival: 54% | θ: 21% vs. 3.7%;Pneumonectomy: 33%;Lobectomy: 17% |
| Chiyo, J Thoracic Cardiovasc Surg 2003 | 36/931 | I, n=10; II, n=4; III, n=18; IV, n=3 | IPF, n=26; Pneumoconiosis, n=5;CTD, n=5 | ND | 5-year survival: 35.6% vs. 62.5% | θ: 25% vs. 2% |
| Iyoda, Exp Therapeutic Med 2011 | 22 | I, n=19; II, n=2; III, n=1 | ND | 0/17/5 | ? | 22.7% |
| Chida, Ann Thor Cardiovasc Surg 2012 | 52/443 | ND | ND | ND | ND | 11.5% |
| Yano, Inter Cardiovasc Thor Surg 2012 | 62 | IA, n=24 | CT-scan: IPF, n=7; NSIPf, n=25 | 0/44/18 | 1-year survival: 83.8%; 3-year survival: 60.3%; 5-year survival: 55.7% | 9.7% |
| IB, n=13; IIA, n=7; IIB, n=9; IIIA, n=8; IV, n=1 | NSIPc, n=16;Unclassifiable, n=14 | |||||
| Voltolini, Eur J Cardio Thoracic Surg 2013 | 37/775 | I, n=16; II, n=10; III, n=7; IV, n=3; | Histology: Pneumoconiosis, n=18; IPF, n=11; NSIP, n=8 (including 4 CTD) | 4/30/3 | 5-year survival: 52% vs. 63%; overall survival: 29±5.4 vs. 47±4.2 months | θ: 13.5% vs. 2.3% |
| Sato, J Thorac Cardiovasc Surg 2014 | 1,763 | I, n=1,028; II, n=311; III, n=358; IV, n=34 | CT-scan:UIP, n=1,300; Non-UIP, n=463 | 33/1,297γ/425 | 9.3% | |
| Sato, J Thorac Cardiovasc Surg 2015 | 1,763 | idem | idem | 5-year survival; FVC >80%: 43.8%; FVC ≤80%: 20.8%; stage IA: 59%; FVC >80%: 64.3%; FVC ≤80%: 20%; wedge: 33.2%; segment: 61%; lobe: 68.4%; stage IB: 42%; stage IIA: 43%; stage IIB: 29%; stage IIIA: 25%; stage IIIB: 17%; stage IV: 17% | ||
| Omori, Ann Thor Surg 2015 | 103 | I, n=51; II, n=27; III, n=25 | IPF, n=46; non-IPF, n=57 | 2/79/22 | 5-year survival: IPF: 22.1%; non-IPF: 53.2% | IPF: 17.5%; Non-IPF: 8.7% |
| Sato, Ann Thorac Cardiovasc Surg 2016 | 100 | I, n=59; II, n=16; III, n=20; IV, n=5 | IPF, n=45; CPFE, n=55 | 0/62/38 | 5-year survival: CPFE: 24.9%; IPF: 36.8% | CPFE: 5.5%; IPF: 6.7% |
| Hata, Ann Thor Surg 2016 | 104/1,160 | IA, n=22; IB, n=41; IIA, n=13; IIB, n=8; IIIA, n=12; IV, n=8 | IPF, n=77; CTD-ILD, n=10; NSIP, n=2; OP, n=1; Smoker ILD, n=2; NA, n=12 | 1/73/30 | 5-year survival; stage IA: 70.3% vs. 93.9% | NA |
| Sekihara, Interact Cardiovasc Thorac Surg 2017 | 106/1,948 | I, n=62; II, n=31; III, n=13 | CT-scan: UIP, n=79; non-UIP, n=27 | 3/89/14 | 5-year survival: 40.4% vs. 72%; stage I:44% vs. 84.6% | 4% |
Prognosis of operated NSCLCs
The survival of operated LC patients with concomitant ILD was inferior to that of those without ILD. A large Japanese surgical cohort compared the prognosis of 1,763 LC-ILD to that of LC registry patients (91,92). The 5-year survival rates for stage IA, IB, IIA, IIB, IIIA, IIIB, and IV were: 59% vs. 86.8%; 42% vs. 73.9%; 43% vs. 61.6%; 29% vs. 49.8%; 25% vs. 40.9%; 17% vs. 27.8%; 17% vs. 27.9%, respectively. Other authors compared 5-year survival rates of patients with and without ILD in three series for stage IA (65,69) and I (93), as follows: 54.2% vs. 88.3%, 70.3% vs. 93.9%, 61.6% vs. 83% (65,93), respectively. In three other studies not differenciating LC staging, 5-year survival rates were: 52% vs. 63%; 35.6% vs. 62.5%; 37.5% vs. 72.5% (67,68,94).
Mortality attributable to ILD-related complications (see below)
Postoperative complication rates were in the 6.2–32% range, respiratory-related mortality being higher in patients with ILD. A Korean study comparing 33 LC-IPF to 66 LC patients reported respiratory mortality rates of 21% vs. 3% (94).
Cancer-related mortality
In 1,763 Japanese LC-ILD patients, 50.2% of deaths were related to cancer, 26.8% to respiratory aggravation, and 23% to another cause (91). In the Saito study comparing 28 LC-ILD to 322 LC patients, deaths were cancer-related in 17.9% vs. 3.7% in controls (65).
Second cancer after first intervention was more common, affecting 36% of cases in one series (95). In the Yano study, limited resections for small-sized tumors were associated with a 2.78-times higher recurrence risk in ILD (96). Of the aforementioned 1,763 operated LC-ILD cases, 425 (24.1%) were treated using infra-lobar resection. AE-related death risk was significantly inferior in patients treated using wedge surgery than those undergoing lobectomy, yet with cancer-related death risk significantly increased (91).
Post-operative complications and AE
NSCLC surgery causes prolonged hospitalization and increased morbidity due to pneumopathy, prolonged drainage, and postoperative AEs (65-67). In the Sato study, CPFE (n=55) was associated with more cardio-pulmonary complications compared to IPF without emphysema (n=45), without between-group mortality differences (97).
Postoperative AEs consisted of respiratory deterioration and parenchymal opacities, without infectious or cardiac causes. In the operated Japanese LC-ILD series (n=1,763), the AE rate was 9.3%, with 43.9% mortality. AEs mainly occurred on day 10 post-surgery (98), in line with previously reported data (99). AEs may also occur in infra-clinical or infra-radiological ILD (63,100), with several risk factors identified (Table 1).
Table 1. Risk factors for postoperative exacerbation.
| Patient-related |
|---|
| Gender, age |
| FVC |
| UIP on radiography |
| History of AE |
| Preop. corticosteroids |
| KL-6 |
| Surgery-related |
| Resection volume |
| Duration of surgery |
| O2 concentration |
| Fluid intake/loss balance |
Patient-related risk factor
Age >75 years (101), IPF versus other ILDs (65,98,102), honeycomb pattern on CT (103,104), fibrosis in the non-operated lung (105), and functional limitation were risk factors for post-operative AEs (66,68,98). In the Voltolini study, patients with FVC >90% did not exhibit post-operative acute respiratory insufficiency (68). A Japanese study described no early AEs among 21 operated LC-IPF patients, possibly due to highly-selected patients (95).
The other risk factors included elevated KL6 or CRP levels, preoperative corticosteroids in IPF, and hyperfixation on PET (98,106-108).
Surgery-related risk factor
Increased post-operative AE risk was associated with >4-hour-long operations, high pre-operative oxygen concentrations (104), parenchyma resection extent (66,98), and relevant volemia variations (107).
Risk scoring
Using a risk scoring system should enable surgery to be performed more often in LC-ILD (109), with patients classed as low-risk if a <10% AE probability, intermediate risk if 10–25%, and high-risk if >25%.
AE prevention
Iwata et al. retrospectively compared 31 patients receiving preoperative pirfenidone versus 19 untreated, reporting one AE with pirfenidone versus four without (110). A phase II study assessed pirfenidone’s pre-operative efficacy in 43 LC patients, 39 exhibiting IPF (111,112), with AEs observed in two IPF cases (5.1%). Potential benefits of systematic post-operative corticotherapy were suggested from 22 operated patients, with 3/4 patients without corticotherapy exhibiting AEs versus 2/18 corticoid-treated subjects (101).
Pooled data analysis in INPULSIS studies demonstrated a decrease in AEs under nintedanib (7.7% vs. 14.9%) in patients IPF with FVC <70% (113). Perioperative CT could enable both infra-clinical AE detection and early treatment (105).
Inoperable localized NSCLC
Radiotherapy is contra-indicated in severe ILD, producing radiation pneumonitis (RP) rates of up to 43% (95). Ozawa et al. reported RP in 9/651 patients undergoing radiotherapy, extended ILD affecting >10% of lung parenchyma identified as sole independent risk factor (82).
Radiotherapy in infra-clinical ILD
Infraclinical ILD is considered an RP risk factor. Evaluating thoracic radiotherapy for LC (n=40) or esophageal cancer (n=19), Yamagushi et al. reported RP in 36% of cases with infraclinical ILD versus 13% without (114), as confirmed by another study (115).
Stereotaxic radiotherapy
Stereotaxic radiotherapy spares healthy tissues surrounding the tumor, with yet isolated fatal RP cases reported (116,117). Yamaguschi et al. assessed RP risk in 100 patients, 16 with infraclinical ILD (118). Though grade 2–5 RP risk was not enhanced, 3/16 infraclinical ILD patients versus 0/84 controls displayed extensive pneumonitis beyond the radiation field. In another study involving stage I NSCLC patients, ILD on CT (n=20/157) was an independent grade >2 RP risk factor (119).
Proton therapy
Proton therapy was evaluated in 16 ILD patients, with disappointing results (120).
Stage IV NSCLC
Like for surgery, some patients with ILD seem to be too frail to receive chemotherapy. In four published series, 20–25% patients were deemed unable to undergo chemotherapy (37,121-123). Kashiwabara et al., however, reported that patients solely receiving comfort care exhibited poorer median OS than those undergoing chemotherapy (2.6 vs. 10.9 months) (83).
Efficacy of first-line therapies
All studies assessing first-line therapy efficacy in LC-IPF/ILD are listed in Tables S5,S6. A recent meta-analysis covered seven studies involving 251 patients with stage IIIA, IIIB, or IV LC-ILD. Response and control rates were 41.3% and 77.7%, respectively, with a PFS of 4.4 months and OS of 8.5 months (124).
Table S5. Efficacy of chemotherapy in various interstitial lung diseases.
| Citations | n | Histology, n | Stage | Chemotherapy | ILD | Response, % | Control, % | PFS, months | Survival, months | 1-year survival, % | AE, n (1st line) | Hematological complications | 2nd line (n) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AD | SCC | Other | |||||||||||||
| Shukuya, Anticancer Res 2010 | 15 | 10 | 5 | IIIA/IIIB/IV/POR: 1/5/7/2 | Carbo (AUC5)-Pacli, n=7;Carbo (AUC2)-Pacli, n=8 | IPF, n=4; NSIP, n=9; DIP, n=1; CTD, n=1 | 33 | 53 | 2.5 | 7 | 29 | 4 | ND | ||
| Minegishi, Lung Cancer 2011 | 18 | 6 | 7 | IIIA/IIIB/IV/POR: 2/3/13 | Carbo-Pacli | IPF, n=6; Non-IPF, n=12 | 61 | 83 | 5.3 | 10.6 | 22 | 4 [1] | 33% | 13 | |
| Okuda, Anticancer Res 2012 | 19 | 10 | 7 | 2 | IIIA/IIIB/IV/POR: 4/6/9/5 | Carbo-Vino, n=9; CDDP-Vino, n=10 | IPF, n=16; Other, n=3 | 42.1 | 73.7 | 4.4 | 7.4 | 36.8 | 15.8 [15.8] | 63.2 | 6 |
| Kinoshita, Oncol Lett 2012 | 22 | 11 | 7 | 4 | IIIA/IIIB/IV/POR: 1/6/15 | Carbo-Pacli, n=19; CDDP-Vino, n=2CDDP-Doce, n=1 | IPF and NSIPi | 36.4 | 77.3 | 3.2 | 5.4 | ND | 3 [3] | ND | ND |
| Choi, Cancer Chemoth Pharm 2014 | 52 | 32 | 13 | 8 | ND | Carbo, n=33; CDDP, n=19;GEM, n=39; PEM, n=13 | IILD, n=42; Pneumoconiosis, n=5; CTD, n=5 | 42.3 | 78.8 | 5.4 | 7.9 | 7 [3] | ND | 35 | |
| Shimizu, Cancer Chemoth Pharm 2014¶ | 21 | 21 | IIIA/IIIB/IV: 3/2/16 | Carbo-Pacli, n=11;Carbo-Pacli-Beva, n=10 | CT-scan: UIP, n=5;Non-UIP, n=16 | 27 vs. 40 (ns) | 90 vs. 82 | 4.4 vs. 5.5 | 9.7 vs. 16.1 | 1 (placebo group) | ND | ? | |||
| Enomoto, Anticancer Res 2015 | 25 | 21 | 0 | 4 | IIIA/IIIB/IV/POR: 1/3/16/5 | Carbo-Pacli-Beva | IPF, n=13; Non-IPF, n=12 | 72 | 7.2 | 8.5 | 40 | 3 including 1 during maintenance Beva | 72% | ||
| Watanabee, Anticancer Res 2015 | 67 | 26 | 21 | 20 | IIIB/IV/POR: 20/42/5 | CDDP-Vino | ND | 34.3 | 73.1 | 3.7 | 7.4 | 22.4 | 7 [7] | 60% | 34 |
| Kenmotsu, Cancer Chemoth Pharm 2015 | 104 | 50 | 47 | 7 | III A and B/IV/POR: 41/55/5 | Carbo-, n=85; (Pacli, n=63; Pacli-Beva, n=5; S1, n=7; GEM, n=6; other, n=4); CDDP-, n=19 (Vino, n=6; Doce, n=4; S1, n=3; Eto, n=3; other, n=3) | CT-scan: confirmed UIP, n=70; poss. or incompat. UIP, n=34 | 38 | 80 | 4.8 | 9.9 | 26 (9 including 2 deaths) | 57 (55%) | ||
| Kashiwabara, anticancer Research 2015 | 14/109 | ND | ND | ND | ? | ND | ND | ND | ND | ND | 10.6 vs. 27.9 | ND |
Table S6. Small-cell lung cancers treated with chemotherapy.
| Citations | n | Localized | Disseminated | Chemotherapy | IPF/other | Response, % | Control, % | PFS, months | Survival, months | AE, n (1st line) | Hematological complications | 2nd line (n) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Minegishi, JTO 2011 | 17 | 8 | 9 | Carbo/Eto, n=17 | 8/9 | 88.2 | 94 | 5.5 | 8.7 | 3 [1] | 88.2 | 8 |
| Watanabee, Int J Clin Oncol 2013 | 11 | 11 | Carbo/Eto, n= 8; CDDP/Eto, n=3 | 11/0 | 62.5 | 75 | 4.7 | 7 | 4 [3] | 72.7 | 5 | |
| Yoshida, Anticancer Res 2013 | 52 | 29 | 23 | Carbo/Eto, n=22; CDDP/Eto, n=30 | ND | 69 | ND | 4.5 | 9.4 | 6 [1] | ND | 33 |
Few studies compared chemotherapy efficacy and prognosis of LC-ILD versus LC. ILD seems to be an independent poor prognostic factor, with HRs of 2.33 for PFS and 2.87 for OS (123).
Carboplatin-paclitaxel is the most common regimen (Tables S5,S7), with bevacizumab at times employed in combination with carboplatin-paclitaxel (125), offering similar efficacy without more toxicity (126).
Table S7. Efficacy of chemotherapy in idiopathic pulmonary fibrosis.
| Citations | n | Histology, n | Stage | Chemo-therapy | ILD | Response, % | Control, % | PFS, months | Survival, months | 1-year survival, % | AE, n (1st line) | Hematological complications | 2nd line (n) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AD | SCC | Other | |||||||||||||
| Watanabee, Respiration 2012 or 2013 | 21 | ND | ND | ND | IIIB/IV: 11/10 | Carbo+Pacli, n=16; Carbo+Doce, n=3; Vino, n=2 | IPF | 42.9 | 80.9 | 5.4 | 11.4 | 28.6 | 10 [9] | 38.1% | |
| Kanaji, J Cancer Res Clin Oncol 2016¶ | 34/218 | 12 | 12 | 10 | IIIB/IV: 3/31 | ND | IPF | 31 vs. 55 | 53 vs. 87 | 92 vs. 196 days | 223 vs. 539 days | ND | 6 [6] (17.6%) | ND | ND |
Several hypotheses could explain the poorer survival in ILD despite similar response rates, such as poor prognostic factors (i.e., age, male predominance), lower proportion of ADC, lower oncogenic addictions, and increased risk of chemotherapy-induced AE-ILD.
Second-line chemotherapy
In published series, 31.6–72.2% received second-line chemotherapy (Tables S5,S7). In Kashiwabara’s study, LC-ILD patients underwent a median of one line versus two for LC patients (83). Investigating second-line docetaxel therapy in stage IIIB–IV (n=35) patients, Watanabe reported an 8.6% response rate, 37.5% control rate, 1.6-month PFS, and 5.1-month OS (127). Choi et al. reported a 1.2-month PFS and 4-month OS following second-line chemotherapy (128). Nintedanib, a tyrosine kinase inhibitor (TKI), was demonstrated efficient in both IPF and NSCLC progressing after first-line therapy (129,130). In this last setting, a subgroup analysis revealed that nintedanib’s benefits on OS were limited to the ADC subgroup (130).
Chemotherapy-induced ILD or chemotherapy-induced increased AE-ILD risk?
Chemotherapies may provoke drug-induced interstitial pneumonitis, with pre-existing ILD as known risk factor. Two retrospective studies revealed drug-induced ILD to be more common with underlying ILD, 29% vs. 10% and 22.4% vs. 4.6%, respectively (83,131). Sakurada et al. reporting a 5.38 OR in pre-existing ILD (n=161) (131).
Drug-induced ILD responsible for aggravating pre-existing ILD could be considered as chemotherapy-induced AE, one meta-analysis reporting an 8.47% AE incidence.
Patient-related risk factors
The ILD subtype appears paramount, with IPF and radiological UIP pattern identified as risk factors. In two series, AE incidence was 22% vs. 8% and 50% vs. 18% (132) in patients with radiological UIP pattern versus without, respectively. The Minegishi cohort reported a 3.19 RR of AE (P=0.13) in patients with IPF versus other ILDs (133,134), with decreased FVC as an independent risk factor for drug-induced ILD (135).
Treatment-related risk factors
In Kenmotsu’s series, vinorelbin and paclitaxel were rarely responsible drug-induced ILDs. Patients receiving cisplatin or carboplatin exhibited similar AE incidence (10% vs. 13%), which was higher for docetaxel (28%) and etoposide (24%), whilst lower for paclitaxel (3%) (132). A trial is currently ongoing evaluating nintedanib’s role in preventing AEs induced by carboplatin plus Nab-paclitaxel regimens (136).
Similar results were reported with another series (72) involving 53 LC-ILD patients undergoing 96 chemotherapies: docetaxel caused more AEs than others (18.4% vs. 5.2%), while AEs were similar with or without pemetrexed (13.3% vs. 9.9%), whereas paclitaxel caused no aggravation. Pemetrexed/cisplatin is the preferred first-line regime for extended ADC treatment versus gemcitabine for SCC, with little data available for LC-ILD. In 459 consecutive patients, Sakurada et al. reported 33 chemotherapy-induced ILDs, 11 with underlying ILD. Of 88 pemetrexed-treated patients, no drug-induced ILD occurred (131). For stage IV NSCLC-ILD, of 39 patients receiving first-line cisplatine/gemcitabine, one AE was noted, versus two in 13 receiving cisplatin/pemetrexed (128).
TKI gefitinib, erlotinib, and afatinib are indicated after detecting activating EGFR gene mutations. In the general population, TKI-EGFR carries the risk of drug-induced ILD, especially for gefitinib versus erlotinib (137). Underlying LC-ILD is an independent risk factor of drug-induced ILD (OR: 2.89) (138).
Crizotinib, a TKI used in LC patients with ALK rearrangements, was responsible for drug-induced ILD in 2.4% of cases, with one fatal AE on preexisting ILD reported (139,140). Nivolumab, a novel agent that binds to the PD1 receptor, is used in LC, and particularly SCC. Two studies (phase I and II) reported 7% and 5% drug-induced ILD, respectively (141,142), while a retrospective study revealed nivolumab-induced ILD in 43/915 (5%) (143). A pilot trial of nivolumab in six mild ILD patients did not reveal any pulmonary complication (144).
Small-cell LC
Table S6 lists data from three series comprising 80 patients xith small-cell LC-ILD, 27 localized-stage (133,145,146). Carboplatin-etoposide chemotherapy was administered to 47 patients, cisplatin-etoposide to 33. Tumor response and control rates were 62.5%, 88.2%, 75% and 94%, respectively, with 4.5–5.5-month PFS, 7–9-month OS. Grade 3 and 4 hematological complication rates from two studies were 72.7% and 88.2% (133,145).
Concerning second-line chemotherapy, the Fujimoto series comprised 23 patients receiving paclitaxel-carboplatin, paclitaxel alone, or topotecan (147), resulting in response and control rates of 22% and 52%, respectively, with a median PFS and OS of 2.1- and 7.1-month, comparable to ILD-free patients, with an AE rate of 13%. Enomoto et al. evaluated topetecan safety as second-line in 11 patients and third-line in 12, with 5 (21.7%) exhibiting AEs after last topotecan injection (148).
Conclusions
LC-ILD association is common in clinical practice. While patient prognosis appaers poorer than with LC alone, most therapeutic regimens can be applied, with therapeutic management properly adapted taking AE-ILD risk into account.
Our review emphasizes the multidisciplinary relevance of LC-ILD management. Surgery and radiotherapy decisions must consider patients’ limited respiratory function and risk of post-operative/-radiotherapy acute exacerbation (AE)-ILD. Risk/benefit assessments of early AE-induced mortality versus late LC-recurrence must be performed case-by-case. Chemotherapy for advanced-disease raises the issue of feasibility and AE-ILD risk, with the exact role of ILD and LC therapies be better defined.
Studies are warranted to further our understanding of underlying cancer development in the fibrotic areas, with prospective clinical studies conducted to better define the optimal therapeutic strategies.
Acknowledgements
We thank Simon Cadranel for his graphic contribution.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Raghu G, Nyberg F, Morgan G. The epidemiology of interstitial lung disease and its association with lung cancer. Br J Cancer 2004;91 Suppl 2:S3-10. 10.1038/sj.bjc.6602061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ma Y, Seneviratne CK, Koss M. Idiopathic pulmonary fibrosis and malignancy. Curr Opin Pulm Med 2001;7:278-82. 10.1097/00063198-200109000-00005 [DOI] [PubMed] [Google Scholar]
- 3.Karampitsakos T, Tzilas V, Tringidou R, et al. Lung cancer in patients with idiopathic pulmonary fibrosis. Pulm Pharmacol Ther 2017;45:1-10. 10.1016/j.pupt.2017.03.016 [DOI] [PubMed] [Google Scholar]
- 4.King TE, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet 2011;378:1949-61. 10.1016/S0140-6736(11)60052-4 [DOI] [PubMed] [Google Scholar]
- 5.Malhotra J, Malvezzi M, Negri E, et al. Risk factors for lung cancer worldwide. Eur Respir J 2016;48:889-902. 10.1183/13993003.00359-2016 [DOI] [PubMed] [Google Scholar]
- 6.Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013;188:733-48. 10.1164/rccm.201308-1483ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Onishi A, Sugiyama D, Kumagai S, et al. Cancer incidence in systemic sclerosis: meta-analysis of population-based cohort studies. Arthritis Rheum 2013;65:1913-21. 10.1002/art.37969 [DOI] [PubMed] [Google Scholar]
- 8.Kawasaki H, Ogura T, Yokose T, et al. p53 gene alteration in atypical epithelial lesions and carcinoma in patients with idiopathic pulmonary fibrosis. Hum Pathol 2001;32:1043-9. 10.1053/hupa.2001.28246 [DOI] [PubMed] [Google Scholar]
- 9.Hironaka M, Fukayama M. Pulmonary fibrosis and lung carcinoma: a comparative study of metaplastic epithelia in honeycombed areas of usual interstitial pneumonia with or without lung carcinoma. Pathol Int 1999;49:1060-6. 10.1046/j.1440-1827.1999.00989.x [DOI] [PubMed] [Google Scholar]
- 10.Khan KA, Kennedy MP, Moore E, et al. Radiological characteristics, histological features and clinical outcomes of lung cancer patients with coexistent idiopathic pulmonary fibrosis. Lung 2015;193:71-7. 10.1007/s00408-014-9664-8 [DOI] [PubMed] [Google Scholar]
- 11.Korfei M, Skwarna S, Henneke I, et al. Aberrant expression and activity of histone deacetylases in sporadic idiopathic pulmonary fibrosis. Thorax 2015;70:1022-32. 10.1136/thoraxjnl-2014-206411 [DOI] [PubMed] [Google Scholar]
- 12.Chen Z, Liu M, Li L, et al. Involvement of the Warburg effect in non-tumor diseases processes. J Cell Physiol 2018;233:2839-49. 10.1002/jcp.25998 [DOI] [PubMed] [Google Scholar]
- 13.Choe C, Shin YS, Kim C, et al. Crosstalk with cancer-associated fibroblasts induces resistance of non-small cell lung cancer cells to epidermal growth factor receptor tyrosine kinase inhibition. Onco Targets Ther 2015;8:3665-78. 10.2147/OTT.S89659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu XQ, Kiefl R, Roskopf C, et al. Interactions among Lung Cancer Cells, Fibroblasts, and Macrophages in 3D Co-Cultures and the Impact on MMP-1 and VEGF Expression. PloS One 2016;11:e0156268. 10.1371/journal.pone.0156268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pirozzi G, Tirino V, Camerlingo R, et al. Epithelial to mesenchymal transition by TGFβ-1 induction increases stemness characteristics in primary non small cell lung cancer cell line. PloS One 2011;6:e21548. 10.1371/journal.pone.0021548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schottenfeld D, Beebe-Dimmer J. Chronic inflammation: a common and important factor in the pathogenesis of neoplasia. CA Cancer J Clin 2006;56:69-83. 10.3322/canjclin.56.2.69 [DOI] [PubMed] [Google Scholar]
- 17.Scrimini S, Pons J, Agustí A, et al. Expansion of myeloid-derived suppressor cells in chronic obstructive pulmonary disease and lung cancer: potential link between inflammation and cancer. Cancer Immunol Immunother CII 2015;64:1261-70. 10.1007/s00262-015-1737-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Demopoulos K, Arvanitis DA, Vassilakis DA, et al. MYCL1, FHIT, SPARC, p16(INK4) and TP53 genes associated to lung cancer in idiopathic pulmonary fibrosis. J Cell Mol Med 2002;6:215-22. 10.1111/j.1582-4934.2002.tb00188.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vancheri C, Failla M, Crimi N, et al. Idiopathic pulmonary fibrosis: a disease with similarities and links to cancer biology. Eur Respir J 2010;35:496-504. 10.1183/09031936.00077309 [DOI] [PubMed] [Google Scholar]
- 20.Caraci F, Gili E, Calafiore M, et al. TGF-beta1 targets the GSK-3beta/beta-catenin pathway via ERK activation in the transition of human lung fibroblasts into myofibroblasts. Pharmacol Res 2008;57:274-82. 10.1016/j.phrs.2008.02.001 [DOI] [PubMed] [Google Scholar]
- 21.Mazieres J, He B, You L, et al. Wnt signaling in lung cancer. Cancer Lett 2005;222:1-10. 10.1016/j.canlet.2004.08.040 [DOI] [PubMed] [Google Scholar]
- 22.Takenaka K, Gemma A, Yoshimura A, et al. Reduced transcription of the Smad4 gene during pulmonary carcinogenesis in idiopathic pulmonary fibrosis. Mol Med Rep 2009;2:73-80. [DOI] [PubMed] [Google Scholar]
- 23.Du G, Jin L, Han X, et al. Naringenin: a potential immunomodulator for inhibiting lung fibrosis and metastasis. Cancer Res 2009;69:3205-12. 10.1158/0008-5472.CAN-08-3393 [DOI] [PubMed] [Google Scholar]
- 24.Maitra M, Cano CA, Garcia CK. Mutant surfactant A2 proteins associated with familial pulmonary fibrosis and lung cancer induce TGF-β1 secretion. Proc Natl Acad Sci U S A 2012;109:21064-9. 10.1073/pnas.1217069110 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Wang Y, Kuan PJ, Xing C, et al. Genetic defects in surfactant protein A2 are associated with pulmonary fibrosis and lung cancer. Am J Hum Genet 2009;84:52-9. 10.1016/j.ajhg.2008.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nathan N, Giraud V, Picard C, et al. Germline SFTPA1 mutation in familial idiopathic interstitial pneumonia and lung cancer. Hum Mol Genet 2016;25:1457-67. 10.1093/hmg/ddw014 [DOI] [PubMed] [Google Scholar]
- 27.Terasaki Y, Akuta T, Terasaki M, et al. Guanine nitration in idiopathic pulmonary fibrosis and its implication for carcinogenesis. Am J Respir Crit Care Med 2006;174:665-73. 10.1164/rccm.200510-1580OC [DOI] [PubMed] [Google Scholar]
- 28.Carpagnano GE, Lacedonia D, Crisetti E, et al. New panel of microsatellite alterations detectable in the EBC for lung cancer prognosis. J Cancer 2016;7:2266-9. 10.7150/jca.15921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Antoniou KM, Margaritopoulos GA, Soufla G, et al. Expression analysis of Akt and MAPK signaling pathways in lung tissue of patients with idiopathic pulmonary fibrosis (IPF). J Recept Signal Transduct Res 2010;30:262-9. 10.3109/10799893.2010.489227 [DOI] [PubMed] [Google Scholar]
- 30.Navarro S, Driscoll B. Regeneration of the Aging Lung: A Mini-Review. Gerontology 2017;63:270-80. 10.1159/000451081 [DOI] [PubMed] [Google Scholar]
- 31.Selman M, López-Otín C, Pardo A. Age-driven developmental drift in the pathogenesis of idiopathic pulmonary fibrosis. Eur Respir J 2016;48:538-52. 10.1183/13993003.00398-2016 [DOI] [PubMed] [Google Scholar]
- 32.Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol 2013;75:685-705. 10.1146/annurev-physiol-030212-183653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mizuno S, Takiguchi Y, Fujikawa A, et al. Chronic obstructive pulmonary disease and interstitial lung disease in patients with lung cancer. Respirology 2009;14:377-83. 10.1111/j.1440-1843.2008.01477.x [DOI] [PubMed] [Google Scholar]
- 34.Hubbard R, Venn A, Lewis S, et al. Lung cancer and cryptogenic fibrosing alveolitis. A population-based cohort study. Am J Respir Crit Care Med 2000;161:5-8. 10.1164/ajrccm.161.1.9906062 [DOI] [PubMed] [Google Scholar]
- 35.Le Jeune I, Gribbin J, West J, et al. The incidence of cancer in patients with idiopathic pulmonary fibrosis and sarcoidosis in the UK. Respir Med 2007;101:2534-40. 10.1016/j.rmed.2007.07.012 [DOI] [PubMed] [Google Scholar]
- 36.Ozawa Y, Suda T, Naito T, et al. Cumulative incidence of and predictive factors for lung cancer in IPF. Respirology 2009;14:723-8. 10.1111/j.1440-1843.2009.01547.x [DOI] [PubMed] [Google Scholar]
- 37.Tomassetti S, Gurioli C, Ryu JH, et al. The impact of lung cancer on survival of idiopathic pulmonary fibrosis. Chest 2015;147:157-64. 10.1378/chest.14-0359 [DOI] [PubMed] [Google Scholar]
- 38.Park J, Kim DS, Shim TS, et al. Lung cancer in patients with idiopathic pulmonary fibrosis. Eur Respir J 2001;17:1216-9. 10.1183/09031936.01.99055301 [DOI] [PubMed] [Google Scholar]
- 39.Kawasaki H, Nagai K, Yoshida J, et al. Postoperative morbidity, mortality, and survival in lung cancer associated with idiopathic pulmonary fibrosis. J Surg Oncol 2002;81:33-7. 10.1002/jso.10145 [DOI] [PubMed] [Google Scholar]
- 40.Miyazaki K, Satoh H, Kurishima K, et al. Impact of interstitial lung disease on survival for patients with non-small cell lung cancer. Anticancer Res 2009;29:2671-4. [PubMed] [Google Scholar]
- 41.Kawasaki H, Nagai K, Yokose T, et al. Clinicopathological characteristics of surgically resected lung cancer associated with idiopathic pulmonary fibrosis. J Surg Oncol 2001;76:53-7. [DOI] [PubMed] [Google Scholar]
- 42.Turner-Warwick M, Lebowitz M, Burrows B, et al. Cryptogenic fibrosing alveolitis and lung cancer. Thorax 1980;35:496-9. 10.1136/thx.35.7.496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Girard N, Marchand-Adam S, Naccache JM, et al. Lung cancer in combined pulmonary fibrosis and emphysema: a series of 47 Western patients. J Thorac Oncol 2014;9:1162-70. 10.1097/JTO.0000000000000209 [DOI] [PubMed] [Google Scholar]
- 44.Kitaguchi Y, Fujimoto K, Hanaoka M, et al. Clinical characteristics of combined pulmonary fibrosis and emphysema. Respirology 2010;15:265-71. 10.1111/j.1440-1843.2009.01676.x [DOI] [PubMed] [Google Scholar]
- 45.Enomoto Y, Inui N, Yoshimura K, et al. Lung cancer development in patients with connective tissue disease-related interstitial lung disease: A retrospective observational study. Medicine (Baltimore) 2016;95:e5716. 10.1097/MD.0000000000005716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hashimoto A, Arinuma Y, Nagai T, et al. Incidence and the risk factor of malignancy in Japanese patients with systemic sclerosis. Intern Med 2012;51:1683-8. 10.2169/internalmedicine.51.7686 [DOI] [PubMed] [Google Scholar]
- 47.Hill CL, Nguyen AM, Roder D, et al. Risk of cancer in patients with scleroderma: a population based cohort study. Ann Rheum Dis 2003;62:728-31. 10.1136/ard.62.8.728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosenthal AK, McLaughlin JK, Gridley G, et al. Incidence of cancer among patients with systemic sclerosis. Cancer 1995;76:910-4. [DOI] [PubMed] [Google Scholar]
- 49.Smitten AL, Simon TA, Hochberg MC, et al. A meta-analysis of the incidence of malignancy in adult patients with rheumatoid arthritis. Arthritis Res Ther 2008;10:R45. 10.1186/ar2404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Archontogeorgis K, Steiropoulos P, Tzouvelekis A, et al. Lung cancer and interstitial lung diseases: a systematic review. Pulm Med 2012;2012:315918. 10.1155/2012/315918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lu X, Yang H, Shu X, et al. Factors predicting malignancy in patients with polymyositis and dermatomyostis: a systematic review and meta-analysis. PloS One 2014;9:e94128. 10.1371/journal.pone.0094128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.So MW, Koo BS, Kim YG, et al. Idiopathic inflammatory myopathy associated with malignancy: a retrospective cohort of 151 Korean patients with dermatomyositis and polymyositis. J Rheumatol 2011;38:2432-5. 10.3899/jrheum.110320 [DOI] [PubMed] [Google Scholar]
- 53.Wang J, Guo G, Chen G, et al. Meta-analysis of the association of dermatomyositis and polymyositis with cancer. Br J Dermatol 2013;169:838-47. 10.1111/bjd.12564 [DOI] [PubMed] [Google Scholar]
- 54.Kurihara N, Wada O. Silicosis and smoking strongly increase lung cancer risk in silica-exposed workers. Ind Health 2004;42:303-14. 10.2486/indhealth.42.303 [DOI] [PubMed] [Google Scholar]
- 55.Henderson DW, Rödelsperger K, Woitowitz HJ, et al. After Helsinki: a multidisciplinary review of the relationship between asbestos exposure and lung cancer, with emphasis on studies published during 1997-2004. Pathology 2004;36:517-50. 10.1080/00313020400010955 [DOI] [PubMed] [Google Scholar]
- 56.Hessel PA, Gamble JF, McDonald JC. Asbestos, asbestosis, and lung cancer: a critical assessment of the epidemiological evidence. Thorax 2005;60:433-6. 10.1136/thx.2004.037267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kuramochi J, Inase N, Miyazaki Y, et al. Lung cancer in chronic hypersensitivity pneumonitis. Respiration 2011;82:263-7. 10.1159/000327738 [DOI] [PubMed] [Google Scholar]
- 58.Bonifazi M, Bravi F, Gasparini S, et al. Sarcoidosis and cancer risk: systematic review and meta-analysis of observational studies. Chest 2015;147:778-91. 10.1378/chest.14-1475 [DOI] [PubMed] [Google Scholar]
- 59.Sadoun D, Vaylet F, Valeyre D, et al. Bronchogenic carcinoma in patients with pulmonary histiocytosis X. Chest 1992;101:1610-3. 10.1378/chest.101.6.1610 [DOI] [PubMed] [Google Scholar]
- 60.Vassallo R, Ryu JH, Schroeder DR, et al. Clinical outcomes of pulmonary Langerhans’-cell histiocytosis in adults. N Engl J Med 2002;346:484-90. 10.1056/NEJMoa012087 [DOI] [PubMed] [Google Scholar]
- 61.Harris JM, Johnston IDA, Rudd R, et al. Cryptogenic fibrosing alveolitis and lung cancer: the BTS study. Thorax 2010;65:70-6. 10.1136/thx.2009.121962 [DOI] [PubMed] [Google Scholar]
- 62.Kreuter M, Ehlers-Tenenbaum S, Palmowski K, et al. Impact of Comorbidities on Mortality in Patients with Idiopathic Pulmonary Fibrosis. PloS One 2016;11:e0151425. 10.1371/journal.pone.0151425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chida M, Ono S, Hoshikawa Y, et al. Subclinical idiopathic pulmonary fibrosis is also a risk factor of postoperative acute respiratory distress syndrome following thoracic surgery. Eur J Cardiothorac Surg 2008;34:878-81. 10.1016/j.ejcts.2008.07.028 [DOI] [PubMed] [Google Scholar]
- 64.Watanabe A, Miyajima M, Mishina T, et al. Surgical treatment for primary lung cancer combined with idiopathic pulmonary fibrosis. Gen Thorac Cardiovasc Surg 2013;61:254-61. 10.1007/s11748-012-0180-6 [DOI] [PubMed] [Google Scholar]
- 65.Saito Y, Kawai Y, Takahashi N, et al. Survival after surgery for pathologic stage IA non-small cell lung cancer associated with idiopathic pulmonary fibrosis. Ann Thorac Surg 2011;92:1812-7. 10.1016/j.athoracsur.2011.06.055 [DOI] [PubMed] [Google Scholar]
- 66.Kumar P, Goldstraw P, Yamada K, et al. Pulmonary fibrosis and lung cancer: risk and benefit analysis of pulmonary resection. J Thorac Cardiovasc Surg 2003;125:1321-7. 10.1016/S0022-5223(03)00028-X [DOI] [PubMed] [Google Scholar]
- 67.Chiyo M, Sekine Y, Iwata T, et al. Impact of interstitial lung disease on surgical morbidity and mortality for lung cancer: analyses of short-term and long-term outcomes. J Thorac Cardiovasc Surg 2003;126:1141-6. 10.1016/S0022-5223(03)00791-8 [DOI] [PubMed] [Google Scholar]
- 68.Voltolini L, Bongiolatti S, Luzzi L, et al. Impact of interstitial lung disease on short-term and long-term survival of patients undergoing surgery for non-small-cell lung cancer: analysis of risk factors. Eur J Cardiothorac Surg 2013;43:e17-23. 10.1093/ejcts/ezs560 [DOI] [PubMed] [Google Scholar]
- 69.Hata A, Suzuki H, Nakajima T, et al. Concomitant Interstitial Lung Disease Is a Risk Factor for Pleural Invasion in Lung Cancer. Ann Thorac Surg 2017;103:967-74. 10.1016/j.athoracsur.2016.08.074 [DOI] [PubMed] [Google Scholar]
- 70.Usui K, Tanai C, Tanaka Y, et al. The prevalence of pulmonary fibrosis combined with emphysema in patients with lung cancer. Respirology 2011;16:326-31. 10.1111/j.1440-1843.2010.01907.x [DOI] [PubMed] [Google Scholar]
- 71.Goto T, Maeshima A, Oyamada Y, et al. Idiopathic pulmonary fibrosis as a prognostic factor in non-small cell lung cancer. Int J Clin Oncol 2014;19:266-73. 10.1007/s10147-013-0566-1 [DOI] [PubMed] [Google Scholar]
- 72.Kanaji N, Tadokoro A, Kita N, et al. Impact of idiopathic pulmonary fibrosis on advanced non-small cell lung cancer survival. J Cancer Res Clin Oncol 2016;142:1855-65. 10.1007/s00432-016-2199-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kishi K, Homma S, Kurosaki A, et al. High-resolution computed tomography findings of lung cancer associated with idiopathic pulmonary fibrosis. J Comput Assist Tomogr 2006;30:95-9. 10.1097/01.rct.0000188650.66527.d2 [DOI] [PubMed] [Google Scholar]
- 74.Song DH, Choi IH, Ha SY, et al. Usual Interstitial Pneumonia with Lung Cancer: Clinicopathological Analysis of 43 Cases. Korean J Pathol 2014;48:10-6. 10.4132/KoreanJPathol.2014.48.1.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Oh SY, Kim MY, Kim JE, et al. Evolving Early Lung Cancers Detected During Follow-Up of Idiopathic Interstitial Pneumonia: Serial CT Features. AJR Am J Roentgenol 2015;204:1190-6. 10.2214/AJR.14.13587 [DOI] [PubMed] [Google Scholar]
- 76.Fujita J, Yamadori I, Namihira H, et al. Increased intensity of lung infiltrates at the side of lung cancer in patients with lung cancer associated with pulmonary fibrosis. Lung Cancer 1999;26:169-74. 10.1016/S0169-5002(99)00086-0 [DOI] [PubMed] [Google Scholar]
- 77.Fukui M, Takamochi K, Matsunaga T, et al. Risk of the preoperative underestimation of tumour size of lung cancer in patients with idiopathic interstitial pneumonias. Eur J Cardiothorac Surg 2016;50:428-32. 10.1093/ejcts/ezw065 [DOI] [PubMed] [Google Scholar]
- 78.Jeon TY, Lee KS, Yi CA, et al. Incremental value of PET/CT Over CT for mediastinal nodal staging of non-small cell lung cancer: Comparison between patients with and without idiopathic pulmonary fibrosis. AJR Am J Roentgenol 2010;195:370-6. 10.2214/AJR.09.3772 [DOI] [PubMed] [Google Scholar]
- 79.Mizushima Y, Kobayashi M. Clinical characteristics of synchronous multiple lung cancer associated with idiopathic pulmonary fibrosis. A review of Japanese cases. Chest 1995;108:1272-7. 10.1378/chest.108.5.1272 [DOI] [PubMed] [Google Scholar]
- 80.Oshikawa K, Sugiyama Y. Serum anti-p53 autoantibodies from patients with idiopathic pulmonary fibrosis associated with lung cancer. Respir Med 2000;94:1085-91. 10.1053/rmed.2000.0917 [DOI] [PubMed] [Google Scholar]
- 81.Goto T, Maeshima A, Akanabe K, et al. Acute exacerbation of idiopathic pulmonary fibrosis of microscopic usual interstitial pneumonia pattern after lung cancer surgery. Ann Thorac Cardiovasc Surg 2011;17:573-6. 10.5761/atcs.cr.10.01619 [DOI] [PubMed] [Google Scholar]
- 82.Ozawa Y, Abe T, Omae M, et al. Impact of Preexisting Interstitial Lung Disease on Acute, Extensive Radiation Pneumonitis: Retrospective Analysis of Patients with Lung Cancer. PloS One 2015;10:e0140437. 10.1371/journal.pone.0140437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kashiwabara K, Semba H, Fujii S, et al. Difference in benefit of chemotherapy between small cell lung cancer patients with interstitial pneumonia and patients with non-small cell lung cancer. Anticancer Res 2015;35:1065-71. [PubMed] [Google Scholar]
- 84.Usui K, Ushijima T, Tanaka Y, et al. The Frequency of Epidermal Growth Factor Receptor Mutation of Nonsmall Cell Lung Cancer according to the Underlying Pulmonary Diseases. Pulm Med 2011;2011:290132. 10.1155/2011/290132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sekine A, Tamura K, Satoh H, et al. Prevalence of underlying lung disease in smokers with epidermal growth factor receptor-mutant lung cancer. Oncol Rep 2013;29:2005-10. 10.3892/or.2013.2320 [DOI] [PubMed] [Google Scholar]
- 86.Fujimoto D, Tomii K, Otoshi T, et al. Preexisting interstitial lung disease is inversely correlated to tumor epidermal growth factor receptor mutation in patients with lung adenocarcinoma. Lung Cancer 2013;80:159-64. 10.1016/j.lungcan.2013.01.017 [DOI] [PubMed] [Google Scholar]
- 87.Masai K, Tsuta K, Motoi N, et al. Clinicopathological, Immunohistochemical, and Genetic Features of Primary Lung Adenocarcinoma Occurring in the Setting of Usual Interstitial Pneumonia Pattern. J. Thorac Oncol 2016;11:2141-9. 10.1016/j.jtho.2016.07.034 [DOI] [PubMed] [Google Scholar]
- 88.Guyard A, Danel C, Théou-Anton N, et al. Morphologic and molecular study of lung cancers associated with idiopathic pulmonary fibrosis and other pulmonary fibroses. Respir Res 2017;18:120. 10.1186/s12931-017-0605-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tzouvelekis A, Spagnolo P, Bonella F, et al. Patients with IPF and lung cancer: diagnosis and management. Lancet Respir Med 2018;6:86-8. 10.1016/S2213-2600(17)30478-2 [DOI] [PubMed] [Google Scholar]
- 90.Zhang Q, Zhang S, Xu X, et al. Value of radial probe endobronchial ultrasound-guided transbronchial biopsy and computer tomography-guided transthoracic needle aspiration in the diagnosis of peripheral pulmonary lesions. Medicine (Baltimore) 2017;96:e7843. 10.1097/MD.0000000000007843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sato T, Watanabe A, Kondo H, et al. Long-term results and predictors of survival after surgical resection of patients with lung cancer and interstitial lung diseases. J Thorac Cardiovasc Surg 2015;149:64-9,70.e1-2. [DOI] [PubMed]
- 92.Sawabata N, Miyaoka E, Asamura H, et al. Japanese lung cancer registry study of 11,663 surgical cases in 2004: demographic and prognosis changes over decade. J Thorac Oncol 2011;6:1229-35. 10.1097/JTO.0b013e318219aae2 [DOI] [PubMed] [Google Scholar]
- 93.Watanabe A, Higami T, Ohori S, et al. Is lung cancer resection indicated in patients with idiopathic pulmonary fibrosis? J Thorac Cardiovasc Surg 2008;136:1357-63, 1363.e1-2. [DOI] [PubMed]
- 94.Lee T, Park JY, Lee HY, et al. Lung cancer in patients with idiopathic pulmonary fibrosis: clinical characteristics and impact on survival. Respir Med 2014;108:1549-55. 10.1016/j.rmed.2014.07.020 [DOI] [PubMed] [Google Scholar]
- 95.Fujimoto T, Okazaki T, Matsukura T, et al. Operation for lung cancer in patients with idiopathic pulmonary fibrosis: surgical contraindication? Ann Thorac Surg 2003;76:1674-8; discussion 1679. [DOI] [PubMed]
- 96.Yano M, Yoshida J, Koike T, et al. Survival of 1737 lobectomy-tolerable patients who underwent limited resection for cStage IA non-small-cell lung cancer. Eur J Cardiothorac Surg 2015;47:135-42. 10.1093/ejcts/ezu138 [DOI] [PubMed] [Google Scholar]
- 97.Sato S, Koike T, Hashimoto T, et al. Surgical Outcomes of Lung Cancer Patients with Combined Pulmonary Fibrosis and Emphysema and Those with Idiopathic Pulmonary Fibrosis without Emphysema. Ann Thorac Cardiovasc Surg 2016;22:216-23. 10.5761/atcs.oa.15-00315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sato T, Teramukai S, Kondo H, et al. Impact and predictors of acute exacerbation of interstitial lung diseases after pulmonary resection for lung cancer. J Thorac Cardiovasc Surg 2014;147:1604-11.e3. 10.1016/j.jtcvs.2013.09.050 [DOI] [PubMed] [Google Scholar]
- 99.Chida M, Kobayashi S, Karube Y, et al. Incidence of acute exacerbation of interstitial pneumonia in operated lung cancer: institutional report and review. Ann Thorac Cardiovasc Surg 2012;18:314-7. 10.5761/atcs.oa.11.01839 [DOI] [PubMed] [Google Scholar]
- 100.Saito H, Minamiya Y, Nanjo H, et al. Pathological finding of subclinical interstitial pneumonia as a predictor of postoperative acute respiratory distress syndrome after pulmonary resection. Eur J Cardiothorac Surg 2011;39:190-4. 10.1016/j.ejcts.2010.05.017 [DOI] [PubMed] [Google Scholar]
- 101.Iyoda A, Jiang SX, Amano H, et al. Prediction of postoperative exacerbation of interstitial pneumonia in patients with lung cancer and interstitial lung disease. Exp Ther Med 2011;2:1073-6. 10.3892/etm.2011.342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Omori T, Tajiri M, Baba T, et al. Pulmonary Resection for Lung Cancer in Patients With Idiopathic Interstitial Pneumonia. Ann Thorac Surg 2015;100:954-60. 10.1016/j.athoracsur.2015.03.094 [DOI] [PubMed] [Google Scholar]
- 103.Sugiura H, Takeda A, Hoshi T, et al. Acute exacerbation of usual interstitial pneumonia after resection of lung cancer. Ann Thorac Surg 2012;93:937-43. 10.1016/j.athoracsur.2011.12.010 [DOI] [PubMed] [Google Scholar]
- 104.Ito H, Nakayama H, Tsuboi M, et al. Subpleural honeycombing on high resolution computed tomography is risk factor for fatal pneumonitis. Ann Thorac Surg 2011;91:874-8. 10.1016/j.athoracsur.2010.10.062 [DOI] [PubMed] [Google Scholar]
- 105.Suzuki H, Sekine Y, Yoshida S, et al. Risk of acute exacerbation of interstitial pneumonia after pulmonary resection for lung cancer in patients with idiopathic pulmonary fibrosis based on preoperative high-resolution computed tomography. Surg Today 2011;41:914-21. 10.1007/s00595-010-4384-z [DOI] [PubMed] [Google Scholar]
- 106.Yano M, Sasaki H, Moriyama S, et al. Post-operative acute exacerbation of pulmonary fibrosis in lung cancer patients undergoing lung resection. Interact Cardiovasc Thorac Surg 2012;14:146-50. 10.1093/icvts/ivr029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mizuno Y, Iwata H, Shirahashi K, et al. The importance of intraoperative fluid balance for the prevention of postoperative acute exacerbation of idiopathic pulmonary fibrosis after pulmonary resection for primary lung cancer. Eur J Cardiothorac Surg 2012;41:e161-5. 10.1093/ejcts/ezs147 [DOI] [PubMed] [Google Scholar]
- 108.Maniwa T, Isaka M, Nakagawa K, et al. Chest-tube drainage is a sign of acute exacerbation of interstitial lung disease associated with lung cancer. Surg Today 2013;43:408-11. 10.1007/s00595-012-0428-x [DOI] [PubMed] [Google Scholar]
- 109.Sato T, Kondo H, Watanabe A, et al. A simple risk scoring system for predicting acute exacerbation of interstitial pneumonia after pulmonary resection in lung cancer patients. Gen Thorac Cardiovasc Surg 2015;63:164-72. 10.1007/s11748-014-0487-6 [DOI] [PubMed] [Google Scholar]
- 110.Iwata T, Yoshida S, Fujiwara T, et al. Effect of Perioperative Pirfenidone Treatment in Lung Cancer Patients With Idiopathic Pulmonary Fibrosis. Ann Thorac Surg 2016;102:1905-10. 10.1016/j.athoracsur.2016.05.094 [DOI] [PubMed] [Google Scholar]
- 111.Iwata T, Yoshida S, Nagato K, et al. Experience with perioperative pirfenidone for lung cancer surgery in patients with idiopathic pulmonary fibrosis. Surg Today 2015;45:1263-70. 10.1007/s00595-014-1071-5 [DOI] [PubMed] [Google Scholar]
- 112.Iwata T, Yoshino I, Yoshida S, et al. A phase II trial evaluating the efficacy and safety of perioperative pirfenidone for prevention of acute exacerbation of idiopathic pulmonary fibrosis in lung cancer patients undergoing pulmonary resection: West Japan Oncology Group 6711 L (PEOPLE Study). Respir Res 2016;17:90. 10.1186/s12931-016-0398-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Costabel U, Inoue Y, Richeldi L, et al. Efficacy of Nintedanib in Idiopathic Pulmonary Fibrosis across Prespecified Subgroups in INPULSIS. Am J Respir Crit Care Med 2016;193:178-85. 10.1164/rccm.201503-0562OC [DOI] [PubMed] [Google Scholar]
- 114.Yamaguchi S, Ohguri T, Matsuki Y, et al. Radiotherapy for thoracic tumors: association between subclinical interstitial lung disease and fatal radiation pneumonitis. Int J Clin Oncol 2015;20:45-52. 10.1007/s10147-014-0679-1 [DOI] [PubMed] [Google Scholar]
- 115.Lee YH, Kim YS, Lee SN, et al. Interstitial Lung Change in Pre-radiation Therapy Computed Tomography Is a Risk Factor for Severe Radiation Pneumonitis. Cancer Res Treat 2015;47:676-86. 10.4143/crt.2014.180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Takeda A, Sanuki N, Enomoto T, et al. Subclinical interstitial lung disease: is it a risk factor for fatal radiation pneumonitis following stereotactic body radiotherapy? Lung Cancer 2014;83:112. 10.1016/j.lungcan.2013.10.009 [DOI] [PubMed] [Google Scholar]
- 117.Nagano T, Kotani Y, Fujii O, et al. A case of acute exacerbation of idiopathic pulmonary fibrosis after proton beam therapy for non-small cell lung cancer. Jpn J Clin Oncol 2012;42:965-9. 10.1093/jjco/hys115 [DOI] [PubMed] [Google Scholar]
- 118.Yamaguchi S, Ohguri T, Ide S, et al. Stereotactic body radiotherapy for lung tumors in patients with subclinical interstitial lung disease: the potential risk of extensive radiation pneumonitis. Lung Cancer 2013;82:260-5. 10.1016/j.lungcan.2013.08.024 [DOI] [PubMed] [Google Scholar]
- 119.Ueki N, Matsuo Y, Togashi Y, et al. Impact of pretreatment interstitial lung disease on radiation pneumonitis and survival after stereotactic body radiation therapy for lung cancer. J Thorac Oncol 2015;10:116-25. 10.1097/JTO.0000000000000359 [DOI] [PubMed] [Google Scholar]
- 120.Ono T, Hareyama M, Nakamura T, et al. The clinical results of proton beam therapy in patients with idiopathic pulmonary fibrosis: a single center experience. Radiat Oncol 2016;11:56. 10.1186/s13014-016-0637-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Watanabe N, Taniguchi H, Kondoh Y, et al. Efficacy of chemotherapy for advanced non-small cell lung cancer with idiopathic pulmonary fibrosis. Respiration 2013;85:326-31. 10.1159/000342046 [DOI] [PubMed] [Google Scholar]
- 122.Okuda K, Hirose T, Oki Y, et al. Evaluation of the safety and efficacy of combination chemotherapy with vinorelbine and platinum agents for patients with non-small cell lung cancer with interstitial lung disease. Anticancer Res 2012;32:5475-80. [PubMed] [Google Scholar]
- 123.Kinoshita T, Azuma K, Sasada T, et al. Chemotherapy for non-small cell lung cancer complicated by idiopathic interstitial pneumonia. Oncol Lett 2012;4:477-82. 10.3892/ol.2012.753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chen YJ, Chen LX, Han MX, et al. The Efficacy and Safety of Chemotherapy in Patients With Nonsmall Cell Lung Cancer and Interstitial Lung Disease: A PRISMA-Compliant Bayesian Meta-Analysis and Systematic Review. Medicine (Baltimore) 2015;94:e1451. 10.1097/MD.0000000000001451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Enomoto Y, Kenmotsu H, Watanabe N, et al. Efficacy and Safety of Combined Carboplatin, Paclitaxel, and Bevacizumab for Patients with Advanced Non-squamous Non-small Cell Lung Cancer with Pre-existing Interstitial Lung Disease: A Retrospective Multi-institutional Study. Anticancer Res 2015;35:4259-63. [PubMed] [Google Scholar]
- 126.Shimizu R, Fujimoto D, Kato R, et al. The safety and efficacy of paclitaxel and carboplatin with or without bevacizumab for treating patients with advanced nonsquamous non-small cell lung cancer with interstitial lung disease. Cancer Chemother Pharmacol 2014;74:1159-66. 10.1007/s00280-014-2590-x [DOI] [PubMed] [Google Scholar]
- 127.Watanabe N, Niho S, Kirita K, et al. Second-line docetaxel for patients with platinum-refractory advanced non-small cell lung cancer and interstitial pneumonia. Cancer Chemother. Pharmacol 2015;76:69-74. 10.1007/s00280-015-2775-y [DOI] [PubMed] [Google Scholar]
- 128.Choi MK, Hong JY, Chang W, et al. Safety and efficacy of gemcitabine or pemetrexed in combination with a platinum in patients with non-small-cell lung cancer and prior interstitial lung disease. Cancer Chemother Pharmacol 2014;73:1217-25. 10.1007/s00280-014-2458-0 [DOI] [PubMed] [Google Scholar]
- 129.Richeldi L, du Bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 2014;370:2071-82. 10.1056/NEJMoa1402584 [DOI] [PubMed] [Google Scholar]
- 130.Reck M, Kaiser R, Mellemgaard A, et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial. Lancet Oncol 2014;15:143-55. 10.1016/S1470-2045(13)70586-2 [DOI] [PubMed] [Google Scholar]
- 131.Sakurada T, Kakiuchi S, Tajima S, et al. Characteristics of and risk factors for interstitial lung disease induced by chemotherapy for lung cancer. Ann Pharmacother 2015;49:398-404. 10.1177/1060028014566446 [DOI] [PubMed] [Google Scholar]
- 132.Kenmotsu H, Naito T, Kimura M, et al. The risk of cytotoxic chemotherapy-related exacerbation of interstitial lung disease with lung cancer. J Thorac Oncol 2011;6:1242-6. 10.1097/JTO.0b013e318216ee6b [DOI] [PubMed] [Google Scholar]
- 133.Minegishi Y, Kuribayashi H, Kitamura K, et al. The feasibility study of Carboplatin plus Etoposide for advanced small cell lung cancer with idiopathic interstitial pneumonias. J Thorac Oncol 2011;6:801-7. 10.1097/JTO.0b013e3182103d3c [DOI] [PubMed] [Google Scholar]
- 134.Minegishi Y, Sudoh J, Kuribayasi H, et al. The safety and efficacy of weekly paclitaxel in combination with carboplatin for advanced non-small cell lung cancer with idiopathic interstitial pneumonias. Lung Cancer 2011;71:70-4. 10.1016/j.lungcan.2010.04.014 [DOI] [PubMed] [Google Scholar]
- 135.Enomoto Y, Inui N, Kato T, et al. Low forced vital capacity predicts cytotoxic chemotherapy-associated acute exacerbation of interstitial lung disease in patients with lung cancer. Lung Cancer 2016;96:63-7. 10.1016/j.lungcan.2016.03.017 [DOI] [PubMed] [Google Scholar]
- 136.Otsubo K, Kishimoto J, Kenmotsu H, et al. Treatment Rationale and Design for J-SONIC: A Randomized Study of Carboplatin Plus Nab-paclitaxel With or Without Nintedanib for Advanced Non-Small-cell Lung Cancer With Idiopathic Pulmonary Fibrosis. Clin Lung Cancer 2018;19:e5-9. 10.1016/j.cllc.2017.06.003 [DOI] [PubMed] [Google Scholar]
- 137.Min JH, Lee HY, Lim H, et al. Drug-induced interstitial lung disease in tyrosine kinase inhibitor therapy for non-small cell lung cancer: a review on current insight. Cancer Chemother Pharmacol 2011;68:1099-109. 10.1007/s00280-011-1737-2 [DOI] [PubMed] [Google Scholar]
- 138.Ando M, Okamoto I, Yamamoto N, et al. Predictive factors for interstitial lung disease, antitumor response, and survival in non-small-cell lung cancer patients treated with gefitinib. J Clin Oncol 2006;24:2549-56. 10.1200/JCO.2005.04.9866 [DOI] [PubMed] [Google Scholar]
- 139.Watanabe N, Nakahara Y, Taniguchi H, et al. Crizotinib-induced acute interstitial lung disease in a patient with EML4-ALK positive non-small cell lung cancer and chronic interstitial pneumonia. Acta Oncol 2014;53:158-60. 10.3109/0284186X.2013.802838 [DOI] [PubMed] [Google Scholar]
- 140.Créquit P, Wislez M, Fleury Feith J, et al. Crizotinib Associated with Ground-Glass Opacity Predominant Pattern Interstitial Lung Disease: A Retrospective Observational Cohort Study with a Systematic Literature Review. J Thorac Oncol 2015;10:1148-55. 10.1097/JTO.0000000000000577 [DOI] [PubMed] [Google Scholar]
- 141.Gettinger SN, Horn L, Gandhi L, et al. Overall Survival and Long-Term Safety of Nivolumab (Anti-Programmed Death 1 Antibody, BMS-936558, ONO-4538) in Patients With Previously Treated Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2015;33:2004-12. 10.1200/JCO.2014.58.3708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol 2015;16:908-18. 10.1016/S1470-2045(15)00083-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Naidoo J, Wang X, Woo KM, et al. Pneumonitis in Patients Treated With Anti-Programmed Death-1/Programmed Death Ligand 1 Therapy. J Clin Oncol 2017;35:709-17. 10.1200/JCO.2016.68.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fujimoto D, Morimoto T, Ito J, et al. A pilot trial of nivolumab treatment for advanced non-small cell lung cancer patients with mild idiopathic interstitial pneumonia. Lung Cancer 2017;111:1-5. 10.1016/j.lungcan.2017.06.008 [DOI] [PubMed] [Google Scholar]
- 145.Watanabe N, Taniguchi H, Kondoh Y, et al. Chemotherapy for extensive-stage small-cell lung cancer with idiopathic pulmonary fibrosis. Int J Clin Oncol 2014;19:260-5. 10.1007/s10147-013-0554-5 [DOI] [PubMed] [Google Scholar]
- 146.Yoshida T, Yoh K, Goto K, et al. Safety and efficacy of platinum agents plus etoposide for patients with small cell lung cancer with interstitial lung disease. Anticancer Res 2013;33:1175-9. [PubMed] [Google Scholar]
- 147.Fujimoto D, Shimizu R, Kato R, et al. Second-line Chemotherapy for Patients with Small Cell Lung Cancer and Interstitial Lung Disease. Anticancer Res 2015;35:6261-6. [PubMed] [Google Scholar]
- 148.Enomoto Y, Inui N, Imokawa S, et al. Safety of topotecan monotherapy for relapsed small cell lung cancer patients with pre-existing interstitial lung disease. Cancer Chemother Pharmacol 2015;76:499-505. 10.1007/s00280-015-2816-6 [DOI] [PubMed] [Google Scholar]