Increased Prevalence of Immunoglobulin A Deficiency in Patients with the Chromosome 22q11.2 Deletion Syndrome (DiGeorge Syndrome/Velocardiofacial Syndrome) (original) (raw)
Abstract
We wished to determine the prevalence of immunoglobulin A (IgA) deficiency in patients with the chromosome 22q11.2 deletion syndrome. A total of 32 patients with the chromosome 22q11.2 deletion were examined for IgA deficiency. We report a 13% (n = 4) prevalence of IgA deficiency in patients with this syndrome. The odds ratio of IgA deficiency in this population is 14.20 (P < 0.0001). This confirms the occurrence of significant humoral deficits in this predominantly cellular immunodeficiency.
Hemizygous deletion of chromosome 22q11.2 is associated with DiGeorge syndrome, velocardiofacial syndrome, and conotruncal anomaly face syndrome (8, 12). The prevalence of the 22q11.2 deletion syndrome in the general population has been estimated to range from 1:3,000 to 1:4,000. This syndrome stems from aberrant development of the structures arising from the 3rd and 4th pharyngeal pouches, resulting in hypoplastic parathyroid glands, conotruncal cardiac defects, palatal abnormalities, and craniofacial dysmorphism (5). Immunologic characteristics include a hypoplastic thymus, T-cell lymphopenia, and invariably decreased T-cell function. The prevalence of immunoglobulin A (IgA) deficiency in this population has not been previously described.
IgA deficiency is the most common primary immunodeficiency, with a prevalence ranging from 0.03 to 0.3% (4, 10, 15, 22). Patients with IgA deficiency have an increased risk of bacterial infections, an increased risk of a variety of autoimmune disorders, and are at increased risk of anaphylaxis from blood products containing IgA. IgA deficiency results from impaired B-lymphocyte differentiation into IgA plasma cells due to either intrinsic B-cell defects or extrinsic T-cell defects (17). Many cellular immunodeficiencies have secondary humoral defects presumably on the basis of impaired T-cell help. We hypothesized that patients with the 22q11.2 deletion syndrome might have defective T-cell help retarding B-lymphocyte differentiation into IgA-producing plasma cells. Therefore, we specifically evaluated the prevalence of IgA deficiency in patients with the chromosome 22q11.2 deletion syndrome.
Patients with developmental delay, palatal abnormalities, hypocalcemia, or conotruncal cardiac disease at The Children’s Hospital of Philadelphia were screened for chromosome 22q11.2 deletions by fluorescent in situ hybridization with the N25 (D22575) probe (Oncor, Gaithersburg, Md.). Patients identified with the 22q11.2 deletion were then referred for an immunologic evaluation. These data reflect immunologic evaluations of 32 patients over 2 years of age seen from May 1989 to April 1997. The immunologic evaluation included lymphocyte subset enumeration, lymphocyte function assays, quantitative Ig determinations, and assessment of ability to make functional antibodies. IgA levels in serum were determined by nephelometry. The lowest detectable IgA level varied, but was between 6 and 8 mg/dl. We therefore defined IgA deficiency as an IgA level in serum of less than 10 mg/dl in patients older than 2 years of age, which is comparable to standard textbook definitions of IgA deficiency (2, 7, 13). We defined developmental delay as an intelligence quotient less than 80. The intelligence quotient was estimated by using the Bayley Scales of Infant Development, 2nd ed., the Wexler Preschool and Primary Scales of Intelligence—Revised, or the Wexler Intelligence Scale for Children III, depending on age. We defined cardiac defects as any structural abnormality involving the cardiovascular system, including truncus arteriosus, tetralogy of fallot, atrial septal defects, interrupted aortic arch, ventricular septal defects, vascular rings, and patent ductus arteriosus. We defined hypocalcemia as a sustained level of calcium in serum of less than 7.5 mg/dl occurring more than 48 h from any operative procedure and requiring calcium supplementation. We defined palatal abnormalities as any palate defect, including palatal insufficiency, cleft palate, submucous cleft palate, and bifid uvula. Based on normative data (16), we set levels of IgG in serum of 553 mg/dl and IgM in serum of 35 mg/dl as the lower limits of normal. Based on normative data from Comans-Bitter et al. (6), we set an absolute CD4 count of 900 and an absolute CD8 count of 400 as the lower limits of normal.
This study used a cross-sectional design. The odds ratio of IgA deficiency in the study population was calculated by using the approximation of Katz, and the P value was determined with Fisher’s exact test. Patients ranged in age from 24 months to 29.5 years (mean age, 6 years; median age, 3.6 years). A total of 16 of 32 (50%) patients were male; 28 of 32 (87%) were Caucasian. None of the patients had macroscopic deletions, and >90% of the chromosome 22q11.2 deletion syndrome patients seen at our institution have the identical 2.5-Mbp deletion (9).
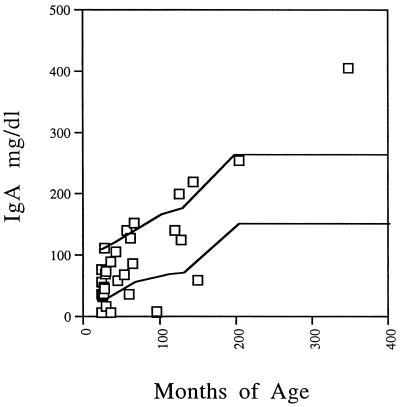
Cellular and humoral immune evaluations were performed for patients identified with the chromosome 22q11.2 deletion. A total of 4 of 32 (13%) patients had IgA levels in serum of less than 10 mg/dl. One 8-year-old had an IgA level of <6 mg/dl, one 2-year-old had an IgA level of <6 mg/dl, one 3-year-old had an IgA level of <6 mg/dl, and one 8-year-old had an IgA level of <8 mg/dl. All but one of these studies were repeated a minimum of 6 months later, and the results were found to be stable over time. None of the patients were receiving medication known to affect IgA levels. Two of the IgA-deficient patients had juvenile rheumatoid arthritis, one patient had chronic sinusitis, and one patient had a history of chronic otitis media but had improved by 8 years of age. An additional three patients had IgA levels below the normal range, and five patients had levels exceeding the normal range (Fig. 1). A community survey study of 3,212 Americans with a threshold level of IgA of <10 mg/dl demonstrated a prevalence of 0.10% in the general population (3). The 13% prevalence of IgA deficiency we report in our study population is substantially higher than the national prevalence. The odds ratio of IgA deficiency in our chromosome 22q11.2 deletion syndrome population is 14.2 (4.7 to 42.8) with P < 0.0001 (with the survey mentioned above used for comparison).
FIG. 1.
IgA levels in patients with the chromosome 22q11.2 deletion syndrome. The solid lines designate the normal range (16).
We examined whether IgA deficiency occurred more frequently in patients with other types of immunologic deficits. There was no association between IgA deficiency and any other laboratory assessment of cellular or humoral immune function (Table 1). Although it is likely that the IgA deficiency seen in this population is due to impaired T-cell help, we were not able to identify any T-cell correlates with the IgA deficiency. This could reflect the nonspecific nature of the T-cell laboratory studies or failure to perform the tests during some critical developmental window. We also examined the relationship of IgA deficiency to other phenotypic characteristics associated with the 22q11.2 deletion syndrome. There was no association found between IgA deficiency and either developmental delay, conotruncal cardiac defects, hypocalcemia, and palatal abnormalities (Table 2).
TABLE 1.
Immunologic analyses of patients with the chromosome 22q11.2 deletion syndromea
| Immunologic parameter | No. (%) of patients with IgA level/no. tested | P value | |
|---|---|---|---|
| <10 mg/dl | >10 mg/dl | ||
| CD4, <900 cells/mm3 | 1/4 (25) | 10/28 (36) | 1.00 |
| CD8, <400 cells/mm3 | 1/4 (25) | 11/28 (39) | 1.00 |
| Phytohemagglutinin, <50% of control | 0/4 (0) | 6/28 (21) | 0.57 |
| IgG in serum, <553 mg/dl | 0/4 (0) | 3/28 (11) | 1.00 |
| IgM in serum, <35 mg/dl | 0/4 (0) | 2/28 (7) | 1.00 |
| Nonprotective tetanus or diphtheria titerb | 0/3 (0) | 1/24 (4) | 1.00 |
TABLE 2.
Relationship of IgA deficiency to other features associated with the chromosome 22q11.2 deletion syndromea
| Phenotypic feature | No. (%) of patients with IgA level/no. tested | P value | |
|---|---|---|---|
| <10 mg/dl | >10 mg/dl | ||
| Developmental delay | 2/4 (50) | 10/14 (71) | 0.57 |
| Cardiac defects | 2/4 (50) | 14/28 (50) | 1.40 |
| Hypocalcemiab | 1/4 (25) | 13/27 (48) | 0.61 |
| Palatal abnormalities | 3/4 (75) | 13/28 (46) | 0.60 |
The chromosome 22q11.2 deletion syndrome is typically associated with various combinations of developmental delay, palatal abnormalities, conotruncal heart defects, hypocalcemia, and deficient cellular immunity (5, 19). Aberrant T-cell function in other immunodeficiencies may be associated with secondary humoral deficits; therefore, we sought to establish the prevalence of IgA deficiency in our chromosome 22q11.2 deletion syndrome population. IgA deficiency is the most prevalent immune deficiency recognized in general, occurring in 0.03 to 0.3% of the population (4, 15, 22). In our patient population with the chromosome 22q11.2 deletion syndrome, we report a 13% prevalence of IgA deficiency (odds ratio, 14.20; P < 0.0001). It has been previously noted that patients with DiGeorge syndrome may occasionally have decreased IgA levels (2), and another study of 13 patients with the chromosome 22q11.2 deletion syndrome found a mildly delayed acquisition of protective titers to immunization in 3 patients and transiently low levels of IgG in 2 patients. No patients in this series were IgA deficient (11). There are also two case reports of patients with different chromosome 22 anomalies (monosomy 22 and ring 22) associated with IgA deficiency (14, 20). These studies suggest that humoral deficits may be more common in the chromosome 22q11.2 deletion syndrome than previously appreciated. The association of IgA deficiency and the chromosome 22q11.2 deletion syndrome is clinically relevant, because patients with both the chromosome 22q11.2 deletion syndrome and IgA deficiency may have unique management considerations.
The 22q11.2 deletion syndrome results from a hemizygous chromosome deletion at position 22q11.2. Over 90% of patients carry an identical deletion in spite of widely varied phenotypic manifestations (9, 19). Management issues may include frequent surgical procedures for cardiac lesions, palatal defects, tympanostomy tubes, and sinusitis. Reflecting the immunodeficiency, recurrent upper respiratory infections are common, and there is an increased prevalence of autoimmune disease (18, 19). These problems are also common in patients with IgA deficiency. Clinical features of IgA deficiency include recurrent sinopulmonary infections, gastrointestinal infections, autoimmune diseases, and atopy (2, 13, 17). Management of IgA deficiency may include antibiotic prophylaxis for recurrent bacterial upper respiratory infections and aggressive management of chronic gastrointestinal infections.
Patients with the chromosome 22q11.2 deletion syndrome and IgA deficiency may have special management issues. Patients with the chromosome 22q11.2 deletion syndrome may have deficient cellular immunity, which can result in more severe and prolonged viral infections (1). Patients with IgA deficiency lack IgA antibodies directed against pathogens on mucosal surfaces, and they may be more susceptible to bacterial superinfection.
Patients with IgA deficiency are also at increased risk of anaphylaxis from IgA-containing blood products (21). The conotruncal heart defects and palate defects commonly associated with this syndrome frequently require surgical repair. Surgery increases the possibility that these patients may require blood transfusions. Patients with IgA deficiency can develop anti-IgA antibodies following transfusion with IgA-containing blood products. Subsequent transfusions with IgA-containing blood products may result in anaphylaxis. In IgA-deficient individuals, it possible to assess risk of anaphylaxis by screening for anti-IgA antibodies prior to surgery.
This is the first report of increased prevalence of IgA deficiency in patients with the chromosome 22q11.2 deletion syndrome. This association may be important clinically. This subgroup of patients may be prone to more frequent and severe viral infections with associated secondary bacterial superinfections. Screening of patients with the chromosome 22q11.2 deletion syndrome for IgA deficiency is diagnostically important and relevant to the medical management of this patient population.
Acknowledgments
This work was supported in part by MO1-RR00240 and The Wallace chair.
We acknowledge the assistance of the families, residents, and fellows at The Children’s Hospital of Philadelphia.
REFERENCES
- 1.Bastian J, Law S, Vogler L, Lawton A, Herrod H, Anderson S, Horowitz S, Hong R. Prediction of persistent immunodeficiency in the DiGeorge anomaly. J Pediatr. 1989;115:391–396. doi: 10.1016/s0022-3476(89)80837-6. [DOI] [PubMed] [Google Scholar]
- 2.Buckley R H. Primary immunodeficiency diseases. In: Paul W E, editor. Fundamental immunology. 3rd ed. New York, N.Y: Raven Press; 1993. pp. 1353–1374. [Google Scholar]
- 3.Cassidy J T, Nordby G L. Human serum immunoglobulin concentrations: prevalence of immunoglobulin deficiencies. J Allergy Clin Immunol. 1975;55:35–48. doi: 10.1016/s0091-6749(75)80006-6. [DOI] [PubMed] [Google Scholar]
- 4.Clark J A, Callicoat P A, Brenner N A, Bradley C A, Smith D M. Selective IgA deficiency in blood donors. Am J Clin Pathol. 1983;80:210–213. doi: 10.1093/ajcp/80.2.210. [DOI] [PubMed] [Google Scholar]
- 5.Conley M E, Beckwith J B, Mancer J F K, Tenckhoff L. The spectrum of DiGeorge syndrome. J Pediatr. 1979;94:883–890. doi: 10.1016/s0022-3476(79)80207-3. [DOI] [PubMed] [Google Scholar]
- 6.Comans-Bitter W M, de Groot R, van Dongen J M. Immunophenotyping of blood lymphocytes in childhood. J Pediatr. 1996;130:388–393. doi: 10.1016/s0022-3476(97)70200-2. [DOI] [PubMed] [Google Scholar]
- 7.Cunningham-Rundles C. Diseases of the IgA system. In: Stiehm E R, editor. Immunologic disorders in infants and children. W. B. Philadelphia, Pa: Saunders; 1996. pp. 423–442. [Google Scholar]
- 8.Driscoll D A, Budarf M L, Emanuel B S. A genetic etiology for DiGeorge syndrome: consistent deletions and microdeletions of 22q11. Am J Hum Genet. 1992;50:924–933. [PMC free article] [PubMed] [Google Scholar]
- 9.Driscoll D A, Li M, Chien P, Capuano S, Zackai E H, McDonald-McGinn D M, Christensen K M, Cuneo B F, Saal H M, Gold R, Spector E B, Emanuel B S, Budarf M L. Familial 22q11 deletions: phenotypic variability and determination of deletion boundaries by FISH. Am J Hum Genet. 1995;57:A163. [Google Scholar]
- 10.Holt P D J, Tandy N P, Anstee D J. Screening of blood donors for IgA deficiency: a study of the donor population of southwest England. J Clin Pathol. 1977;30:1007–1010. doi: 10.1136/jcp.30.11.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Junker A K, Driscoll D A. Humoral immunity in DiGeorge syndrome. J Pediatr. 1995;127:231–237. doi: 10.1016/s0022-3476(95)70300-4. [DOI] [PubMed] [Google Scholar]
- 12.Kelley R I, Zackai E H, Emanuel B S, Kistenmacher M, Greenberg F, Punnett H H. The association of the DiGeorge anomalad with partial monosomy of chromosome 22. J Pediatr. 1982;101:197–200. doi: 10.1016/s0022-3476(82)80116-9. [DOI] [PubMed] [Google Scholar]
- 13.Lawton A R, Hummel D S. Primary antibody deficiencies. In: Rich R R, editor. Clinical immunology principles and practice. St. Louis, Mo: Mosby-Year Book, Inc.; 1996. pp. 621–626. [Google Scholar]
- 14.Miranda J L G, Gomez A O, Ansedes H V, Torres N R, Espinosa C G, Cortabarria C, Salgado G S. Monosomy 22 with humoral immunodeficiency: is there an immunoglobulin chain deficit? J Med Genet. 1983;20:69–72. doi: 10.1136/jmg.20.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ropars C, Muller A, Paint N, Beige D, Avenard G. Large scale detection of IgA deficient blood donors. J Immunol Methods. 1982;54:183–189. doi: 10.1016/0022-1759(82)90059-x. [DOI] [PubMed] [Google Scholar]
- 16.Stiehm E R, Fudenberg H H. Serum levels of immune globulins in health and disease: a survey. Pediatrics. 1966;37:715–727. [PubMed] [Google Scholar]
- 17.Strober W, Sneller M C. IgA deficiency. Ann Allergy. 1991;66:363–375. [PubMed] [Google Scholar]
- 18.Sullivan K, McDonald-McGinn D, Driscoll D, Reed L, Emanuel B S, Zackai E, Athreya B H, Keenan G. Juvenile rheumatoid arthritis-like polyarthritis in chromosome 22q11.2 deletion syndrome (DiGeorge anomalad/velocardiofacial syndrome/conotruncal anomaly face syndrome) Arthritis Rheum. 1997;40:430–436. doi: 10.1002/art.1780400307. [DOI] [PubMed] [Google Scholar]
- 19.Sullivan K E, Jawad A F, Randall P, Driscoll D A, Emanuel B S, McDonald-McGinn D M, Zackai E H. Lack of correlation between impaired T cell production, immunodeficiency and other phenotypic features in chromosome 22q11.2 deletions syndrome (DiGeorge syndrome/velocardiofacial syndrome) Clin Immunol Immunopathol. 1998;86:141–146. doi: 10.1006/clin.1997.4463. [DOI] [PubMed] [Google Scholar]
- 20.Taalman R D F M, Weemaes C M R, Hustinx T W J, Scheres J M J C, Clement J M E, Stoelinga G B A. Chromosome studies in IgA-deficient patients. Clin Genet. 1987;32:81–87. doi: 10.1111/j.1399-0004.1987.tb03330.x. [DOI] [PubMed] [Google Scholar]
- 21.Vyas G N, Perkins H A, Fudenberg H H. Anaphylactoid transfusion reactions associated with anti-IgA. Lancet. 1968;ii:312–315. doi: 10.1016/s0140-6736(68)90527-8. [DOI] [PubMed] [Google Scholar]
- 22.Wells J V, McNally M P, King M A. Selective IgA deficiency in Australian blood donors. Aust N Z J Med. 1980;10:410–413. doi: 10.1111/j.1445-5994.1980.tb04091.x. [DOI] [PubMed] [Google Scholar]