Structural and Functional Heterogeneity of Nuclear Bodies (original) (raw)
Abstract
The nuclear body is a cellular structure that appears to be involved in the pathogenesis of acute promyelocytic leukemia and viral infection. In addition, the nuclear body is a target of autoantibodies in patients with the autoimmune disease primary biliary cirrhosis. Although the precise function of the nuclear body in normal cellular biology is unknown, this structure may have a role in the regulation of gene transcription. In a previous investigation, we identified a leukocyte-specific, gamma interferon (IFN-γ)-inducible autoantigen designated Sp140. The objectives of the present study were to investigate the cellular location of Sp140 with respect to the nuclear-body components PML and Sp100 and to examine the potential role of Sp140 in the regulation of gene transcription. We used adenovirus-mediated gene transfer to express Sp140 in human cells and observed that the protein colocalized with PML and Sp100 in resting cells and associated with structures containing PML during mitosis. In cells infected with the adenovirus expressing Sp140 and incubated with IFN-γ, the number of PML-Sp100 nuclear bodies per cell increased but immunoreactive Sp140 was not evenly distributed among the nuclear bodies. Sp140 associated with a subset of IFN-γ-induced PML-Sp100 nuclear bodies. To examine the potential effect of Sp140 on gene transcription, a plasmid encoding Sp140 fused to the DNA-binding domain of GAL4 was cotransfected into COS cells with a chloramphenicol acetyltransferase (CAT) reporter gene containing five GAL4-binding sites and a simian virus 40 enhancer region. The GAL4-Sp140 fusion protein increased the expression of the reporter gene. In contrast, Sp100 fused to the GAL4 DNA-binding domain inhibited CAT activity in transfected mammalian cells. The results of this study demonstrate that Sp140 associates with a subset of PML-Sp100 nuclear bodies in IFN-γ-treated cells and that Sp140 may activate gene transcription. Taken together, these observations suggest that the nuclear bodies within a cell may be heterogeneous with respect to both composition and function.
The nuclear body (also known as nuclear domain 10, the PML oncogenic domain, and the Kr body) is a subcellular domain that appears to be involved in the pathogenesis of a variety of human diseases including acute promyelocytic leukemia and viral infections. In addition, the nuclear body is a target of autoantibodies in the serum of patients with the autoimmune disease primary biliary cirrhosis (reviewed in reference 30). After immunohistochemical staining, nuclear bodies appear as 5 to 30 discrete, punctate regions within the nucleus. They are distinct from other subnuclear domains including centromeres, kinetochores, coiled bodies, spliceosomes, and interchromatin granules (6). The number of nuclear bodies in the cell increases in response to stimuli including interferons (IFNs), heat shock, and viral infection (3). In a recent study, LaMorte et al. demonstrated that nascent RNA associates with some but not all nuclear bodies (22). The authors concluded that nuclear bodies may play a role in transcriptional events and may have more than one functional state.
The nuclear-body component promyelocytic leukemia protein (PML) is fused to the retinoic acid receptor α in the majority of patients with acute promyelocytic leukemia. The fusion protein appears to disrupt the normal differentiation of promyelocytes (5, 9, 17, 26). In addition, PML-retinoic acid receptor α alters the structure of nuclear bodies such that instead of the usual 5 to 30 discrete domains in the nucleus, staining for nuclear-body components reveals numerous smaller speckles. Treatment of promyelocytic leukemia cells with retinoic acid results in differentiation of myeloid precursor cells and re-formation of nuclear bodies (12, 21, 35). Previous investigators suggested that PML may function as a suppressor of cellular growth and transformation (2, 24). Wang et al. observed that homozygous disruption of the PML gene in mice altered cellular proliferation, enhanced tumorigenesis, and inhibited the differentiation of myeloid precursor cells (34). The authors suggested that PML may mediate the growth-suppressive and differentiating activities of retinoic acid.
The nuclear body is a target of autoantibodies in approximately 40% of patients with primary biliary cirrhosis. These autoantibodies are rarely detected in normal individuals or in patients with other autoimmune diseases (13, 33). Szostecki et al. used serum from patients with primary biliary cirrhosis to identify a cDNA encoding the nuclear-body component Sp100 (32). Seeler et al. reported that Sp100 interacts with the heterochromatin protein 1 (HP1) family of nonhistone chromosomal proteins (29). Both Sp100 and HP1, when tethered to DNA, behaved as transcriptional repressors in transfected cells. The authors suggested that the PML-Sp100 nuclear body may regulate gene transcription by modifying chromatin or heterochromatin structure.
In a previous study, we used serum from a patient with primary biliary cirrhosis to identify a cDNA encoding a nuclear body autoantigen designated Sp140 (4). The amino-terminal portion of Sp140 was 49% identical to the amino-terminal region in Sp100. The carboxyl portion of Sp140 contained a plant homeobox domain and bromodomain and was 39% identical to the carboxyl portion of murine nuclear hormone receptor transcription intermediary factor 1α (TIF1α) (25). These structural features suggested that Sp140 may have a role in the regulation of gene transcription. High levels of mRNA encoding Sp140 were detected in human spleen and peripheral blood leukocytes; much lower levels were expressed in all other tissues examined. Using immunohistochemistry, we demonstrated that Sp140 localized to nuclear bodies containing PML in HL60 cells. These results suggested that Sp140 is a leukocyte-specific component of the nuclear body.
Dent et al., as part of a study to identify novel lymphocyte- restricted transcription factors, identified two cDNAs encoding splice variants of Sp140, which were designated lymphoid-restricted homologues of Sp100 (LySp100A and LySp100B) (8). Using antiserum directed against LySp100, these investigators observed a nuclear-body staining pattern in MBB1 cells, an Epstein-Barr virus (EBV)-transformed, human lymphoblastoid cell line. Interestingly, in MBB1 cells, LySp100 and PML staining patterns were largely nonoverlapping. The authors designated the LySp100-containing nuclear bodies as “LySp100-associated nuclear domains.”
The objectives of this study were to further investigate the cellular location of Sp140 with respect to the PML-Sp100 nuclear body and to examine the potential role of Sp140 in the regulation of gene transcription. The small amount of Sp140 in peripheral blood leukocytes and myeloid cell lines made it difficult to clearly establish the cellular location of this protein by indirect immunofluorescence. In this study, we used a replication-deficient adenovirus vector to express Sp140 in human cell lines. We confirm that Sp140 colocalizes with PML-Sp100 nuclear bodies in resting cells and demonstrate that Sp140 associates with a subset of PML-Sp100 nuclear bodies in IFN-γ-treated cells. To examine the potential effect of Sp140 on gene expression, a plasmid encoding Sp140 fused to the GAL4 DNA-binding domain (GAL4-Sp140) was cotransfected into mammalian cells with a chloramphenicol acetyltransferase (CAT) reporter plasmid containing five GAL4 binding sites and a simian virus 40 (SV40) enhancer domain. In contrast to Sp100, which inhibited gene transcription when tethered to DNA, the GAL4-Sp140 fusion protein activated expression of the reporter gene. Taken together, our data suggest that nuclear bodies may be heterogeneous with respect to both composition and function.
MATERIALS AND METHODS
Antiserum, affinity-purified antibodies, and monoclonal antibodies.
A monoclonal antibody directed against β-galactosidase was obtained from Sigma Chemical Co. (St. Louis, Mo.). A monoclonal antibody directed against PML was obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, Calif.), and was used in indirect immunofluorescence to identify nuclear bodies in HeLa cells. The preparation of rat antibodies directed against Sp140 was described previously (4).
Antibodies in serum from patients with primary biliary cirrhosis were used to identify nuclear bodies in HEp-2 cells. The diagnosis of primary biliary cirrhosis in these patients was based on the presence of elevated liver function enzyme levels and high-titer antibodies directed against the mitochondrial antigen E2 pyruvate dehydrogenase complex (E2 PDC). These antibodies are present in approximately 95% of patients with primary biliary cirrhosis and are rarely seen in normal individuals or in patients with other autoimmune diseases (reviewed in reference 18). Antibodies directed against PML and Sp100 were immunoaffinity purified from human serum by using recombinant proteins prepared as described below.
To prepare recombinant Sp100, DNA encoding Sp100 was produced by PCR with two oligonucleotides (5′-TTGAATTCGGTGGGAAGATGGCAGGTGGG-3′ and 5′-TTGAATTCCTGACATTCTGCAGGCCA-3′) and cDNA prepared from human spleen (Clontech, Palo Alto, Calif.). The nucleotide sequence of the PCR product was determined and confirmed to encode Sp100. The DNA fragment was treated with _Eco_RI and ligated into the _Eco_RI site of prokaryotic expression vector pGEX (Pharmacia, Piscataway, N.J.). The plasmid was used to transform Escherichia coli, and expression of the fusion protein was induced by treatment with isopropyl-1-thio-β-d-galactopyranoside. E. coli extracts containing the glutathione _S_-transferase–Sp100 fusion protein were fractionated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and transferred to nitrocellulose. The membrane was incubated in blocking solution (phosphate-buffered saline [PBS] containing 5% nonfat dry milk) and then in human serum diluted 1:10 with blocking solution. Bound antibodies were eluted and concentrated as previously described (4). Successful purification of anti-Sp100 antibodies from other antibodies present in patient serum was confirmed by the absence of reactivity with E2 PDC on immunoblotting and indirect immunofluorescence.
To prepare a DNA fragment encoding PML in frame with glutathione _S_-transferase in the pGEX vector, the cDNA encoding PML (a generous gift of H. de Thé, Hôpital St. Louis, Paris, France) and two oligonucleotides (5′-TTGAATTCATGGAGCCTGCACCCGCCCGATCT 3′ and 5′-TTGAATTCGAGCTGCTGATCACCACAACGCGT 3′) were used in the PCR. The PCR product was treated with _Eco_RI and ligated into the _Eco_RI site of pGEX. Recombinant protein and affinity-purified human antibodies were prepared as described above.
Construction of an E1-deleted recombinant adenovirus vector containing Sp140.
The cDNA encoding Sp140 was cloned into the _Not_I and _Bam_HI sites of pAd.RSV4 (provided by D. Dichek, Gladstone Institute for Cardiovascular Diseases, San Francisco, Calif.), which contains the Rous sarcoma virus long terminal repeat promoter and the SV40 polyadenylation signal. The plasmid containing Sp140 was cotransfected into 293 cells with pJM17 (provided by F. L. Graham, McMaster University, Hamilton, Ontario, Canada). Homologous recombination between the two plasmids resulted in an adenovirus that contained Sp140 sequences in place of E1 sequences. Recombinant viruses in a plaque were amplified in 293 cells, and a high-titer stock (strain Ad.Sp140) was prepared, as previously described (14). The absence of replication-competent adenovirus in the viral stock was confirmed by the failure of Ad.Sp140 to produce cytopathic changes in A549 lung carcinoma cells. In addition, PCR failed to amplify a DNA fragment corresponding to the E1 region of adenovirus by using oligonucleotides and the Ad.Sp140 stock. A control virus carrying a nucleus-targeted form of β-galactosidase (strain Ad.βgal) (10) was provided by D. Dichek.
Cell culture, fixation and staining.
HEp-2, HeLa, and COS cells (American Type Culture Collection, Rockville, Md.) were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum, l-glutamine (2 mM), penicillin (200 U/ml), and streptomycin (200 μg/ml).
To detect β-galactosidase in HEp-2 cells infected with Ad.βgal, the cells were fixed with glutaraldehyde and incubated with 5 mM K4Fe(CN)6, 5 mM K3Fe(CN)6, 1 mM MgCl2, and 1 mg of 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside per ml in PBS for 4 to 6 h. Under light microscopy, cells containing β-galactosidase appeared blue.
For immunofluorescence staining, HEp-2 and HeLa cells were grown in tissue culture chambers (Nunc Inc., Naperville, Ill.), fixed in 4% paraformaldehyde in PBS at room temperature for 10 min, and permeabilized by treatment with acetone at −20°C for 2 min. Rat anti-Sp140 antiserum and human antibodies were incubated with substrate for 1 h at room temperature. Unbound antibodies were removed by three successive washes with PBS. Bound rat antibodies were detected with species-specific fluorescein isothiocyanate (FITC)-conjugated goat anti-rat immunoglobulin G (IgG) antiserum (Boehringer Mannheim, Indianapolis, Ind.). Bound human antibodies were detected with species-specific Texas Red ITC-conjugated goat anti-human IgG Fc antiserum. A species-specific FITC-conjugated sheep anti-mouse Ig antiserum (Amersham) was used in studies with murine monoclonal antibodies.
To detect the cellular location of nuclear-body components with respect to chromosomes in dividing cells, the cells were stained with the antibodies described above as well as with 4′,6-diamidino-2-phenylindole (DAPI; Sigma Chemical Co.).
SDS-polyacrylamide gel electrophoresis and immunoblotting.
HEp-2 cells were harvested in cold PBS and lysed by boiling for 5 min in sample buffer (0.125 M Tris-HCl, 4% SDS, 20% glycerol, 10% β-mercaptoethanol). The proteins were fractionated by SDS-polyacrylamide gel electrophoresis (8% polyacrylamide) and transferred to nitrocellulose membranes. Membranes were incubated in blocking solution and then with human antibodies or rat antiserum. Bound human antibodies were visualized by incubation with horseradish peroxidase (HRP)-conjugated protein A (Amersham) and enhanced chemiluminescence (Amersham). For studies with the rat antiserum, bound antibodies were detected by using HRP-conjugated goat anti-rat antiserum (Amersham) and chemiluminescence.
CAT assays.
A plasmid encoding Sp140 fused to the DNA-binding domain of GAL4 (amino acids 1 to 147) was prepared by ligating a cDNA encoding Sp140 into expression plasmid pBXG (pBXG-Sp140) (19). A cDNA encoding Sp100 was prepared by PCR, as described above, and ligated into pBXG (pBXG-Sp100). A plasmid encoding KRAB-A-interacting protein 1 (KRIP-1)/TIF1β fused to the GAL4 DNA-binding domain (pBXG–KRIP-1/TIF1β), previously shown to inhibit expression of the CAT gene, was used as a control (19). A plasmid encoding CAT under the control of five GAL4-binding sites, an SV40 enhancer, and an E1b TATA promoter (pG5SV-BCAT) was used as a reporter construct. Plasmids pBXG, pBXG-KRIP-1/TIF1β, and pG5SV-BCAT were kindly provided by S. Shu and J. Bonventre (Massachusetts General Hospital, Boston, Mass.) (19).
COS cells were transfected with a total of 11 μg of DNA by using the SuperFect transfection system (Qiagen Inc., Valencia, Calif.). At 48 h after transfection, the cells were washed twice with PBS, harvested in 0.25 M Tris Cl (pH 7.8), and disrupted by alternate freezing and thawing. The cell supernatants were assayed for CAT activity as described previously (27).
A plasmid encoding growth hormone was included in each transfection for normalization of transfection efficiencies. The amount of growth hormone in tissue culture medium 48 h after transfection was determined by a radioimmunoassay (Nichols Institute, San Juan Capistrano, Calif.).
RESULTS
Sp140 expression in cells infected with Ad.Sp140.
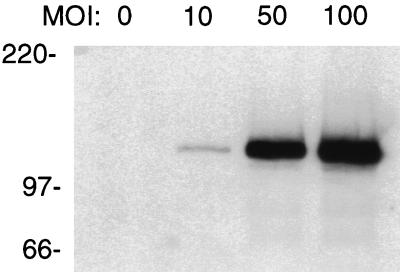
Adenovirus-mediated gene transfer was used to express Sp140 in HEp-2 cells. These cells were chosen because they are easily infected by adenovirus and because they express the nuclear-body components PML and Sp100. To determine whether Sp140 was expressed in HEp-2 cells exposed to Ad.Sp140, cells were incubated with Ad.Sp140 for 48 h at multiplicities of infection (MOIs) of 10, 50, and 100 PFU/cell. There was a dose-dependent increase in the amount of immunoreactive Sp140 in HEp-2 cells infected with Ad.Sp140 (Fig. 1). Sp140 was not detected in uninfected cells (lane 0) or in cells infected with a control virus (Ad.βgal) at the same MOIs (data not shown). In the following experiments, HEp-2 cells were infected with Ad.Sp140 at a MOI of 100 PFU/cell.
FIG. 1.
Immunoblot of Ad.Sp140-infected and control HEp-2 cells. Rat anti-Sp140 antiserum was used to detect Sp140 in uninfected HEp-2 cells or cells incubated with Ad.Sp140 at MOIs of 10, 50, and 100 PFU/cell. There was a dose-dependent increase in the level of Sp140 in infected HEp-2 cells.
Cellular distribution of Sp140 in Ad.Sp140-infected cells.
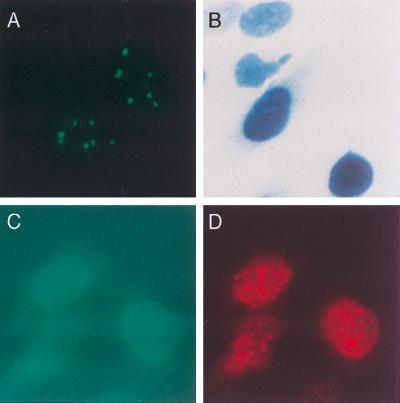
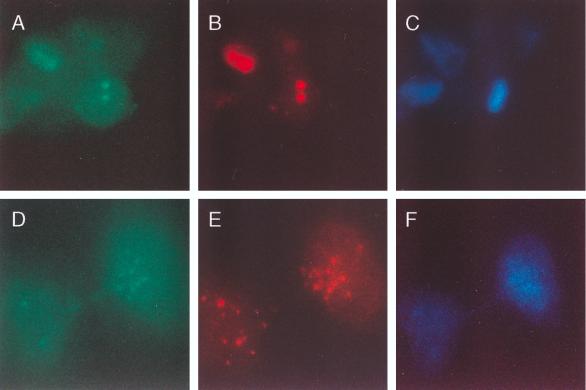
To determine the cellular location of Sp140 in cells infected with Ad.Sp140, HEp-2 cells were exposed to the virus for 48 h, fixed, and stained with rat anti-Sp140 antiserum. Sp140 was detected in a typical nuclear-body staining pattern (Fig. 2A). To demonstrate that the adenovirus vector did not direct the encoded protein to the nuclear body, cells were infected with Ad.βgal (which expresses β-galactosidase fused to a nucleus-targeting sequence) and stained for β-galactosidase. In Ad.βgal-infected cells, β-galactosidase was detected diffusely throughout the nucleus (Fig. 2B). To confirm that the replication-deficient virus vector did not disrupt nuclear bodies, HEp-2 cells were infected with Ad.βgal and stained with monoclonal antibody directed against β-galactosidase and with human serum from patient F111, which contained antibodies directed against PML and Sp100 (see below). Staining for β-galactosidase was observed throughout the nuclei of infected cells (Fig. 2C). Staining for PML and Sp100 (Fig. 2D) revealed the same nuclear-body pattern as that seen in uninfected cells (results not shown).
FIG. 2.
Cellular distribution of Sp140 or β-galactosidase expressed by an adenovirus vector. (A) When rat anti-Sp140 antiserum was used, a typical nuclear-body staining pattern was observed in Ad.Sp140-infected HEp-2 cells. (B) To demonstrate that the adenovirus vector did not direct the encoded protein to the nuclear body, Ad.βgal was used to infect HEp-2 cells. β-Galactosidase was observed diffusely throughout the nucleus of these cells. (C and D) To confirm that the replication-deficient adenovirus vector did not alter the cellular location of nuclear-body components, cells were infected with Ad.βgal and then stained with monoclonal antibody directed against β-galactosidase and with human serum containing antibodies directed against PML and Sp100. Staining for β-galactosidase was observed diffusely throughout the nucleus (C, green). Staining for PML and Sp100 revealed the same nuclear body pattern (D, red) as that seen in uninfected cells (results not shown).
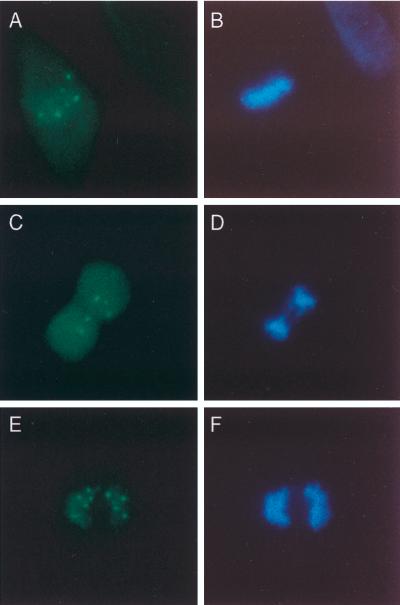
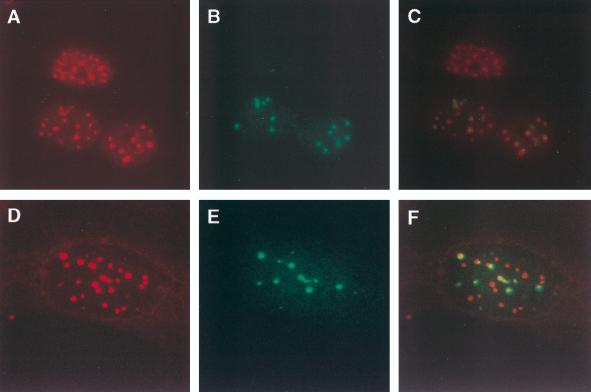
The high efficiency of gene transfer with the adenovirus vector permitted determination of the cellular location of Sp140 in the relatively few cells undergoing mitosis. Sp140-containing nuclear bodies were present near chromosomes during metaphase (Fig. 3A and B), anaphase (Fig. 3C and D), and telophase (Fig. 3E and F).
FIG. 3.
Sp140 associates with chromosomes in dividing cells. To determine the cellular location of Sp140 during mitosis, cells were infected with Ad.Sp140 and stained with rat anti-Sp140 antiserum (green) and DAPI (blue), which selectively binds to DNA. Sp140-containing nuclear bodies localized near chromosomes during metaphase (A and B), anaphase (C and D), and telophase (E and F).
Relationship of Sp140 to other nuclear-body components.
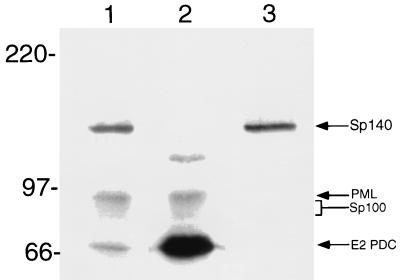
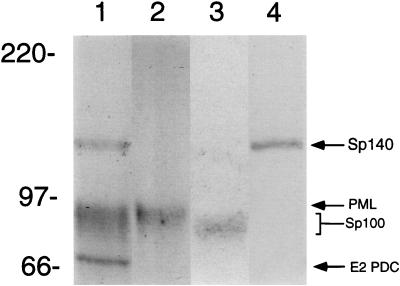
To determine the location of Sp140 with respect to the nuclear bodies recognized by antibodies in a patient with primary biliary cirrhosis, Ad.Sp140-infected HEp-2 cells were stained with rat anti-Sp140 antiserum and serum from patient F111. This serum was chosen because it contained antibodies directed against PML and Sp100 but did not react with Sp140 as determined by immunoblotting (Fig. 4, lane 2) and by immunoprecipitation of in vitro-translated proteins (data not shown). Antibodies in F111 serum reacted with E2 PDC (70 kDa), PML (∼90 kDa), Sp100 (∼80 kDa), and an as yet unidentified 120-kDa protein. In contrast, serum from a second patient (K142) reacted with E2 PDC, PML, Sp100, and Sp140 (lane 1). Rat anti-Sp140 antibodies reacted only with the 140-kDa protein (lane 3). Indirect immunofluorescence showed that rat anti-Sp140 antibodies colocalized with F111 serum antibodies in the nuclear bodies of Ad.Sp140-infected HEp-2 cells (see Fig. 6A to C). Note that the nuclear bodies identified by F111 serum did not differ in Ad.Sp140-infected cells and adjacent, uninfected cells, confirming that infection with the replication-deficient adenovirus did not alter the structure of the nuclear body. Sp140 was also observed to localize to PML-Sp100 nuclear bodies in Ad.Sp140-infected T24 cells (data not shown) and HeLa cells (see below).
FIG. 4.
Immunoblot of Ad.Sp140-infected HEp-2 cells with sera from patients with primary biliary cirrhosis or with rat anti-Sp140 antiserum. Antibodies in serum from patient K142 with primary biliary cirrhosis (lane 1) reacted with Sp140 (140 kDa), PML (90 kDa), Sp100 (∼80 kDa), and E2 PDC (70 kDa). Antibodies in serum from patient F111 (lane 2) reacted with PML, Sp100, E2 PDC, and an unidentified 120-kDa protein but did not react with Sp140. Rat anti-Sp140 antiserum (lane 3) reacted only with the 140-kD protein. Note that we and others have observed that Sp100 migrates as an ∼80-kDa protein (31, 36).
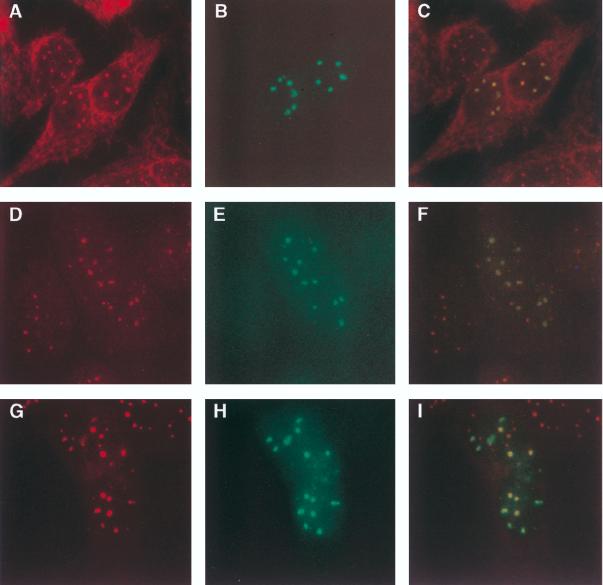
FIG. 6.
Immunofluorescence microscopy of Ad.Sp140-infected HEp-2 cells. (A to C) Cells were incubated with serum from primary biliary cirrhosis patient F111 (A, red) and rat anti-Sp140 antiserum (B, green). (D to F) Affinity-purified anti-Sp100 antibodies reacted with nuclear bodies in Ad.Sp140-infected cells (D), and Sp140 was detected within these structures (E). (G to I) Anti-Sp140 antibodies (H) also colocalized with affinity-purified anti-PML antibodies (G) in Ad.Sp140-infected cells. Colocalization of green and red fluorescence yields a yellow image (C, F, and I). To confirm the species specificity of the secondary antibodies used in this study, Ad.Sp140-infected cells were stained with normal rat serum and serum from primary biliary cirrhosis patient F111 and were subsequently incubated with both secondary antibodies. In these cells red but not green nuclear bodies were observed. In addition, infected cells were stained with rat anti-Sp140 antiserum and normal human serum and were subsequently incubated with both secondary antibodies. In these cells green but not red nuclear bodies were observed (data not shown).
To investigate the relationship between Sp140 and nuclear bodies containing Sp100, Ad.Sp140-infected HEp-2 cells were stained with anti-Sp140 antiserum and human affinity-purified anti-Sp100 antibodies. The affinity-purified antibodies reacted with Sp100 but not E2 PDC by immunoblotting (Fig. 5, lane 3). In addition, in indirect immunofluorescence, affinity-purified anti-Sp100 antibodies reacted with HEp-2 cell nuclear bodies but not with cytoplasmic E2 PDC (Fig. 6D), confirming a successful purification of anti-Sp100 antibodies from anti-E2 PDC antibodies. Sp140 colocalized with Sp100 in resting cells (Fig. 6D to F).
FIG. 5.
Immunoblot of Ad.Sp140-infected HEp-2 cells demonstrating the specificity of affinity-purified human antibodies. Antibodies in K142 serum (lane 1) reacted with Sp140 (140 kDa), PML (90 kDa), Sp100 (∼80 kDa), and E2 PDC (70 kDa). In contrast, anti-PML antibodies (lane 2) and anti-Sp100 antibodies (lane 3), affinity purified from human serum, reacted only with the corresponding proteins. Rat anti-Sp140 antibodies (lane 4) reacted only with the 140-kDa protein.
To examine the relationship between Sp140 and nuclear bodies containing PML, Ad.Sp140-infected HEp-2 cells were stained with anti-Sp140 antiserum and a commercially available mouse monoclonal antibody directed against PML. The monoclonal antibody did not detect endogenous PML in HEp-2 cells (data not shown). As an alternative approach, human anti-PML antibodies were affinity purified from patient serum with a recombinant protein. Affinity-purified anti-PML antibodies reacted with PML but not E2 PDC, as shown by immunoblotting (Fig. 5, lane 2). In addition, indirect immunofluorescence showed that the antibodies reacted with nuclear bodies but not E2 PDC in the cytoplasm of HEp-2 cells (Fig. 6G), confirming that anti-PML antibodies had been purified from anti-E2 PDC antibodies in the human serum. Sp140 colocalized with nuclear bodies containing PML in Ad.Sp140-infected HEp-2 cells (Fig. 6G to I).
To determine the cellular location of Sp140 with respect to PML in dividing cells, HeLa cells were infected with Ad.Sp140 and stained with antibodies directed against Sp140 and PML. The relatively high level of PML in HeLa cells permitted the detection of PML with the commercially available monoclonal antibody. Sp140 localized to PML-containing aggregates near the chromosomal mass in metaphase (Fig. 7A to C). In addition, Sp140 associated with PML-containing nuclear bodies in anaphase (Fig. 7D to F). These results demonstrated that Sp140 associates with structures containing PML in both resting and dividing cells. As noted by other investigators, Sp100 did not associate with chromosomes in dividing cells (reference 31 and data not shown).
FIG. 7.
Sp140 colocalizes with PML in dividing cells. To determine the cellular location of Sp40 during mitosis, HeLa cells were infected with Ad.Sp140 and stained with antibodies directed against Sp140 and PML. (A and B) Sp140 (green, A) localized to PML-containing aggregates (red, B) near the chromosomal mass in cells in metaphase. (D and E) In addition, Sp140 (green, D) associated with PML-containing nuclear bodies (E) in late anaphase. (C and F) DAPI was used to determine the stage of cellular division.
Sp140 interacts with a subset of nuclear bodies in IFNγ-treated, Ad.Sp140-infected cells.
To investigate the effect of IFN-γ on the cellular location of Sp140, HEp-2 cells were infected with Ad.Sp140 and treated with IFN-γ (1,000 U/ml of culture medium). As expected, IFN-γ treatment increased the number of nuclear bodies that were identified by using either anti-PML (Fig. 8A) or anti-Sp100 (Fig. 8D) antibodies. In contrast, the number of nuclear bodies that contained Sp140 did not increase (Fig. 8B and E). Similar results were observed independent of the order of treatment: IFN-γ treatment of cells for 48 h before Ad.Sp140 infection and infection of cells 48 h before addition of IFN-γ produced the same results (data not shown). These findings demonstrated that Sp140 associates with a subset of PML-Sp100 nuclear bodies in Ad.Sp140-infected cells treated with IFN-γ.
FIG. 8.
Immunofluorescence microscopy of IFN-γ-treated, Ad.Sp140-infected cells. HEp-2 cells were infected with Ad.Sp140 and incubated with IFN-γ for 48 h. (A and B) They were subsequently fixed and stained with affinity-purified anti-PML antibodies (A) and rat anti-Sp140 antiserum (B). (D and E) In addition, cells were stained with affinity-purified anti-Sp100 antibodies (D) and rat anti-Sp140 antiserum (E). (C and F) Colocalization of green and red fluorescence yields a yellow image. As expected, IFN-γ treatment increased the number of PML-Sp100-containing nuclear bodies (see, for comparison, Fig. 6D and G). The number of Sp140-containing nuclear bodies did not increase.
Transcriptional activation by Sp140.
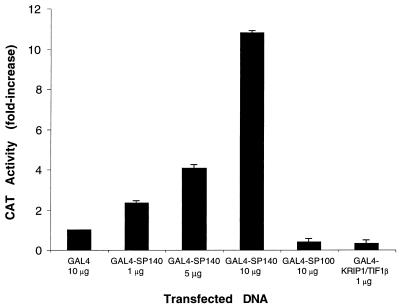
To examine the potential effect of Sp140 on gene transcription, a eukaryotic expression plasmid encoding Sp140 fused to the DNA-binding domain of GAL4 (pBXG-Sp140) was cotransfected with a CAT reporter plasmid containing five GAL4 binding sites and an SV40 enhancer region (pG5SV-BCAT) into COS cells. There was a dose-dependent increase in CAT activity in cells transfected with increasing amounts of pBXG-Sp140. In contrast, as previously described (19, 29), both pBXG-Sp100 and pBXG-KRIP-1/TIF1β inhibited CAT activity when cotransfected with the reporter plasmid (Fig. 9). These results demonstrated that Sp140, when bound to a promoter, behaves as a transcriptional activator in transfected mammalian cells.
FIG. 9.
Sp140 functions as a transcriptional enhancer when tethered to DNA. The GAL4 DNA-binding domain or GAL4 DNA-binding domain fusions of Sp140, Sp100, or KRIP-1/TIF1β were transfected into COS cells together with a reporter plasmid. When 1, 5, and 10 μg of pBXG-Sp140 were used, there was a dose-dependent increase in CAT activity. In contrast, 10 μg of pBXG-Sp100 and 1 μg of pBXG-KRIP-1/TIF1β markedly inhibited CAT activity. CAT reporter activity was expressed as a percentage of the activity obtained with the GAL4 DNA-binding domain control. Transfections were performed in triplicate, and 0.5 μg of reporter plasmid was used in each transfection. The total amount of plasmid DNA was the same in each transfection. Results are presented as means and standard errors of the means. To control for transfection efficiency, a plasmid encoding growth hormone was cotransfected with the reporter plasmid and the growth hormone levels in the tissue culture medium were measured 48 h after transfection. There was no significant difference in transfection efficiency of pBXG, pBXG-Sp140, pBXG-Sp100, or pBXG-KRIP-1/TIF1β.
DISCUSSION
The objectives of this study were to determine the cellular location of Sp140 with respect to the PML-Sp100 nuclear body and to investigate the potential role of Sp140 in the regulation of gene expression. The small amount of Sp140 in primary cells and myeloid cell lines was difficult to detect by indirect immunofluorescence. We therefore used gene transfer to enhance the expression of Sp140 in human cell lines. We chose the method of adenovirus-mediated gene transfer because previous investigators demonstrated that replication-deficient adenovirus vectors did not, by themselves, disrupt the nuclear body (1, 16). In addition, the high efficiency of gene transfer afforded by the adenovirus vector permitted the localization of Sp140 in nearly all cells in a population, including the relatively few cells undergoing mitosis.
Sp140 colocalized with PML-Sp100 nuclear bodies in resting cells, confirming our previous observations in HL60 cells (4) but differing from the results of Dent et al., who observed that Sp140 rarely colocalized with PML and Sp100 in the EBV-transformed human lymphoblastoid cell line MBB1 (8). At least two possibilities may explain the differences between the two studies: EBV transformation may alter the relationship between Sp140 and PML, or colocalization of Sp140 and PML may be cell type specific. With respect to the second possibility, we observed that Sp140 also colocalized with PML-Sp100 nuclear bodies in Ad.Sp140-infected T24 cells and HeLa cells, demonstrating that colocalization of Sp140 with PML-Sp100 is not unique to HEp-2 cells.
The number of nuclear bodies and the composition of these structures change during the course of the cell cycle. Koken et al. (20) observed that the smallest number of nuclear bodies is present in G0 and that the number increases until S phase. Using immunofluorescence and immunoelectron microscopy, Koken et al. observed staining for PML in “two or three perichromosomal dots” during mitosis (20). Sternsdorf et al. confirmed that “aggregates” containing PML but not Sp100 localized near chromosomes in dividing cells (31). In this study, we demonstrated that Sp140 localized with PML in dividing cells. The observation that Sp140-containing nuclear bodies associate with chromosomes throughout mitosis suggests that these structures are directly “inherited” by daughter cells. In view of the recent findings suggesting that nuclear bodies are involved in gene transcription (22) and our results that Sp140 may activate gene transcription, it is possible that Sp140-containing nuclear bodies have a role in the epigenetic control of gene expression.
Previous investigators demonstrated that the number of nuclear bodies increased in cells treated with IFN-γ (15, 23). In this study, the number of PML-Sp100-containing nuclear bodies in Ad.Sp140-infected, IFN-γ-treated cells increased but only a subset of these nuclear bodies contained Sp140. Thus, although the structure of Sp140 contains all of the information required to localize to the nuclear body in resting cells, Sp140 associated with only a subset of nuclear bodies in IFN-γ-treated cells. The composition of the nuclear bodies appears to vary both during the course of the cell cycle and in response to IFN-γ.
LaMorte et al. observed that nascent RNA associates with some but not all nuclear bodies and suggested that individual nuclear bodies within the cell may have different functions (22). In this study, we demonstrated that Sp140, when tethered to DNA, activated gene transcription. In contrast, Sp100 and KRIP-1/TIF1β (proteins that contain structural similarity to Sp140) both inhibited gene transcription when cotransfected into mammalian cells with a reporter plasmid. The observations that Sp140 associates with some but not all of the IFN-γ-inducible nuclear bodies and that Sp140 may activate gene expression suggest that both the composition and the function of nuclear bodies may be heterogeneous.
One potential limitation of this study relates to the use of adenovirus-mediated gene transfer to express Sp140 in cells, because proteins derived from the adenovirus vector may interact with and disrupt the nuclear body. Several investigators demonstrated that overexpression of adenovirus protein E4 ORF3 was sufficient to disrupt the nuclear body (7, 11, 28). Although adenovirus E4 ORF3 is present within the genome of Ad.Sp140, the virus lacks E1 and is replication deficient. At the MOIs used in this study, neither Ad.Sp140 nor the control adenovirus, Ad.βgal, appeared to alter the structure of the nuclear body. It seems likely that the level of E4 ORF3 expressed by these viruses was not sufficient to disrupt the nuclear body. Of note, He et al. demonstrated that adenovirus-mediated expression of PML also did not disrupt the nuclear body in prostate cancer cell lines (16). In addition, an adenovirus vector encoding cytomegalovirus protein IE2 did not disrupt nuclear bodies in human diploid fibroblasts and Vero cells (1).
A second potential limitation of this study concerns the overexpression of Sp140 in cells that do not normally contain this protein. HeLa or HEp-2 cells expressing Sp140 may not completely reflect the in vivo situation in human leukocytes.
In summary, we have used adenovirus-mediated gene transfer to demonstrate that Sp140 colocalized with PML-Sp100 nuclear bodies in resting cells and with structures containing PML in dividing cells. In IFN-γ-treated cells, Sp140 associated with a subset of PML-Sp100 nuclear bodies. In addition, when bound to a promoter, Sp140 but not Sp100 enhanced the expression of a reporter gene. These results demonstrate that nuclear bodies may be heterogeneous with respect to both composition and function.
ACKNOWLEDGMENTS
This work was supported by grants from the Arthritis Foundation (to D.B.B.), Massachusetts Biomedical Research Corporation and the Phillippe Foundation (to J.-D.C.), and the National Institutes of Health (grants AR-01866 and DK-051179 to D.B.B.; grants HL-54202, HL-59521, and AI-40970 to A.R.; and grant HL-55377 to K.D.B.). K. D. Bloch and A. Rosenzweig are Established Investigators of the American Heart Association.
We thank K. J. Bloch and L. Diller for advice, S. M. Schlutsmeyer and A. Brown for technical assistance, and H. de The, J. Bonventre, S. Shu, and D. Dichek for gifts of plasmids and adenovirus.
REFERENCES
- 1.Ahn J-H, Hayward G S. The major immediate-early proteins IE1 and IE2 of human cytomegalovirus colocalize with and disrupt PML-associated nuclear bodies at very early times in infected permissive cells. J Virol. 1997;71:4599–4613. doi: 10.1128/jvi.71.6.4599-4613.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahn M-J, Nason-Burchenal K, Moasser M M, Dmitrovsky E. Growth suppression of acute promyelocytic leukemia cells having increased expression of the non-rearranged alleles; RARα or PML. Oncogene. 1995;10:2307–2314. [PubMed] [Google Scholar]
- 3.Ascoli C A, Maul G G. Identification of a novel nuclear domain. J Cell Biol. 1991;112:785–795. doi: 10.1083/jcb.112.5.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bloch D B, de la Monte S M, Guigaouri P, Filippov A, Bloch K D. Identification and characterization of a leukocyte-specific component of the nuclear body. J Biol Chem. 1996;46:29198–29204. doi: 10.1074/jbc.271.46.29198. [DOI] [PubMed] [Google Scholar]
- 5.Borrow J, Goddard A, Sheer D, Solomon E. Molecular analysis of acute promyelocytic leukemia breakpoint cluster region on chromosome 17. Science. 1990;249:1577–1580. doi: 10.1126/science.2218500. [DOI] [PubMed] [Google Scholar]
- 6.Brasch K, Ochs R L. Nuclear bodies (NBs): a newly “rediscovered” organelle. Exp Cell Res. 1992;202:211–223. doi: 10.1016/0014-4827(92)90068-j. [DOI] [PubMed] [Google Scholar]
- 7.Carvalho T, Seeler J-S, Ohman K, Jordan P, Petterson U, Akusjarvi G, Carmo-Fonseca M, Dejean A. Targeting of adenovirus E1A and E4-ORF3 proteins to nuclear matrix-associated PML bodies. J Cell Biol. 1995;131:45–56. doi: 10.1083/jcb.131.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dent L D, Yewdell J, Puvion-Dutilleul F, Koken M H M, de The H, Staudt L M. LySp100-associated nuclear domains (LANDS): description of a new class of subnuclear structures and their relationship to PML nuclear bodies. Blood. 1996;88:1423–1436. [PubMed] [Google Scholar]
- 9.de The H, Lavau C, Marchio A, Chomienne C, Degos L, Dejean A. The PML-RARα fusion mRNA generated by the t(15;17) translocation in acute promyelocytic leukemia encodes a functionally altered RAR. Cell. 1991;66:675–684. doi: 10.1016/0092-8674(91)90113-d. [DOI] [PubMed] [Google Scholar]
- 10.Dong G, Schulick A, De Young M B, Dichek D A. Identification of a cis-acting sequence in the human PAI-1 gene that mediates TGF-1 responsiveness in endothelium in vivo. J Biol Chem. 1996;271:29969–29977. doi: 10.1074/jbc.271.47.29969. [DOI] [PubMed] [Google Scholar]
- 11.Doucas V, Ishov A M, Romo A, Juguilon H, Weitzman M D, Evans R M, Maul G G. Adenovirus replication is coupled with the dynamic properties of the PML nuclear structure. Genes Dev. 1996;10:196–207. doi: 10.1101/gad.10.2.196. [DOI] [PubMed] [Google Scholar]
- 12.Dyck J A, Maul G G, Miller W H, Chen J D, Kakizuka A, Evans R M. A novel macromolecular structure is a target of the promyelocyte-retinoic acid receptor oncoprotein. Cell. 1994;76:333–343. doi: 10.1016/0092-8674(94)90340-9. [DOI] [PubMed] [Google Scholar]
- 13.Evans J, Reuben A, Craft J. PBC 95K, a 95-kilodalton nuclear autoantigen in primary biliary cirrhosis. Arthritis Rheum. 1991;34:731–736. doi: 10.1002/art.1780340614. [DOI] [PubMed] [Google Scholar]
- 14.Graham F L, Preyec L. Manipulation of adenovirus vectors. In: Murray E, editor. Methods in molecular biology: gene transfer and expression protocols. Clifton, N.J: Humana Press; 1991. pp. 109–128. [DOI] [PubMed] [Google Scholar]
- 15.Grotzinger T, Sternsdorf T, Jensen K, Will H. Interferon-modulated expression of genes encoding the nuclear-dot-associated proteins Sp100 and promyelocytic leukemia protein (PML) Eur J Biochem. 1996;238:554–560. doi: 10.1111/j.1432-1033.1996.0554z.x. [DOI] [PubMed] [Google Scholar]
- 16.He D, Mu Z-M, Le X, Hsieh J-T, Pong R-C, Chung L W K, Chang K-S. Adenovirus-mediated expression of PML suppresses growth and tumorigenicity of prostate cancer cells. Cancer Res. 1997;57:1868–1872. [PubMed] [Google Scholar]
- 17.Kakizuka A, Miller W H, Umesono K, Warrell R P, Frankel S R, Murty V V, Dmitrovsky E, Evans R M. Chromosomal translocation t(15;17) in human acute promyelocytic leukemia fuses RARα with a novel putative transcription factor, PML. Cell. 1991;66:663–674. doi: 10.1016/0092-8674(91)90112-c. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan M M. Primary biliary cirrhosis. N Engl J Med. 1996;335:1570–1580. doi: 10.1056/NEJM199611213352107. [DOI] [PubMed] [Google Scholar]
- 19.Kim S-S, Chen Y-M, O’Leary E, Witzgall R, Vidal M, Bonventre J V. A novel member of the RING finger family, KRIP-1, associates with the KRAB-A transcriptional repressor domain of zinc finger proteins. Proc Natl Acad Sci USA. 1996;93:15299–15304. doi: 10.1073/pnas.93.26.15299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koken M H M, Linares-Cruz G, Quignon F, Viron A, Chelbi-Alix M K, Sobczak-Thepot J, Juhlin L, Degos L, Calvo F, de The H. The PML growth-suppressor has an altered expression in human oncogenesis. Oncogene. 1995;10:1315–1324. [PubMed] [Google Scholar]
- 21.Koken M H M, Puvion-Dutilleul F, Guillemin M C, Viron A, Linares-Cruz G, Stuurman N, de Jong L, Szostecki C, Calvo F, Chomienne C, Degos L, Puvion E, de The H. The t(15;17) translocation alters a nuclear body in a retinoic acid-reversible fashion. EMBO J. 1994;13:1073–1083. doi: 10.1002/j.1460-2075.1994.tb06356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LaMorte V J, Dyck J A, Ochs R L, Evans R E. Localization of nascent RNA and CREB binding protein with the PML-containing nuclear body. Proc Natl Acad Sci USA. 1998;95:4991–4996. doi: 10.1073/pnas.95.9.4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lavau C, Marchio A, Fagioli M, Jansen J, Falini B, Lebon P, Grosveld F, Pandolfi P P, Pelicci P G, Dejean A. The acute promyelocytic leukaemia-associated PML gene is induced by interferon. Oncogene. 1995;11:871–876. [PubMed] [Google Scholar]
- 24.Le X-F, Yang P, Chang K-S. Analysis of growth and transformation suppressor domains of promyelocytic leukemia gene, PML. J Biol Chem. 1996;271:130–135. doi: 10.1074/jbc.271.1.130. [DOI] [PubMed] [Google Scholar]
- 25.Le Douarin B, Zechel C, Garnier J-M, Lutz Y, Tora L, Pierrat B, Heery D, Gronemeyer H, Chambon P, Losson R. The N-terminal part of TIF1, a putative mediator of the ligand-dependent activation function (AF-2) of nuclear receptors, is fused to B-raf in the oncogenic protein T18. EMBO J. 1995;14:2020–2033. doi: 10.1002/j.1460-2075.1995.tb07194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Longo L, Pandolfi P P, Biondi A, Rombaldi A, Mencarelli A, Lo Coco F, Direrio D, Pegoraro L, Avanzi G, Tabilio A, Zangrilli D, Alcalay M, Donti E, Grignani F, Pelicci P G. Rearrangements and aberrant expression of the retinoic acid receptor α gene in acute promyelocytic leukemias. J Exp Med. 1990;172:1571–1575. doi: 10.1084/jem.172.6.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neumann J R, Mornecy C A, Russian K O. A novel rapid assay for chloramphenicol acetyltransferase gene expression. BioTechniques. 1987;5:444–447. [Google Scholar]
- 28.Puvion-Dutilleul F, Chelbi-Alix M K, Koken M, Quignon F, Puvion E, de The H. Adenovirus infection induces rearrangement in the intranuclear distribution of the nuclear body-associated PML protein. Exp Cell Res. 1995;218:9–16. doi: 10.1006/excr.1995.1125. [DOI] [PubMed] [Google Scholar]
- 29.Seeler J-S, Marchia A, Sitterlin D, Transy C, Dejean A. Interaction of Sp100 and HP1 proteins: a link between the promyelocytic leukemia-associated nuclear bodies and the chromatin compartment. Proc Natl Acad Sci USA. 1998;95:7316–7321. doi: 10.1073/pnas.95.13.7316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sternsdorf T, Grotzinger T, Jensen K, Will H. Nuclear dots: actors on many stages. Immunoblotting. 1997;198:307–331. doi: 10.1016/S0171-2985(97)80051-4. [DOI] [PubMed] [Google Scholar]
- 31.Sternsdorf T, Jensen K, Will H. Evidence for covalent modification of the nuclear dot-associated proteins PML and Sp100 by PIC1/SUMO-1. J Cell Biol. 1997;139:1621–1634. doi: 10.1083/jcb.139.7.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szostecki C, Guldner H H, Netter H J, Will H. Isolation and characterization of cDNA encoding a human nuclear antigen predominantly recognized by autoantibodies from patients with primary biliary cirrhosis. J Immunol. 1990;145:4338–4347. [PubMed] [Google Scholar]
- 33.Szostecki C, Guldner H H, Wills H. Autoantibodies against “nuclear dots” in primary biliary cirrhosis. Semin Liver Dis. 1997;17:71–77. doi: 10.1055/s-2007-1007184. [DOI] [PubMed] [Google Scholar]
- 34.Wang Z G, Delva L, Gaboli M, Rivi R, Giorgio M, Cordon-Cardo C, Grosveld F, Pandolfi P P. Role of PML in cell growth and the retinoic acid pathway. Science. 1998;279:1547–1551. doi: 10.1126/science.279.5356.1547. [DOI] [PubMed] [Google Scholar]
- 35.Weis K, Rambaud S, Lavau C, Jansen J, Carvalho T, Carmo-Fonseca M, Lamond A, Dejean A. Retinoic acid regulates aberrant nuclear localization of PML-RARα in acute promyelocytic leukemia cells. Cell. 1994;76:345–356. doi: 10.1016/0092-8674(94)90341-7. [DOI] [PubMed] [Google Scholar]
- 36.Xie K, Lambie E J, Snyder M. Nuclear dot antigens may specify transcriptional domains in the nucleus. Mol Cell Biol. 1993;13:6170–6179. doi: 10.1128/mcb.13.10.6170. [DOI] [PMC free article] [PubMed] [Google Scholar]