Pleiotropic Contributions of Phospholipase C-γ1 (PLC-γ1) to T-Cell Antigen Receptor-Mediated Signaling: Reconstitution Studies of a PLC-γ1-Deficient Jurkat T-Cell Line (original) (raw)
Abstract
Phospholipase C-γ1 (PLC-γ1) plays a crucial role in the coupling of T-cell antigen receptor (TCR) ligation to interleukin-2 (IL-2) gene expression in activated T lymphocytes. In this study, we have isolated and characterized two novel, PLC-γ1-deficient sublines derived from the Jurkat T-leukemic cell line. The P98 subline displays a >90% reduction in PLC-γ1 expression, while the J.gamma1 subline contains no detectable PLC-γ1 protein. The lack of PLC-γ1 expression in J.gamma1 cells caused profound defects in TCR-dependent Ca2+ mobilization and NFAT activation. In contrast, both of these responses occurred at normal levels in PLC-γ1-deficient P98 cells. Unexpectedly, the P98 cells displayed significant and selective defects in the activation of both the composite CD28 response element (RE/AP) and the full-length IL-2 promoter following costimulation with anti-TCR antibodies and phorbol ester. These transcriptional defects were reversed by transfection of P98 cells with a wild-type PLC-γ1 expression vector but not by expression of mutated PLC-γ1 constructs that lacked a functional, carboxyl-terminal SH2 [SH2(C)] domain or the major Tyr783 phosphorylation site. On the other hand, the amino-terminal SH2 [SH2(N)] domain was not essential for reconstitution of RE/AP- or IL-2 promoter-dependent transcription but was required for the association of PLC-γ1 with LAT, as well as the tyrosine phosphorylation of PLC-γ1 itself, in activated P98 cells. These studies demonstrate that the PLC-γ1 SH2(N) and SH2(C) domains play functionally distinct roles during TCR-mediated signaling and identify a non-Ca2+-related signaling function linked to the SH2(C) domain, which couples TCR plus phorbol ester-CD28 costimulation to the activation of the IL-2 promoter in T lymphocytes.
Ligation of the T-cell antigen receptor (TCR) triggers a cascade of biochemical events that culminates in cytokine gene expression, cellular proliferation, and the execution of T-cell effector functions (10, 14, 64). The initiation of signal output from the TCR involves the activation of three families of nonreceptor protein tyrosine kinases (PTKs). Src family members Lck and Fyn are responsible for the phosphorylation of immunoreceptor-based tyrosine activation motifs, which are found in multiple copies in the cytoplasmic domains of the CD3 and ζ subunits of the TCR complex. In mature T cells, the phosphotyrosine-containing immunoreceptor-based tyrosine activation motifs serve as docking sites for the Syk family PTK, ZAP-70, to the activated receptor complex (60, 66). The activation of Src family kinases during TCR engagement also leads to the phosphorylation and activation of the Tec family members Itk and Rlk (2, 16, 22, 40). The concerted activities of the Src, Syk, and Tec family PTKs result in the phosphorylation of a series of intracellular enzymes and adapter proteins which, in turn, propagate T-cell regulatory signals through the cytoplasm and into the nucleus.
A key substrate for the TCR-coupled PTKs is phospholipase C-gamma 1 (PLC-γ1). TCR engagement provokes rapid increases in both the tyrosine phosphorylation and the catalytic activity of PLC-γ1 (32, 44, 52, 67). The activated enzyme hydrolyzes phosphatidylinositol-4,5-bisphosphate (PIP2) to inositol-1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). These metabolites act as second messengers to stimulate the release of Ca2+ from intracellular stores and activate protein kinase C, respectively (6). The increase in the intracellular free Ca2+ concentration ([Ca2+]i) triggered by IP3 plays crucial roles in the induction of numerous T-cell activation-associated responses (17, 61). A pivotal target of the Ca2+ signaling pathway is NFAT, a transcription factor that regulates the expression of several T-cell activation-associated genes, including the gene for interleukin-2 (IL-2) (47). The importance of the [Ca2+]i increase during the early stages of T-cell activation has raised considerable interest in the mechanism whereby the TCR activates PLC-γ1, as well as the interactions of PLC-γ1 with other components of the TCR-linked signaling machinery.
Mammalian cells express at least 10 different PLC family members, which are grouped into three subfamilies, β, γ, and δ (34, 37, 48). The PLC-γ subfamily contains two members, PLC-γ1 and -γ2, both of which bear structural motifs that confer regulation by PTKs. PLC-γ1 is widely expressed in mammalian tissues, while PLC-γ2 expression is largely restricted to hematopoietic and lymphoid lineage cells (13, 35). Among lymphoid cells, T cells express predominantly PLC-γ1 while NK and B cells express PLC-γ2 in amounts similar to or greater than those of PLC-γ1 (62). Although some evidence suggests that the two PLC-γ isoforms are subject to different modes of regulation (4, 7), the functional significance of PLC-γ1 versus PLC-γ2 activation in various lymphoid subpopulations remains unclear.
PLC-γ1 is largely responsible for the increase in PIP2 hydrolysis induced by stimulation of receptor tyrosine kinases (34), as well as multichain antigen receptors, which lack intrinsic PTK domains but employ nonreceptor PTKs as proximal signaling elements (32, 52, 67). Targeted disruptions of both Plcg1 alleles in mice result in early embryonic lethality, indicating an essential role for this enzyme during organismal development (30). The lethal consequences of Plcg1 gene disruption have so far precluded analyses of the signaling functions of PLC-γ1 in developing thymocytes or mature peripheral T cells in vivo. However, the availability of a _Plcg1_−/− DT40 chicken B-cell line has allowed some valuable insights into the regulation and function role of PLC-γ1 during B-cell antigen receptor (BCR) stimulation (15).
A common structural feature of the PLCs is a split catalytic domain comprised of conserved X and Y subdomains. According to current models, PLC activation hinges, in part, on a conformational change that juxtaposes the X and Y subdomains to create a contiguous catalytic domain (19, 37, 48). In the PLC-γ subfamily, the X and Y domains are separated by the SH region, a stretch of approximately 500 amino acids that encodes two SH2 domains and one SH3 domain. The dual SH2 domains permit PLC-γ recruitment to specific phosphotyrosine-containing target sequences (57), which not only positions the enzyme for phosphorylation by membrane-associated PTKs but also allows associations with other cytoplasmic signaling proteins. On the other hand, the SH3 domain may mediate associations between PLC-γ1 and proline-rich motifs found in c-Cbl (25), as well as cytoskeletal proteins (5). In addition to its role in the regulation of PLC-γ1 catalytic activity (26, 27), the SH region may allow PLC-γ1 to act as a scaffold for the assembly of multimolecular signaling complexes during TCR signaling.
Stimulation of the platelet-derived growth factor (PDGF) receptor (39) or TCR (44) triggers the rapid phosphorylation of PLC-γ1 on at least three tyrosine residues. Studies performed with PDGF-responsive cells indicated that phosphorylation of Tyr783 (Y783) is essential for activation of PLC-γ1, while phosphorylation at a second site, Tyr1254, was needed for maximal stimulation of phosphoinositide breakdown by PDGF (39). Interestingly, a third Phe substitution, at Tyr771, actually increased the PDGF-dependent activation of PLC-γ1 in these cells. The mechanisms through which these phosphorylation events modulate the activities of the catalytic X and Y domains have not been defined.
The biochemical events that link TCR stimulation to the phosphorylation and catalytic activation of PLC-γ1 are only partially understood. Results obtained with genetically deficient Jurkat T-cell lines and gene-targeted mice indicate that optimal tyrosine phosphorylation of PLC-γ1 requires the concerted activities of Lck, ZAP-70, and Itk/Rlk (50, 59, 69). Moreover, studies performed with LAT (20, 72)- or SLP-76 (70)-deficient Jurkat T-cell lines indicate that both of these adapter proteins are needed for optimal coupling of these upstream PTKs to PLC-γ1. Although the sequence of events that leads to PLC-γ1 activation remains unclear, both the amino- and carboxyl-terminal SH2 [SH2(N) and SH2(C), respectively] domains are required for phosphorylation and activation of the enzyme during PDGF receptor (29) or BCR stimulation (15). In addition to mediating associations with phosphotyrosine-containing proteins, the PLC-γ1 SH2(C) domain binds to phosphatidylinositol-3,4,5-trisphosphate, which suggests that this region might also receive a regulatory input from phosphoinositide 3-kinase (3, 46).
Efforts to define both the activation mechanism and function of PLC-γ1 in T cells have been hindered by the lack of a genetically manipulatable model system in which TCR signaling is compromised due to deficient expression of PLC-γ1. In this report, we describe the isolation of two Jurkat T-cell-derived sublines, designated P98 and J.gamma1, which display partial to complete loss of PLC-γ1 protein expression, respectively. While TCR-dependent Ca2+ signaling is seriously compromised in J.gamma1 cells, the P98 cell line displays wild-type patterns of Ca2+ mobilization and NFAT activation during TCR stimulation. However, the P98 cells show significant defects in the activation of IL-2 promoter-dependent transcription, together with a specific defect in the activation of the composite CD28 response element—the AP-1 (RE/AP) site (54) found in the IL-2 promoter. These transcriptional defects were reversed by expression of wild-type PLC-γ1 but not by that of mutant PLC-γ1 bearing loss-of-function mutations in the catalytic domain, the SH2(C) domain, or the Tyr783 phosphorylation site. The results of these studies indicate that the SH2(N) and SH2(C) domains make differential contributions to the regulation and function of PLC-γ1 and point toward a previously unrecognized role for this enzyme in the activation of the RE/AP element of the IL-2 promoter in T lymphocytes.
MATERIALS AND METHODS
Reagents and cell lines.
The human CD3 ɛ-specific monoclonal antibody (MAb) OKT3 (65) was purified from murine ascites by chromatography over a protein G-agarose affinity column. The antiphosphotyrosine MAb 4G10 was obtained from Upstate Biotechnology, Inc. (Lake Placid, N.Y.). The anti-AU1 MAb, which recognizes the epitope tag sequence DTYRYI (single-letter amino acid code), was obtained from BabCo (Richmond, Calif.). The anti-FLAG M2 MAb, which recognizes the epitope tag sequence DYKDDDDK, was obtained from Sigma (Saint Louis, Mo.). The rabbit polyclonal anti-PLC-γ1 antibody used was described previously (62). Rabbit polyclonal anti-ZAP-70, PLC-γ2, and -SLP-76 antibodies were kindly provided by Paul J. Leibson (Mayo Foundation, Rochester, Minn.), and the polyclonal anti-LAT antibody used was a gift from Larry Samelson (National Institutes of Health, Bethesda, Md.). Mouse ascites fluid containing the anti-CD28 MAb 9.3 was provided by David McKean (Mayo Foundation).
The P98 subline was derived by treatment of Jurkat E6 cells with the frameshift mutagen ICR-191, followed by repetitive rounds of selection for cells with defects in pervanadate (PV)-induced Ca2+ mobilization (69). The J.gamma1 subline was isolated by repeating the mutagenesis-mutant selection procedure with P98 cells as the starting cell population. The parental Jurkat E6 cell line and all mutant cell lines were maintained in standard growth medium (RPMI 1640 medium buffered to pH 7.4 with 10 mM HEPES and supplemented with 10% fetal bovine serum). Cells were maintained in culture at densities below 8 × 105/ml. K562 erythroleukemia cells were obtained from the American Type Culture Collection (Manassas, Va.) and were maintained as described above.
Plasmids.
The full-length bovine PLC-γ1 cDNA was obtained from John Knopf (Genetics Institute, Cambridge, Mass.). The PLC-γ1 open reading frame was connected at the 5′ terminus with a nucleotide sequence encoding two tandem AU1 epitope tags and was cloned into the mammalian expression vector pcDNA3 (Invitrogen, San Diego, Calif.). The resulting expression vector was designated pcaPLC-γ1. Mutagenesis of the PLC-γ1 insert in this plasmid was performed with the Transformer Site-Directed Mutagenesis kit (Clontech, Palo Alto, Calif.). The mutagenized PLC-γ1 expression vectors were named as follows (the introduced amino acid substitutions are in parentheses): pcaPLC-γ1 LI (His335→Phe), pcaPLC-γ1 SH2(N)* (Arg586→Lys), pcaPLC-γ1 SH2(C)* (Arg694→Lys), pcaPLC-γ1 SH2(N,C)* (Arg586→Lys, Arg694→Lys), pcaPLC-γ1 SH3* (Pro842→Lys), pcaPLC-γ1 Y783F (Tyr783→Phe), and pcaPLC-γ1 SH3*,Y783F (Pro842→Leu, Tyr783→Phe).
The pIL-2-luciferase (Luc) reporter plasmid was obtained from by Ellen Rothenberg (California Institute of Technology, Pasadena). The 3× pNFAT- and 3× NFκB-Luc(33) reporter plasmids were provided by David McKean (Mayo Foundation). The 4× pAP-1 Luc reporter plasmid (71) was provided by Xiao-Fan Wang (Duke University, Durham, N.C.). 4× pRE/AP Luc was provided by Arthur Weiss (University of California at San Francisco) (54). The Myc-tagged wild-type ZAP-70 expression plasmid was described previously (68). The FLAG-tagged SLP-76 expression vector and the glutathione _S_-transferase (GST)–Grb2 SH2-encoding bacterial expression vector were provided by Gary Koretzky (University of Pennsylvania, Philadelphia). The GST-γ1 SH2(N) and GST-γ1 SH2(C) bacterial expression plasmids and the methods used for the purification of bacterially expressed GST fusion proteins were described previously (68).
Transient-transfections and luciferase assays.
P98 or K562 cells were harvested and resuspended at 5.7 × 107/ml in standard growth medium. The cells (2 × 107 per sample) were transfected by electroporation with the indicated PLC-γ1 expression plasmids, together with LAT-, ZAP-70- or SLP-76-encoding plasmids. The plasmid DNA amounts varied among experiments and are noted in the figure legends. The total amount of plasmid DNA used in each transfection was brought up to 40 μg with empty vector pcDNA3. Cells were transfected by square-wave electroporation as previously described (68).
For luciferase reporter gene assays, Jurkat or P98 cells were resuspended in growth medium at 4 × 107/ml. The cells (107 per sample) were cotransfected with the different pcaPLC-γ1 expression plasmids (amounts are indicated in the figure legends) plus 10 μg of pIL2 Luc, pNFAT Luc, pNFκB, pAP-1 Luc, or pRE/AP Luc reporter plasmid DNA. The total amount of DNA in each transfection was brought up to 30 μg with the filler pcDNA3. The cells were transfected as described previously (38). After transfection, the cells were diluted with 20 ml of medium and divided into four equivalent aliquots. After 16 to 18 h in culture, the cells were stimulated with 20 ng of phorbol myristate acetate (PMA; Sigma) per ml plus either 1 μg of MAb OKT3 per ml or 2 μM ionomycin (Calbiochem, La Jolla, Calif.). Where indicated, CD28 costimulation was done by addition of MAb 9.3-containing ascites fluid, at a volume ratio of 1:5,000, to each sample. Unstimulated control samples received the appropriate solvent vehicles only. After 6 h, the stimulated cells were harvested and lysed with Promega passive lysis buffer (Promega, Madison, Wis.). Luciferase activities were determined with Luciferase Assay Reagent II (Promega) using an EG&G Berthold Lumat LB 9507 luminometer (Wallac Inc., Gaithersburg, Md.).
Cellular stimulations, protein precipitations, and immunoblot analyses.
Cells were stimulated at 37°C in a final volume of 200 μl of solution 2 (Hanks balanced salt solution buffered to pH 7.4 with 10 mM HEPES and supplemented with 5 mM glucose) containing either 1 μg of MAb OKT3 per ml cross-linked with 10 μg of goat anti-mouse immunoglobulin G (Pierce, Rockford, Ill.) per ml or 100 μM PV as described previously (51).
For immunoprecipitations, the stimulated cell suspensions were lysed with 800 μl of MT lysis buffer (25 mM HEPES, 150 mM NaCl, 5 mM EDTA, 0.5 mM CaCl2, 1 mM Na3VO4, 10% glycerol, 0.2% [wt/vol] NP-40, 0.2% Tween 20, 10 μg of leupeptin per ml, 10 μg of aprotinin per ml, 5 μg of pepstatin per ml). The cleared extracts were mixed for 1 h at 4°C with 1 μl of anti-AU1 antibody-containing ascites fluid plus 10 μl (packed volume) of protein A-Sepharose and 10 μl of protein G-agarose beads. Immunoprecipitates were washed two times in MT lysis buffer and then boiled in 30 μl of 2× sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer (36). An aliquot (30 μl) of each whole-cell extract was set aside for direct immunoblotting before immunoprecipitation. The cellular extracts were mixed with 30 μl of 4× SDS-PAGE sample buffer and denatured by heating for 5 min at 100°C. The denatured proteins were resolved by SDS-PAGE and transferred electrophoretically to an Immobilon P membrane (Millipore, Bedford, Mass.). Membranes were blocked in Tris-buffered saline containing 0.2% Tween 20 supplemented with either 5% milk (for anti-AU1 and anti-FLAG immunoblotting) or 2% bovine serum albumin (for anti-Ptyr, anti-PLC-γ1, anti-ZAP-70, anti-SLP-76, and anti-LAT immunoblotting). Immunoblotting was carried out with the specified antibodies, followed by treatment of the membranes with either horseradish peroxidase-coupled protein A (Amersham, Piscataway, N.J.) for anti-PLC-γ1, anti-ZAP-70, anti-SLP-76, and anti-LAT immunoblots or with horseradish peroxidase-coupled sheep anti-mouse immunoglobulin G (Amersham) for anti-AU1, anti-FLAG, or anti-Ptyr immunoblots. Immunoreactive proteins were visualized with the Renaissance enhanced-chemiluminescence reagent (NEN, Boston, Mass.).
Precipitations with GST fusion proteins were performed with extracts prepared from either Jurkat cell lines or K562 cells. The cells were suspended in solution 2 as described above and stimulated for various times with 100 μM PV. The reactions were terminated with 800 μl of lysis buffer B (25 mM Tris HCl, 150 mM NaCl, 5 mM EDTA [pH 7.4] containing 1% Triton X-100, 1 mM Na3VO4, leupeptin at 10 μg/ml, aprotinin at 10 μg/ml, and pepstatin A at 5 μg/ml). The cellular extracts were precleared by mixing for 30 min at 4°C with 2 μg of GST immobilized on 15 μl of packed glutathione (GSH)-agarose beads. The cleared extracts were transferred to tubes containing 15 μl of packed GSH-agarose beads loaded with 2.5 μg of the indicated GST fusion proteins. After mixing for 2 h at 4°C, the beads were precipitated by centrifugation and washed three times with lysis buffer B. The precipitated proteins were solubilized in 30 μl of 2× SDS-PAGE sample buffer, separated by SDS-PAGE, and immunoblotted as described above.
Intracellular free-Ca2+ measurements.
Cells were loaded with the Ca2+ indicator dye indo-1/AM and stimulated with 1 μg of OKT3 per ml as described previously (69). Stimulus-induced changes in the intracellular Ca2+ concentration were determined by monitoring the fluorescence emission ratio of the Ca2+-bound versus the free form of Indo-1 at 405 and 495 nM, respectively, on a FACStar Plus cell sorter (Becton Dickinson). Time-dependent changes in the 405-nm/495-nm fluorescence emission ratio of Indo-1 are directly related to changes in the intracellular free-Ca2+ concentration.
PLC activity measurements.
K562 cells were transiently transfected with the indicated pcaPLC-γ1 expression plasmids, and after 24 h in culture, the transfected cells were washed, resuspended in solution 2, and stimulated with 100 μM PV as described above. After stimulation, the cells were lysed with 800 μl of PLC lysis buffer (20 mM Tris, 100 mM NaCl, 50 mM β-glycerophosphate, 10% glycerol, 0.5 mM dithiothreitol, 1 mM Na3VO4, 1% Triton X-100, 10 μg of leupeptin per ml, 10 μg of aprotinin per ml, 5 μg of pepstatin per ml). The cellular extracts were immunoprecipitated with anti-AU1 antibody as described above. The immunoprecipitates were washed twice in PLC lysis buffer and once with reaction buffer (35 mM sodium phosphate, 70 mM KCl, 0.8 mM CaCl2, 0.8 mM EGTA, 0.5 mM dithiothreitol, 0.1% Triton X-100 [pH 6.8]). The PLC activity in the immunoprecipitates was assayed under linear reaction conditions with micelles containing phosphatidylinositol-4,5-bisphosphate [inositol-2-3H(N); specific activity, 12 Ci/mmol] (NEN) as the substrate (52).
RESULTS
Isolation of PLC-γ1-deficient Jurkat T-cell sublines.
The P98 cell line emerged from a mutant selection strategy designed to enrich for Jurkat somatic mutants bearing defects in TCR-dependent Ca2+ mobilization (71). This strategy has previously yielded Jurkat-derived mutants that fail to express several key signaling proteins, including ZAP-70, LAT, and Lck (69, 72; B.L.W. and R.T.A., unpublished results). In this study, we subjected a panel of sublines from the primary Ca2+-based screen to secondary immunoblotting assays with the goal of identifying PLC-γ1-deficient variant subclones. The P98 subline emerged from this secondary screen as a somatic mutant that displayed a >90% reduction in PLC-γ1 expression (Fig. 1A). The drastic decrease in anti-PLC-γ1 immunoreactive protein observed in P98 cell extracts was not simply explained by a mutation-induced loss of the epitope recognized by this particular antiserum, as immunoblot analyses with a commercially available polyclonal antibody (product no. sc-426, Santa Cruz Biotechnology, Inc.) directed against a distinct region of the protein yielded identical results (not shown). After three additional rounds of mutagenesis, the Ca2+-based cell sorting protocol was used to isolate a clonal derivative of the P98 subline, designated J.gamma1, which expressed no detectable PLC-γ1 protein (Fig. 1A).
FIG. 1.
Phenotypic characterization of P98 cells. (A) PLC-γ1 expression. Jurkat E6, P98, and J.gamma1 cells and J.gamma1 cells stably expressing wild-type aPLC-γ1 (WT-1) were lysed in detergent-containing buffer. One hundred micrograms of whole-cell extract proteins was separated by SDS-PAGE and immunoblotted with PLC-γ1- and ZAP-70-specific antisera. (B) TCR-dependent calcium mobilization. Jurkat E6, P98, J.gamma1, and J.gamma1-derived WT-1 cells were loaded with Indo-1/AM and stimulated with anti-CD3 MAb OKT3. The ratio of fluorescence emission at 405 nm to that at 495 nm, which is directly proportional to the concentration of intracellular free Ca2+, is plotted as a function of time. (C) PLC-γ2 phosphorylation. The indicated Jurkat cell lines were stimulated with MAb OKT3, and cellular extracts were immunoprecipitated (IP) with anti-PLC-γ1 or anti-PLC-γ2 antibodies. The immunoprecipitated proteins were resolved by SDS-PAGE and immunoblotted sequentially with anti-phosphotyrosine (pY) and either anti-PLC-γ1 or anti-PLC-γ2 antibodies. (D) NFAT-dependent transcription. Jurkat E6 cells or the indicated Jurkat subclones were transiently transfected with a pNFAT-Luc reporter plasmid plus pcDNA3 or 3 μg of the pcaPLC-γ1 WT expression plasmid. Cells were stimulated with medium only (unstimulated), OKT3, or PMA plus ionomycin. Luciferase activities were first normalized to the maximal activity obtained with PMA plus ionomycin in each sample, and then the value obtained with Jurkat E6 cells was arbitrarily set at 100%. All other data are expressed as percentages of the maximal activity obtained with Jurkat E6 cells. The data presented are the mean ± the standard error of the mean of six independent trials.
Surprisingly, in spite of the severe reduction in total PLC-γ1 protein expression, TCR stimulation evoked a wild-type increase in [Ca2+]i in the P98 cells (Fig. 1B). As the concentration of MAb OKT3 (1 μg/ml) used in these studies represented a supramaximal stimulus for TCR-mediated signaling, we compared the patterns of Ca2+ mobilization in Jurkat E6 and P98 cells after stimulation with MAb OKT3 at concentrations ranging from 0.05 to 1 μg/ml. Again, we obtained no evidence for an acute defect in TCR-mediated Ca2+ release or extracellular Ca2+ influx in P98 cells (results not shown). In addition, we found that the population-averaged Ca2+ mobilization patterns in Jurkat E6 and P98 cells were identical when measured over an extended time frame of 0 to 120 min (results not shown). Given the above results, the mechanism whereby the P98 clone emerged from the Ca2+-based negative selection procedure remains puzzling. One rational possibility is that the P98 cell displays a more subtle alteration in [Ca2+]i oscillations at the single-cell level, which caused the P98 progenitor cell to be negatively selected during cell sorting. Regardless of the mechanism, the results obtained with P98 cells indicate that the level of PLC-γ1 protein expressed in the parental Jurkat E6 cell line is considerably in excess of that needed to trigger the normal [Ca2+]i increase during TCR stimulation.
In contrast to P98 cells, the PLC-γ1-null J.gamma1 cells showed a drastically altered pattern of [Ca2+]i changes in response to TCR cross-linkage. To confirm that this defect was related to the lack of PLC-γ1 expression, J.gamma1 cells were stably transfected with a plasmid vector encoding AU1 epitope-tagged PLC-γ1 (aPLC-γ1 WT) and the WT-1 subclone was selected for further analysis based on its expression of PLC-γ1 at levels similar to those found in Jurkat E6 cells (Fig. 1A). As shown in Fig. 1B, reintroduction of PLC-γ1 into J.gamma1 cells fully corrected the defect in TCR-dependent Ca2+ mobilization (Fig. 1B). Although TCR-dependent Ca2+ mobilization was clearly abnormal in J.gamma1 cells, we consistently noted that MAb OKT3 stimulation provoked a transient rise in [Ca2+]i (Fig. 1B). A possible explanation for this finding is that the cells express PLC-γ2 and that this second PLC-γ isoform is also activated by TCR stimulation. In preliminary immunoblotting studies, we found that Jurkat E6, P98, and J.gamma1 cells express similar levels of PLC-γ2 (results not shown). Moreover, TCR ligation triggered rapid increases in the tyrosine phosphorylation of both PLC-γ1 and PLC-γ2 in Jurkat E6 cells. The loss of PLC-γ1 expression had no effect on either the magnitude or the time course of PLC-γ2 phosphorylation in J.gamma1 cells (Fig. 1C). These findings suggest, but do not prove, that the residual TCR-dependent Ca2+ signal in J.gamma1 cells stems from the activation of PLC-γ2.
Defective IL-2 promoter activation in P98 cells.
Expression of the gene for IL-2 in activated T cells is controlled by a series of interactive transcription factors, including NFAT, NFκB, AP-1, and the Oct1-OAP complex (49, 53). Although PLC-γ1 activity may modulate the transactivating functions of many of these factors, the tight linkage between Ca2+ signaling and NFAT activation prompted speculation that NFAT-dependent transcription might be impaired in P98 and/or J.gamma1 cells. To test this possibility, transient-transfection assays were performed with an NFAT-regulated luciferase reporter gene (pNFAT Luc) (Fig. 1D). The results were consistent with those obtained in the Ca2+ mobilization assays described above. While P98 cells mounted a wild-type NFAT activation response following anti-CD3 antibody stimulation, PLC-γ1-negative J.gamma1 cells showed no increase in NFAT-dependent luciferase expression under these conditions. The NFAT activation defect was reversed by cotransfection of J.gamma1 cells with the pcaPLC-γ1 WT expression vector, which verifies that this abnormality is causally related to the absence of PLC-γ1 activity in these cells.
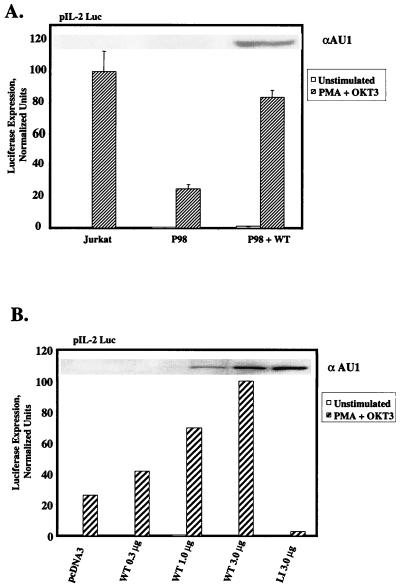
As stated above, transcriptional activation of the IL-2 gene requires multiple pathways of nuclear signaling, which are initiated by concomitant engagement of the TCR and the CD28 coreceptor (28). Consequently, we tested the hypothesis that the quantitative deficiency in PLC-γ1 expression in P98 cells might impair the delivery of a signal(s) (presumably unrelated to the [Ca2+]i elevation) required for the assembly and/or full functional activation of a multiprotein transactivating complex in the IL-2 promoter region. P98 cells were transiently transfected with the pIL2-Luc reporter plasmid, which places the luciferase cDNA under the transcriptional control of the IL-2 promoter-enhancer region. As shown in Fig. 2A, stimulation of Jurkat E6 cells with MAb OKT3 plus PMA provoked a strong increase in IL-2 promoter-dependent luciferase expression. In contrast, luciferase expression was reduced by approximately 80% in transiently transfected P98 cells under identical stimulation conditions. This transcriptional defect was largely reversed by cotransfection of the cells with an expression plasmid encoding aPLC-γ1 WT. The level of functional reconstitution in this assay was directly related to the amount of pcaPLC-γ1 plasmid DNA transfected into the cells (Fig. 2B). Furthermore, correction of the defect in IL-2 promoter transactivation depended on the introduction of catalytically active PLC-γ1, as cotransfection of the cells with a lipase-inactive mutant form of aPLC-γ1 (LI; see Fig. 4A for description) actually decreased luciferase activity below the residual level observed in P98 cells. Thus, while the reduced level of PLC-γ1 expressed in P98 cells was sufficient to support a normal, TCR-dependent NFAT activation response, PLC-γ1 expression was limiting for the delivery of an additional signal(s) needed for optimal activation of the IL-2 promoter in these cells.
FIG. 2.
Rescue of IL-2 promoter activation defect in P98 cells with wild-type PLC-γ1. (A) IL-2 promoter transactivation. Jurkat and P98 cells were transiently transfected with the pIL-2 Luc reporter plasmid plus pcDNA3 only or 3 μg of the pcaPLC-γ1 WT expression plasmid. Cells were treated with medium only (unstimulated) or stimulated with PMA plus OKT3 or PMA plus ionomycin. Luciferase activities are presented as described in the legend to Fig. 1D. The results shown are the mean ± the standard errors of the mean of three independent experiments. The inset shows the level of aPLC-γ1 expressed in each cell population. (B) Correction of the transcriptional defect in P98 cells by ectopically expressed PLC-γ1. P98 cells were transiently transfected with the pIL-2 Luc reporter plasmid plus the indicated amounts of pcaPLC-γ1 WT or lipase-inactive pcaPLC-γ1 (LI). The luciferase activities were normalized as described in the legend to Fig. 1D, except that the result obtained with cells transfected with 3 μg of pcaPLC-γ1 WT was set at 100% and all of the other values were normalized to this response. The inset shows levels of aPLC-γ1 expression in each transfected cell population.
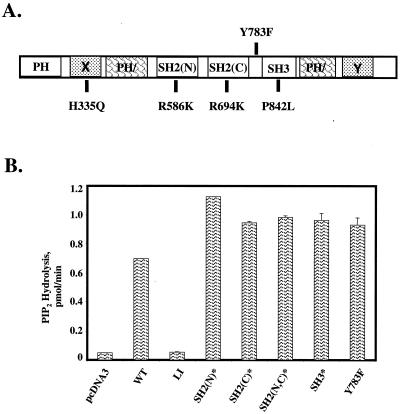
FIG. 4.
In vitro catalytic activities of PLC-γ1 mutants. (A) Structural domains of PLC-γ1 include the pleckstrin homology (PH) domain, the split PH (PH/) domain, the SH2(N), SH2(C), and SH3 domains, and the X and Y boxes, which comprise the split catalytic domain. The single amino acid substitutions used to generate the PLC-γ1 mutants used in this study are also shown. (B) Catalytic activities of wild-type (WT) and mutated aPLC-γ1 proteins. K562 cells were transiently transfected with empty plasmid pcDNA3 or with expression vectors encoding the indicated wild-type or mutated aPLC-γ1 polypeptides. The cells were stimulated for 5 min with 100 μM PV, and the recombinant PLC-γ1 proteins were immunoprecipitated with anti-AU1 antibodies. Reactions were terminated at 5 min, and the release of radioactive IP3 from 3H-labeled PIP2-containing vesicles was determined by liquid scintillation counting. The results are presented as the mean ± the variance of two experiments.
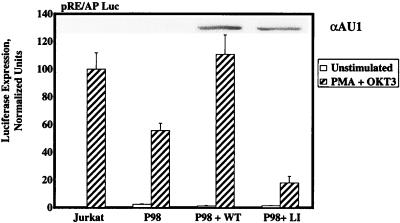
The defect in IL-2-dependent transcription in P98 cells prompted an extended series of studies to determine whether this abnormality could be attributed to any specific component of the multiprotein complex that inducibly binds to the IL-2 promoter-enhancer region. Transient-transfection studies were carried out with luciferase reporter genes containing concatemerized binding sites for AP-1 or NFκB family members, as well as the composite CD28 response element (RE/AP) from the IL-2 promoter. As observed in the pNFAT-Luc reporter assays, P98 cells were fully competent to support the transcription of AP-1- or NFκB-dependent reporter genes following stimulation with MAb OKT3, in the absence or presence of PMA, respectively (data not shown). However, these cells displayed a significant reduction in RE/AP-dependent transcription under identical stimulation conditions (Fig. 3). In accordance with the earlier report (54), stimulation of either Jurkat or P98 cells with an anti-CD3 MAb only was insufficient to activate the RE/AP element (data not shown), indicating that activation of this composite element depends on synergistic signals provided by the TCR and PMA. The defective induction of RE/AP-dependent luciferase expression observed in OKT3-plus-PMA-stimulated P98 cells was largely reversed by cotransfection of the cells with a wild-type pcaPLC-γ1 expression vector, while the catalytically inactive aPLC-γ1 LI mutant actually reduced this transcriptional response. These results suggest that the IL-2 promoter activation defect displayed by P98 cells may be attributed, at least in part, to a PLC-γ1-related decrease in transcription through the RE/AP element.
FIG. 3.
RE/AP promoter-dependent gene expression in Jurkat-derived subclones. Jurkat E6 cells or P98 cells were transiently transfected with the pRE/AP-Luc reporter plasmid plus pcDNA3, 1 μg of pcaPLC-γ1 WT, or 3 μg of the pcaPLC-γ1 LI expression plasmid. Cells were treated with medium only (unstimulated) or stimulated with PMA plus OKT3 or PMA plus ionomycin. Luciferase activities are reported as described in the legend to Fig. 1D. The histogram represents the mean ± the standard errors of the mean of six independent trials. The inset shows levels of aPLC-γ1 expression in whole-cell extracts.
Effects of SH region mutations on PLC-γ1 activity in vitro.
To determine the roles of various PLC-γ1 subdomains in the complementation of the defect in IL-2 promoter activation in P98 cells, we generated a series of PLC-γ1 mutants (indicated by an asterisk) bearing single amino acid substitutions that disabled the SH2(N), SH2(C), or SH3 domain (Fig. 4A). The SH2(N)* and SH2(C)* domain mutants contained Arg→Lys substitutions in the conserved Phe-Leu-Val-Arg motif (the mutated residue is underlined) that functions in the binding of phosphotyrosine (41), while the SH2(N,C)* double mutant contained inactivating point mutations in both SH2 domains. The PLC-γ1 SH3* mutant contains a Pro842→Leu substitution that alters a conserved proline residue in the SH3 ligand recognition subdomain (9). An identical substitution was previously shown to inactivate the SH3 domain of the Caenorhabditis elegans Grb2 homolog (12). The previously mentioned aPLC-γ1 LI mutant contained a single amino acid substitution in the catalytic X box (His335→Phe) which disables the phospholipase activity of this protein (57). Finally, we converted the known Tyr783 phosphorylation site in PLC-γ1 (39, 44) to a nonphosphorylatable Phe residue (PLC-γ1 Y783F).
As a first step toward the characterization of the functional competencies of these mutated forms of PLC-γ1, we determined the in vitro phosphoinositide-hydrolyzing activities of the mutated PLC-γ1 proteins with 3H-labeled PIP2 as the substrate. The wild-type and mutated PLC-γ1 constructs were transiently expressed in K562 cells, and the transfected cells were stimulated with PV to induce maximal tyrosine phosphorylation of each protein. Detergent extracts from each cell population were immunoprecipitated with an anti-AU1 MAb, and the immunoprecipitates were assayed for PLC activity with PIP2-containing detergent micelles as the substrate. The extracts were immunoblotted to ensure approximately equivalent expression of the recombinant aPLC-γ1 proteins (results not shown). With the exception of the catalytically inactive PLC-γ1 LI mutant (56), the wild-type and mutated forms of PLC-γ1 displayed similar levels of PIP2-hydrolyzing activity in vitro (Fig. 4B). These results suggested that the intrinsic phosphoinositide-hydrolyzing activity of PLC-γ1 does not depend on either phosphorylation at Tyr783 or the binding of protein ligands to the SH2 and SH3 domains.
Role of the PLC-γ1 SH3 domain in IL-2 promoter-driven transcription.
To examine the role of the PLC-γ1 SH3 domain in TCR-dependent signaling, we transiently cotransfected P98 cells with the pcaPLC-γ1 SH3* expression plasmid and the pIL2-Luc reporter plasmid. The aPLC-γ1 SH3* mutant fully restored IL-2 promoter-driven luciferase expression in these cells compared to that in wild-type PLC-γ1-transfected cells (Fig. 5A). Indeed, aPLC-γ1 SH3* expression increased basal luciferase activity by 2- to 10-fold in repeated trials (Fig. 5A, inset) and significantly enhanced the transcriptional responses induced by either OKT3-plus-PMA or ionomycin-plus-PMA costimulation. Although the immunoblot shown in Fig. 5A indicates that aPLC-γ1 SH3* was expressed at a slightly higher level than its wild-type counterpart, the results of four independent trials demonstrated that the apparent hyperactivity of the aPLC-γ1 SH3* mutant was not explained by the relative expression levels of the aPLC-γ1 WT and SH3* proteins (results not shown). Given that aPLC-γ1 SH3* showed no indication of elevated catalytic activity in the in vitro PLC assays (Fig. 4B), this result suggests that the SH3 domain negatively regulates PLC-γ1 activity in intact Jurkat cells. Whether the dampening effect of the SH3 domain on PLC activity involves an interaction with a heterologous regulatory protein such as Cbl (18, 24) or an intramolecular, autoinhibitory mechanism similar to that described for the Src family PTKs (42, 55) remains unclear.
FIG. 5.
Effects of PLC-γ1 mutants on IL-2 promoter activation. (A) IL-2 promoter transactivation. P98 cells were transiently transfected with the pIL-2 Luc reporter plasmid plus one of the following pcaPLC-γ1 expression plasmids: 3 μg of WT, 3 μg of SH3*, 5 μg of Y783F, or 5 μg of SH3*,Y783F. The amounts of pcaPLC-γ1 plasmid DNAs were adjusted to yield approximately equivalent expression of the various PLC-γ1 polypeptides (inset). The transfected cells were treated with medium only (unstim.) or stimulated with OKT3 plus PMA (striped bars) or with ionomycin plus PMA (solid bars), and IL-2 promoter-dependent luciferase activities were determined and reported as relative light units (R.L.U.). The luciferase activities measured in OKT3-plus-PMA-stimulated samples were also normalized to the maximal responses obtained with parallel samples stimulated with ionomycin plus PMA. The actual percentages for each transfected population are as follows: pcDNA3, 16%; WT, 94%; SH3*, 65%; Y783F, 2%; SH3*, Y783F, 3%. (B) Inhibition of IL-2 promoter activation by aPLC-γ1 Y783F. Jurkat E6 cells were transiently transfected with the pIL-2 Luc reporter plasmid plus the indicated amounts of the pcaPLC-γ1 Y783F expression plasmid (Y783F). Luciferase activities are normalized as described in the legend to Fig. 1D. The values shown are the means ± the standard errors of the mean of three experiments. The inset shows the expression level of aPLC-γ1 Y783F in each sample.
Role of Tyr783 phosphorylation in PLC-γ1 activation.
Phosphorylation of Tyr783 in the SH region of PLC-γ1 is required for full activation of this enzyme in PDGF-stimulated fibroblasts (39). As Tyr783 is also known to undergo phosphorylation in response to TCR stimulation (44), we were interested in determining whether the aPLC-γ1 Y783F mutant complemented the IL-2 promoter activation defect in P98 cells. In contrast to aPLC-γ1 WT, aPLC-γ1 Y783F failed to restore OKT3-plus-PMA-induced luciferase expression from the pIL2-Luc reporter plasmid, indicating that phosphorylation at this site was essential for coupling of TCR stimulation to IL-2 promoter activation in P98 cells (Fig. 5A). Based on the mechanism proposed for the regulation of Src kinase activity (42, 55), we reasoned that phosphorylation of Tyr783 might be required to overcome an SH3 domain-mediated intramolecular interaction that interferes with the access of substrates to the catalytic domain. Consequently, we prepared a PLC-γ1 double mutant that contains the Tyr783→Phe substitution plus the SH3 domain-disabling Pro842→Leu substitution described above. However, when expressed in P98 cells, the aPLC-γ1 SH3*,Y783F double mutant was as defective as the aPLC-γ1 Y783F single mutant, indicating that Tyr783 phosphorylation does not counteract a negative regulatory influence of the SH3 domain on PLC-γ1 activity in P98 cells.
In repeated trials, transfection of pcaPLC-γ1 Y783F into P98 cells actually decreased IL-2 promoter-dependent luciferase expression below that observed in mock-transfected cells. To determine whether this phosphorylation site mutant might be a dominant-acting suppressor of PLC-γ1 signaling functions, we cotransfected wild-type Jurkat E6 cells with the pIL2-Luc reporter and progressively increasing amounts of pcaPLC-γ1 Y783F plasmid DNA. Expression of increasing amounts of aPLC-γ1 Y783F caused progressive inhibition of OKT3-plus-PMA-induced luciferase expression in Jurkat E6 cells (Fig. 5B). Transfection of these cells with only 0.5 μg of pcaPLC-γ1 Y783F plasmid DNA inhibited reporter gene expression by approximately 50%, indicating that aPLC-γ1 Y783F is a potent suppressor of TCR-dependent IL-2 promoter activation. In contrast, parallel titration experiments with the pcaPLC-γ1 WT expression vector demonstrated that the nonmutated PLC-γ1 WT protein did not inhibit the OKT3-plus-PMA-induced luciferase expression in Jurkat E6 cells (data not shown).
Effects of SH2(N) and SH2(C) mutations on PLC-γ1-dependent signaling.
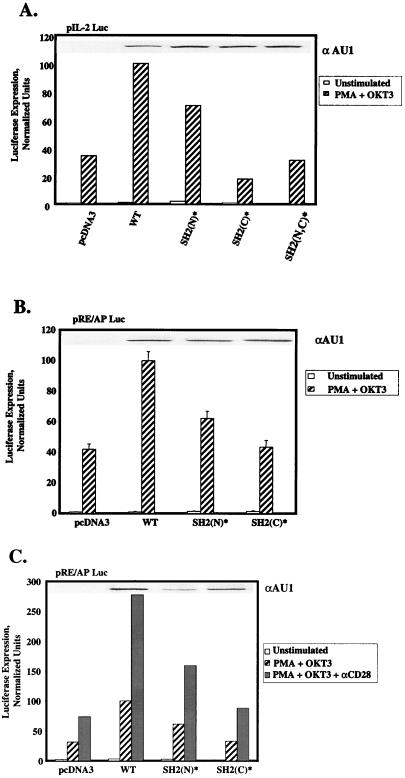
Previous studies indicated that the SH2 domains of PLC-γ1 contribute to the plasma membrane localization and tyrosine phosphorylation of this protein during cell surface receptor stimulation (15, 29). In order to examine the roles of the SH2(N) and SH2(C) domains of PLC-γ1 in TCR-mediated PLC-γ1 activation, we determined whether the PLC-γ1 SH2(N)* and SH2(C)* single mutants, as well as a PLC-γ1 SH2(N,C)* double mutant, complemented the IL-2 promoter activation defect in P98 cells. Expression of aPLC-γ1 SH2(N)* in P98 cells significantly enhanced OKT3-plus-PMA-induced luciferase expression from the pIL2-Luc reporter plasmid in P98 cells (Fig. 6A). However, the transcriptional response to OKT3-plus-PMA stimulation in aPLC-γ1 SH2(N)*-expressing cells was consistently reduced (5 to 35% reduction in four independent trials) relative to that observed in cells transfected with the aPLC-γ1 WT expression vector. In contrast, the PLC-γ1 SH2(C)* mutant completely failed to reverse the defect in IL-2 promoter-driven transcription in these cells. Over four independent trials, OKT3-plus-PMA-stimulated luciferase expression in aPLC-γ1 SH2(C)*-expressing P98 cells was not significantly different than that observed in mock-transfected cells. The aPLC-γ1 SH2(N,C)* double mutant also showed no complementing activity in these assays. Immunoblot analyses of detergent-soluble proteins with the tag-specific anti-AU1 MAb ruled out the possibility that the dramatic functional difference between the aPLC-γ1 SH2(N)* and SH2(C)* mutants was due to differences in the expression levels of these proteins. Collectively, these results suggest that the PLC-γ1 SH2(C) domain plays a unique role in the delivery of an undefined signal(s) leading to IL-2 promoter transactivation in P98 cells.
FIG. 6.
Effects of PLC-γ1 SH2(N) and SH2(C) domain mutations on IL-2 promoter- and RE/AP-dependent transcription. (A) P98 cells were transiently transfected with the pIL-2 Luc reporter plasmid plus pcDNA3 only or one of the following pcaPLC-γ1 expression plasmids: 3 μg of WT, 3 μg of SH2(N)*, 5 μg of SH2(C)*, or 5 μg of SH2(N,C)*. Cellular stimulations were performed, and luciferase activities were determined as described in the legend to Fig. 1D, except that the value obtained with the pcaPLC-γ1 WT-transfected sample was set at 100% and all of the remaining values were normalized to this sample. The expression level of each aPLC-γ1 polypeptide was determined by immunoblotting (inset). (B) P98 cells were transiently transfected with the pRE/AP-Luc reporter plasmid plus pcDNA3 or one of the pcaPLC-γ1 expression plasmids as described for panel A. Cellular stimulations were performed, and luciferase activities were determined as described above. The values shown are the mean ± the standard error of the mean of three experiments. The expression level of each aPLC-γ1 polypeptide was determined by immunoblotting (inset). (C) P98 cells were transiently transfected with the RE/AP reporter and various aPLC-γ1 expression vectors as described for panel A. The cells were stimulated with MAb OKT3 plus PMA, minus or plus an anti-CD28 MAb. Luciferase activities were determined as described above. The expression level of each aPLC-γ1 polypeptide was determined by immunoblotting (inset).
The results presented in Fig. 3 indicate that P98 cells exhibit a specific defect in the activation of the IL-2 promoter-derived RE/AP element. To determine whether this response was also dependent on an intact SH2(C) domain, we cotransfected the P98 cells with the RE/AP reporter plasmid, together with a pcaPLC-γ1 WT, pcaPLC-γ1 SH2(N)*, or pcaPLC-γ1 SH2(C)* expression vector (Fig. 6B). Expression of aPLC-γ1 SH2(N)* increased RE/AP activation by an average of 37% (three experiments) relative to that obtained in aPLC-γ1 WT-transfected cells. In contrast, the aPLC-γ1 SH2(C)* mutant exhibited no reconstituting activity in this assay. Maximal activation of the RE/AP element in Jurkat cells is induced by cellular stimulation with a cocktail of anti-CD3 and anti-CD28 antibodies plus PMA (55). To investigate the possibility that CD28 signaling modified the requirement for the PLC-γ1 SH2(C) domain, the RE/AP reporter gene studies were repeated with P98 cells stimulated with MAb OKT3 plus PMA in the absence or presence of anti-CD28 MAb 9.3 (Fig. 6C). Although CD28 stimulation strongly increased RE/AP-dependent luciferase expression, the enhancement of this response by exogenously introduced PLC-γ1 remained fully dependent on the presence of an intact SH2(C) domain.
Roles of SH2 domains in TCR-dependent PLC-γ1 phosphorylation.
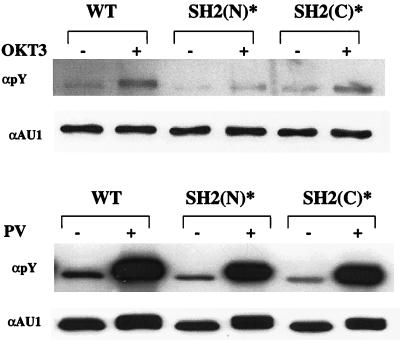
A predicted function of one or both of the PLC-γ1 SH2 domains is to promote the phosphorylation of PLC-γ1 by colocalizing the enzyme with TCR-regulated Src and/or Syk family PTKs. To examine this notion in further detail, we transiently expressed the pcaPLC-γ1 WT, pcaPLC-γ1 SH2(N)*, or pcaPLC-γ1 SH2(C)* expression plasmid in P98 cells and stimulated these cells with MAb OKT3 for 3 min, a time at which maximal PLC-γ1 phosphorylation is observed in nontransfected wild-type Jurkat T cells (results not shown). The cells were lysed, and detergent-soluble proteins were immunoprecipitated with an anti-AU1 MAb. The immunoprecipitated proteins were resolved by SDS-PAGE and immunoblotted with an antiphosphotyrosine antibody (Fig. 7, top). Relative to the aPLC-γ1 WT protein, the tyrosine phosphorylation of aPLC-γ1 SH2(C)* was moderately reduced in MAb OKT3 stimulated P98 cells. However, as reported previously (58), mutation of the SH2(N) domain virtually abrogated the phosphorylation of aPLC-γ1 under the same stimulation conditions. The severe defect in aPLC-γ1 SH2(N)* phosphorylation was not explained by a difference in the time course of this response relative to that observed with the aPLC-γ1 WT (results not shown). However, the aPLC-γ1 SH2(N)* mutant was not completely refractory to PTK-dependent phosphorylation in P98 cells, as both the wild-type and mutated aPLC-γ1 proteins were phosphorylated at approximately equivalent levels in PV-stimulated P98 cells (Fig. 7, bottom). PV is a potent, cell membrane-permeable protein tyrosine phosphatase inhibitor that is capable of activating Src and Syk family PTKs in Jurkat T cells (51). Thus, the SH2(N) domain appears to be particularly important for the effective presentation of PLC-γ1 to the upstream PTKs that are activated during ligand-induced TCR aggregation.
FIG. 7.
Roles of SH2(N) and SH2(C) domains in PLC-γ1 phosphorylation. P98 cells were transiently transfected with pcaPLC-γ1 WT (5 μg), SH2(N)* (5 μg), or SH2(C)* (15 μg). The transfected cells were stimulated for 3 min with MAb OKT3 (top) or for 5 min with 100 μM PV (bottom). Detergent-soluble proteins were immunoprecipitated with an anti-AU1 MAb, separated by SDS-PAGE, and immunoblotted with an anti-phosphotyrosine (pY) antibody. The membrane was stripped and reprobed with an anti-AU1 MAb.
Binding of LAT to the SH2(N) domain of PLC-γ1.
The importance of the SH2(N) domain in the phosphorylation of PLC-γ1 by TCR-linked PTKs prompted a search for candidate ligands that might promote localization of PLC-γ1 to the activated TCR complex. A potential SH2(N) domain interactor is the membrane-localized adapter protein LAT, which undergoes tyrosine phosphorylation and association with PLC-γ1 during TCR stimulation (73). In the initial studies, we transfected K562 erythroleukemia cells with a LAT expression vector and stimulated the cells with PV to induce intracellular protein tyrosine phosphorylation. Detergent extracts were prepared from these cells, and the soluble proteins were precipitated with purified GST fusion proteins containing the isolated SH2(N) or SH2(C) domain of PLC-γ1. A GST fusion protein containing the SH2 domain of Grb2, which is known to bind to LAT, served as a positive control (73). The results showed that GST fusion proteins containing the SH2(N) or SH2(C) domain of PLC-γ1 precipitated LAT from PV-stimulated cell extracts, while precipitates with GST alone showed no inducible LAT binding activity (Fig. 8A). Although this assay does not allow comparisons of the actual LAT binding affinities of the PLC-γ1 SH2(N) and SH2(C) domains, we consistently observed that the GST-γ1 SH2(C) fusion protein precipitated more LAT from the cellular extracts than did the GST-γ1 SH2(N) protein. Nonetheless, the precipitation studies with GST fusion proteins indicated that either SH2 domain of PLC-γ1 is capable of recognizing tyrosine-phosphorylated LAT in detergent extracts prepared from K562 cells.
FIG. 8.
Binding of LAT to PLC-γ1 SH2(N) and SH2(C) domains. (A) Precipitations with GST fusion proteins. K562 erythroleukemia cells were transiently transfected with 10 μg of Myc-tagged LAT expression plasmid, and the transfected cells were stimulated for 3 min with either medium only or 100 μM PV. The cells were lysed in detergent-containing buffer, and the extracts were precipitated with 2.5 μg of GST only, GST-γ1 SH2(N), or GST-γ1 SH2(C) fusion proteins bound to GSH-agarose beads. After resolution by SDS-PAGE, the precipitated proteins were immunoblotted with anti-LAT and anti-GST antibodies. (B) Binding of LAT to full-length PLC-γ1. K562 cells were transiently transfected with 10 μg of the indicated pcaPLC-γ1 expression plasmids, together with 10 μg of pcDNA3 or the Myc-tagged LAT expression plasmid. The transfected cells were stimulated for 3 min with either medium only or 100 μM PV. Detergent-soluble proteins were immunoprecipitated (IP) with an anti-AU1 MAb, separated by SDS-PAGE, and immunoblotted sequentially with LAT-specific antiserum and the anti-AU1 MAb. An aliquot of the whole-cell extract (WCE) was also immunoblotted with the anti-LAT serum to verify equivalent LAT protein expression in each sample (bottom).
In order to determine whether the PLC-γ1 SH2(N) and SH2(C) domains served as redundant LAT-binding regions in the context of full-length PLC-γ1, we cotransfected K562 cells with LAT and the pcaPLC-γ1 WT, pcaPLC-γ1 SH2(N)*, pcaPLC-γ1 SH2(C)*, or pcaPLC-γ1 SH2(N,C)* expression plasmid. After cellular stimulation with PV, detergent-soluble proteins were immunoprecipitated with an anti-AU1 MAb. The immunoprecipitates were then analyzed for the presence of coprecipitating LAT by immunoblotting. In contrast to the fusion protein experiments described above, these coprecipitation experiments revealed that the inducible binding of full-length PLC-γ1 to LAT was strictly dependent on the integrity of the SH2(N) domain (Fig. 8B). On the other hand, mutation of the SH2(C) domain did not impair, and may have actually increased, the PV-inducible interaction of PLC-γ1 with LAT.
Binding of ZAP-70 and SLP-76 to the PLC-γ1 SH2(C) domain.
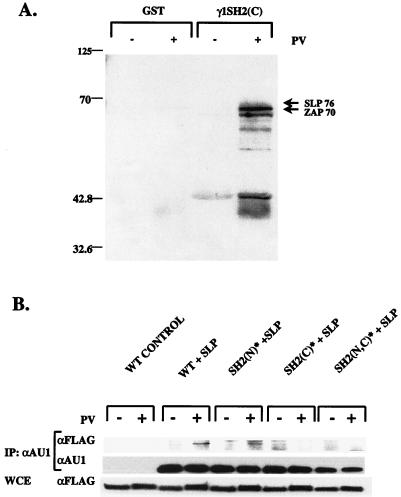
Potential ligands for the PLC-γ1 SH2(C) domain were first identified in precipitation experiments with GST fusion proteins. Cellular extracts from unstimulated or PV-stimulated Jurkat E6 cells were mixed with either immobilized GST or GST-γ1 SH2(C) fusion protein, and the bound proteins were separated by SDS-PAGE and immunoblotted with anti-phosphotyrosine antibodies (Fig. 9A). As previously reported (58), several phosphotyrosyl proteins were specifically precipitated with the GST-γ1 SH2(C) fusion protein but not by GST alone or by the GST-γ1 SH2(N) fusion protein (results not shown). Two of the major GST-γ1 SH2(C)-binding phosphoproteins were identified as ZAP-70 and SLP-76 by reprobing of the membrane with specific antibodies. Although the possibility of indirect interactions with the fusion protein cannot be ruled out, it is interesting that both ZAP-70 and SLP-76 appear to lie upstream of PLC-γ1 in the TCR signaling pathway (68–70).
FIG. 9.
Binding of phosphotyrosyl proteins to the PLC-γ1 SH2(C) domain. (A) Precipitations with GST fusion proteins. P98 cells were stimulated for 2 min with medium only or 100 μM PV. Detergent extracts were precipitated with 2.5 μg of immobilized GST only or GST-γ1 SH2(C) fusion protein. The bound proteins were separated by SDS-PAGE and immunoblotted with an anti-phosphotyrosine antibody. The protein bands indicated with arrows were identified as SLP-76 and ZAP-70 by subsequent immunoblotting of the same membrane with the respective protein-specific antisera. The numbers on the left indicate molecular weights of protein calibration markers. (B) SH2(C) domain-dependent binding of SLP-76. P98 cells were transiently transfected with pcaPLC-γ1 WT (5 μg), SH2(N)* (5 μg), or SH2(C)* (15 μg) plasmid DNA plus either pcDNA3 or FLAG-tagged SLP-76-encoding plasmid DNA (5 μg). Samples were stimulated for 5 min with either medium only or 100 μM PV. Detergent-soluble proteins were immunoprecipitated (IP) with an anti-AU1 MAb, separated by SDS-PAGE, and immunoblotted with an anti-FLAG MAb. The membrane was stripped and reprobed with the anti-AU1 MAb to determine the amount of PLC-γ1 immunoprecipitated in each sample. An aliquot of the whole-cell extract (WCE) was immunoblotted with the anti-FLAG antibody to determine the expression level of FLAG–SLP-76 in each sample (bottom).
The analysis of PLC-γ1 SH2(C)-interacting proteins was next extended to the full-length polypeptide by transfection of the AU1-tagged PLC-γ1 WT, SH2(N)*, SH2(C)*, and SH2(N,C)* expression vectors into P98 cells. The various aPLC-γ1 constructs were cotransfected into P98 cells, together with FLAG epitope-tagged SLP-76 or Myc-tagged ZAP-70. Detergent extracts from unstimulated or PV-stimulated cells were immunoprecipitated with an anti-AU1 MAb and were immunoblotted with an anti-FLAG or an anti-Myc antibody in order to detect coprecipitating SLP-76 or ZAP-70, respectively. We detected a constitutive, low level of coprecipitating Myc–ZAP-70 in aPLC-γ1 WT immunoprecipitates, the significance of which is uncertain due to the overexpression of both proteins (results not shown). However, in parallel experiments, PV stimulation clearly induced the association of FLAG–SLP-76 with both aPLC-γ1 WT and the aPLC-γ1 SH2(N)* mutant (Fig. 9B). This interaction depended on an intact SH2(C) domain, as the aPLC-γ1 SH2(C)* and aPLC-γ1 SH2 (N,C)* mutants showed no detectable increase in binding to FLAG–SLP-76 in extracts from PV-stimulated P98 cells. Thus, the signaling functions of the PLC-γ1 SH2(C) domain may be mediated, at least in part, through an inducible association with the adapter protein SLP-76.
DISCUSSION
In this study, we used a random somatic mutagenesis-mutant selection protocol to isolate two novel, PLC-γ1-deficient Jurkat T-cell lines. The first cell line, P98, expresses PLC-γ1 at <10% of the level found in the parental Jurkat E6 cell line. The J.gamma1 subline was subsequently derived from P98 cells and expresses no detectable PLC-γ1 protein. In accordance with existing models of TCR signaling, J.gamma1 cells display severe defects in TCR-dependent Ca2+ mobilization and NFAT activation. In contrast, both of these responses occur at wild-type levels in P98 cells, in spite of the severe reduction in PLC-γ1 expression. However, the level of PLC-γ1 expression in P98 cells proved to be limiting for transcriptional activation of the IL-2 promoter by the synergistic stimuli anti-CD3 antibodies and PMA. We took advantage of the “partial” mutant phenotype to uncover a unique function for the PLC-γ1 SH2(C) domain in the transmission of signals from the TCR to both the IL-2 promoter and the RE/AP element contained within this promoter.
An unexpected outcome of the initial studies with P98 cells was that a >90% reduction in PLC-γ1 expression led to no detectable impairment of the TCR-dependent [Ca2+]i increase. These findings were corroborated by measurements of TCR-mediated IP3 formation, which also revealed no difference between P98 cells and the parental Jurkat E6 cell line (unpublished results). Consistent with the normal pattern of [Ca2+]i changes, TCR stimulation triggered a wild-type increase in NFAT-dependent transcription in P98 cells. In contrast, the complete loss of PLC-γ1 in J.gamma1 cells rendered these cells unable to activate NFAT in response to TCR cross-linkage. The simplest interpretation of these results is that while PLC-γ1 activity is essential for TCR-mediated NFAT activation, the expression level of this enzyme in Jurkat E6 cells is considerably in excess of that needed for efficient triggering of the Ca2+-calcineurin-dependent pathway leading to NFAT nuclear translocation.
The results of the NFAT-dependent reporter gene assays contrasted sharply with those obtained with an IL-2 promoter-linked luciferase reporter construct. The activation of this reporter was reduced by ∼80% in MAb OKT3-plus-PMA-stimulated P98 cells, and this transcriptional defect was rescued by ectopic expression of wild-type, but not catalytically inactive, PLC-γ1. These results suggested that the reduced level of PLC-γ1 expression in P98 cells was limiting for the DNA-binding activity and/or function of one or more of the transcription factors involved in IL-2 gene transcription in activated T cells (49, 53). With the exception of NFAT, the contributions of PLC-γ1 to the activation of IL-2 gene transcription are poorly understood. The present findings suggest that the critical functions of PLC-γ1 extend beyond the activation of the Ca2+-calcineurin-NFAT pathway and that this protein is required for the initiation of an additional signaling pathway needed for maximal activation of the IL-2 promoter.
A screen of known TCR-regulated transactivating factors revealed no defects in the activation of NFAT, AP-1, or NFκB in P98 cells. However, strikingly different results were obtained with the RE/AP reporter construct, which contains both the CD28RE and vicinal NF-IL-2B sites from the IL-2 promoter region (54). The activated P98 cells displayed a significant defect in RE/AP-dependent transcription, which was reversed by transfection of the cells with wild-type PLC-γ1. Previous findings indicated that CD28RE binds to the NFκB family member c-Rel, while the NF-IL-2B element contains a nonconsensus binding site for AP-1. The strong dependence of RE/AP activation on synergistic signals provided by the TCR plus CD28-PMA costimulation, as well as the interactive nature of c-Rel and AP1 binding to this site, strongly suggests that the RE/AP element functions as a true composite element in the IL-2 promoter (54). Although the mechanism underlying the RE/AP activation defect in P98 cells is currently under active investigation, recent results indicate that these cells fail to induce the expression of c-Rel protein in response to anti-CD3 antibody-plus-PMA costimulation and that ectopic expression of c-Rel rescues the RE/AP activation defect in these cells (unpublished results). Collectively, these results suggest that the expression level of PLC-γ1 in P98 cells limits the delivery of a signal(s) needed for the induction c-Rel and, hence, RE/AP activation in P98 cells.
An obvious candidate for this limiting signal is DAG, which is produced concomitantly with the Ca2+-mobilizing second messenger IP3 during TCR-dependent PLC-γ1 activation. However, it is difficult to imagine that DAG production limits IL-2 promoter-dependent transcription in P98 cells, because these assays were performed in the presence of an optimal concentration of PMA, which is a potent agonist for DAG-dependent signaling processes. A more plausible model is that PLC-γ1 does not function solely as a generator of phosphoinositide-derived second messengers during TCR signaling. Like the Rac guanine nucleotide exchange factor Vav1 (8), PLC-γ1 bears structural features, including SH2, SH3, and pleckstrin homology domains, which suggest that this enzyme serves as both a second-messenger generator and a molecular scaffold during T-cell activation.
The reversible defect in IL-2 promoter-dependent transcription in P98 cells facilitated the implementation of a mutagenesis-based approach to the examination of the roles of the SH region in signaling through PLC-γ1 during T-cell activation. The partially defective phenotype of P98 cells was an advantage for these studies in that we were able to focus on potential signaling functions of PLC-γ1 other than those stemming from IP3-mediated intracellular Ca2+ mobilization. Interestingly, we observed that mutation of the PLC-γ1 SH3 domain strongly enhanced the signaling activity of this enzyme in intact P98 cells but had no effect on the intrinsic PIP2-hydrolyzing activity of PLC-γ1 in vitro. These results suggest that the SH3 domain mediates an interaction between PLC-γ1 and a negative regulatory protein in T cells. A provocative candidate is Cbl (24, 25), a multifunctional protein that dampens signaling through PTK-coupled receptors, possibly by promoting the ubiquitin-dependent proteolysis of receptors and/or receptor-associated signaling proteins (1, 31). If Cbl proves to be the relevant ligand for the PLC-γ1 SH3 domain, then it is tempting to speculate that elimination of a Cbl-mediated “brake” on PLC-γ1 activity in vivo plays a role in the overall hyperresponsiveness of thymocytes from Cbl−/− mice (43).
In contrast to the SH3 domain, loss-of-function mutations in either the PLC-γ1 SH2(N) or SH2(C) domain compromised the phosphorylation and/or activity of the enzyme in P98 cells. As reported previously by Stoica et al. (58), we found that an intact SH2(N) domain was critical for the optimal tyrosine phosphorylation of PLC-γ1 in response to anti-CD3 antibody stimulation. These findings are also in general agreement with results obtained with PDGF and epidermal growth factor receptor-expressing fibroblasts, as well as in the DT40 chicken B-cell line, a model system for studies of BCR signaling (11, 15, 29, 45). Thus, in both lymphoid and nonlymphoid cells, the SH2(N) domain plays a particularly important role in coupling the activation of receptor-linked PTKs to the tyrosine phosphorylation of PLC-γ1. However, SH2(N) domain function was not essential for rescue of IL-2 promoter activation by PLC-γ1 in P98 cells. This outcome is likely explained by the fact that P98 cells retain sufficient endogenous PLC-γ1 to support the TCR-dependent Ca2+ signaling pathway, which obviates the requirement for a functional SH2(N) domain. In J.gamma1 cells, which lack endogenous PLC-γ1, we found that the PLC-γ1 SH2(N)* mutant fails to rescue TCR-dependent NFAT or IL-2 promoter activation (unpublished result). The latter result is fully consistent with the findings obtained with _Plcg_−/− DT40 B cells (15). Thus, the SH2(N) domain of PLC-γ1 apparently plays essential roles in positioning the enzyme for phosphorylation by receptor-activated PTKs and in stimulating the hydrolysis of PIP2 to the Ca2+-mobilizing second messenger IP3.
On the other hand, the results obtained with the PLC-γ1 SH2(C)* mutant suggest that the SH2(C) domain is not principally involved in the coupling of TCR ligation to the tyrosine phosphorylation of PLC-γ1. Again, these findings support earlier results obtained with wild-type Jurkat cells and PLC-γ1−/− DT40 B cells (15, 58). Nonetheless, expression of the PLC-γ1 SH2(C)* mutant failed to support BCR-dependent phosphoinositide breakdown or Ca2+ mobilization in DT40 cells (15). In preliminary studies with J.gamma1 cells, we have shown that the same SH2(C)* mutant also fails to rescue MAb OKT3-stimulated NFAT activation, which is triggered by an increase in [Ca2+]i (unpublished results). Collectively, these results suggest that while the SH2(N) domain is more important for optimal PLC-γ1 phosphorylation in response to BCR or TCR stimulation, both of the SH2 domains of PLC-γ1 are required for the generation of IP3-dependent Ca2+ signals downstream of these receptors. Only in the P98 cell background, in which the requirement for Ca2+ signaling is fulfilled in trans by endogenous PLC-γ1, do we observe a unique role for the SH2(C) domain in the delivery of additional signals needed for RE/AP and IL-2 promoter activation.
The differential contributions of the SH2(N) and SH2(C) domains to the phosphorylation and function of PLC-γ1 likely reflect the distinct binding specificities of these domains for phosphotyrosyl-containing proteins in activated T cells. Ligand-binding studies with a degenerate phosphotyrosyl peptide library revealed that the SH2(N) domain prefers a pYLEL target sequence, while the SH2(C) domain favors pY(V/I)IP (57). The membrane-localized adapter protein LAT contains a phosphorylation site at Tyr132 whose sequence (YLVV) could be recognized by either the SH2(N) or the SH2(C) domain of PLC-γ1 (73, 74). Although studies with GST fusion proteins indicated that both SH2 domains are capable of binding to phosphorylated LAT, an intact SH2(N) domain was both necessary and sufficient for the interaction of full-length PLC-γ1 with this adapter protein. Thus, the defective tyrosine phosphorylation of the PLC-γ1 SH2(N)* mutant in P98 cells is a probable consequence of the failure of this protein to associate with LAT during TCR stimulation. A similar SH2(N) domain-adapter protein interaction appears to be important for PLC-γ1 phosphorylation in B cells, although the SLP-76-related protein BLNK, rather than LAT, is the cognate SH2(N) ligand in B lymphocytes (15, 21).
The situation with respect to SH2(C) domain ligands seems considerably more complex. First, in contrast to the isolated SH2(N) domain, we and others (58) found that a GST–PLC-γ1 SH2(C) fusion protein precipitated multiple phosphotyrosyl proteins from activated T-cell extracts. Two notable ligands for the SH2(C) domain are ZAP-70 and SLP-76. To date, we have been unable to demonstrate an inducible interaction between full-length PLC-γ1 and activated ZAP-70 in Jurkat T cells. On the other hand, the binding of SLP-76 to PLC-γ1 was both inducible and strongly dependent on the PLC-γ1 SH2(C) domain. A recent report provided genetic evidence that SLP-76 lies upstream of PLC-γ1 in the TCR-linked pathway leading to PLC-γ1 phosphorylation (70). Interestingly, we observed that mutation of the SH2(C) domain caused only a partial reduction in the tyrosine phosphorylation of PLC-γ1 in anti-CD3 antibody-stimulated P98 cells. Therefore, the SH2(C) domain may not be the only channel through which SLP-76 promotes TCR-dependent PLC-γ1 phosphorylation. Moreover, our findings with P98 cells suggest that the binding of SLP-76 to the SH2(C) domain is also crucial for downstream signaling from PLC-γ1. In this case, the direct interaction with SLP-76 may mediate the colocalization of PLC-γ1 with other signaling proteins, including Vav1, Grb2, and Gads. Because the tandem SH2 domains of PLC-γ1 are capable of interacting with both LAT and SLP-76, this enzyme may act as a bridge between LAT- and SLP-76-associated signaling proteins during TCR stimulation. A similar bridging function has been proposed for the Grb2-related Grap adapter protein in T cells (63). The failure of the PLC-γ1 SH2(C)* mutant to reverse the IL-2 promoter activation defect in P98 cells may reflect the disruptive effect of this mutation on the dynamic interplay between the different LAT–SLP-76 complexes during T-cell activation (20, 70, 72).
Phosphorylation of PLC-γ1 at Tyr783 represents a critical step in the activation of this enzyme during PDGF receptor stimulation (39). Although TCR cross-linkage also triggers the phosphorylation of Tyr783 in PLC-γ1 (44), the functional significance of this phosphorylation event in T cells has not been evaluated. We observed that the PLC-γ1 Y783F mutant was completely inactive in our IL-2 promoter-based reconstitution assay in P98 cells and potently inhibited TCR-mediated IL-2 promoter transactivation when expressed in wild-type Jurkat cells. Thus, the Tyr783→Phe mutation may generate a form of PLC-γ1 that persistently, but nonproductively, occupies critical signaling proteins, e.g., LAT and SLP-76, during TCR engagement. Phosphorylation of Tyr783 is not essential for the PIP2-hydrolyzing activity of PLC-γ1, based on the in vitro phospholipase assays. However, tyrosine phosphorylation at this site may alter the recognition of the PIP2 substrate in vivo (23). A mutually nonexclusive possibility is that Tyr783 phosphorylation triggers the recruitment of an SH2 domain-containing protein that functionally activates PLC-γ1 in intact cells.
In summary, our results obtained with the P98 model system hint that the signaling functions of PLC-γ1 in T cells are considerably more pleiotropic than predicted by the existing models of TCR signaling. The PIP2-hydrolyzing activity of PLC-γ1 is clearly central to the activating function of this protein in T cells; however, accumulating evidence suggests that this enzyme may also act as a molecular scaffold during TCR signaling. Intermolecular interactions involving the SH2 and SH3 domains, as well as the Tyr783 phosphorylation site, may place PLC-γ1 in a key position to integrate signals from the TCR and costimulatory receptors during the early stage of T-cell activation.
ACKNOWLEDGMENTS
We thank Weiguo Zhang, Paul Leibson, and David McKean for helpful discussions and reagents. We also thank Joel Ross for his expert assistance with flow cytometry and Art Weiss for kindly providing the RE/AP reporter construct.
This work was supported by National Institutes of Health grant GM47286 and by the Mayo Foundation. R.T.A. is a Glaxo-Wellcome Professor of Molecular Cancer Biology.
REFERENCES
- 1.Andoniou C E, Lill N L, Thien C B, Lupher M L J, Ota S, Bowtell D D, Scaife R M, Langdon W Y, Band H. The Cbl proto-oncogene product negatively regulates the Src-family tyrosine kinase Fyn by enhancing its degradation. Mol Cell Biol. 2000;20:851–867. doi: 10.1128/mcb.20.3.851-867.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.August A, Sadra A, Dupont B, Hanafusa H. Src-induced activation of inducible T cell kinase (ITK) requires phosphatidylinositol 3-kinase activity and the pleckstrin homology domain of inducible T cell kinase. Proc Natl Acad Sci USA. 1997;94:11227–11232. doi: 10.1073/pnas.94.21.11227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bae Y S, Cantley L G, Chen C S, Kim S R, Kwon K S, Rhee S G. Activation of phospholipase C-gamma by phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:4465–4469. doi: 10.1074/jbc.273.8.4465. [DOI] [PubMed] [Google Scholar]
- 4.Barker S A, Caldwell K K, Pfeiffer J R, Wilson B S. Wortmannin-sensitive phosphorylation, translocation, and activation of PLCgamma1, but not PLCgamma2, in antigen-stimulated RBL-2H3 mast cells. Mol Biol Cell. 1998;9:483–496. doi: 10.1091/mbc.9.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bar-Sagi D, Rotin D, Batzer A, Mandiyan V, Schlessinger J. SH3 domains direct cellular localization of signaling molecules. Cell. 1993;74:83–91. doi: 10.1016/0092-8674(93)90296-3. [DOI] [PubMed] [Google Scholar]
- 6.Berridge M J. Inositol trisphosphate and calcium signaling. Ann N Y Acad Sci. 1995;766:31–43. doi: 10.1111/j.1749-6632.1995.tb26646.x. [DOI] [PubMed] [Google Scholar]
- 7.Blake R A, Schieven G L, Watson S P. Collagen stimulates tyrosine phosphorylation of phospholipase C-gamma 2 but not phospholipase C-gamma 1 in human platelets. FEBS Lett. 1994;353:212–216. doi: 10.1016/0014-5793(94)01037-4. [DOI] [PubMed] [Google Scholar]
- 8.Bonnefoy-Berard N, Munshi A, Yron I, Wu S, Collins T L, Deckert M, Shalom-Barak T, Giampa L, Herbert E, Hernandez J, Meller N, Couture C, Altman A. Vav: function and regulation in hematopoietic cell signaling. Stem Cells. 1996;14:250–268. doi: 10.1002/stem.140250. [DOI] [PubMed] [Google Scholar]
- 9.Booker G W, Gout I, Downing A K, Driscoll P C, Boyd J, Waterfield M D, Campbell I D. Solution structure and ligand-binding site of the SH3 domain of the p85 alpha subunit of phosphatidylinositol 3-kinase. Cell. 1993;73:813–822. doi: 10.1016/0092-8674(93)90259-s. [DOI] [PubMed] [Google Scholar]
- 10.Cantrell D A. T cell antigen receptor signal transduction pathways. Cancer Surv. 1996;27:165–175. [PubMed] [Google Scholar]
- 11.Chattopadhyay A, Vecchi M, Ji Q, Mernaugh R, Carpenter G. The role of individual SH2 domains in mediating association of phospholipase C-gamma1 with the activated EGF receptor. J Biol Chem. 1999;274:26091–26097. doi: 10.1074/jbc.274.37.26091. [DOI] [PubMed] [Google Scholar]
- 12.Clark S G, Stern M J, Horvitz H R. C. elegans cell-signalling gene sem-5 encodes a protein with SH2 and SH3 domains. Nature. 1992;356:340–344. doi: 10.1038/356340a0. [DOI] [PubMed] [Google Scholar]
- 13.Coggeshall K M, McHugh J C, Altman A. Predominant expression and activation-induced tyrosine phosphorylation of phospholipase C-gamma 2 in B lymphocytes. Proc Natl Acad Sci USA. 1992;89:5660–5664. doi: 10.1073/pnas.89.12.5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crabtree G R, Clipstone N A. Signal transmission between the plasma membrane and nucleus of T lymphocytes. Annu Rev Biochem. 1994;63:1045–1083. doi: 10.1146/annurev.bi.63.070194.005145. [DOI] [PubMed] [Google Scholar]
- 15.DeBell K E, Stoica B A, Veri M C, Di B, Miscia S, Graham L J, Rellahan B L, Ishiai M, Kurosaki T, Bonvini E. Functional independence and interdependence of the Src homology domains of phospholipase C-gamma 1 in B-cell receptor signal transduction. Mol Cell Biol. 1999;19:7388–7398. doi: 10.1128/mcb.19.11.7388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Debnath J, Chamorro M, Czar M J, Schaeffer E M, Lenardo M J, Varmus H E, Schwartzberg P L. rlk/TXK encodes two forms of a novel cysteine string tyrosine kinase activated by Src family kinases. Mol Cell Biol. 1999;19:1498–1507. doi: 10.1128/mcb.19.2.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Desai D M, Newton M E, Kadlecek T, Weiss A. Stimulation of the phosphatidylinositol pathway can induce T-cell activation. Nature. 1990;348:66–69. doi: 10.1038/348066a0. [DOI] [PubMed] [Google Scholar]
- 18.Donovan J A, Wange R L, Langdon W Y, Samelson L E. The protein product of the c-cbl protooncogene is the 120-kDa tyrosine-phosphorylated protein in Jurkat cells activated via the T cell antigen receptor. J Biol Chem. 1994;269:22921–22924. [PubMed] [Google Scholar]
- 19.Essen L O, Perisic O, Cheung R, Katan M, Williams R L. Crystal structure of a mammalian phosphoinositide-specific phospholipase C delta. Nature. 1996;380:595–602. doi: 10.1038/380595a0. [DOI] [PubMed] [Google Scholar]
- 20.Finco T S, Kadlecek T, Zhang W, Samelson L E, Weiss A. LAT is required for TCR-mediated activation of PLCgamma1 and the Ras pathway. Immunity. 1998;9:617–626. doi: 10.1016/s1074-7613(00)80659-7. [DOI] [PubMed] [Google Scholar]
- 21.Fu C, Turck C W, Kurosaki T, Chan A C. BLNK: a central linker protein in B cell activation. Immunity. 1998;9:93–103. doi: 10.1016/s1074-7613(00)80591-9. [DOI] [PubMed] [Google Scholar]
- 22.Gibson S, August A, Kawakami Y, Kawakami T, Dupont B, Mills G B. The EMT/ITK/TSK (EMT) tyrosine kinase is activated during TCR signaling: LCK is required for optimal activation of EMT. J Immunol. 1996;156:2716–2722. [PubMed] [Google Scholar]
- 23.Goldschmidt-Clermont P J, Kim J W, Machesky L M, Rhee S G, Pollard T D. Regulation of phospholipase C-gamma 1 by profilin and tyrosine phosphorylation. Science. 1991;251:1231–1233. doi: 10.1126/science.1848725. [DOI] [PubMed] [Google Scholar]
- 24.Graham L J, DeBell K E, Veri M, Stoica B, Mostowski H, Bonvini E, Rellahan B. Differential effects of Cbl and 70Z/3 Cbl on T cell receptor-induced phospholipase Cgamma-1 activity. FEBS Lett. 2000;470:273–280. doi: 10.1016/s0014-5793(00)01341-7. [DOI] [PubMed] [Google Scholar]
- 25.Graham L J, Stoica B A, Shapiro M, DeBell K E, Rellahan B, Laborda J, Bonvini E. Sequences surrounding the Src-homology 3 domain of phospholipase Cgamma-1 increase the domain's association with Cbl. Biochem Biophys Res Commun. 1998;249:537–541. doi: 10.1006/bbrc.1998.9177. [DOI] [PubMed] [Google Scholar]
- 26.Horstman D A, Chattopadhyay A, Carpenter G. The influence of deletion mutations on phospholipase C-gamma 1 activity. Arch Biochem Biophys. 1999;361:149–155. doi: 10.1006/abbi.1998.0978. [DOI] [PubMed] [Google Scholar]
- 27.Horstman D A, DeStefano K, Carpenter G. Enhanced phospholipase C-gamma1 activity produced by association of independently expressed X and Y domain polypeptides. Proc Natl Acad Sci USA. 1996;93:7518–7521. doi: 10.1073/pnas.93.15.7518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jain J, Loh C, Rao A. Transcriptional regulation of the IL-2 gene. Curr Opin Immunol. 1995;7:333–342. doi: 10.1016/0952-7915(95)80107-3. [DOI] [PubMed] [Google Scholar]
- 29.Ji Q S, Chattopadhyay A, Vecchi M, Carpenter G. Physiological requirement for both SH2 domains for phospholipase C-gamma1 function and interaction with platelet-derived growth factor receptors. Mol Cell Biol. 1999;19:4961–4970. doi: 10.1128/mcb.19.7.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ji Q S, Winnier G E, Niswender K D, Horstman D, Wisdom R, Magnuson M A, Carpenter G. Essential role of the tyrosine kinase substrate phospholipase C-gamma1 in mammalian growth and development. Proc Natl Acad Sci USA. 1997;94:2999–3003. doi: 10.1073/pnas.94.7.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joazeiro C A, Wing S S, Huang H, Leverson J D, Hunter T, Liu Y C. The tyrosine kinase negative regulator c-Cbl as a RING-type, E2-dependent ubiquitin-protein ligase. Science. 1999;286:309–312. doi: 10.1126/science.286.5438.309. [DOI] [PubMed] [Google Scholar]
- 32.June C H, Fletcher M C, Ledbetter J A, Samelson L E. Increases in tyrosine phosphorylation are detectable before phospholipase C activation after T cell receptor stimulation. J Immunol. 1990;144:1591–1599. [PubMed] [Google Scholar]
- 33.Kalli K, Huntoon C, Bell M, McKean D J. Mechanism responsible for T-cell antigen receptor- and CD28- or interleukin 1 (IL-1) receptor-initiated regulation of IL-2 gene expression by NF-κB. Mol Cell Biol. 1998;18:3140–3148. doi: 10.1128/mcb.18.6.3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kamat A, Carpenter G. Phospholipase C-gamma1: regulation of enzyme function and role in growth factor-dependent signal transduction. Cytokine Growth Factor Rev. 1997;8:109–117. doi: 10.1016/s1359-6101(97)00003-8. [DOI] [PubMed] [Google Scholar]
- 35.Kang J S, Kohlhuber F, Hug H, Marme D, Eick D, Ueffing M. Cloning and functional analysis of the hematopoietic cell-specific phospholipase C(gamma)2 promoter. FEBS Lett. 1996;399:14–20. doi: 10.1016/s0014-5793(96)01276-8. [DOI] [PubMed] [Google Scholar]
- 36.Karnitz L, Sutor S L, Torigoe T, Reed J C, Bell M P, McKean D J, Leibson P J, Abraham R T. Effects of p56lck deficiency on the growth and cytolytic effector function of an interleukin-2-dependent cytotoxic T-cell line. Mol Cell Biol. 1992;12:4521–4530. doi: 10.1128/mcb.12.10.4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Katan M. Families of phosphoinositide-specific phospholipase C: structure and function. Biochim Biophys Acta. 1998;1436:5–17. doi: 10.1016/s0005-2760(98)00125-8. [DOI] [PubMed] [Google Scholar]
- 38.Kennedy A P, Sekulic A, Irvin B J, Nilson A E, Dilworth S M, Abraham R T. Polyomavirus middle T antigen as a probe for T cell antigen receptor-coupled signaling pathways. J Biol Chem. 1998;273:11505–11513. doi: 10.1074/jbc.273.19.11505. [DOI] [PubMed] [Google Scholar]
- 39.Kim H K, Kim J W, Zilberstein A, Margolis B, Kim J G, Schlessinger J, Rhee S G. PDGF stimulation of inositol phospholipid hydrolysis requires PLC-gamma 1 phosphorylation on tyrosine residues 783 and 1254. Cell. 1991;65:435–441. doi: 10.1016/0092-8674(91)90461-7. [DOI] [PubMed] [Google Scholar]
- 40.Liao X C, Littman D R. Altered T cell receptor signaling and disrupted T cell development in mice lacking Itk. Immunity. 1995;3:757–769. doi: 10.1016/1074-7613(95)90065-9. [DOI] [PubMed] [Google Scholar]
- 41.Mayer B J, Jackson P K, Van E, Baltimore D. Point mutations in the abl SH2 domain coordinately impair phosphotyrosine binding in vitro and transforming activity in vivo. Mol Cell Biol. 1992;12:609–618. doi: 10.1128/mcb.12.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moarefi I, LaFevre-Bernt M, Sicheri F, Huse M, Lee C H, Kuriyan J, Miller W T. Activation of the Src-family tyrosine kinase Hck by SH3 domain displacement. Nature. 1997;385:650–653. doi: 10.1038/385650a0. [DOI] [PubMed] [Google Scholar]
- 43.Murphy M A, Schnall R G, Venter D J, Barnett L, Bertoncello I, Thien C B, Langdon W Y, Bowtell D D. Tissue hyperplasia and enhanced T-cell signalling via ZAP-70 in c-Cbl-deficient mice. Mol Cell Biol. 1998;18:4872–4882. doi: 10.1128/mcb.18.8.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park D J, Rho H W, Rhee S G. CD3 stimulation causes phosphorylation of phospholipase C-gamma 1 on serine and tyrosine residues in a human T-cell line. Proc Natl Acad Sci USA. 1991;88:5453–5456. doi: 10.1073/pnas.88.12.5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poulin B, Sekiya F, Rhee S G. Differential roles of the Src homology 2 domains of phospholipase C-gamma1 (PLC-gamma1) in platelet-derived growth factor-induced activation of PLC-gamma1 in intact cells. J Biol Chem. 2000;275:6411–6416. doi: 10.1074/jbc.275.9.6411. [DOI] [PubMed] [Google Scholar]
- 46.Rameh L E, Rhee S G, Spokes K, Kazlauskas A, Cantley L C, Cantley L G. Phosphoinositide 3-kinase regulates phospholipase Cgamma-mediated calcium signaling. J Biol Chem. 1998;273:23750–23757. doi: 10.1074/jbc.273.37.23750. [DOI] [PubMed] [Google Scholar]
- 47.Rao A, Luo C, Hogan P G. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- 48.Rhee S G, Bae Y S. Regulation of phosphoinositide-specific phospholipase C isozymes. J Biol Chem. 1997;272:15045–15048. doi: 10.1074/jbc.272.24.15045. [DOI] [PubMed] [Google Scholar]
- 49.Rothenberg E V, Ward S B. A dynamic assembly of diverse transcription factors integrates activation and cell-type information for interleukin 2 gene regulation. Proc Natl Acad Sci USA. 1996;93:9358–9365. doi: 10.1073/pnas.93.18.9358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schaeffer E M, Debnath J, Yap G, McVicar D, Liao X C, Littman D R, Sher A, Varmus H E, Lenardo M J, Schwartzberg P L. Requirement for Tec kinases Rlk and Itk in T cell receptor signaling and immunity. Science. 1999;284:638–641. doi: 10.1126/science.284.5414.638. [DOI] [PubMed] [Google Scholar]
- 51.Secrist J P, Burns L A, Karnitz L, Koretzky G A, Abraham R T. Stimulatory effects of the protein tyrosine phosphatase inhibitor, pervanadate, on T-cell activation events. J Biol Chem. 1993;268:5886–5893. [PubMed] [Google Scholar]
- 52.Secrist J P, Karnitz L, Abraham R T. T-cell antigen receptor ligation induces tyrosine phosphorylation of phospholipase C-gamma 1. J Biol Chem. 1991;266:12135–12139. [PubMed] [Google Scholar]
- 53.Serfling E, Avots A, Neumann M. The architecture of the interleukin-2 promoter: a reflection of T lymphocyte activation. Biochim Biophys Acta. 1995;1263:181–200. doi: 10.1016/0167-4781(95)00112-t. [DOI] [PubMed] [Google Scholar]
- 54.Shapiro V S, Truitt K E, Imboden J B, Weiss A. CD28 mediates transcriptional upregulation of the interleukin-2 (IL-2) promoter through a composite element containing the CD28RE and NF-IL-2B AP-1 sites. Mol Cell Biol. 1997;17:4051–4058. doi: 10.1128/mcb.17.7.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sicheri F, Moarefi I, Kuriyan J. Crystal structure of the Src family tyrosine kinase Hck. Nature. 1997;385:602–609. doi: 10.1038/385602a0. [DOI] [PubMed] [Google Scholar]
- 56.Smith M R, Liu Y L, Matthews N T, Rhee S G, Sung W K, Kung H F. Phospholipase C-gamma 1 can induce DNA synthesis by a mechanism independent of its lipase activity. Proc Natl Acad Sci USA. 1994;91:6554–6558. doi: 10.1073/pnas.91.14.6554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Songyang Z, Shoelson S E, Chaudhuri M, Gish G, Pawson T, Haser W G, King F, Roberts T, Ratnofsky S, Lechleider R J. SH2 domains recognize specific phosphopeptide sequences. Cell. 1993;72:767–778. doi: 10.1016/0092-8674(93)90404-e. [DOI] [PubMed] [Google Scholar]
- 58.Stoica B, DeBell K E, Graham L, Rellahan B L, Alava M A, Laborda J, Bonvini E. The amino-terminal Src homology 2 domain of phospholipase C gamma 1 is essential for TCR-induced tyrosine phosphorylation of phospholipase C gamma 1. J Immunol. 1998;160:1059–1066. [PubMed] [Google Scholar]
- 59.Straus D B, Weiss A. Genetic evidence for the involvement of the lck tyrosine kinase in signal transduction through the T cell antigen receptor. Cell. 1992;70:585–593. doi: 10.1016/0092-8674(92)90428-f. [DOI] [PubMed] [Google Scholar]
- 60.Straus D B, Weiss A. The CD3 chains of the T cell antigen receptor associate with the ZAP-70 tyrosine kinase and are tyrosine phosphorylated after receptor stimulation. J Exp Med. 1993;178:1523–1530. doi: 10.1084/jem.178.5.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Timmerman L A, Clipstone N A, Ho S N, Northrop J P, Crabtree G R. Rapid shuttling of NF-AT in discrimination of Ca2+ signals and immunosuppression. Nature. 1996;383:837–840. doi: 10.1038/383837a0. [DOI] [PubMed] [Google Scholar]
- 62.Ting A T, Karnitz L M, Schoon R A, Abraham R T, Leibson P J. Fc gamma receptor activation induces the tyrosine phosphorylation of both phospholipase C (PLC)-gamma 1 and PLC-gamma 2 in natural killer cells. J Exp Med. 1992;176:1751–1755. doi: 10.1084/jem.176.6.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Trub T, Frantz J D, Miyazaki M, Band H, Shoelson S E. The role of a lymphoid-restricted, Grb2-like SH3-SH2-SH3 protein in T cell receptor signaling. J Biol Chem. 1997;272:894–902. doi: 10.1074/jbc.272.2.894. [DOI] [PubMed] [Google Scholar]
- 64.van Leeuwen J, Samelson L E. T cell antigen-receptor signal transduction. Curr Opin Immunol. 1999;11:242–248. doi: 10.1016/s0952-7915(99)80040-5. [DOI] [PubMed] [Google Scholar]
- 65.Van Wauwe J, De M, Goossens J G. OKT3: a monoclonal anti-human T lymphocyte antibody with potent mitogenic properties. J Immunol. 1980;124:2708–2713. [PubMed] [Google Scholar]
- 66.Wange R L, Malek S N, Desiderio S, Samelson L E. Tandem SH2 domains of ZAP-70 bind to T cell antigen receptor zeta and CD3 epsilon from activated Jurkat T cells. J Biol Chem. 1993;268:19797–19801. [PubMed] [Google Scholar]
- 67.Weiss A, Koretzky G, Schatzman R C, Kadlecek T. Functional activation of the T-cell antigen receptor induces tyrosine phosphorylation of phospholipase C-gamma 1. Proc Natl Acad Sci USA. 1991;88:5484–5488. doi: 10.1073/pnas.88.13.5484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Williams B L, Irvin B J, Sutor S L, Chini C C, Yacyshyn E, Bubeck W, Dalton M, Chan A C, Abraham R T. Phosphorylation of Tyr319 in ZAP-70 is required for T-cell antigen receptor-dependent phospholipase C-gamma1 and Ras activation. EMBO J. 1999;18:1832–1844. doi: 10.1093/emboj/18.7.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Williams B L, Schreiber K L, Zhang W, Wange R L, Samelson L E, Leibson P J, Abraham R T. Genetic evidence for differential coupling of Syk family kinases to the T-cell receptor: reconstitution studies in a ZAP-70-deficient Jurkat T-cell line. Mol Cell Biol. 1998;18:1388–1399. doi: 10.1128/mcb.18.3.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yablonski D, Kuhne M R, Kadlecek T, Weiss A. Uncoupling of nonreceptor tyrosine kinases from PLC-gamma-1 in an SLP-76-deficient T cell. Science. 1998;281:413–416. doi: 10.1126/science.281.5375.413. [DOI] [PubMed] [Google Scholar]
- 71.Yingling J M, Datto M B, Wong C, Frederick J P, Liberati N T, Wang X F. Tumor suppressor Smad4 is a transforming growth factor beta-inducible DNA binding protein. Mol Cell Biol. 1997;17:7019–7028. doi: 10.1128/mcb.17.12.7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang W, Irvin B J, Trible R P, Abraham R T, Samelson L E. Functional analysis of LAT in TCR-mediated signaling pathways using a LAT-deficient Jurkat cell line. Int Immunol. 1999;11:943–950. doi: 10.1093/intimm/11.6.943. [DOI] [PubMed] [Google Scholar]
- 73.Zhang W, Sloan-Lancaster J, Kitchen J, Trible R P, Samelson L E. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell. 1998;92:83–92. doi: 10.1016/s0092-8674(00)80901-0. [DOI] [PubMed] [Google Scholar]
- 74.Zhang W, Trible R P, Zhu M, Liu S K, McGlade C J, Samelson L E. Association of Grb2, Gads, and phospholipase C-gamma1 with phosphorylated LAT tyrosine residues. J Biol Chem. 2000;275:23355–23361. doi: 10.1074/jbc.M000404200. [DOI] [PubMed] [Google Scholar]