Human Immunodeficiency Virus Types 1 and 2 Differ in the Predominant Mechanism Used for Selection of Genomic RNA for Encapsidation (original) (raw)
Abstract
Retroviral RNA encapsidation is a highly selective process mediated through recognition by the viral Gag proteins of _cis_-acting RNA packaging signals in genomic RNA. This RNA species is also translated, producing the viral gag gene products. The relationship between these processes is poorly understood. Unlike that of human immunodeficiency virus type 1 (HIV-1), the dominant packaging signal of HIV-2 is upstream of the major splice donor and present in both unspliced and spliced viral RNAs, necessitating additional mechanisms for preferential packaging of unspliced genomic RNA. Encapsidation studies of a series of HIV-2-based vectors showed efficient packaging of viral genomes only if the unspliced, encapsidated RNA expressed full-length Gag protein, including functional nucleocapsid. We propose a novel encapsidation initiation mechanism, providing selectivity, in which unspliced HIV-2 RNA is captured in cis by the Gag protein. This has implications for the use of HIV-2 and other lentiviruses as vectors.
Newly transcribed unspliced retroviral RNA has two possible roles in the cytoplasm of the infected cell: it has a coding function as message for translation of the viral gag and (in all except foamy viruses) pol gene products and it harbors _cis_-acting signals that allow it to be encapsidated by Gag polyprotein during virus assembly. This latter requires interactions between sequence- and structure-specific motifs on the unspliced RNA (known as psi or E) and the Gag polyprotein. Mutational analyses of the nucleocapsid (NC) domain of Gag have demonstrated the pivotal importance of the zinc finger motifs and basic flanking residues for encapsidation of the viral genomic RNA (6, 9, 11, 12, 36). However, newer data have implicated the P2 domain of human immunodeficiency virus type 1 (HIV-1) as contributing to RNA selection (18). In vitro binding studies have shown that the Gag polyprotein and the NC domain bind to RNA containing HIV-1 packaging sequences (2, 3, 5, 6, 35).
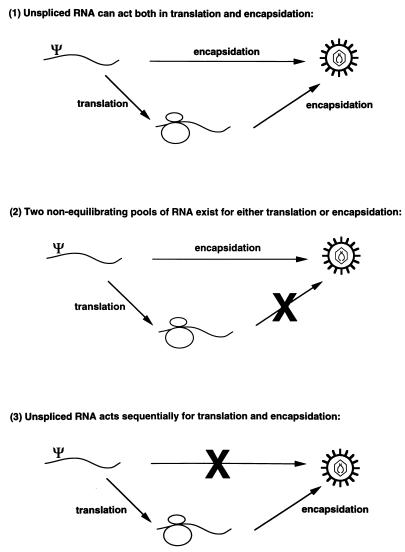
Three possible models can be invoked to explain the relationship between translation and encapsidation of unspliced retroviral RNA (Fig. 1). Model 1, any unspliced RNA can be either translated or encapsidated. Model 2, unspliced RNA is sorted into two nonequilibrating pools, one for translation and one for encapsidation. Model 3, unspliced RNA can only be encapsidated after it has been translated. Studies with Moloney murine leukemia virus (M-MuLV) suggested that unspliced M-MuLV RNA exists in two nonequilibrating pools (23, 24) (i.e., model 2). Recent studies of the relationship between translation and encapsidation in HIV-1 suggest that unspliced HIV-1 RNA can act as both mRNA and genomic RNA (3a). McBride and coauthors reported that translation of gag in cis was not necessary for efficient encapsidation of HIV-1 RNA (27), and HIV-1 is successfully used as a vector (33, 32, 29). These results indicate that unspliced HIV-1 RNA can act as both message and genomic RNA (i.e., model 1).
FIG. 1.
Possible sorting mechanisms involved in translation and encapsidation of full-length retroviral RNA.
The packaging signal region of HIV-1 has been extensively mapped by deletion mutagenesis and secondary structure analysis (1, 4, 14, 16, 22, 26). The core packaging signal is composed of a series of stem-loops spanning the major splice donor (15). One of these (SL3) is essential for efficient encapsidation of HIV-1 genomic RNA. The packaging signal region of HIV-2 has been less fully characterized. Deletion analyses of the 5′ untranslated region of HIV-2 showed that sequences upstream of the major splice donor were required for efficient encapsidation of HIV-2 RNA, whereas sequences downstream of the splice donor appeared to be less important (28). Other studies of the HIV-2 packaging signal region have suggested that sequences downstream of the major splice donor function both in packaging (30) and as a negative regulatory element (10).
The packaging signal regions of HIV-1 and HIV-2 have little sequence homology, although conserved GGNGR motifs can be identified (15). By contrast, the amino acid sequences of their NC domains are very similar and they exhibit conservative substitutions. We previously demonstrated that there is a nonreciprocal relationship between the RNA packaging of HIV-1 and HIV-2 (18). HIV-1 helper virus was able to encapsidate both HIV-1- and HIV-2-based vectors. However, unspliced HIV-1 vector RNA was preferentially packaged, whereas both unspliced and spliced HIV-2 vector RNAs were packaged relative to their respective levels in the cell. This is consistent with the HIV-2 packaging signal being present on both species of RNA and that of HIV-1 being on the unspliced RNA alone. HIV-2 helper virus did not package HIV-1 vector RNA but, surprisingly, was also unable to act as a helper virus to package HIV-2-based vectors. In the present study, we have further mapped sequences required for efficient encapsidation of HIV-2 RNA and, in addition, confirmed the lack of important _cis_-acting functions at the 3′ ends of the gag and pol genes. We find that efficient encapsidation of HIV-2 RNA requires cotranslation of Gag protein containing a functional NC domain. We propose that efficient packaging of HIV-2 RNA begins via a mechanism in which the unspliced RNA is translated and the nascent Gag polyprotein binds to the packaging signal on the translated RNA. Further Gag polyprotein recruitment then follows. This model, in which selection of RNA for packaging occurs preferentially in cis, would provide the required selectivity for unspliced RNA in a system in which the packaging signal is present on all of the HIV-2 messages.
MATERIALS AND METHODS
Plasmid construction.
pSVR is an infectious proviral clone of HIV-2 ROD containing a simian virus 40 origin of replication (28). Restriction sites, where given, refer to the first nucleotide of the viral RNA. Proviral constructs with deletions in their 5′ untranslated regions, pSVRΔ1, pSVRΔ2, pSVRΔ3, and pSVRΔ4, have been previously described (28) and are shown in Fig. 2A. pSVRΔAX has been previously described (18). Briefly, the sequences between _Acc_I (position 912) and _Xba_I (position 5067) were deleted. This and other vectors are shown in Fig. 3A. pSVRΔHX was constructed by deleting the sequences between _Hin_dIII (position 1458) and _Xba_I (position 5067). pSVRΔpol was constructed by deletion of the sequences between _Xho_I (position 2032) and _Xba_I (position 5067). A translation stop codon was introduced at the _Hin_dIII site (position 1458) of pSVRΔpol by filling in the 5′ overhanging ends of the _Hin_dIII site with Klenow polymerase and religating the blunt ends. The resulting construct was called pSVRΔHΔpol. The NC mutant, pSVRΔpolNCm, was constructed by oligonucleotide-directed mutagenesis (21) using the mutagenic oligonucleotides 5′ AGAAAGGCATTTAAAGCCTGGAACGCTGGAAAGGAAGGGGCCTCGGCAAGACAAGCCCGAGCACCTA 3′ and 5′ GAAGGCAGGG CGCCTGGAAGGCTGGTAAGCCAGGAGCCATCATGACAAACGCCCC AGATAGACAGGCA 3′. The underlined sequences in the mutagenic oligonucleotides substituted alanines for cysteine and histidine residues in the two zinc fingers of the HIV-2 NC domain. The resulting construct was subjected to nucleotide sequencing to confirm the mutated sequences. All HIV-2-based plasmids were grown in TOP10F′ (Invitrogen) at 30°C to minimize recombination. All other plasmids were grown in DH5α.
FIG. 2.
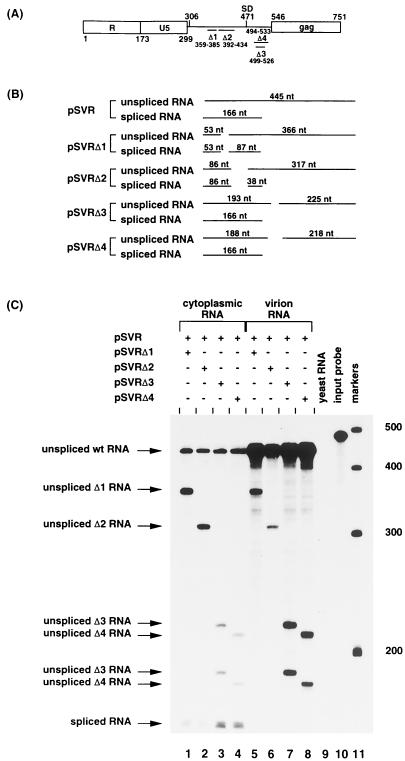
Sequences upstream of the splice donor are required for efficient encapsidation of HIV-2 RNA. (A) Leader sequence of HIV-2 showing sites of deletions: Δ1 (nt 359 to 385), Δ2 (nt 392 to 434), Δ3 (nt 499 to 526), and Δ4 (nt 494 to 533). SD, splice donor. (B) Predicted sizes of the protected fragments in RPA of wild-type and deletion mutant RNA. (C) RNase protection analysis, using the KS2ΨKE riboprobe. Protection with control RNA (yeast RNA) and with the riboprobe without RNase treatment (input probe) is shown (lanes 9 and 10, respectively). The positions of RNA size markers (lane 11) are shown in nucleotides.
FIG. 3.
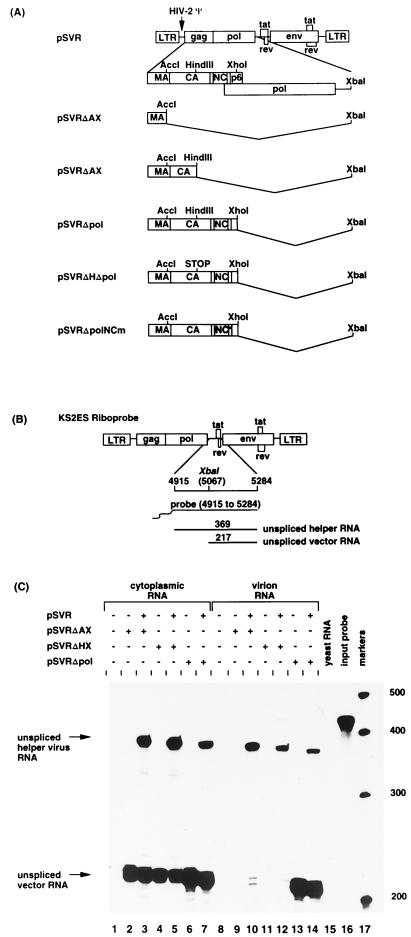
(A) Schematic representation of helper virus and vector constructs. LTR, long terminal repeat; Ψ, encapsidation sequences. (B) Predicted sizes of the protected fragments for riboprobe KS2ES. (C) RNase protection analysis using riboprobe KS2ES. The positions of unspliced helper virus RNA (nt 369) and unspliced vector RNA (nt 217) are indicated. Protection with control RNA (yeast RNA) and with the riboprobe without RNase treatment (input probe) is shown (lanes 15 and 16, respectively). The positions of RNA size markers (lane 17) are shown in nucleotides.
Plasmids used as templates for production of riboprobes were constructed as follows. KS2ES and KS2ΨKE have been previously described (18). They contain HIV-2 sequences from positions 4915 to 5284 and 306 to 751, respectively, cloned into the polylinker of Bluescript KSII (Stratagene). Plasmid KS2HIV-2 contains the HIV-2 sequences from _Pst_I (position −107) to _Bgl_I (position 503) cloned into the polylinker site of Bluescript KSII. In vitro transcription of the linearized plasmids using T3 RNA polymerase yields antisense riboprobes for use in RNase protection assays (RPA).
Cell culture and transfections.
COS-1 cells were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum, penicillin, and streptomycin. Transient transfection of COS-1 cells was performed by the DEAE-dextran method (37). Cells and supernatants were harvested 48 to 72 h later. Viral particle production was measured by reverse transcriptase assay (31).
Protein analysis.
COS-1 cells were metabolically labelled with [35S]methionine (>1,000 Ci/mmol) from 44 to 48 h after transfection. Labelled cells were lysed in radioimmunoprecipitation assay (RIPA) buffer (140 mM NaCl, 8 mM Na2HPO4, 2 mM NaH2PO4, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.05% sodium dodecyl sulfate [SDS]). Virions released into the supernatant were pelleted by centrifugation for 15 min at 4°C and 80,000 rpm in a Beckman TLA-100 rotor. Pelleted virions were lysed in RIPA buffer, and cell and virion lysates were immunoprecipitated with serum from a panel of HIV-2-infected individuals (MRC AIDS Reagent Project) prior to analysis through 5 to 20% acrylamide-SDS gradient gels (Bio-Rad).
RNA isolation.
Cytoplasmic RNA was obtained by rapid lysis at 4°C in Nonidet P-40 buffer (50 mM Tris-Cl [pH 8.0], 100 mM NaCl, 5 mM MgCl2, 0.5% [vol/vol] Nonidet P-40). Cell debris and nuclei were removed by a 2-min centrifugation step in a microcentrifuge. The supernatant was adjusted to 0.2% SDS and 125 μg of proteinase K per ml, incubated at 37°C for 15 min, and extracted twice with acid-buffered phenol-chloroform (pH 4.7) and once with chloroform. Nucleic acids were collected by ethanol precipitation, and RNA was stored at −80°C. For RNA extraction from virions, particles released from the cell culture supernatant were pelleted by polyethylene glycol (PEG) precipitation by the addition of 0.5 volumes of 30% PEG 8000 in 0.4 M NaCl for 16 h at 4°C. The precipitate was collected by centrifugation at 2,000 rpm in an MSE 43124-129 rotor at 4°C for 45 min and resuspended in 0.5 ml of TNE (10 mM Tris-Cl, 150 mM NaCl, 1 mM EDTA [pH 7.5]). This material was layered over an equal volume of TNE containing 20% sucrose and centrifuged at 98,000 × g for 2 h at 4°C. Virus particles were lysed in proteinase K buffer (50 mM Tris-Cl [pH 7.5], 100 mM NaCl, 10 mM EDTA, 1% SDS, 100 μg of proteinase K per ml, 100 μg of tRNA per ml) for 30 min at 37°C. After two extractions with acid-buffered phenol-chloroform and one extraction with the chloroform, the RNA was precipitated with ethanol and stored at −80°C. The isolated RNA was resuspended in 100 μl of a buffer containing 10 mM Tris-Cl (pH 8.0), 1 mM EDTA, 10 mM MgCl2, 1 mM dithiothreitol, 5 U of RNase-free DNase I (Promega), and 4 U of RNase inhibitor (Promega) and incubated at 37°C for 15 min. The reaction was stopped by the addition of 25 μl of a solution containing 50 mM EDTA, 1.5 M sodium acetate, and 1% SDS, and the samples were extracted once with acid-buffered phenol-chloroform and once with chloroform. The RNA was precipitated with ethanol.
RPA.
32P-labelled riboprobes were synthesized by in vitro transcription of linearized plasmids, using T3 RNA polymerase (Promega). Riboprobes were purified from 5% polyacrylamide–8 M urea gels prior to use in RPA.
Reagents for RPA were obtained from a commercially available kit (Ambion, Austin, Tex.). Cytoplasmic RNA or RNA extracted from pelleted particles representing 1/3 of the transfected cells was incubated with 2 × 105 cpm of 32P-labelled probe in 10 μl of hybridization buffer (Ambion) for 10 min at 68°C. Unhybridized regions of the probe were then degraded by the addition of 0.5 U of RNase A and 20 U of RNase T1 in 100 μl of RNase digestion buffer (Ambion). Protected fragments were precipitated in ethanol, resuspended in RNA loading buffer, and separated on 5% polyacrylamide–8 M urea gels. For size determination, 32P-labelled RNA markers synthesized with the RNA Century Marker template set (Ambion) were run in parallel. The gels were subjected to autoradiography, and the radioactivity was counted by real-time analysis with an Instant Imager (Packard).
RESULTS
Sequences upstream of the splice donor of HIV-2 are required for efficient genomic RNA encapsidation.
Deletion analyses of the 5′ untranslated region of HIV-2 have previously identified sequences that are required for efficient encapsidation of HIV-2 RNA (28). However, there remains controversy over the exact location of the packaging signal in HIV-2 (10). Consequently, we further characterized the effect of deletions in the HIV-2 5′ untranslated region by using competitive RPA. Competitive RPA measure the relative levels of encapsidation of coexpressed vector and helper constructs and thus have the benefit of controlling for intersample variations. Proviral clones containing mutated 5′ untranslated regions (Fig. 2A) were cotransfected into COS-1 cells with wild-type virus as an internal control, and cytoplasmic and virion RNAs were subjected to RPA to determine the effects of the mutations on RNA encapsidation efficiency. The results are shown in Fig. 2C and Table 1. The relative levels of wild-type and mutant unspliced RNA in each virion sample were normalized to the relative levels in the corresponding transfected cells. Deletion of the region upstream of the splice donor is seen to cause a significantly greater packaging defect than that downstream. Although this has been demonstrated previously, the quantitative nature of the RPA consolidates this observation. In addition, it is important to observe that the presence of cotransfected helper virus does not increase packaging of the deletion mutants nor does the presence of the deletion mutants affect packaging of wild-type virus. This supports the scheme outlined below that packaging initiation is occurring in cis and is unaffected by the presence of Gag available in trans.
TABLE 1.
Effects of mutations in the 5′ untranslated region on RNA encapsidation efficiencya
| Mutant | Relative encapsidation efficiency | SD |
|---|---|---|
| pSVRΔ1 | 0.17 | 0.04 |
| pSVRΔ2 | 0.22 | 0.06 |
| pSVRΔ3 | 0.54 | 0.12 |
| pSVRΔ4 | 0.48 | 0.10 |
HIV-2 vectors with deletions at the 3′ end of gag are not efficiently packaged in trans.
We have previously reported the failure of an HIV-2-based vector lacking gag and pol sequences to be encapsidated in trans by wild-type HIV-2 helper virus (18). To address the reason for the failure of encapsidation of such a vector, we constructed a series of vectors containing increasing amounts of gag coding sequence (Fig. 3A). The HIV-2-based vectors, pSVRΔAX, pSVRΔHX, and pSVRΔpol, were transfected either alone or with a wild-type HIV-2 proviral construct, pSVR, into COS-1 cells. Cytoplasmic and virion RNAs were extracted and subjected to RPA (results shown in Fig. 3C and Table 2). The different vectors were expressed to similar levels in the cytoplasm of the transfected cells (compare lanes 2 to 7). The vectors with the largest truncations of gag sequence, pSVRΔAX and pSVRΔHX, were not efficiently packaged by HIV-2 helper virus (lanes 10 and 12). However, virus-like particles produced from a vector containing almost the entire gag gene, pSVRΔpol, were able to encapsidate the vector RNA efficiently (lane 13). In addition, the level of vector packaged did not dramatically increase when cotransfected HIV-2 helper virus (lane 14) was present. Therefore, there was a requirement for the region between the _Hin_dIII and _Xho_I sites (positions 1458 and 2032) for efficient encapsidation of HIV-2 vector RNA.
TABLE 2.
Relative encapsidation efficiencies of various HIV-1 vectors compared to that of the wild typea
| Vector | Relative encapsidation efficiency | SD |
|---|---|---|
| pSVRΔAX | 0.27 | 0.12 |
| pSVRΔHX | 0.19 | 0.04 |
| pSVRΔHΔpol | 0.31 | 0.11 |
| pSVRΔpol | 1.41 | 0.20 |
| pSVRΔpolNCm | 0.47 | 0.08 |
Requirement for translation of the C terminus of gag for efficient encapsidation of HIV-2 RNA.
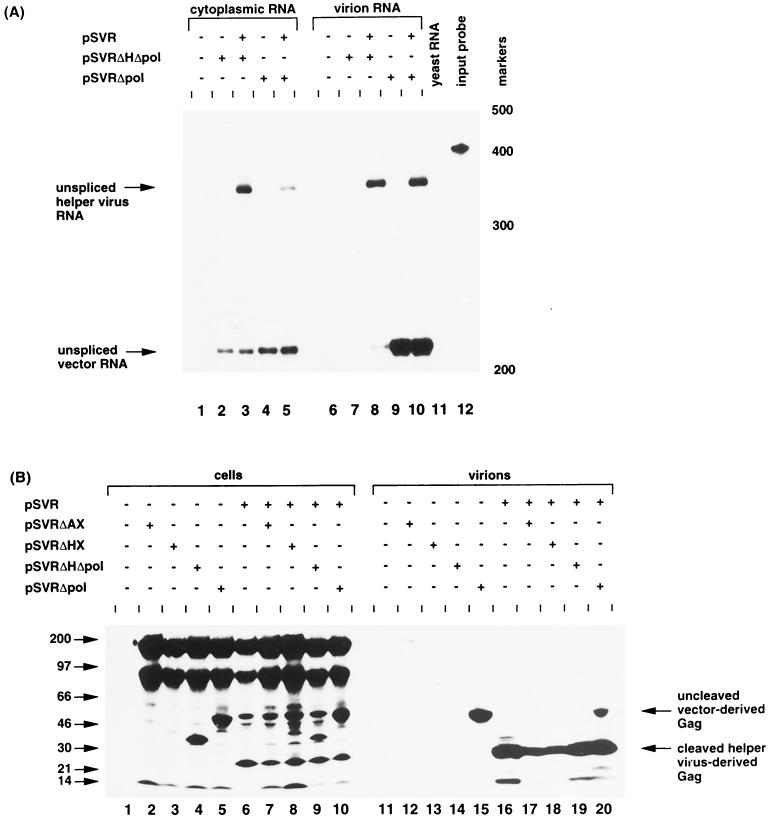
Two possible explanations exist for the failure of efficient encapsidation of HIV-2 vectors lacking sequences at the 3′ end of gag: (i) the presence of _cis_-acting packaging sequences located at the 3′ end of gag (between positions 1458 and 2032) or (ii) a _trans_-acting requirement for translation of the Gag protein. To address these issues, we constructed an HIV-2 vector with a stop codon at the _Hin_dIII site (see Materials and Methods). The resulting vector, pSVRΔHΔpol (Fig. 3A), was transfected, along with pSVRΔpol, either alone or with HIV-2 helper virus. Cytoplasmic and virion RNAs extracted from the transfectants were subjected to RPA (results are shown in Fig. 4A and Table 2). The different vectors were expressed to similar levels in the cytoplasm of the transfected cells (lanes 2 to 5). As previously observed, pSVRΔpol was able to efficiently encapsidate its own RNA. However, pSVRΔHΔpol did not package its RNA when transfected alone (lane 7). This was not surprising, as the Gag protein produced from this vector would not be expected to assemble into virion particles (25). Unspliced pSVRΔHΔpol vector RNA was packaged to a low level by HIV-2 helper virus; however, the level of encapsidation was greatly reduced compared to that observed for pSVRΔpol (compare lanes 8 and 10). The ability of the various vectors to produce virus-like particles was analyzed by immunoprecipitation of cell and virion proteins using human anti-sera to HIV-2 (Fig. 4B). The vectors were transfected either alone or with HIV-2 helper virus. Only pSVRΔpol was able to produce virions (Fig. 4B, lanes 15 and 20). A truncated form of Gag was expressed in the cytoplasm of cells transfected with pSVRΔHΔpol (Fig. 4B, lanes 4 and 9); however, this was not released from the cell (Fig. 4B, lanes 14 and 19). The vectors pSVRΔHΔpol and pSVRΔpol contain the same _cis_-acting sequences and differ only in their ability to translate the C terminus of Gag, hence, the efficiency of vector RNA encapsidation correlates with the ability of the vector to produce particles. Expression of a truncated form of Gag protein did not affect the formation of wild-type particles, as protein expression, processing, and helper virus RNA encapsidation were normal (Fig. 4A, lane 8, and 4B, lane 19).
FIG. 4.
(A) RNase protection analysis using riboprobe KS2ES of cytoplasmic and virion RNAs from transfections of vector competent to make viral particles (pSVRΔpol) against translational stop control (pSVRΔHΔpol) with or without helper virus. The positions of unspliced helper virus RNA (nt 369) and of unspliced vector RNA (nt 217) are indicated. Protection with control RNA (yeast RNA) and with the riboprobe without RNase treatment (input probe) is shown (lanes 11 and 12, respectively). The positions of RNA size markers are shown (in nucleotides) to the right of the figure. (B) Immunoprecipitation analysis of cellular and virion proteins produced from vectors with and without helper virus. The molecular mass markers are shown to the left in kilodaltons. The predicted positions of uncleaved vector-derived Gag and cleaved helper virus-derived Gag are indicated.
Translation of Gag protein containing a functional NC domain is required for efficient encapsidation of HIV-2 RNA.
The previous experiments compared the encapsidation efficiencies of a vector which could produce virus-like particles with one which could not. We wanted to compare two vectors which could both produce virus-like particles, one of which would be able and another which would be unable to package its own RNA. We therefore mutated the cysteine and histidine residues in the two zinc fingers of HIV-2 NC to alanines (Fig. 5A). This would be expected to disrupt the formation of the zinc fingers and impair RNA binding by the mutant NC (6, 9, 11, 12, 36). The ability of a proviral construct containing a mutated NC domain to package its unspliced viral RNA was studied by RPA of cells transfected with either wild-type or mutant proviral constructs (pSVR or pSVRNCm, respectively), and the results are shown in Fig. 5C. Unspliced and spliced RNAs were expressed in the cytoplasm of cells transfected with either wild-type or NC mutant constructs (Fig. 5C, lanes 2 and 3). Wild-type HIV-2 was able to efficiently encapsidate unspliced RNA; however, as expected, the NC mutant was unable to package viral RNA (Fig. 5C, lanes 5 and 6). Thus, mutation of the cysteine and histidine residues in the NC domain of HIV-2 Gag was sufficient to abrogate viral RNA packaging. The failure of the mutant to package its viral RNA was not due to a failure to produce viral particles, as the NC mutant produced levels of reverse transcriptase activity similar to those of wild-type HIV-2 (data not shown).
FIG. 5.
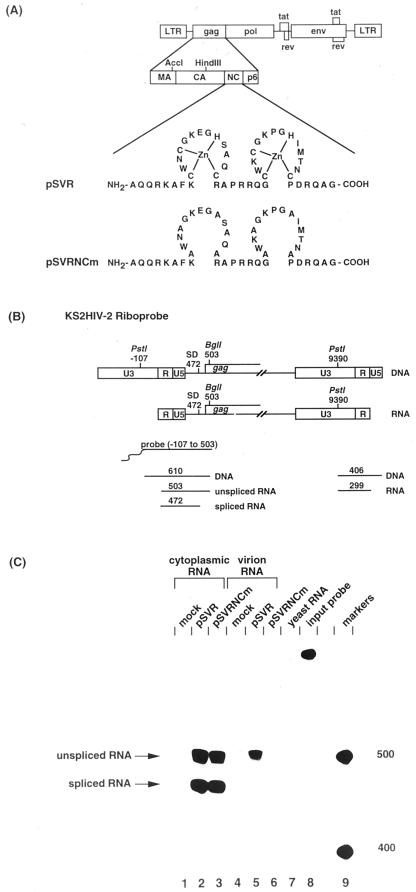
(A) Alanine substitution of cysteine and histidine residues in the NC region of pSVR, producing pSVRNCm. LTR, long terminal repeat; MA, matrix; CA, capsid; NC, nucleocapsid. (B) Predicted sizes of protected fragments using KS2HIV-2 riboprobe. SD, splice donor. (C) RNase protection analysis of cytoplasmic and virion RNA from cells transfected with wild-type virus or pSVRNCm. The positions of unspliced RNA (nt 503) and spliced RNA (nt 472) are shown. Protection with control RNA (yeast RNA) and with the riboprobe without RNase treatment (input probe) is shown (lanes 7 and 8, respectively). The positions of RNA size markers (lane 9) are shown in nucleotides.
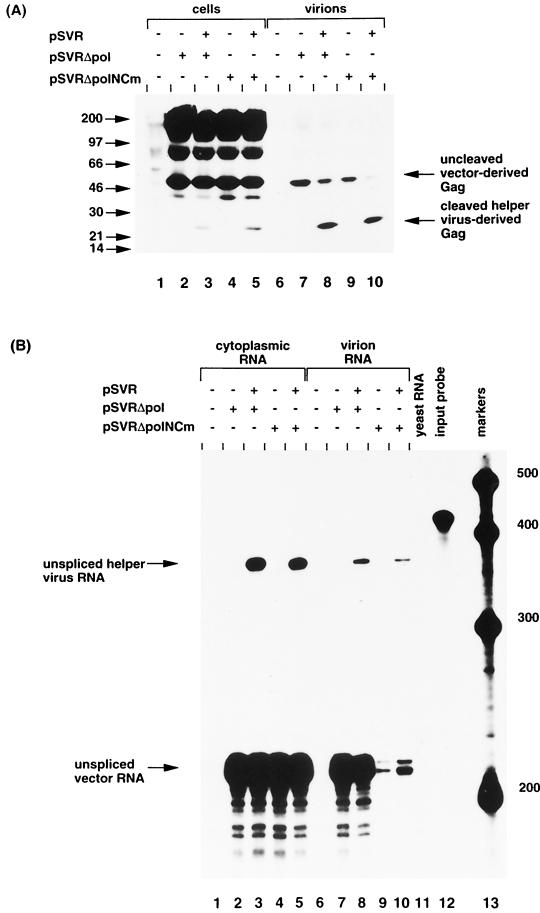
To compare the ability of the NC mutant and pSVRΔpol to be encapsidated in trans by HIV-2 helper virus, we deleted the sequences between the _Xho_I and _Xba_I sites (positions 2032 and 5067) from pSVRNCm. The ability of the vectors to produce virus-like particles was assessed by immunoprecipitation of cell and virion proteins from cells transfected with pSVRΔpol or pSVRΔpolNCm either alone or with HIV-2 helper virus (Fig. 6A). Uncleaved vector-derived Gag was expressed in the cell and virion samples of cells transfected with either pSVRΔpol or pSVRΔpolNCm (Fig. 6A, lanes 2 to 5 and 7 to 10).
FIG. 6.
(A) Immunoprecipitation of cell and virion proteins produced from cells transfected with pSVRΔpol or pSVRΔpolNCm with or without helper virus. The positions of molecular mass markers are shown to the left in kilodaltons. The positions of uncleaved vector-derived Gag and cleaved helper virus-derived Gag are indicated. (B) RNase protection analysis of cytoplasmic and virion RNA from cells transfected with pSVRΔpol or pSVRΔpolNCm with or without helper virus using riboprobe KS2ES. The positions of unspliced helper virus RNA (nt 369) and unspliced vector RNA (nt 217) are indicated. Protection with control RNA (yeast RNA) and with riboprobe without RNase treatment (input probe) is shown (lanes 11 and 12, respectively). The positions of RNA size markers (lane 13) are shown in nucleotides.
The ability of HIV-2 to encapsidate in trans the NC mutant vector and pSVRΔpol RNAs was compared. These two constructs were transfected either alone or with HIV-2 helper virus, and the levels of RNA encapsidation were studied by RPA (Fig. 6B). A very low level of encapsidation of NC mutant vector RNA was observed when the vector was transfected alone (Fig. 6B, lane 9), indicating that the NC mutant packaged its viral RNA to a greatly reduced level compared to wild-type NC (compare lanes 7 and 9). There was a low level of encapsidation of NC mutant vector RNA in trans by HIV-2 helper virus (Fig. 6B, lane 10). Thus, efficient encapsidation of HIV-2 RNA requires cotranslation of Gag protein containing a functional NC domain.
DISCUSSION
There have been relatively few studies addressing how the unspliced species of retroviral RNA is sorted to fulfill its two functions of coding and (uniquely) forming the viral genome. Packaging of subgenomic RNA would be highly disadvantageous for the virus and, in some viruses, it is excluded from the packaging process by having _cis_-acting signals spliced out. Some viral messages such as that of the env mRNA are targeted by their leader sequence to be translated on the rough endoplasmic reticulum, sequestering them away from the site of production of Gag and from viral assembly.
Levin et al. showed, in a simple retroviral system, that unspliced RNA which was being actively translated was unable subsequently to be encapsidated (23, 24), suggesting that two nonequilibrating pools of RNA exist (model 2, Fig. 1). These studies did not however exclude the possibility that the production of other cellular proteins essential to the encapsidation process had been inhibited by actinomycin D. Another study proposed that Gag polyproteins competed with ribosomes to determine the fate of the nascent retroviral RNA (38). The competition model proposed for avian sarcoma and leukosis virus (ASLV) (38) would be consistent with the MuLV results, suggesting the existence of two pools of unspliced RNA, one for translation and one for encapsidation (23, 24).
The efficiency of MuLV as a vector would argue that encapsidation in cis is not required. The fact that HIV-1-based vector systems also work implies that RNA encapsidation in trans is possible, although many vector systems include variable lengths of gag coding region in the vector to enhance packaging efficiency and the effect of these in targeting the RNA to polyribosomes when Gag protein is being produced has not been evaluated. McBride et al. analyzed an HIV-1 construct which contained a translation stop codon in the capsid domain of Gag (27). The unspliced RNA produced from this construct was encapsidated by helper virus with an efficiency of 0.93 compared to that of wild-type RNA, indicating that translation of Gag in cis was not essential for efficient encapsidation of HIV-1 RNA.
In addition to HIV-2, a notable example of a group of retroviruses in which the packaging signal is located upstream of the major splice donor are the ASLV group retroviruses (17, 19, 20). The 5′ leader region of ASLV RNA contains three short open reading frames (ORFs) whose size and position are conserved (13). These ORFs have been shown to be translated (7), and translation and encapsidation appear to be functionally linked (7, 8).
Unlike the ASLV family, HIV-2 does not possess ORFs upstream of the gag initiation codon. The location of the packaging signal upstream of the major splice donor necessitates a mechanism for selection of unspliced viral RNA for encapsidation into progeny virions. We previously observed the failure of an HIV-2-based vector lacking gag and pol sequences to be encapsidated by HIV-2 helper virus. Interestingly, both unspliced and spliced HIV-2 vector RNAs were packaged by HIV-1 helper virus (18), confirming the existence of a fully competent packaging signal in both species upstream of the splice donor, and suggesting that the two viruses might use different mechanisms to select unspliced RNA for encapsidation. In the present study, we have further analyzed the requirements for efficient encapsidation of HIV-2 RNA and propose that HIV-2 uses a previously undescribed mechanism for selection of unspliced viral RNA for encapsidation. Analysis of a series of HIV-2-based vectors which contained increasing amounts of gag sequence revealed that until the 3′ end of gag was included, vector RNA was packaged only poorly in virus particles. To further exclude _cis_-acting signals in these regions, we compared the packaging efficiencies of two vectors which contained identical 3′ _cis_-acting sequences, one of which was able to produce full-length Gag protein and the other which produced a truncated Gag protein. The latter did not produce viral particles and was packaged to a greatly reduced level compared to a vector, pSVRΔpol, which contained identical _cis_-acting sequences but could produce full-length gag and export particles. Efficient RNA encapsidation thus correlated with the ability to form virus-like particles. Analysis of an HIV-2-based vector which produced virus-like particles but which failed to package its own RNA, pSVRΔpolNCm, confirmed that a functional NC domain was required for efficient HIV-2 RNA encapsidation.
Translation per se was not responsible for efficient encapsidation of HIV-2 RNA, as vectors with C-terminal truncations of Gag or a translational stop codon, were translated but were not efficiently encapsidated. Thus, directing the unspliced viral RNA to polyribosomes for translation is not sufficient for its efficient selection for encapsidation. The data are thus most consistent with the hypothesis that HIV-2 uses a novel mechanism for selection of unspliced RNA in which Gag polyprotein translated off the unspliced viral RNA binds to the packaging signal on the unspliced RNA that is being translated and then directs that RNA for encapsidation into progeny virions. This model (model 3, Fig. 1) would provide an additional selectivity for unspliced RNA in a system in which the packaging signal is located on both unspliced and spliced viral RNA. The stoichiometry of the interaction involving 2 genomes/∼3,000 Gag proteins clearly would not allow every full-length viral RNA to be encapsidated, and we therefore propose a scheme in which an initial Gag-RNA interaction occurs in cis and provides a nucleation site for further assembly of Gag polyproteins. These would assemble on the initial complex at an as yet undefined subcellular location between the polyribosomes and the plasma membrane. The interaction would involve Gag monomers binding to each other through their major homology regions and aligning their NC subdomains, which are known to bind RNA without sequence specificity, along the viral genome. A recent study (30) suggests that HIV-2-based vectors can be packaged in trans. These studies, however, did not compare spliced and unspliced helper virus RNA packaging and did not quantitate vector packaging directly but would be consistent with the low level of packaging of Gag-deleted vectors that we have reported here.
One of the striking features of studies of lentivirus RNA encapsidation has been the difficulty in precisely locating a single packaging signal region. Deletion mutations of a magnitude shown to abolish encapsidation in murine viruses rarely completely abrogate lentivirus packaging, suggesting that there is functional redundancy in the system and that more than one site can act to contribute to packaging. Conceivably, this may be explained by the presence of a number of similar motifs, for example the GGNGR motifs previously identified, any one of which may act as a nucleation site for the Gag-RNA interaction. Highly efficient encapsidation may therefore depend on a nucleation event of a small number of Gag monomers on some or all of these _cis_-acting sites. Close approximation of a number of packaging signal sequences could occur by their being present in adjacent locations on the same RNA strand but would also be achieved by the close association of two RNA strands, each of which has one or more of these sequences. This model would explain the intimate association between retroviral RNA dimerization and encapsidation which has been documented so frequently and also why disrupting RNA dimerization in lentiviruses almost inevitably leads to impaired encapsidation efficiency. HIV-1 has become established as a lentivirus vector of promise, and several other lentivirus vectors are also under development. If the nucleic acid recognition process in some lentiviruses requires encoding of structural proteins to begin encapsidation in cis, this could severely limit their potential for use as viral vectors.
ACKNOWLEDGMENTS
We thank Nijsje Dorman for the riboprobe template, KS2HIV-2, and the Medical Research Council AIDS Reagent Project for human sera to HIV-2.
This work was funded by the Royal Society and the Medical Research Council (United Kingdom) and supported by grant 960675 (Biomed II). Jane F. Kaye is funded by a Royal Society Dorothy Hodgkin Research Fellowship.
REFERENCES
- 1.Aldovini A, Young R A. Mutations of RNA and protein sequences involved in human immunodeficiency virus type 1 packaging result in production of noninfectious virus. J Virol. 1990;64:1920–1926. doi: 10.1128/jvi.64.5.1920-1926.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berkowitz R D, Goff S P. Analysis of binding elements in the human immunodeficiency virus type 1 genomic RNA and nucleocapsid protein. Virology. 1994;202:233–246. doi: 10.1006/viro.1994.1339. [DOI] [PubMed] [Google Scholar]
- 3.Berkowitz R D, Luban J, Goff S P. Specific binding of human immunodeficiency virus type 1 Gag polyprotein and nucleocapsid protein to viral RNAs detected by RNA mobility shift assays. J Virol. 1993;67:7190–7200. doi: 10.1128/jvi.67.12.7190-7200.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3a.Boris-Lawrie, K. Personal communication.
- 4.Clavel F, Orenstein J M. A mutant of human immunodeficiency virus with reduced RNA packaging and abnormal particle morphology. J Virol. 1990;64:5230–5234. doi: 10.1128/jvi.64.10.5230-5234.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clever J, Sassetti C, Parslow T G. RNA secondary structure and binding sites for gag gene products in the 5′ packaging signal of human immunodeficiency virus type 1. J Virol. 1995;69:2101–2109. doi: 10.1128/jvi.69.4.2101-2109.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dannull J, Surovoy A, Jung G, Moelling K. Specific binding of HIV-1 nucleocapsid protein to PSI RNA in vitro requires N-terminal zinc finger and flanking basic amino acid residues. EMBO J. 1994;13:1525–1533. doi: 10.1002/j.1460-2075.1994.tb06414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donze O, Damay P, Spahr P F. The first and third uORFs in RSV leader RNA are efficiently translated: implications for translational regulation and viral RNA packaging. Nucleic Acids Res. 1995;23:861–868. doi: 10.1093/nar/23.5.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donze O, Spahr P F. Role of the open reading frames of Rous sarcoma virus leader RNA in translation and genome packaging. EMBO J. 1992;11:3747–3757. doi: 10.1002/j.1460-2075.1992.tb05460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dorfman T, Luban J, Goff S P, Haseltine W A, Göttlinger H G. Mapping of functionally important residues of a cysteine-histidine box in the human immunodeficiency virus type 1 nucleocapsid protein. J Virol. 1993;67:6159–6169. doi: 10.1128/jvi.67.10.6159-6169.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garzino Demo A, Gallo R C, Arya S K. Human immunodeficiency virus type 2 (HIV-2): packaging signal and associated negative regulatory element. Hum Gene Ther. 1995;6:177–184. doi: 10.1089/hum.1995.6.2-177. [DOI] [PubMed] [Google Scholar]
- 11.Gorelick R J, Chabot D J, Rein A, Henderson L E, Arthur L O. The two zinc fingers in the human immunodeficiency virus type 1 nucleocapsid protein are not functionally equivalent. J Virol. 1993;67:4027–4036. doi: 10.1128/jvi.67.7.4027-4036.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorelick R J, Nigida S M, Jr, Bess J W, Jr, Arthur L O, Henderson L E, Rein A. Noninfectious human immunodeficiency virus type 1 mutants deficient in genomic RNA. J Virol. 1990;64:3207–3211. doi: 10.1128/jvi.64.7.3207-3211.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hackett P B, Dalton M W, Johnson D P, Petersen R B. Phylogenetic and physical analysis of the 5′ leader RNA sequences of avian retroviruses. Nucleic Acids Res. 1991;19:6929–6934. doi: 10.1093/nar/19.24.6929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harrison G P, Lever A M L. The human immunodeficiency virus type 1 packaging signal and major splice donor region have a conserved stable secondary structure. J Virol. 1992;66:4144–4153. doi: 10.1128/jvi.66.7.4144-4153.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harrison G P, Miele G, Hunter E, Lever A M L. Functional analysis of the core HIV-1 packaging signal in a permissive cell line. J Virol. 1998;72:5886–5896. doi: 10.1128/jvi.72.7.5886-5896.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayashi T, Shioda T, Iwakura Y, Shibuta H. RNA packaging signal of human immunodeficiency virus type 1. Virology. 1992;188:590–599. doi: 10.1016/0042-6822(92)90513-o. [DOI] [PubMed] [Google Scholar]
- 17.Katz R A, Terry R W, Skalka A M. A conserved cis-acting sequence in the 5′ leader of avian sarcoma virus RNA is required for packaging. J Virol. 1986;59:163–167. doi: 10.1128/jvi.59.1.163-167.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaye J F, Lever A M L. Nonreciprocal packaging of human immunodeficiency virus type 1 and type 2 RNA: a possible role for the p2 domain of Gag in RNA encapsidation. J Virol. 1998;72:5877–5885. doi: 10.1128/jvi.72.7.5877-5885.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knight J B, Si Z H, Stoltzfus C M. A base-paired structure in the avian sarcoma virus 5′ leader is required for efficient encapsidation of RNA. J Virol. 1994;68:4493–4502. doi: 10.1128/jvi.68.7.4493-4502.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koyama T, Harada F, Kawai S. Characterization of a Rous sarcoma virus mutant defective in packaging its own genomic RNA: biochemical properties of mutant TK15 and mutant-induced transformants. J Virol. 1984;51:154–162. doi: 10.1128/jvi.51.1.154-162.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kunkel T A, Roberts J D, Zakour R A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- 22.Lever A, Göttlinger H, Haseltine W, Sodroski J. Identification of a sequence required for efficient packaging of human immunodeficiency virus type 1 RNA into virions. J Virol. 1989;63:4085–4087. doi: 10.1128/jvi.63.9.4085-4087.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levin J G, Grimley P M, Ramseur J M, Berezesky I K. Deficiency of 60 to 70S RNA in murine leukemia virus particles assembled in cells treated with actinomycin D. J Virol. 1974;14:152–161. doi: 10.1128/jvi.14.1.152-161.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levin J G, Rosenak M J. Synthesis of murine leukemia virus proteins associated with virions assembled in actinomycin D-treated cells: evidence for persistence of viral messenger RNA. Proc Natl Acad Sci USA. 1976;73:1154–1158. doi: 10.1073/pnas.73.4.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luo L, Li Y, Dales S, Kang C Y. Mapping of functional domains for HIV-2 gag assembly into virus-like particles. Virology. 1994;205:496–502. doi: 10.1006/viro.1994.1670. [DOI] [PubMed] [Google Scholar]
- 26.McBride M S, Panganiban A T. The human immunodeficiency virus type 1 encapsidation site is a multipartite RNA element composed of functional hairpin structures. J Virol. 1996;70:2963–2973. doi: 10.1128/jvi.70.5.2963-2973.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McBride M S, Schwartz M D, Panganiban A T. Efficient encapsidation of human immunodeficiency virus type 1 vectors and further characterization of cis elements required for encapsidation. J Virol. 1997;71:4544–4554. doi: 10.1128/jvi.71.6.4544-4554.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCann E M, Lever A M L. Location of cis-acting signals important for RNA encapsidation in the leader sequence of human immunodeficiency virus type 2. J Virol. 1997;71:4133–4137. doi: 10.1128/jvi.71.5.4133-4137.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naldini L, Blomer U, Gallay P, Ory D, Mulligan P, Gage F H, Verma I M, Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 30.Poeschla E, Gilbert J, Li X, Huang S, Ho A, Wong-Staal F. Identification of human immunodeficiency virus type 2 (HIV-2) encapsidation determinant and transduction of nondividing human cells by HIV-2 based lentivirus vectors. J Virol. 1998;72:6527–6536. doi: 10.1128/jvi.72.8.6527-6536.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Potts B J. ‘Mini’ reverse transcriptase (RT) assay. In: Aldovini A, Walker B D, editors. Techniques in HIV research. New York, N.Y: Stockton Press; 1990. pp. 102–106. [Google Scholar]
- 32.Poznansky M, Lever A M L, Bergeron L, Haseltine W, Sodroski J. Gene transfer into human lymphocytes by a defective human immunodeficiency virus type 1 vector. J Virol. 1991;65:532–536. doi: 10.1128/jvi.65.1.532-536.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richardson J H, Child L A, Lever A M L. Packaging of HIV-1 genomic RNA requires cis-acting sequences outside the 5′ leader region. J Virol. 1993;67:3097–4005. doi: 10.1128/jvi.67.7.3997-4005.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rizvi T A, Panganiban A T. Simian immunodeficiency virus RNA is efficiently encapsidated by human immunodeficiency virus type 1 particles. J Virol. 1993;67:2681–2688. doi: 10.1128/jvi.67.5.2681-2688.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sakaguchi K, Zambrano N, Baldwin E T, Shapiro B A, Erickson J W, Omichinski J G, Clore G M, Gronenborn A M, Appella E. Identification of a binding site for the human immunodeficiency virus type 1 nucleocapsid protein. Proc Natl Acad Sci USA. 1993;90:5219–5223. doi: 10.1073/pnas.90.11.5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmalzbauer E, Strack B, Dannull J, Guehmann S, Moelling K. Mutations of basic amino acids of NCp7 of human immunodeficiency virus type 1 affect RNA binding in vitro. J Virol. 1996;70:771–777. doi: 10.1128/jvi.70.2.771-777.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Selden R F. Transfection using DEAE-dextran. In: Ausubel R, Brent R E, Kingston D D, Moore J G, Seidman J A, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: Wiley Interscience; 1987. pp. 9.21–9.22. [Google Scholar]
- 38.Sonstegard T S, Hackett P B. Autogenous regulation of RNA translation and packaging by Rous sarcoma virus Pr76gag. J Virol. 1996;70:6542–6552. [PMC free article] [PubMed] [Google Scholar]