Early discharge after lung resection is safe: 10-year experience (original) (raw)
Abstract
Background
The average hospitalization after lung resection is 6 days, but some patients are discharged early in the post-operative period. The patient factors associated with early discharge (ED) and the safety of this approach are unknown. We hypothesized that specific patient populations are associated with ED, and that complications in this practice are low.
Methods
A prospective database of lung resections performed at an academic medical center between Jan 1, 2007 and Jan 1, 2017 was queried. Demographic and outcome variables were assessed using standard techniques. ED was defined as the length of stay (LOS) for the quintile with the lowest LOS for patients with anatomic resection (AR) or patients with wedge resection (WR). We then compared clinical factors between patients with ED to those patients discharged by day 7, to determine factors associated with ED (relative to “average” discharge).
Results
During the study period, there were 922 AR and 1,150 WR performed. A total of 448 (39.0%) patients had WRED and 211 patients (22.9%) had ARED. The rate of WRED varied by surgeon, but ARED did not. ARED and WRED patients was associated with several factors, including younger age, better lung function, and were less likely to have elevated American Society of Anesthesiologist (ASA) class. Multivariable analysis suggested that patient factors and primary surgeon influence ED. WRED was associated with 30-day mortality of 0.22% vs. 1.14% for longer LOS (P=0.08). After AR, there were no post-operative deaths within 30 days among 211 patients discharged on postoperative day 1 or 2 [(vs. 2/541, 0.4%, P=0.376) with longer LOS, P=0.048].
Conclusions
ED after lung resection is multifactorial but is safe among selected patients. Age, lung function, procedure duration, and surgeon all influence ED. Complications after ED were rare. Individual surgeon comfort with ED likely impacts LOS, and education or enhanced recovery protocols may help overcome this barrier. Standardized pathways would likely help identify low-risk patients for expeditious discharge.
Keywords: Thoracic surgery, postoperative complications, length of stay (LOS), patient discharge, risk factors
Introduction
Minimally invasive techniques in anatomic lung resection have been associated with reduced post-operative complications and shorter length of hospitalization (1,2). Although the average length of stay (LOS) after thoracoscopic lung resection is 6 days, a subset of patients is likely eligible for accelerated discharge (3,4). Accelerated care pathways in thoracic surgery have been proposed to facilitate early discharge (ED) after thoracic surgery (5,6). Some of these protocols have proposed discontinuing the chest tube on post-operative day (POD) 2 or 3 as a method to reduce hospitalization (7). Other studies have advocated for discharge on the first POD after lung resection, but the safety of this approach is unknown (8,9). Despite anecdotal support for ED, this approach has not been studied.
Fewer than 1% patients of patients with anatomic lung resection are discharged on POD 1 (10), but small studies suggest that discharge on the first POD can be safely performed (8,9). We believe that ED should be the appropriate goal in selected patient populations, as a short period of post-operative monitoring of respiratory status will identify patients who will have complications.
We hypothesized that ED is associated with specific patient populations. Although no formal enhanced recovery pathway was used during the study period, many patients were discharged early in the postoperative period. We performed a retrospective review of our institutional thoracic surgery database to examine factors associated with discharge as well as outcomes of patients who have been discharged in an expedited fashion after lung resection.
Methods
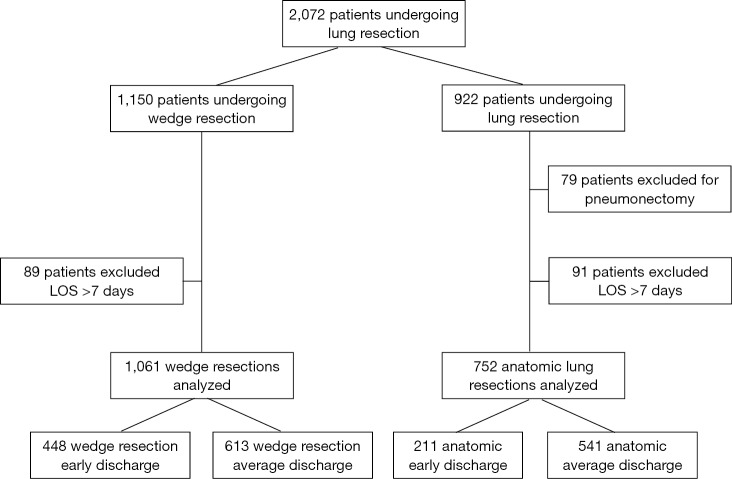
All patients undergoing lung resection between January 1, 2007 and January 1, 2017 at an academic medical center were identified via a prospectively maintained database. Exclusion criteria included patients with pneumonectomy or patients with LOS greater than 7 days. To ensure that patients with severe complications were not included in our comparisons, we excluded patients with post-operative LOS (POLOS) greater than 7 days, thereby comparing patients with ED to patients with “average” POLOS. Clinical data were extracted from the institution’s database and retrospective review from the electronic medical record. This study was approved by the institution’s Institutional Review Board (IRB Number: CC439). Study data were collected and managed using REDCap electronic data capture tools hosted at University Hospitals Cleveland Medical Center (11). All data were analyzed using STATA/SE, Version 13.0 (Stata Corp, College Station, TX).
Variables extracted from the clinical database included demographics, preoperative comorbidities, procedure characteristics, and postoperative complications. Preoperative characteristics extracted included age, body mass index (BMI), and hypertension, coronary artery disease (CAD), prior chemotherapy or radiation, chronic obstructive pulmonary disease (COPD), diabetes, congestive heart failure (CHF), Zubrod class, and American Society of Anesthesiologists (ASA) class. Procedures were characterized as wedge resection (WR), segmentectomy, lobectomy, bi-lobectomy, or pneumonectomy. Anatomic lung resection included segmental resection, lobectomy, bi-lobectomy, or pneumonectomy, but not WR. Patients who received multiple procedures on the same side were characterized by the largest extent of resection. Surgery technique was classified as video-assisted thoracoscopic surgery (VATS), VATS converted to open, robotic, robotic converted to VATS, robotic converted to open, and open (thoracotomy). Full explanations of these procedures are available elsewhere (12,13). Complications were extracted from the database and were verified with chart review and represent at least 30-day follow-up for all patients. Complications recorded included return to the operating room, postoperative infections, arrhythmias, respiratory issues (including reintubation and pneumonia), postoperative transfusions, and unexpected admission to the intensive care unit, readmission to any hospital in the 30-day post-operative period, and were categorized as defined by the Society of Thoracic Surgeons (STS) (14). Patients who had any recorded complication, regardless of complication severity, were categorized as having a complication.
The primary outcome of interest was ED. POLOS was defined as the number of “midnights” which occurred between the procedure and discharge and is reported as an integer number of days. Descriptive analysis was performed to determine distribution of LOS. To define the primary outcome, we examined the distribution of LOS for WR and anatomic resection (AR) patients. We expected that patients with WR would have a shorter LOS than patients with anatomic lung resection. We then defined ED as the LOS for patients with the lowest quintile of POLOS for WR (WRED) and AR (ARED).
Based on an a priori hypothesis that surgeon preferences rather than patient factors may affect ED, we performed a secondary analysis to determine the effects of surgeon on ED. The attending surgeons of record were responsible for clinical decisions, including operative approach and chest tube management. In general, reoperation and/or comorbidity were not contraindications to performing a minimally invasive approach. Chest tubes were placed on “water seal” in the recovery room if there was no significant air leak or pneumothorax on initial post-operative radiographic imaging. Chest tube were removed when there was no evidence of air leak and drainage from the tube was not sanguineous. There was no formal protocol regarding an absolute volume of chest tube drainage necessary for chest tube removal. All patients had Foley catheters removed on POD 1 unless they demonstrated hypovolemia or hemodynamic instability. All patients received an intravenous “patient controlled anesthesia” device, which was discontinued when they could tolerate oral pain medications, typically on POD 1 or 2.
Data are presented as median and interquartile range. Chi-square or Fisher’s Exact test, as appropriate, was utilized to determine differences between groups for categorical variables. Kruskal-Wallis one-way analysis of variance was performed to determine if there were differences between groups; pairwise comparisons between groups were then performed when differences were identified. Logistic regression was preformed among patients with complete datasets to determine factors associated with ED. Otherwise, P value less than 0.05 was considered significant.
Results
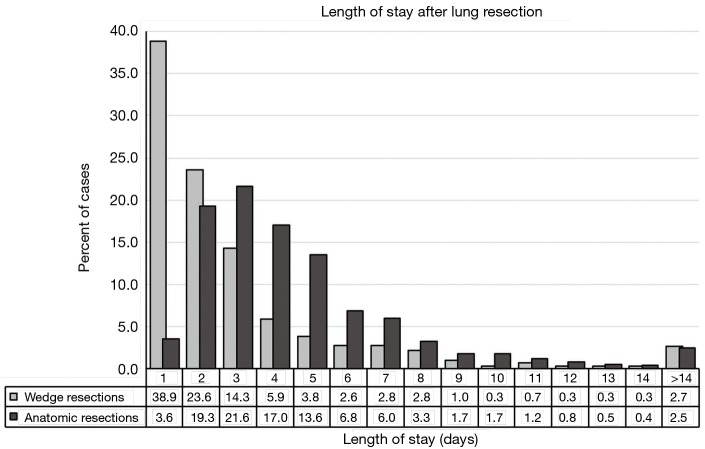
During the study period, 2,072 lung resections were performed by seven surgeons. There were 922 AR and 1,150 WR. The characteristics of the cohort are shown in Table 1. The distribution of hospital LOS is shown in Figure 1.
Table 1. Description of cohort: patients who underwent lung resection from Jan 1, 2007–Jan 1, 2017.
| Variables | Wedge (n=1,150) | Anatomic (n=922) |
|---|---|---|
| Age (years), mean (standard error) | 63.6 (0.42) | 67.4 (0.37) |
| Male, n (%) | 531 (46.2) | 417 (45.2) |
| Caucasian race, n (%) | 928 (80.7) | 739 (80.2) |
| Preoperative characteristic | ||
| BMI >35 kg/m2, n (%) | 164 (14.4)(n=1,140) | 89 (9.7) (n=921) |
| Hypertension, n (%) | 583 (52.3) (n=1,115) | 537 (58.3) (n=921) |
| Steroid, n (%) | 74 (6.6) (n=1,115) | 20 (2.2) (n=921) |
| CHF, n (%) | 48 (4.3) (n=1,115) | 33 (3.6) (n=921) |
| CAD, n (%) | 181 (16.2) (n=1,115) | 169 (18.3) (n=921) |
| PVD, n (%) | 52 (4.7) (n=1,115) | 67 (7.3) (n=920) |
| Diabetes, n (%) | 180 (15.6) | 139 (15.1) |
| Any comorbidity, n (%) | 715 (62.2) | 623 (67.6) |
| ppFEV1, mean (standard error) | 78.9 (0.91) | 82.9 (0.71) |
| ppDLCO, mean (standard error) | 77.4 (1.00) | 77.6 (0.77) |
| ASA class >2, n (%) | 986 (85.7) | 846 (91.8) |
| Previous cardiothoracic surgery, n (%) | 205 (18.4) (n=1,114) | 162 (17.9) (n=903) |
| Procedure characteristic | ||
| Right laterality, n (%) | 675 (58.7) | 551 (59.8) |
| Diagnosis of lung cancer, n (%) | 244 (21.2) | 682 (74.0) |
| VATS + robotic, n (%) | 1,105 (96.1) | 657 (71.3) |
| Procedure time (hours), mean (standard error) | 1.27 (0.04) | 3.09 (0.04) |
| POLOS (days), mean (standard error) | 3.2 (0.12) | 4.8 (0.15) |
Figure 1.
Length of stay after lung resection. Histogram of percent of patients’ length of stay among wedge resections (light bars) and anatomic resections (dark bars).
Among 1,150 WR, 448 (39.0%) were discharged on POD 1. Based on methods described above, WRED was defined as discharge within 1 day of surgery. Among WR, 89 patients (7.7%) had POLOS >7 days, and these patients were excluded from further analysis (Figure S1). The characteristics of patients with ED after WR is shown in Table 2. Compared to 613 patients with POLOS 2–7 days, the 448 patients who were discharged on POD 1 after WR tended to be younger, have better pulmonary function test results, and have fewer comorbidities. Patient who were discharged on POD 1 after WR were also more likely to have minimally invasive techniques used (99.6% vs. 94.6% with longer LOS, P<0.001) and tended to have shorter operating room times (1.08 vs. 1.45 hours, P<0.001).
Table 2. Description of cohort: patient characteristics and relationship to day of discharge among patients who received wedge resections (n=1,061 unless otherwise noted).
| Wedge resection | Wedge early discharge (n=448) | Wedge POLOS 2-7 (n=613) | Comparison P value* |
|---|---|---|---|
| Age (years), mean (standard error) | 62.3 (0.62) | 64.8 (0.60) | 0.005 |
| Male, n (%) | 204 (45.5) | 273 (44.5) | 0.746 |
| Caucasian race, n (%) | 381 (85.0) | 487 (79.4) | 0.020 |
| Preoperative characteristic | |||
| BMI >35, n (%) | 57 (12.8) (n=445) | 94 (15.4) (n=609) | 0.229 |
| Hypertension, n (%) | 206 (47.8) (n=431) | 326 (54.6) (n=597) | 0.031 |
| Steroid, n (%) | 23 (5.3) (n=431) | 37 (6.2) (n=597) | 0.561 |
| CHF, n (%) | 13 (3.0) (n=431) | 26 (4.4) (n=597) | 0.322 |
| CAD, n (%) | 57 (13.2) (n=431) | 110 (18.4) (n=597) | 0.026 |
| PVD, n (%) | 11 (2.6) (n=431) | 38 (6.4) (n=597) | 0.005 |
| Diabetes, n (%) | 56 (12.5) | 109 (17.8) | 0.019 |
| Any comorbidity, n (%) | 255 (56.9) | 395 (64.4) | 0.013 |
| ppFEV1, mean (standard error), n=588 | 85.3 (1.23) | 75.6 (1.27) | <0.001 |
| ppDLCO, mean (standard error), n=533 | 83.9 (1.55) | 73.2 (1.31) | <0.001 |
| ASA class >2, n (%) | 372 (83.0) | 531 (86.6) | 0.105 |
| Previous cardiothoracic surgery, n (%) | 61 (14.2) (n=431) | 127 (21.3) (n=597) | 0.004 |
| Procedure characteristic | |||
| Right laterality, n (%) | 247 (55.1) | 369 (60.2) | 0.099 |
| Diagnosis of lung cancer, n (%) | 89 (19.9) | 144 (23.5) | 0.159 |
| VATS + robotic, n (%) | 446 (99.6) | 580 (94.6) | <0.001 |
| Procedure time (hours), mean (standard error) | 1.08 (0.03) | 1.45 (0.04) | <0.001 |
Among 922 AR, 211 patients (22.9%) were discharged on POD 1 or 2. As a result, anatomic ED (AED) was defined as discharge within 2 days of surgery. Among AR, 112 patients (12.1%) had POLOS >7 days and 79 patients received pneumonectomy, and these patients were excluded from further analysis (Figure S1). The comparison of patients with ARED to longer LOS is shown in Table 3. Patients discharged on day 1 or 2 tended to be younger, have better DLCO, and were less likely to have a primary diagnosis of lung cancer. ARED patients were also more likely to have minimally invasive techniques used (95.7% vs. 73.6% with longer LOS, P<0.001) and tended to have shorter operating room times (2.6 vs. 3.1 hours, P<0.001).
Table 3. Description of cohort: patient characteristics and relationship to day of discharge among patients who received non-pneumonectomy anatomic lung resections discharged prior to day 8 (n=752 unless otherwise noted).
| Anatomic resection | POLOS 1–2 (n=211) | POLOS 3–7 (n=541) | Comparison P value* |
|---|---|---|---|
| Age (years), mean (standard error) | 65.3 (0.75) | 67.9 (0.50) | 0.007 |
| Male, n (%) | 84 (39.8) | 237 (43.8) | 0.319 |
| Caucasian race, n (%) | 177 (83.9) | 425 (78.6) | 0.100 |
| Preoperative characteristic | |||
| BMI >35, n (%) | 25 (11.8) | 50 (9.24) | 0.284 |
| Hypertension, n (%) | 114 (54.0) | 326 (60.3) | 0.119 |
| Steroid, n (%) | 4 (1.9) | 12 (2.2) | 0.781 |
| CHF, n (%) | 4 (1.9) | 20 (3.7) | 0.254 |
| CAD, n (%) | 35 (16.6) | 99 (18.3) | 0.574 |
| PVD, n (%) | 7 (3.3) | 45 (8.3) | 0.016 |
| Diabetes, n (%) | 36 (17.1) | 75 (13.9) | 0.275 |
| Any comorbidity, n (%) | 128 (60.7) | 375 (69.3) | 0.023 |
| ppFEV1, mean (standard error), n=713 | 86.7 (1.35) | 83.6 (0.94) | 0.071 |
| ppDLCO, mean (standard error), n=676 | 83.4 (1.59) | 76.7 (1.00) | <0.001 |
| ASA class >2, n (%) | 189 (89.6) | 497 (91.9) | 0.318 |
| Previous cardiothoracic surgery, n (%) | 26 (12.7) (n=205) | 93 (17.6) (n=528) | 0.104 |
| Procedure characteristic | |||
| Right laterality, n (%) | 122 (57.8) | 324 (59.9) | 0.604 |
| Diagnosis of lung cancer, n (%) | 145 (68.7) | 411 (76.0) | 0.042 |
| VATS or robotic, n (%) | 202 (95.7) | 398 (73.6) | <0.001 |
| Procedure time (hours), mean (standard error) | 2.58 (0.06) | 3.11 (0.05) | <0.001 |
Post-operative LOS (P<0.001) varied by surgeon (Table 4). The rate of ED after WRED also varied (P<0.001), while the frequency of ED after AR (ARED) did not differ (P=0.337). One surgeon (“Surgeon 1”), in particular, was associated with increased rate of EDs after WR, but their rate of ED after AR was similar to the others (P=0.844).
Table 4. Length of stay and rate of early discharge by surgeon.
| Surgeon | Surgeon average POLOS (days) | Others average POLOS (days) | Difference | Wedge resection | Anatomic resection | ||||
|---|---|---|---|---|---|---|---|---|---|
| WED POLOS =1, n (%) | WED/POLOS =1 by others, n (%) | Difference | AED POLOS =1–2, n (%) | AED/POLOS =1–2 by others, n (%) | Difference | ||||
| 1 | 3.7 | 4.1 | 0.07 | 220/431 (51.0) | 228/630 (36.2) | <0.001 | 95/336 (28.3) | 116/416 (27.9) | 0.906 |
| 2 | 3.9 | 3.9 | 0.68 | 147 /380 (38.7) | 301/681 (44.2) | 0.081 | 74/252 (29.4) | 137/500 (27.4) | 0.571 |
| 3 | 4.4 | 3.9 | 0.14 | 26/84 (31.0) | 422/977 (43.2) | 0.029 | 14/66 (21.2)) | 197/686 (28.7) | 0.195 |
| 4 | 4.3 | 3.9 | 0.44 | 9/24 (37.5) | 439/1,037 (42.3) | 0.636 | 5/26 (19.2) | 206/726 (28.4) | 0.308 |
| 5 | 7.3 | 3.9 | <0.001 | 7/19 (36.8) | 441/1,042 (42.3) | 0.632 | 1/2 (50.0%) | 210/750 (28.0) | 0.489 |
| 6 | 3.8 | 3.9 | 0.71 | 37/105 (35.2) | 411/956 (43.0) | 0.127 | 22/64 (34.4) | 189/688 (27.5) | 0.240 |
| 7 | 5.1 | 3.9 | 0.14 | 2/18 (11.1) | 446/1,043 (42.8) | 0.007 | 0/6 | 211/746 (28.3) | 0.125 |
Logistic regression was performed to determine which variables were independently associated with ED for both WRED and ARED (Tables 5,6). Covariates for the logistic regression were included based on selection from univariate model and clinical relevance. Patients with missing covariates were excluded from this analysis. To determine the effect of surgical management, a dichotomous variable to account for the surgeon with the most POD 1 discharges was also included. Factors associated with WRED were age, the presence of a comorbidity, lung function result, surgery duration, and whether the procedure was performed by surgeon 1. After AR, age, diffusing capacity of the lung for carbon monoxide (DLCO), and surgery duration were associated with ED.
Table 5. Logistic regression to determine factors associated with early discharge among wedge resections.
| Wedge resections | POD 1 discharge (n=515) | ||
|---|---|---|---|
| Odds ratio | 95% CI | P value | |
| Age* | 0.98 | 0.96–0.99 | 0.004 |
| Any comorbidity | 0.62 | 0.41–0.95 | 0.028 |
| ppFEV1* | 1.01 | 1.00–1.02 | 0.008 |
| ppDLCO* | 1.01 | 1.00–1.02 | 0.002 |
| Previous cardiothoracic surgery | 0.74 | 0.464–1.18 | 0.208 |
| Surgeon 1 vs. others | 2.66 | 1.78–3.97 | <0.001 |
| Procedure time (hours)* | 0.47 | 0.34–0.64 | <0.001 |
Table 6. Logistic regression to determine factors associated with early discharge among anatomic lung resections.
| Anatomic lung resections | POD 1 or 2 discharge (n=676) | ||
|---|---|---|---|
| Odds ratio | 95% CI | P value | |
| Age* | 0.98 | 0.96–0.99 | 0.006 |
| Any comorbidity | 0.99 | 0.68–1.43 | 0.936 |
| ppDLCO* | 1.01 | 1.00–1.02 | 0.003 |
| Surgeon 1 vs. others | 1.20 | 0.84–1.71 | 0.320 |
| Procedure time (hours)* | 0.59 | 0.48–0.71 | <0.001 |
Post-operative complications were infrequent after ED. No patients discharged on POD 1 or 2 were discharged with a chest tube in place. After WR there was one postoperative mortality among 448 POD 1 discharges (0.22% vs. 7/613 1.14%, P=0.08, overall mortality for cohort 8/1,061 0.75%). The one death was among a patient with interstitial lung disease who underwent diagnostic WR of middle and lower lobe. After WR, there was a trend towards fewer readmissions after POD 1 discharge (25/448, 5.6%) compared to patients with average LOS (52/613, 8.5%; P=0.08). After WR, there were also fewer total complications after POD 1 discharge (4/448, 0.9%) compared to patients with average LOS (75/613, 12.2%), P<0.001). The four complications in the WRED group were a surgical site infection, atrial fibrillation, and pneumonia (n=2), and no patient required pleural drainage procedures.
After AR, there were no postoperative deaths among 211 patients discharged on POD 1 or 2 [vs. 2/541 (0.4%) with longer LOS, P=0.376]. Patients with ED had similar readmission rates to patients with longer LOS [7/211 (3.3%) vs. 30/541 (5.5%), P=0.204]. There were also fewer total complications among the ARED group (4/211, 1.9%) compared to patients with average LOS (112/541, 20.7%; P<0.001). Complications in the ARED group included pneumothorax requiring chest tube reinsertion (n=2), pulmonary embolism, and urinary tract infection.
Discussion
Reducing duration of hospitalization and post-operative readmission is crucial to achieving the most effective patient care and the best patient outcomes. Shorter LOS is associated with lower hospitals costs and possibly fewer hospital acquired complications, such as pneumonia and deep vein thrombosis (DVT) (15). For this reason, accelerated care pathways have been proposed after thoracic surgery to reduce length of hospitalization (5,6). For example, Cerfolio’s paper of accelerated discharge after lung surgery suggested that patients routinely be managed with a chest tube for at least 2 days, even after WR (7). Our institution has a robust experience of ED after lung resection. Among 2,072 consecutive lung resections over 10 years, 480 were discharged on POD 1, including 33 AR. Among EDs, complications were infrequent and there were no perioperative deaths.
Very little has been written about ED in thoracic surgery. In other surgical disciplines, POD 1 discharge has become commonplace. For example, open cholecystectomy had an average LOS of 5–7 days prior to laparoscopic techniques (16), but minimally invasive techniques are now associated with a median LOS of 1–1.5 days with 30–37% percent performed as outpatients (17). Laparoscopic Roux-en-Y gastric bypass is another procedure in which minimally invasive techniques have been associated with successful discharge on POD 1 (18). The advent of minimally invasive techniques in lung surgery have not demonstrated such a dramatic effect in hospitalization (19). In fact, the first well-known randomized trial did not show a benefit in LOS (20). More recent reports of VATS lobectomy suggest that it is associated with a reduction in LOS from 4–5 vs. 6–7 days with thoracotomy (2,21). While there is anecdotal support for POD 1 discharge after WR and this is often considered a common practice, there has been no specific study to demonstrate safety of this approach or considerations for patient selection. Mckenna’s report of 1,100 VATS lobectomies suggested that 2.9% of cases were discharged on POD 1, which is far more than suggested from institutions reporting to the STS (2,4). Several studies have tried to address the need for accelerated discharge after thoracic surgery with enhanced recovery pathways with varying degrees of success (5,7,22). One failure of “recovery pathway” studies may be that they applied the same protocol to all of their patients, when different groups of patients likely require specific care. In this study, we demonstrate that younger patients, patients receiving segmental resections or right middle lobe resections, and those without comorbidity can be discharged early in the post-operative period with low rates of complications and readmissions. Based on our findings, we recommend focusing enhanced recovery pathways on these subsets of patients with expectations of discharge by POD 1 or 2 rather than expecting all patient to progress at an equal pace. We feel strongly that delaying discharge as part of a pathway or “formula” (such as keeping a chest tube until POD 2) is not appropriate for these patients.
We believe that POD 1 discharge is an attainable and reasonable goal in a significant subset of patients after WR and perhaps after AR. Our data support this in that there was a significant impact of a single surgeon on POD 1 discharge rate in univariate and multivariate analysis among WR. We believe rate of ED likely reflects the impact of meticulous operative technique, avoiding intraoperative complications and post-operative air leak. Moreover, there is likely a non-technical “preference” or “comfort” for ED which guides this provider to discharge their patient faster than others. In addition to factors related to the procedure, a multimodal approach to perioperative care involving adequate postoperative pain control and resumption of functional status, is essential for ED.
A concern about ED and the possibility of a missed opportunity to “rescue” a patient from a complication which may occur outside of a healthcare system. These data suggest that among selected patients, major complications are rare after ED. These data may not apply to hospital systems which serve patients in very remote areas without access to care.
This retrospective study had several limitations. The study spans 10 years and seven surgeons, who preferences and technique have also varied over time. Therefore, the lack of standardization and the retrospective nature of the study, make physician judgment a significant confounder in the decision regarding timing of discharge. We attempted to mitigate this effect by adjusting for surgeon, which showed how pronounced this effect can be. The logistic regression of WR was performed on the subset of patients with full pulmonary function test data available and this may have affected the result. Furthermore, other clinical factors, such as socioeconomic status, disability, and distance from hospital to home are not included in this analysis and might affect POLOS. Another limitation of the study is that the effect of each comorbidity in the logistic regression model was assumed to be equal. This assumption may have over- or under-estimated the effect of certain conditions.
This study suggests that discharge on ED is safe for select patients after lung resection. As techniques improve to reduce perioperative discomfort and physiologic aberrations, patients will be eligible for earlier discharge after major lung surgery. In our institution, the impact of a single surgeon led to a significantly shorter LOS and higher rates of POD 1 discharge, suggesting that patterns of discharge are surgeon specific and safe early-discharge outcomes are attainable. We also show that complications are rare among select patients after POD 1 discharge, even among patients who received anatomic lung resections. We believe that there is likely considerable variation in ED practice among thoracic surgeons, and believe a multi-institution study to determine characteristics which are associated with safe POD 1 discharge is appropriate. Ultimately these data can be used to create a prospective enhanced recovery pathway to facilitate ED among the appropriate candidates.
Figure S1.
Description of study cohort and exclusion criteria. Patients were divided into wedge resection and anatomic resection groups. Patients were excluded from the study if they received a pneumonectomy or had length of stay (LOS) >7 days.
Acknowledgements
None.
Ethical Statement: This study was approved by the institution’s Institutional Review Board (IRB Number: CC439).
Footnotes
Conflicts of Interest: CW Towe reports that he is a consultant for Atricure and Medtronic, but that these relationships have not affected this manuscript or the accuracy of the data analysis. The other authors have no conflicts of interest to declare.
References
- 1.Kumar A, Asaf BB. Robotic thoracic surgery: The state of the art. J Minim Access Surg 2015;11:60-7. 10.4103/0972-9941.147693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010;139:366-78. 10.1016/j.jtcvs.2009.08.026 [DOI] [PubMed] [Google Scholar]
- 3.Blasberg JD, Seder CW, Leverson G, et al. Video-Assisted Thoracoscopic Lobectomy for Lung Cancer: Current Practice Patterns and Predictors of Adoption. Ann Thorac Surg 2016;102:1854-62. 10.1016/j.athoracsur.2016.06.030 [DOI] [PubMed] [Google Scholar]
- 4.McKenna RJ, Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg 2006;81:421-5; discussion 425-6. 10.1016/j.athoracsur.2005.07.078 [DOI] [PubMed] [Google Scholar]
- 5.Fiore JF, Jr, Bejjani J, Conrad K, et al. Systematic review of the influence of enhanced recovery pathways in elective lung resection. J Thorac Cardiovasc Surg 2016;151:708-15.e6. 10.1016/j.jtcvs.2015.09.112 [DOI] [PubMed] [Google Scholar]
- 6.Madani A, Fiore JF, Jr, Wang Y, et al. An enhanced recovery pathway reduces duration of stay and complications after open pulmonary lobectomy. Surgery 2015;158:899-908; discussion 908-10. 10.1016/j.surg.2015.04.046 [DOI] [PubMed] [Google Scholar]
- 7.Cerfolio RJ, Pickens A, Bass C, et al. Fast-tracking pulmonary resections. J Thorac Cardiovasc Surg 2001;122:318-24. 10.1067/mtc.2001.114352 [DOI] [PubMed] [Google Scholar]
- 8.Tovar EA. One-day admission for major lung resections in septuagenarians and octogenarians: a comparative study with a younger cohort. Eur J Cardiothorac Surg 2001;20:449-53; discussion 453-4. 10.1016/S1010-7940(01)00835-1 [DOI] [PubMed] [Google Scholar]
- 9.Tovar EA, Roethe RA, Weissig MD, et al. One-day admission for lung lobectomy: an incidental result of a clinical pathway. Ann Thorac Surg 1998;65:803-6. 10.1016/S0003-4975(97)01381-7 [DOI] [PubMed] [Google Scholar]
- 10.Wright CD, Gaissert HA, Grab JD, et al. Predictors of prolonged length of stay after lobectomy for lung cancer: a Society of Thoracic Surgeons General Thoracic Surgery Database risk-adjustment model. Ann Thorac Surg 2008;85:1857-65; discussion 1865. [DOI] [PubMed]
- 11.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377-81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andrews WG, Paul S. Techniques of video-assisted thoracoscopic surgery lobectomy and critical review of published data. Future Oncol 2016;12:31-3. 10.2217/fon-2016-0340 [DOI] [PubMed] [Google Scholar]
- 13.Veronesi G. Robotic lobectomy and segmentectomy for lung cancer: results and operating technique. J Thorac Dis 2015;7:S122-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Data Collection STS General Thoracic Surgery Database 2017. Available online: https://www.sts.org/quality-research-patient-safety/national-database/database-managers/general-thoracic-surgery-databa-1
- 15.Khan NA, Quan H, Bugar JM, et al. Association of postoperative complications with hospital costs and length of stay in a tertiary care center. J Gen Intern Med 2006;21:177-80. 10.1007/s11606-006-0254-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roslyn JJ, Binns GS, Hughes EF, et al. Open cholecystectomy. A contemporary analysis of 42,474 patients. Ann Surg 1993;218:129-37. 10.1097/00000658-199308000-00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harboe KM, Bardram L. The quality of cholecystectomy in Denmark: outcome and risk factors for 20,307 patients from the national database. Surg Endosc 2011;25:1630-41. 10.1007/s00464-010-1453-8 [DOI] [PubMed] [Google Scholar]
- 18.Waydia S, Gunawardene A, Gilbert J, et al. 23-hour/next day discharge post-laparoscopic Roux-en-Y gastric bypass (LRYGB) surgery is safe. Obes Surg 2014;24:2007-10. 10.1007/s11695-014-1409-5 [DOI] [PubMed] [Google Scholar]
- 19.Gopaldas RR, Bakaeen FG, Dao TK, et al. Video-assisted thoracoscopic versus open thoracotomy lobectomy in a cohort of 13,619 patients. Ann Thorac Surg 2010;89:1563-70. 10.1016/j.athoracsur.2010.02.026 [DOI] [PubMed] [Google Scholar]
- 20.Kirby TJ, Mack MJ, Landreneau RJ, et al. Lobectomy--video-assisted thoracic surgery versus muscle-sparing thoracotomy. A randomized trial. J Thorac Cardiovasc Surg 1995;109:997-1001; discussion 1001-2. 10.1016/S0022-5223(95)70326-8 [DOI] [PubMed] [Google Scholar]
- 21.Flores RM, Park BJ, Dycoco J, et al. Lobectomy by video-assisted thoracic surgery (VATS) versus thoracotomy for lung cancer. J Thorac Cardiovasc Surg 2009;138:11-8. 10.1016/j.jtcvs.2009.03.030 [DOI] [PubMed] [Google Scholar]
- 22.Brunelli A, Thomas C, Dinesh P, et al. Enhanced recovery pathway versus standard care in patients undergoing video-assisted thoracoscopic lobectomy. J Thorac Cardiovasc Surg 2017;154:2084-90. [DOI] [PubMed] [Google Scholar]