Kinship and Diversification of Bacterial Penicillin-Binding Proteins and β-Lactamases (original) (raw)
The catalytic function of β-lactamases is the primary cause of bacterial resistance to β-lactam antibiotics (e.g., penicillins and cephalosporins). β-Lactamases show diversity in both structure and function (4). Four molecular classes (classes A, B, C, and D) of β-lactamases are recognized; three of these (classes A, C, and D) are active-site serine enzymes, and one (class B) is comprised of zinc-dependent (“EDTA-inhibited”) enzymes. Mounting structural evidence supports the proposal that β-lactamases descended from cell wall biosynthetic enzymes (the so-called penicillin-binding proteins [PBPs] [28]). In light of the large number of β-lactamases and PBPs for which gene sequence information is now available, sequence-based analyses can be used to describe the evolutionary relationships of these important bacterial enzymes. Use of the information obtained by this approach in combination with structural information about the various subgroups of these protein families, along with enzymological information, provides a powerful tool for analysis of the evolution and function of these important microbial proteins. We have attempted such an analysis for both PBPs and β-lactamases.
Procedures.
The amino acid sequences of the proteins studied for this report were taken from either the GenBank or the SWISS-PROT databases. The sources of the enzymes and information on each entry are given in Table 1. We considered only proteins whose sequence similarities were less than 90 to 95%, with very few exceptions. Among those with higher sequence identities, only one was selected as a representative entry in each case. For example, the plasmid-encoded PBP 3r from Enterococcus hirae (44) has a sequence identity of 97% to the low-affinity PBP 5 from Enterococcus faecium (54). Hence, only the latter was included in the analysis. Furthermore, there are more than 50 variants of the class A TEM β-lactamases (3, 46), and many variants of the class A SHV β-lactamases have been identified. Of these, we have kept only the parental enzymes. We felt that to keep many or most of these proteins would complicate the dendrogram, yet it would not add to the information content.
TABLE 1.
Enzymes used in the multiple-sequence analysis and their sources
| β-Lactamase or PBP class and source | Accession no. | Data bank |
|---|---|---|
| Class D β-lactamasesa | ||
| OXA-18, Pseudomonas aeruginosa Mus | U85514 | GenBank |
| OXA-9, Klebsiella pneumoniae | P22070 | SWISS-PROT |
| Aeromonas sobria AER 14 | X80276 | GenBank |
| OXA-1, Escherichia coli K10-35 | P13661 | SWISS-PROT |
| OXA-7, E. coli 7181 | P35695 | SWISS-PROT |
| OXA-11, P. aeruginosa ABD | Q06778 | SWISS-PROT |
| OXA-5, P. aeruginosa 76072601 | Q00982 | SWISS-PROT |
| LCR-1, P. aeruginosa 2293E | Q00983 | SWISS-PROT |
| OXA-2, Salmonella typhimurium type 1A | P05191 | SWISS-PROT |
| High-molecular-weight class C PBPsb | ||
| BLAR1, Bacillus licheniformis 749/C | P12287 | SWISS-PROT |
| BLAR1, Staphylococcus aureus NCTC 9789 | P18357 | SWISS-PROT |
| MECR1, Staphylococcus epidermidis WT55/N315 | P26597 | SWISS-PROT |
| Low-molecular-weight class A PBPsb | ||
| PBP 5, P. aeruginosa PAO1 | P72161 | SWISS-PROT |
| PBP 7, E. coli K-12/BHB2600 | P33364 | SWISS-PROT |
| PBP 7, Haemophilus influenzae RD/KW20 | P44664 | SWISS-PROT |
| E. coli K-12/BHB2600 | P33013 | SWISS-PROT |
| S. typhimurium LT2 | P37604; P37605 | SWISS-PROT |
| PBP 5, E. coli K-12 | P04287 | SWISS-PROT |
| PBP 6, E. coli K-12 | P08506; P77287 | SWISS-PROT |
| PBP 5, H. influenzae RD/KW20 | P44466 | SWISS-PROT |
| Bacillus stearothermophilus ATCC 15952 | Q05523 | SWISS-PROT |
| PBP 5, Bacillus subtilis 168 | P08750 | SWISS-PROT |
| Streptococcus pyogenes M49 | X89237 | GenBank |
| B. subtilis MB24 | P38422 | SWISS-PROT |
| PBP 5*, B. subtilis 168/Marburg | P35150 | SWISS-PROT |
| Streptomyces sp. strain K15 | P39042 | SWISS-PROT |
| Mycobacterium tuberculosis H37Rv | Z74024 | GenBank |
| PBP 4, S. aureus NCTC 8325 | X91786 | GenBank |
| High-molecular-weight class A PBPsb | ||
| PBPA, Mycobacterium leprae | L39923 | GenBank |
| M. tuberculosis H37Rv | Z80775 | GenBank |
| PBP 1a, M. leprae | S82044 | GenBank |
| PBP 1b, E. coli K-12/W3110 | P02919; P75664 | SWISS-PROT |
| PBP 1b, H. influenzae RD/KW20 | P45345 | SWISS-PROT |
| PBP 1a, Streptococcus pneumoniae 2039 | X67870 | GenBank |
| PBP 1a, S. pneumoniae 45607/63915/R6X | Q04707 | SWISS-PROT |
| PBP 1a, Streptococcus oralis | Q00573 | SWISS-PROT |
| PBP 1a/1b, B. subtilis 168 | P39793 | SWISS-PROT |
| PBP 2, S. aureus SRM705 | X62288 | GenBank |
| PBP 1a, E. coli K12/MG1655 | P02918; P76688 | SWISS-PROT |
| PBP 1a, H. influenzae RD/KW20 | P31776 | SWISS-PROT |
| PBP 1a, P. aeruginosa PAO1 | U73780 | GenBank |
| PBP 1, Neisseria gonorrhoeae FA19 | U72876 | GenBank |
| PBP 1a, Synechocystis sp. strain PCC6803 | D64000 | GenBank |
| PBP 1b, Synechocystis sp. strain PCC6803 | D90913 | GenBank |
| PBP 1b, Synechocystis sp. strain PCC6803 | D90904 | GenBank |
| PBP 4, B. subtilis 168 | P38050 | SWISS-PROT |
| PBP 1a, B. subtilis 168 | P40750 | SWISS-PROT |
| PBP 1c, E. coli K-12/MG1655 | P76577 | SWISS-PROT |
| High-molecular-weight class B PBPsb | ||
| PBP 2b, Streptococcus thermophilus Sfi6 | U58210 | GenBank |
| PBP 2b, S. pneumoniae R6 | P10524 | SWISS-PROT |
| PBP 2b, B. subtilis 168 | Q07868 | SWISS-PROT |
| PBP, B. subtilis 168 | Q03524 | SWISS-PROT |
| PBP 2x, S. pneumoniae R6 | P14677 | SWISS-PROT |
| PBP 2, Neisseria meningitidis 1DA | X59628 | GenBank |
| PBP 2, N. gonorrhoeae LM306/CDC84-060384 | P08149 | SWISS-PROT |
| PBP 3, E. coli K-12 | P04286 | SWISS-PROT |
| PBP 3, H. influenzae RD/KW20 | P45059 | SWISS-PROT |
| PBP 3a/PBP 3x, P. aeruginosa PAO1 | X95517 | GenBank |
| PBP 3, P. aeruginosa PAO1 | X84053 | GenBank |
| PBP, Synechocystis sp. strain PCC6803 | D90903 | GenBank |
| PBP 2, M. tuberculosis H37Rv | Z95388 | GenBank |
| PBP 2, M. leprae | Z70722 | GenBank |
| PBP 2, M. tuberculosis H37Rv | Z80233 | GenBank |
| PBP 2, E. coli K-12 | P08150 | SWISS-PROT |
| PBP 2, H. influenzae RD/KW20 | P44469 | SWISS-PROT |
| PBP 5, Enterococcus faecium | X92687 | GenBank |
| PBP 5, Enterococcus hirae R40 | X62280 | GenBank |
| PBP 5, Enterococcus faecalis | X78425 | GenBank |
| PBP 2, B. subtilis 168TrpC2 | D38161 | GenBank |
| PBP 2′, S. aureus | P07944 | SWISS-PROT |
| PBP 2′, S. aureus | X52593 | GenBank |
| Streptomyces clavuligerus NRRL 3585 | U56256 | GenBank |
| Class A β-lactamasesa | ||
| Bacteroides fragilis CS30 | L13472 | GenBank |
| Bacteroides uniformis WAL-7088 | P30898 | SWISS-PROT |
| PER-1, P. aeruginosa RNL-1 | P37321 | SWISS-PROT |
| Bacteroides vulgatus CLA341 | P30899 | SWISS-PROT |
| OHIO-1, Enterobacter cloacae | P18251 | SWISS-PROT |
| SHV-1, K. pneumoniae | P23982 | SWISS-PROT |
| LEN-1, K. pneumoniae LEN-1 | P05192 | SWISS-PROT |
| TEM-1, E. coli | P00810 | SWISS-PROT |
| Proteus mirabilis GN179 | P30897 | SWISS-PROT |
| PSE-4, P. aeruginosa Dalgleish | P16897 | SWISS-PROT |
| Rhodopseudomonas capsulatus SP108 | P14171 | SWISS-PROT |
| NMC, E. cloacae NOR-1 | P52663 | SWISS-PROT |
| Sme-1, Serratia marcescens S6 | P52682 | SWISS-PROT |
| OXY-2, Klebsiella oxytoca D488 | P23954 | SWISS-PROT |
| K. oxytocaE23004/SL781/SL7811 | P22391 | SWISS-PROT |
| S. typhimurium CAS-5 | X92507 | GenBank |
| MEN-1, E. coli MEN | P28585 | SWISS-PROT |
| Serratia fonticola CUV | P80545 | SWISS-PROT |
| Citrobacter diversus ULA27 | P22390 | SWISS-PROT |
| Proteus vulgaris 5E78-1 | P52664 | SWISS-PROT |
| Burkholderia cepacia 249 | U85041 | GenBank |
| Yersinia enterocolitica serotype O:3/Y-56 | Q01166 | SWISS-PROT |
| M. tuberculosis H37RV | Q10670 | SWISS-PROT |
| S. clavuligerus NRRL 3585 | Z54190 | GenBank |
| III, Bacillus cereus 569/H | P06548 | SWISS-PROT |
| B. licheniformis 749/C | P00808 | SWISS-PROT |
| I, Bacillus mycoides NI10R | P28018 | SWISS-PROT |
| I, B. cereus 569/H/9 | P00809 | SWISS-PROT |
| I, B. cereus 5/B | P10424 | SWISS-PROT |
| B. subtilis 168/6GM | P39824 | SWISS-PROT |
| 2, Streptomyces cacaoi DSM40057 | P14560 | SWISS-PROT |
| Streptomyces badius DSM40139 | P35391 | SWISS-PROT |
| Actinomadura sp. strain R39 | X53650 | GenBank |
| Nocardia lactamdurans LC411 | Q06316 | SWISS-PROT |
| S. cacaoi KCC S0352 | Q03680 | SWISS-PROT |
| ROB-1, H. influenzae F990/LNPB51/ serotype A1 | P33949 | SWISS-PROT |
| Streptomyces fradiae DSM40063 | P35392 | SWISS-PROT |
| Streptomyces lavendulae DSM2014 | P35393 | SWISS-PROT |
| Streptomyces albus G | P14559 | SWISS-PROT |
| S. lavendulae KCCS0263 | D12693 | GenBank |
| Streptomyces aureofaciens | P10509 | SWISS-PROT |
| Streptomyces cellulosae KCCS0127 | Q06650 | SWISS-PROT |
| Mycobacterium fortuitum | L25634 | GenBank |
| S. aureus PC1/SK456/NCTC9789 | P00807 | SWISS-PROT |
| BRO-1, Moraxella catarrhalis ATCC 53879 | Z54181 | GenBank; |
| Q59514 | SWISS-PROT | |
| Low-molecular-weight class C PBPsb | ||
| PBP 4, E. coli K-12/MG1655 | P24228 | SWISS-PROT |
| PBP 4, H. influenzae RD/KW20 | P45161 | SWISS-PROT |
| PBP 4, Actinomadura sp. R39 | P39045 | SWISS-PROT |
| B. subtilis 168 | P39844 | SWISS-PROT |
| PBP 4, Synechocystis sp. PCC6803 | D64002 | GenBank |
| PBP 4, M. tuberculosis H37Rv | Z95436 | GenBank |
| Zinc-dependent dd-peptidase (PBP), zinc-dependent PBP, S. albus G/Solvifaciens | P00733 | SWISS-PROT |
| Class C β-lactamasea | ||
| Citrobacter freundii OS60/GN346 | P05193 | SWISS-PROT |
| E. coli K-12/MG1655 | P00811 | SWISS-PROT |
| P99, E. cloacae P99/Q908R/MHN1 | P05364 | SWISS-PROT |
| Y. enterocolitica IP97/serotype O:5B | P45460 | SWISS-PROT |
| Morganella morganii SLM01 | Y10283 | GenBank |
| A. sobria 163a | X80277 | GenBank |
| FOX-3, K. oxytoca 1731 | Y11068 | GenBank |
| K. pneumoniae NU2936 | D13304 | GenBank |
| P. aeruginosa PAO1 | P24735 | SWISS-PROT |
| S. marcescens SR50 | P18539 | SWISS-PROT |
| Psychrobacter immobilis A5 | X83586 | GenBank |
| Low-molecular-weight class B PBPsb | ||
| M. tuberculosis H37Rv | Z84725 | GenBank |
| M. tuberculosis H37Rv | Z84498 | GenBank |
| Streptomyces sp. strain R61 | P15555 | SWISS-PROT |
| PBP 4, M. tuberculosis H37Rv | Z81360 | GenBank |
| N. lactamdurans LC411, PBP4 | Q06317 | SWISS-PROT |
| PBP 4, Synechocystis sp. strain PCC6803 | D90913 | GenBank |
| PBP 4*, B. subtilis W168 | P32959 | SWISS-PROT |
| PBP 4*, E. coli K12/MG1655 | AE000330; U00096 | GenBank |
| Class B β-lactamasea | ||
| II, B. cereus 569/H | P04190 | SWISS-PROT |
| II, Bacillus sp. 170 | P10425 | SWISS-PROT |
| II, B. cereus 5/B/6 | P14488 | SWISS-PROT |
| Chryseobacterium meningosepticum CCUG4310 | X96858 | GenBank |
| IMP-1, S. marcescens AK9373/TN9106 | P52699 | SWISS-PROT |
| B. fragilis TAL3636/TAL2480 | P25910 | SWISS-PROT |
| Aeromonas hydrophila AE036 | P26918 | SWISS-PROT |
| L1, Xanthomonas maltophilia IID 1275 | P52700 | SWISS-PROT |
| Monofunctional transglycosylases | ||
| E. coli K-12/MG1655 | P46022 | SWISS-PROT |
| K. pneumoniae M5A1 | Q48465 | SWISS-PROT |
| H. influenzae RD/KW20 | P44890 | SWISS-PROT |
Multiple alignments of amino acid sequences of PBPs and β-lactamases were made with the program PileUp from the WISCONSIN package, version 9. PileUp uses the algorithm of Needleman and Wunsch (41) for the pairwise comparison of sequences. The relationships between the sequences are characterized by the similarity scores, which are used to build a dendrogram by applying the unweighted pair-group method with arithmetic averages (47). The sequence alignment and dendrogram do not provide information on the parsimony of the bacterial strains, but merely argue for the relationship among these proteins. The sharing of the genetic material among microorganisms takes place via several distinct mechanisms, and its time scale is relatively rapid compared to that of the evolution of function among proteins or the evolution of entire organisms.
The Tripos molecular modeling software Sybyl, version 6.3 (52), and the Molecular Simulations Inc. package InsightII (40) were used for protein visualizations. Energy minimization of the acyl enzyme intermediate of cephalothin in the active site of the Enterobacter cloacae P99 β-lactamase was conducted by using the AMBER force field. The water-accessible Connolly surface was calculated by the use of the “Shaded Surfaces” module of Sybyl. All enzymes shown in Figures 4, 5, and 6 were overlapped on the basis of the Cα of critical residues for catalysis for fixing perspectives. These are Ser-70, Lys-73, Ser-130, Asn-132, and Lys-234 for the TEM-1 β-lactamase; Ser-64, Lys-67, Tyr-150, Asn-152, and Lys-315 for the E. cloacae P99 β-lactamase; and Ser-62, Lys-65, Tyr-159, Asn-161, and His-298 for the bifunctional dd-peptidase–transpeptidase from Streptomyces sp. strain R61.
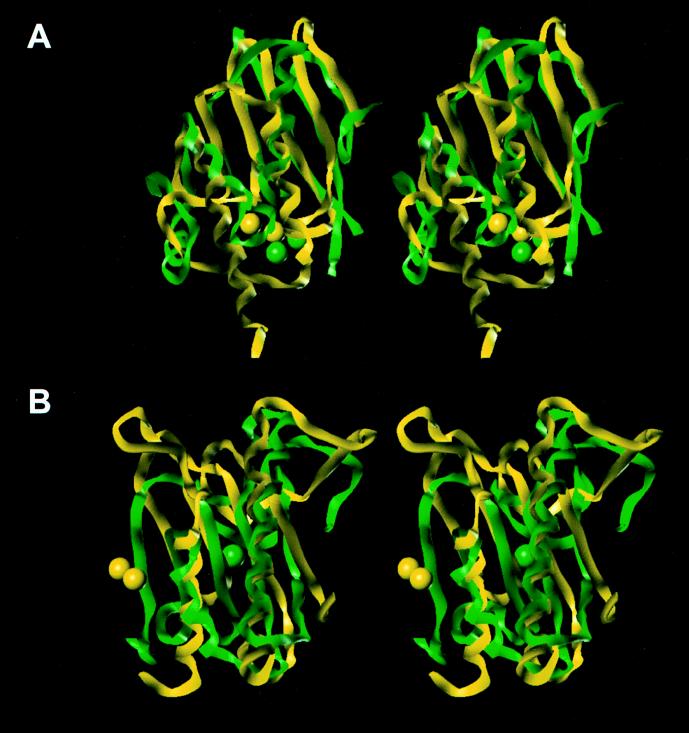
FIG. 4.
Stereoviews of the three-dimensional folds for the superimposed N-terminal (green) and C-terminal (yellow) subdomains of the class B β-lactamase from B. fragilis (A) and of the Leu-91 to Ile-123 stretch of dd-peptidase (a PBP) from S. albus G (green) with the Asn-33 to Leu-155 stretch of thermolysin from B. thermoproteolyticus (yellow) (B). The spheres represent the zinc ions.
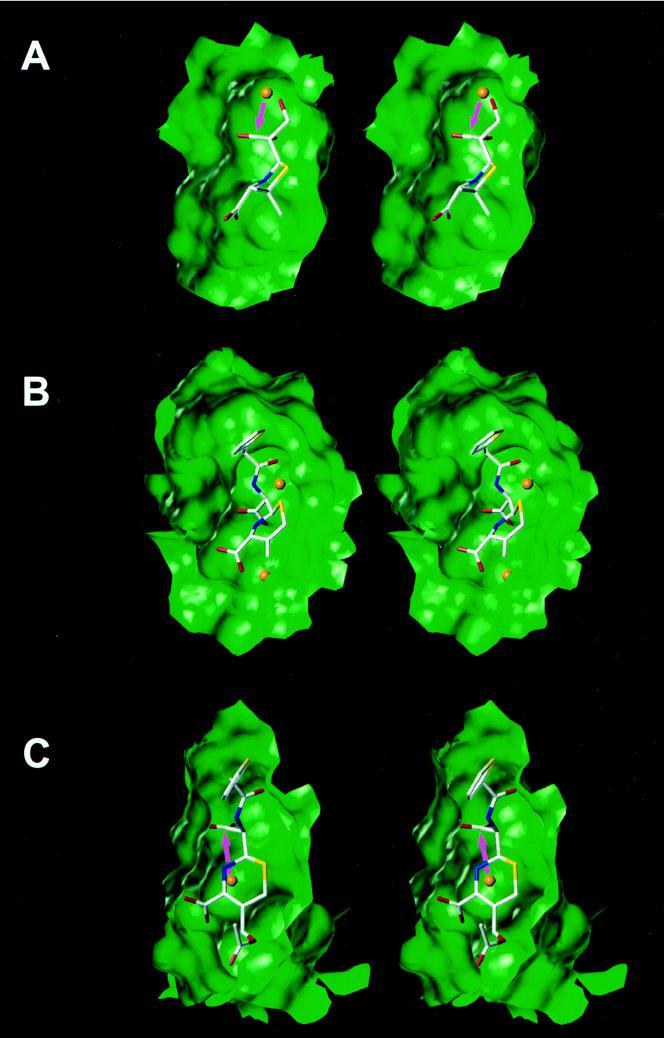
FIG. 5.
Views of the crystal structure of the acyl enzyme intermediate for 6α-(hydroxymethyl)penicillanate in the class A TEM-1 β-lactamase (A), the crystal structure of the dd-peptidase–transpeptidase from Streptomyces sp. strain R61 modified by cephalothin (the C3 substituent is eliminated because of the longevity of the species) (B), and the energy-minimized structure of the acyl enzyme intermediate for cephalothin in the active site of the class C β-lactamase from E. cloacae P99 (the C3 substituent is not eliminated because of the fleeting existence of the species) (C). The active-site cavities are shown as Connolly water-accessible surfaces. Water molecules are represented as orange spheres.
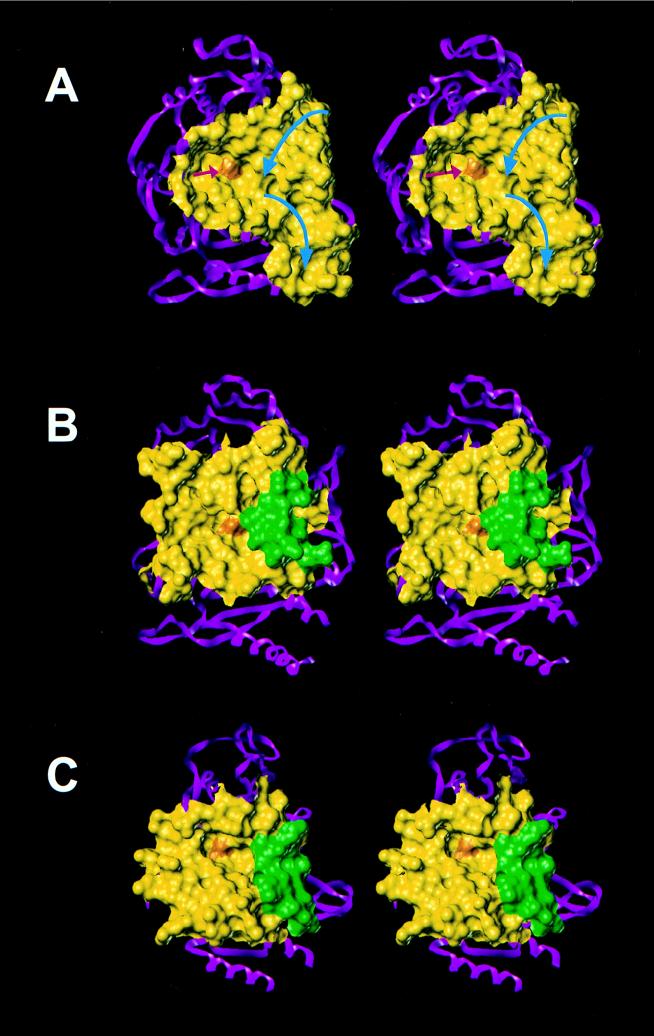
FIG. 6.
Active sites, shown as Connolly water-accessible surfaces, of the bifunctional dd-peptidase–transpeptidase from Streptomyces sp. strain R61 (A), the class C β-lactamase from E. cloacae P99 (B), and the class A TEM-1 β-lactamase from E. coli (C). The red and blue arrows indicate the grooves where the first and second peptidoglycan strands, respectively, bind to the active site. The first peptidoglycan strand (red arrow) would approach the active-site serine, represented by the orange surface, essentially orthogonally to the plain of the figure in the depicted perspective. The green areas of surfaces B and C are contributed by residues Glu-285 to Ile-296 in class C β-lactamases and Asp-214 to Ala-224 residues in class A β-lactamases. The yellow areas constitute the remainder of the active-site regions. The rest of each protein is depicted in the ribbon presentation in magenta.
PBPs and β-lactamases.
Bacteria may have come into existence more than 3.5 billion years ago (24, 36). A primary structural feature of bacteria is the cell wall, whose function is absolutely indispensable for the organism by providing support for the maintenance of bacterial morphology. A major component of the cell wall is the peptidoglycan. In the absence of an effective cell wall, as in bacteria treated with inhibitors of cell wall biosynthetic steps (or those without a cell wall entirely, such as Mycoplasma), the bacteria would be capable of surviving only in media that match their internal osmotic pressure. A family of enzymes collectively known as the PBPs are responsible for the assembly, maintenance, and regulation of the features of the peptidoglycan structure. These proteins are mostly anchored in the bacterial inner membrane, with their active sites made available in the periplasmic space. There are two groups of PBPs, low-molecular-weight and high-molecular-weight PBPs, each of which is subdivided into three classes on the basis of amino acid sequence similarities (16). With the exception of a single protein which appears to be zinc dependent, the remaining known members of this family of enzymes belong to the group of active-site serine proteins. These proteins have neither sequence nor structural similarity to the better-known serine proteases, which also possess an important active-site serine. The fact that these two distinct groups of proteins have evolved a similar strategy in their catalytic mechanisms, namely, the critical involvement of a serine residue, is a clear example of evolutionary forces which came up with a similar strategy via distinct pathways.
The functions of PBPs are indeed quite diverse, and they include transpeptidase, transglycosylase, and carboxypeptidase activities (15, 16, 18, 25). The high-molecular-weight PBPs have a multidomain structure, with a PBP-binding domain which displays the transpeptidase activity. The functions of the other domain(s) of many of these proteins remain unknown. The N-terminal domains of PBP 1a and PBP 1b display the transglycosylase activity (17, 18). BlaR PBPs are signal transducers for the class A β-lactamase in Bacillus licheniformis and Staphylococcus aureus, and in methicillin-resistant Staphylococcus aureus they also stimulate biosynthesis of PBP 2′, which shows a low affinity for β-lactam drugs (21, 32). Low-molecular-weight PBPs show carboxypeptidase activity (18, 25). They probably have single-domain structures, although they may contain in their sequences an insertion(s) which may serve as a membrane linker. Some of these low-molecular-weight PBPs display transpeptidase activity (16, 18).
The reason that these proteins are referred to as PBPs is historic; they are all modified covalently by penicillins in their active-site serine residues. The resultant acyl enzyme species are inactive and are fairly stable compared to the β-lactamases (5), so the bacterium is deprived of the essential functions of these enzymes. Some bacteria have undergone alteration of their PBPs such that they would be less susceptible to the deleterious action of the β-lactam antibiotics (e.g., penicillins). Examples of these modified PBPs, PBP 2′ from methicillin-resistant S. aureus, PBP 2b and PBP 2x from β-lactam-resistant Streptococcus pneumoniae (20), the chromosomal PBP 5 and the plasmid-encoded variant PBP 3r from E. hirae, and the homologous low-affinity PBP 5 from E. faecium, are becoming well known (44, 51), because the organisms which express them generally represent some of the pathogens that are clinically the most difficult to treat with antibiotics (9, 53). To augment the problem, these organisms often exhibit resistance to multiple antibacterial agents (53).
However, by far the most important means for resistance to β-lactam antibiotics is the manifestation of the activity of β-lactamases. These enzymes are related to PBPs, but they have acquired the ability to hydrolyze the β-lactam ring of these antibacterial agents, thereby rendering them inactive. The β-lactam moiety is critical for the biological function, because it is the entity that modifies the active-site serine of PBPs in the step that inactivates the biosynthetic enzymes and impairs cell wall biosynthesis.
The evolutionary pressure for the creation of β-lactamases was presented by microorganisms which biosynthesized the first β-lactam antibiotics (38). These organisms presumably developed antibiotics to gain advantage over nonproducing bacteria in their competition for resources. In return, the nonproducing strains evolved β-lactamases in order to overcome the challenge of the β-lactam antibiotics, which were being exuded into the environment by their competitors. Hence, the evolution of β-lactamases presented a distinct survival advantage to the bacteria. The bacterial producers of β-lactamases were more successful than the nonproducers, and indeed, the presence of the antibiotic created the selection pressure for their survival and dissemination of their genetic materials. This process has been accelerated considerably by the medical use of antibiotics (11, 53), since β-lactam antibiotics are used heavily.
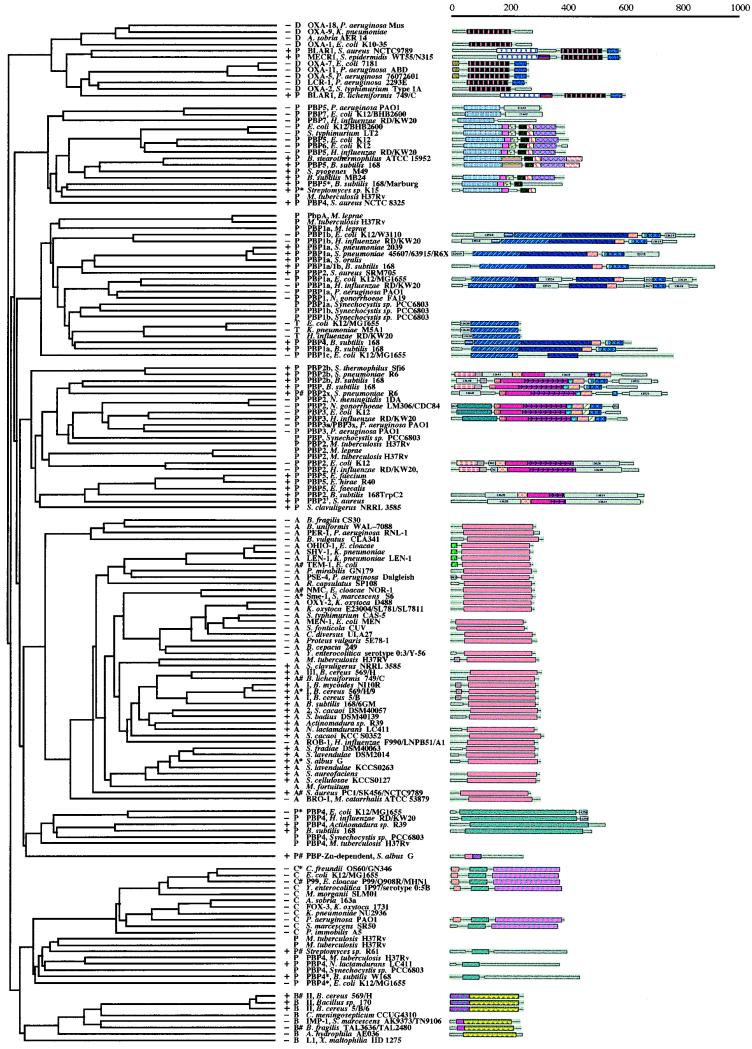
Four structural classes of β-lactamases, classes A, B, C, and D, have been identified to date (4). Of these, classes A, C, and D are active-site serine β-lactamases, whereas class B enzymes are zinc dependent. The history of the classification methodologies for β-lactamases has been outlined well in a recent publication by Bush et al. (4). Those investigators have also carried out an extensive parsimony analysis of the various classes of β-lactamases and have provided a structure-function description of these enzymes, of which just under 200 examples were known by the time of the report (4). We have expanded this study by incorporating the sequences of 77 PBPs, 3 monofunctional transglycosylases, and 73 β-lactamases in the multiple-sequence analysis. The results are shown in Fig. 1. Figure 2 is a simplified version of Fig. 1. A number of features of Fig. 1 and 2 are of special interest.
FIG. 1.
Multiple-sequence alignment of PBPs and β-lactamases made by the use of the program PileUp from the WISCONSIN package. The first column on the left indicates whether the organism is gram positive (+), gram negative (−), or unspecified. The second column states if a given entry is a penicillin-binding protein (P); a class A (A), a class B (B), a class C (C), or a class D (D) β-lactamase; or a monofunctional transglycosylase (T). The pound sign indicates a protein for which a crystal structure is available. The asterisk denotes a protein for which a crystal structure was published but for which the coordinates are not available. The next column indicates the source for the given entry. The last column indicates the domain structures for the proteins from the ProDom library, when they are available. The color and the pattern codes for the domains are arbitrary and were obtained directly from the ProDom library (47a); their utility in this figure is for ready and immediate visualization of homologous domains in different proteins. The scale at the top indicates the lengths of the proteins (in numbers of amino acids).
FIG. 2.
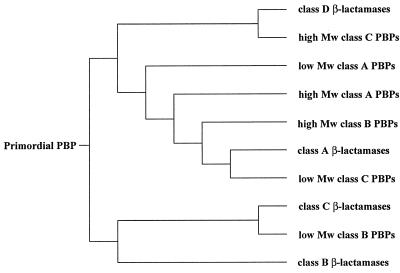
Simplified schematic for the multiple-sequence alignment shown in Fig. 1. Mw, molecular weight.
Throughout this report we use the system proposed by Ghuysen (16) in referring to PBPs. The diversification process clusters the PBPs into six distinct groups (see below), some of which are in turn related to individual classes of β-lactamases. As a result of the way in which they clustered, there is no mixing of the different classes of β-lactamases. The same is true for PBPs, because those of the same class all grouped in the same cluster. Moreover, the trends establish that certain PBPs of a given cluster are more closely related to one class of β-lactamases or another. For example, one can see that the BlaR and MecR transducers (high-molecular-weight class C PBPs) form one cluster with the class D β-lactamases. Interestingly, these transducers exist at branches within the cluster for class D β-lactamases, which suggests that they evolved from these β-lactamases, as first proposed by Ghuysen (16). In essence, while these transducers are considered to be PBPs, they represent examples of how evolution would go from β-lactamases to PBPs, in this case, to facilitate an entirely new reaction, namely, signal transduction for the biosynthesis of class A β-lactamases in bacteria. This would appear to be an oddity, in light of the fact that these proteins are more closely related to class D β-lactamases, yet they participate in signal transduction of biosynthesis of class A β-lactamases. Our second cluster includes the low-molecular-weight class A PBPs (certain examples of which include PBP 5 from Escherichia coli, Pseudomonas aeruginosa, Haemophilus influenzae, and Bacillus subtilis; PBP 6 from E. coli; and PBP 7 from E. coli and H. influenzae). The third cluster is represented by high-molecular-weight class A PBPs, namely, PBP 1a from E. coli, H. influenzae, B. subtilis, S. pneumoniae, P. aeruginosa, Mycobacterium leprae, Streptococcus oralis, and Synechocystis sp.; PBP 1b from E. coli, H. influenzae, and Synechocystis sp.; and PBP 1c from E. coli. The fourth cluster is comprised of the high-molecular-weight class B PBPs, including such examples as PBP 2 from Neisseria gonorrhoeae, Neisseria meningitidis, Mycobacterium tuberculosis, E. coli, B. subtilis, and H. influenzae; PBP 2b from S. pneumoniae, Streptococcus thermophilus, and B. subtilis; PBP 2x from S. pneumoniae; PBP 3 from E. coli, H. influenzae, and P. aeruginosa; PBP 3x from P. aeruginosa; PBP 5 from E. faecium, E. hirae, and E. faecalis; and PBP 2′ from S. aureus; the majority of these show low affinities to β-lactam antibiotics. The fifth cluster is comprised of the class A β-lactamases, representing the largest group of these related enzymes. Class A β-lactamases are most closely akin to the low-molecular-weight class C PBPs of the sixth cluster (PBP 4 from E. coli, H. influenzae, M. tuberculosis, Actinomadura sp., and Synechocystis sp.). The seventh cluster encompasses the members of class C β-lactamases and low-molecular-weight class B PBPs (PBP 4* from B. subtilis and E. coli, PBP 4 from M. tuberculosis and Streptomyces sp., and PBPs related to them), which are closely related. Finally, the eighth cluster belongs to the class B β-lactamases.
The branching point in the pathway which ultimately leads to class A and C β-lactamases (β-lactamases of both of these classes are the most prevalent β-lactamases among β-lactam-resistant pathogens) goes back to the first diversification point for the primordial enzyme and represents two distinct evolutionary pathways, as discerned from the sequences of the extant known proteins (Fig. 1 and 2). In effect, evolution of these enzymes did not progress via a linear process, as suggested previously (35) (a caveat to this has been discussed recently [31]), but rather, several PBPs originated from the primordial enzyme and separately evolved into the various classes of β-lactamases in independent, and perhaps parallel, processes (2, 18).
We also note another important observation from Fig. 1 and 2. It is significant that the dd-peptidase from Streptomyces sp. strain R61, a low-molecular-weight class B PBP, has been considered a representative member of the PBP family. This choice has been based on pragmatic considerations, in that this PBP is not a membrane-anchored protein and has a low molecular weight. Because of these two factors, this protein is relatively well studied, and indeed, it has been amenable to X-ray structure analysis of single crystals (29, 33). It is the only member of the active-site serine family of PBPs which has provided a high-resolution crystal structure (29). However, further analysis of Fig. 1 and 2 reveals that the vast majority of PBPs are indeed more closely related to class A β-lactamases than to the dd-peptidase from Streptomyces sp. strain R61. Hence, there is need for additional structures for other members of this family of proteins. The low-resolution crystal structure for the high-molecular-weight PBP 2x from S. pneumoniae R6 that has recently been reported (43) will prove useful in modeling of the active sites of these closely related PBPs in conjunction with those of the class A β-lactamases.
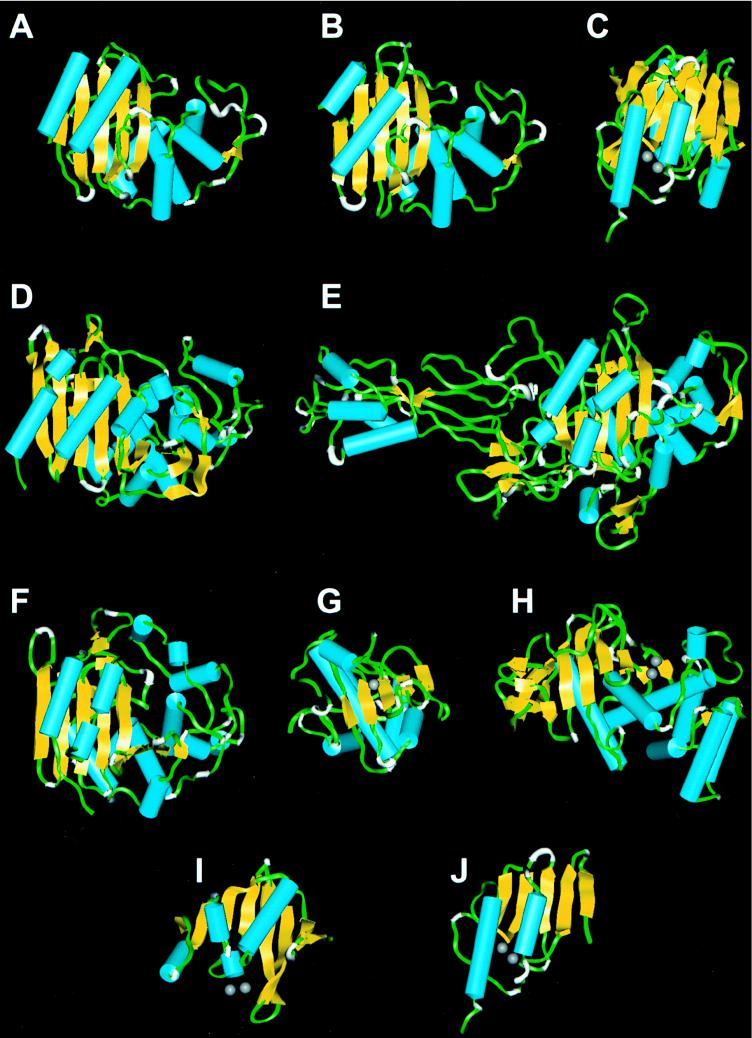
Figures 3A to G show the general topologies of representative members of proteins that interact with β-lactam antibiotics, as discerned from their X-ray crystal structures. These structures share remarkable conformational similarities, reflective of an entirely conserved folding pattern. This is true despite the relatively low amino acid sequence similarities among these proteins, indicating that conservation of topology can tolerate large variations in sequence. The best-studied group of these proteins from a structural aspect is the class A β-lactamases, of which seven members have been crystallized. These are the TEM-1 β-lactamase from E. coli (26, 27, 37, 49) and the enzymes from S. aureus PC1 (8, 23), B. licheniformis 749/C (13, 30, 39), Streptomyces albus G (not available from the Brookhaven Protein Data Bank [12]), NMC E. cloacae NOR-1 (44a), Sme-1 from S. marcescens S6 (not available from the Brookhaven Protein Data Bank [48]), and β-lactamase I from Bacillus cereus 569 (not available from the Brookhaven Protein Data Bank [45]). The general topology is strictly preserved among these enzymes, regardless of whether the enzyme is of gram-positive or gram-negative bacterial origin (Fig. 3A and B, respectively). One hastens to add that although the orientation of the active-site residues critical in the catalytic machinery in these enzymes is strictly identical in all enzymes, subtle differences both in sequence and in structural topology render these enzymes different in their biological responses to substrates and inhibitors (4). In effect, either each enzyme has been fine-tuned for responses to different stimuli or the power of random mutation and selection has taken each enzyme on a different evolutionary tangent. The structures of two representative enzymes of the class C β-lactamases are known. These are enzymes from Citrobacter freundii (not available from the Brookhaven Protein Data Bank [42]) and E. cloacae P99 (34, 35). Although the structure of the Citrobacter enzyme is proprietary, it would appear to be highly similar to the Enterobacter enzyme (there is a sequence identity of 73% between the two).
FIG. 3.
Backbone ribbon presentations for the class A TEM-1 β-lactamase from E. coli (A), the class A β-lactamase from B. licheniformis 749/C (B), the class B β-lactamase from B. fragilis (C), the class C β-lactamase from E. cloacae P99 (D), transpeptidase PBP 2x from S. pneumoniae R6 (E), the dd-peptidase–transpeptidase from Streptomyces sp. strain R61 (F) zinc-dependent dd-peptidase from S. albus G (G), thermolysin from B. thermoproteolyticus (H), the N-terminal half of the class B β-lactamase from B. fragilis (I), and the C-terminal half of the class B β-lactamase from B. fragilis (J). The structures in panels E, F, and G are PBPs. The helices are shown in cyan, the β-strands are in yellow, and the zinc ion is in gray spheres.
Class B β-lactamases are zinc-dependent enzymes. Two members of this group of enzymes have been crystallized (Fig. 3C). These are zinc-dependent β-lactamases from Bacillus cereus (6, 6a, 7) and Bacteroides fragilis (6, 6a, 10). At first glance, it would appear unreasonable to include these metalloenzymes in our multiple-sequence analysis with active-site serine enzymes. It may be a fair proposition that these proteins in fact evolved from different origins. However, comparison of their structures shows us that certain important structural elements are indeed shared by these disparate groups of proteins. The structures of these enzymes consist of two subdomains with a similar fold (7). As depicted in Fig. 4A for the superimposition of the N-terminal and the C-terminal halves of the enzyme from B. fragilis, the similarities of the two subdomains are quite remarkable, as also noted by Carfi et al. (7) for the metallo-β-lactamase from B. cereus. The two metallo-β-lactamases have 34% amino acid sequence identity and the same topology. The structure of the B. fragilis enzyme was reported to have two zinc ions in the active site (10), while the initial report of the structure of the B. cereus enzyme (7) indicated that it contained only one zinc ion. While this minireview was in preparation, Carfi et al. (6, 6a) submitted two more structures for the class B β-lactamases from B. cereus and B. fragilis, and both of them have two zinc atoms in their active sites. We can discern from these crystal structures that the two halves of these enzymes may share a similar origin, arising from a potential gene duplication. Even the relative positions of the zinc-binding regions in these subdomains are clearly the same (Fig. 4A; the two zinc atoms are shown as spheres). The two five-strand sheets within these subdomains, along with their two associated helices (shown in Fig. 3I and J), are highly reminiscent of the same pattern in the other structures shown in Fig. 3A through F.
Three members of the serine PBP family have been crystallized. The bifunctional dd-peptidase–transpeptidases from Streptomyces sp. strain R61 (29) (a low-molecular-weight class B PBP) and Streptomyces K15 (19) (a low-molecular-weight class A PBP; not available from the Brookhaven Protein Data Bank), and PBP 2x from S. pneumoniae R6 (43), (a high-molecular weight class B PBP; only the Cα coordinates are available). The portion of the structure of PBP 2x which shares similarity with the bifunctional PBP is the location of the active site for the transpeptidase reaction. This PBP 2x was clustered together with PBPs of clinical significance, such as PBP 2′ from methicillin-resistant S. aureus, PBP2b from resistant S. pneumoniae, and the chromosomal PBP 5s from E. hirae and E. faecium. The plasmid-encoded variant PBP 3r from E. hirae is 97% homologous to the low-affinity PBP 5 from E. faecium. Therefore, the structural information for PBP 2x can be used to model the active sites of these low-affinity PBPs, as asserted earlier.
The sole known zinc-containing dd-peptidase (a PBP) from S. albus G groups more closely with the low-molecular-weight class C PBPs (of the sixth cluster) and not with the known zinc-dependent class B β-lactamases. We have carried out the analyses that resulted in Fig. 1 using different numbers of the various classes of enzymes that we have assembled. It is important that the number of enzymes used for β-lactamases and PBPs did not alter the outcome of the clustering for the various groups of enzymes. The only exception was when the sequence of the zinc-containing dd-peptidase (a PBP) from S. albus G was included. This protein clustered in different locations depending on the number of enzymes used in the analysis. This observation is indicative of insignificant evolutionary kinship of this dd-peptidase to all PBPs and β-lactamases. It is intriguing that this zinc-containing dd-peptidase shows relatively stronger topological similarity to the bacterial thermolysin-type proteases (also zinc dependent) than to any PBP or β-lactamase. Moreover, the Leu-91 to Ile-213 stretch of this zinc-containing dd-peptidase shares a high degree of topological similarity to the Asn-33 to Leu-155 stretch of thermolysin from Bacillus thermoproteolyticus (Fig. 4B). However, this zinc-dependent dd-peptidase has no sequence similarity either to the thermolysin sequence or to the sequence of any β-lactamases or PBPs. It is interesting that the zinc-containing dd-peptidase, in addition to the two other PBPs, and several class A and C β-lactamases, for all of which crystal structural information is now available, share a similar β-sheet subdomain (usually five strands associated with two or three helices) and another subdomain with a high helix content (Fig. 3). This reveals that the basic template for structural elaboration of these enzymes—despite the lack of a high degree of amino acid sequence similarity—is preserved and may be quite ancient. The conservation of the general topology argues for the versatility of the motif for its various functions in bacteria. It also indicates, however, that nature would tend to be conservative in molecular structural diversity if the functional needs are met by the limited structural repertoire. It is costly to evolve proteins with unique structural folds for the set of the necessary reactions, especially if nature does not have a compelling reason to seek this diversity. These findings reveal that PBPs and β-lactamases constitute an excellent case study for how evolution can repeatedly invent new enzymes by using well-tried motifs to diversify the repertoire of biocatalysts.
Aside from the multiple-sequence alignment for the entire set of the 153 proteins (Fig. 1), we have carried out sequence alignment for each cluster by itself. The information for proteins of known structure was used in conjunction with the alignments to portions of the primary structure critical for the function of the given protein (Tables 2 and 3). These are areas which contain residues directly involved in the transpeptidase-carboxypeptidase activities in PBPs and in hydrolysis of β-lactams by β-lactamases. Table 2 includes the sequences of three loci of amino acid sequences known to be critical for all active-site serine PBPs and β-lactamases. The second column of Table 2 shows the position for the catalytic serine residue. The third column displays the position for the residue corresponding to Ser-130 in class A β-lactamases, which finds a counterpart in Tyr-150 of class C β-lactamases. The fourth column represents the position corresponding to the residue Lys-234 in class A β-lactamases and Lys-315 in class C β-lactamases. The last five columns of Table 2 show the distances between the three loci represented in the table. Variation in these two distances, as well as the position for the catalytic serine listed in the second column, would reflect the possibility of the presence of an additional domain(s) or subdomain(s) as an insertion. Analysis of the residue variability at positions marked by asterisks in Table 2 provides information about the importance of these residues for catalysis. Without any exception, the catalytic serine is followed by a lysine two residues apart (locus I) for all proteins that we have analyzed. Similarly, we observe that the other sole residue, besides the aforementioned serine and lysine, which is absolutely conserved among all these proteins is a glycine found in locus III (Table 2; marked by asterisks). Analysis of the known crystal structures reveals that the backbone carbonyl of this glycine is the carbonyl of the amide which comprises one of the hydrogen bonds for the oxyanion hole. It is interesting that in contrast to this glycine, the residue which contributes the amide nitrogen is indeed quite variable. The common mechanistic feature of all active-site serine β-lactamases and PBPs is that they undergo acylation at the important active-site serine residue. Therefore, it is evident that the acylation machinery has been preserved in these enzymes in the course of evolution and that for this reaction all strictly conserved residues must be significant as a common “denominator.” The conserved serine and lysine residues from locus I and the glycine residue from locus III would appear to be the minimal requisites for the acylation step for either β-lactamases or PBPs. It is the deacylation behavior of these enzymes which makes them distinct mechanistically. This subject is discussed in the next section.
TABLE 2.
Multiple-sequence alignment for the PBPs and β-lactamases in the regions of the primary structure critical for catalysisa
TABLE 3.
Multiple-sequence alignment for the Ω-loop region in the class A β-lactamases
| Source | Sequence of Ω loop, locus IVa |
|---|---|
| * * | |
| B. fragilis CS30 | 162-VIHTENDMHKNLEFCYQNWTTP-183 |
| B. uniformis WAL-7088 | 162-LSETEDGMHSSFEAVYRNWSTP-183 |
| PER-1, P. aeruginosa RNL-1 | 167-VVANEAQMHADDQVQYQNWTSM-188 |
| B. vulgatus CLA341 | 180-IAYTEEEMSADHNKAYSNYTSP-201 |
| OHIO-1, E. cloacae | 158-LDRWETELNEALPGDARDTTTA-179 |
| SHV-1, K. pneumoniae | 158-LDRWETELNEALPGDARDTTTP-179 |
| LEN-1, K. pneumoniae LEN-1 | 158-LDRWETALNEALPGDARDTTTP-179 |
| TEM-1, E. coli | 160-LDRWEPELNEAIPNDERDTTMP-181 |
| P. mirabilis GN179 | 165-LDRWEPELNEAVPGDKRDTTTP-186 |
| PSE-4, P. aeruginosa Dalgleish | 157-LDRIEPDLNEGKLGDLRDTTTP-178 |
| R. capsulatus SP108 | 166-LDRIEPKLNDFASGDERDTTSP-187 |
| NMC, E. cloacae NOR-1 | 164-LDRWELDLNTAIPGDERDTSTP-185 |
| Sme-1, S. marcescens S6 | 166-LDRWELELNTAIPGDKRDTSTP-187 |
| OXY-2, K. oxytoca D488 | 164-LDRTEPTLNTAIPGDERDTSTP-185 |
| K. oxytoca E23004/SL781/SL7811 | 165-LDRTEPALNSAIPGDKRDTTTP-186 |
| S. typhimurium CAS-5 | 165-LDRTEPTLNTAIPGDPRDTTTP-186 |
| MEN-1, E. coli MEN | 137-LDRTEPTLNTAIPGDPRDTTSP-158 |
| S. fonticola CUV | 141-LDRTEPTLNTAIPGDKRDTTSP-162 |
| C. diversus ULA27 | 168-LDRKEPELNTAIPGDERDTTCP-189 |
| P. vulgaris 5E78-1 | 167-LDRKEPELNTAIHGDPRDTTSP-188 |
| B. cepacia 249 | 175-LDRWETELNTALPGDLRDTTTP-196 |
| Y. enterocolitica serotype O:3/Y-56 | 167-LDRWEPDLNTARPNDPRDTTTP-188 |
| M. tuberculosis H37RV | 178-LDAEEPELNRDPPGDERDTTTP-199 |
| S. clavuligerus NRRL 3585 | 186-MDQYEPELNHDPPHDPRDTTTP-207 |
| III, B. cereus 569/H | 185-SERFEPELNEVNPGETHDTSTP-206 |
| B. licheniformis 749/C | 176-PERFEPELNEVNPGETQDTSTA-197 |
| I, B. mycoides NI10R | 179-SDRFETELNEAIPGDIRDTSTA-200 |
| I, B. cereus 569/H/9 | 181-SNRFETELNEAIPGDIRDTSTA-202 |
| I, B. cereus 5/B | 179-SDRFETELNEAIPGDIRDTSTA-200 |
| B. subtilis 168/6GM | 179-AKRFETDLNSAIPGDIRDTSTA-200 |
| 2, S. cacaoi DSM40057 | 186-MERVEPFLSRWEPGSKRDTSTP-207 |
| S. badius DSM40139 | 184-MDREEPELSRWVPGEKRDTSTP-205 |
| Actinomadura sp. strain R39 | 176-ADRIETELNEVPPGETRDTSTP-197 |
| N. lactamdurans LC411 | 175-ADRWEPELNSAVPGDVRDTSTP-196 |
| S. cacaoi KCC S0352 | 194-MDRYEQELGSAVPGDPRDTSTP-215 |
| ROB-1, H. influenzae F990/LNPB51/A1 | 176-TNRLEPDLNQAKPNDIRDTSTP-197 |
| S. fradiae DSM40063 | 178-LDRWEPELNSAEPGRVTDTTSP-199 |
| S. lavendulae DSM2014 | 178-LDRWEPELNSAEPGRLTDTTTP-199 |
| S. albus G | 187-LDRWEPELNSAEPGRVTDTTSP-208 |
| S. lavendulae KCCS0263 | 176-LDRWEPELNSAEPDRVTDVTTP-197 |
| S. aureofaciens | 183-LDRWEPALNSAEPDRVTDTTSP-204 |
| S. cellulosae KCCS0127 | 180-LDRWEPELNSGEPDRRTDTTSP-201 |
| M. fortuitum | 167-LDRWEVELNSAIPGDPRDTSTA-188 |
| S. aureus PC1/SK456/ NCTC 9789 | 153-PVRYEIELNYYSPKSKKDTSTP-174 |
| BRO-1, M. catarrhalis ATCC 53879 | 184-LANNEPLLNRSHYGETSDTAKP-205 |
As will be elaborated in the next section, the Ω loop near the active sites of the class A β-lactamases is critical in catalysis by these enzymes. Specifically, Glu-166 (Table 3) plays the key role of activating the water molecule in the deacylation of the acyl enzyme intermediate (1, 14, 37, 49, 50). Therefore, it is not surprising that Glu-166 is strictly conserved in class A β-lactamases. However, it is remarkable that essentially all other sites in the Ω loop have undergone substitutions in their amino acid contents. This tells us that Glu-166 is indispensable for catalysis, whereas other sites are not. Furthermore, it is worth noting that the Ω loop, despite its size, makes few contacts with the rest of the protein. As such, it may be a flexible element in the protein in the course of catalysis, which may explain the tolerance for substitutions in all positions except position 166.
How a PBP may become a β-lactamase.
As stated earlier, the vast majority of PBPs are active-site serine enzymes, as are the vast majority of β-lactamases. Peptidoglycan acylates PBPs at the active-site serine residue prior to the catalytic step that completes the turnover process for the various functions that they perform (e.g., dd-peptidase and transpeptidase activities). The key feature of inhibition of these enzymes by β-lactams, such as penicillins, is that they also acylate the active-site serine, but the nature of the modified protein is such that it interferes with the function of the enzyme for the subsequent catalytic steps. For a PBP to have evolved into a β-lactamase, the protein needed to acquire the ability to undergo deacylation of the acyl enzyme species. Such a two-step process—acylation followed by deacylation—would complete the hydrolytic pathway for the destruction of β-lactam antibiotics. Indeed, the acquisition of this second step in the reaction profile, namely, hydrolysis of the acyl enzyme species, has been performed deftly by nature. Furthermore, the processes for both enzyme acylation and enzyme deacylation have been refined to approach “perfection” by amino acid substitutions, insertions, and/or deletions on an evolutionary time scale. The approach to catalytic perfection has been achieved such that, indeed, at least for class A β-lactamases, in the catalytic process the chemical steps with a few preferred substrates are no longer the slow steps, but rather, the diffusion of the substrate into the active site and the products away from it are rate limiting (22). Parenthetically, we add that the opposite is seen in the vast majority of enzymes, because the chemical steps—bond making and bond breaking—are typically the more difficult processes.
The assertion that independent evolutionary steps gave rise to different classes of β-lactamases was put on firmer ground by the recent mechanistic studies of β-lactamases of classes A and C (2), as well as by structural comparisons (31). As stated earlier, the diversification of the two classes of PBPs which ultimately gave rise to class A and C β-lactamases took place early in the evolutionary time scale. These two classes of β-lactamases retained the ability of the parental PBP to undergo acylation by β-lactam antibiotics, and it is likely that they refined it further. However, the two enzymes proceeded on different evolutionary paths from that point on. As shown in Fig. 5A, the approach of the hydrolytic water to the acyl enzyme intermediate is from the α face of the antibiotic (down in the perspective depicted), whereas that for the class C enzyme shown in Fig. 5C is from the β face of the antibiotic (up in the perspective depicted). To add to the complexity of the story, the mechanisms for the activation of the hydrolytic water molecules are also distinct for the two families of enzymes (2); a residue on the protein in class A β-lactamases (Glu-166) activates the hydrolytic water (see above), whereas both the substrate nitrogen (the nitrogen of the opened β-lactam ring) and Tyr-150 would appear to facilitate the hydrolytic reaction of the class C enzymes. Therefore, these two classes of β-lactamases use entirely distinct mechanistic strategies for the second step of the turnover process. To add to the intrigue, the X-ray structure of the bifunctional dd-peptidase–transpeptidase from Streptomyces sp. strain R61 shows that two crystallographic water molecules are sequestered in the active site of this PBP (Fig. 5B). The first, the one on the α face of the antibiotic, occupies exactly the same space as the hydrolytic water in all class A β-lactamases, but the protein lacks the mechanism for its activation for the hydrolytic reaction. So, evolution provided the means for deacylation in class A enzymes by the insertion of the secondary structural element recognized as the active-site Ω loop, which bears the strictly conserved general base Glu-166 for the promotion of a water molecule in the hydrolytic step.
The story is different for the class C enzymes. The water molecule on the β face of the antibiotic is activated by the electrostatic environment created by the ring nitrogen (the former β-lactam nitrogen) on the substrate itself, as well as by the residue Tyr-150. Tyr-159 of dd-peptidase–transpeptidase is present in the same space as the Tyr-150 of the class C β-lactamases, but it does not appear to be sufficient to facilitate the deacylation step in this PBP. However, the water molecule is distant from the ring nitrogen of the antibiotic itself—which influences the deacylation step greatly—by as much as 0.5 Å beyond a minimal distance from which the acyl enzyme species could activate it. Therefore, in the case of evolution toward class C β-lactamases, nature had to restructure the active-site surface such that there would not be a physical impediment for the approach of the hydrolytic water to the requisite amine in the acyl enzyme intermediate and Tyr-150 and, eventually after its activation, for its reaction with the acyl carbonyl for promotion of the deacylation reaction.
Among mechanistic enzymologists a salient issue related to β-lactamases has been the apparent lack of symmetry in the catalytic machinery of these enzymes. That is, the catalytic machineries of β-lactamases for the acylation and deacylation steps are distinct (in contrast, for example, to serine proteases). In light of the foregoing discussion, the lack of catalytic symmetry becomes intuitively obvious. The two catalytic steps evolved in response to different selection pressures at different evolutionary time points.
Aside from the acquisition of the hydrolytic step, the nascent β-lactamases had to distance themselves from the reactions which were catalyzed by their parental PBP(s) in order to be most efficient in their function as resistance enzymes. The bacterium would not be served well by a resistance enzyme which would still bind to the peptidoglycan, because it would then not be available as an effective vanguard against the incoming antibiotics. Hence, evolution and selection have incorporated into the sequences of β-lactamases structural elements which disfavor interaction with the peptidoglycan. Consider the case of the bifunctional dd-peptidase–transpeptidase from Streptomyces sp. strain R61. One of the reactions carried out by this enzyme is the critical cross-linking of the peptidoglycan strands in the last step of the cell wall assembly. Figure 6A depicts the crystal structure of this protein. The active site of the enzyme and its surroundings are shown as a Connolly water-accessible surface. The binding site for the first peptidoglycan strand, which acylates the active-site serine (shown by the red arrow), and that for the second strand (shown by the blue arrows), which approaches the first strand for cross-linking by proceeding along a well-defined groove within the active site, are delineated in Fig. 6. In both class A and class C β-lactamases (Fig. 6C and B, respectively) a loop containing portions of helix H10 (30, 35) has been inserted in the location of the binding for the second strand (residues 214 to 224 and 285 to 296 for class A and class C β-lactamases, respectively). Vestigial remnants of the groove may be discerned in this comparative set of stereopictures (Fig. 6B and C), but the binding site for the second strand of the peptidoglycan is effectively obliterated. Furthermore, the divestiture from the parental structure was made complete by the loss of the portions of the structure of the proteins which bind to the first peptidoglycan structure as well. A wall in the active site (at 9 o’clock in Fig. 6A) of the dd-peptidase–transpeptidase defines the binding site for the first peptidoglycan strand. The structures of the class A and C β-lactamases indicate that these β-lactamases have dispensed with this wall entirely (Fig. 6C and B, respectively). This explains the lack of ability of β-lactamases in performing the typical reactions of PBPs, even in vitro. In a comparison of dd-peptidase to the closely related class C β-lactamases, one notes that the β-lactamases have incorporated a loop containing helix H10, which spans Glu-285 to Ile-296. On the other hand, the smaller class A β-lactamases have dispensed with an entire section of the backbone (three strands, B2d, B2e, and B2f, by the nomenclature of Lobkovsky et al. [35]) of the protein by incorporating a loop containing helix H10, which spans from Asp-214 to Ala-224. It is interesting that the directions of the backbone of these insertions in class A and C β-lactamases are opposite one another. This and the earlier discussion on the nature of the inserted segments are indicative of the fact that the immediate precursor PBPs which gave rise to each of these classes of β-lactamases were indeed distinct proteins. Furthermore, nature solved this problem twice by the same strategy at different evolutionary junctures. The examples given in this analysis show how nature has taken the basic conserved protein template (Fig. 3) and elaborated the structure with the powers of mutation and selection with economy as the main bottom line, generating important catalysts which serve such vital functions for the survival of bacteria.
The families of PBPs and β-lactamases have been useful in the study of such evolutionary processes in light of the number of sequences that are known for these proteins, as well as the structural information which is becoming available. It is likely that this type of shared structural template, which gave rise to these distinct functions, is more common in nature, and other examples in the future should shed additional light on the evolution of function in structural biology.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health. I.M. was the recipient of the Rumble and Heller Fellowships.
REFERENCES
- 1.Adachi H, Ohta T, Matsuzawa H. Site-directed mutants, at position 166, of RTEM-1 β-lactamase that form a stable acyl-enzyme intermediate with penicillin. J Biol Chem. 1991;266:3186–3191. [PubMed] [Google Scholar]
- 2.Bulychev A, Massova I, Miyashita K, Mobashery S. Evolution of the versatile β-lactam hydrolase activity: from biosynthetic enzymes to drug resistance factors. J Am Chem Soc. 1997;119:7619–7625. [Google Scholar]
- 3.Bush K, Jacoby G. Nomenclature of TEM β-lactamases. J Antimicrob Chemother. 1997;39:1–3. doi: 10.1093/jac/39.1.1. [DOI] [PubMed] [Google Scholar]
- 4.Bush K, Jacoby G A, Medeiros A A. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob Agents Chemother. 1995;39:1211–1233. doi: 10.1128/aac.39.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bush K, Smith S A, Ohringer S, Tanaka S K, Bonner D P. Improved sensitivity in assays for binding of novel β-lactam antibiotics to penicillin-binding proteins of Escherichia coli. Antimicrob Agents Chemother. 1987;31:1271–1273. doi: 10.1128/aac.31.8.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carfi, A., E. Duee, M. Galleni, J. M. Frère, and O. Dideberg. (1bme Brookhaven Protein Data Bank) Though structures are available, the publications have not appeared in the literature yet.
- 6a.Carfi, A., J. P. Soto, E. Duee, M. Galleni, J. M. Frère, and O. Dideberg. (1bme Brookhaven Protein Data Bank) Though structures are available, the publications have not appeared in the literature yet.
- 7.Carfi A, Pares S, Duee E, Galleni M, Duez C, Frère J M, Dideberg O. The 3-D structure of a zinc metallo-β-lactamase from Bacillus cereus reveals a new type of protein fold. EMBO J. 1995;14:4914. doi: 10.1002/j.1460-2075.1995.tb00174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen C C, Rahil J, Pratt R F, Herzberg O. Structure of a phosphonate-inhibited β-lactamase. An analog of the tetrahedral transition state/intermediate of β-lactam hydrolysis. J Mol Biol. 1993;234:165–178. doi: 10.1006/jmbi.1993.1571. [DOI] [PubMed] [Google Scholar]
- 9.Cohen M L. Epidemiology of drug resistance: implications for a post-antimicrobial era. Science. 1992;257:1050–1055. doi: 10.1126/science.257.5073.1050. [DOI] [PubMed] [Google Scholar]
- 10.Concha N O, Rasmussen B A, Bush K, Herzberg O. Crystal structure of the wide-spectrum binuclear zinc β-lactamase from Bacteroides fragilis. Structure. 1996;4:823–836. doi: 10.1016/s0969-2126(96)00089-5. [DOI] [PubMed] [Google Scholar]
- 11.Davies J. Inactivation of antibiotics and the dissemination of resistance genes. Science. 1994;264:375–382. doi: 10.1126/science.8153624. [DOI] [PubMed] [Google Scholar]
- 12.Dideberg O, Charlier P, Wery J P, Dehottay P, Dusart J, Erpicum T, Frère J M, Ghuysen J M. The crystal structure of the β-lactamase of Streptomyces albus G at 0.3 nm resolution. Biochem J. 1987;245:911–913. doi: 10.1042/bj2450911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dideberg O, Libert M, Frère J M, Charlier P, Zhao H, Knox J R. Crystallization and preliminary X-ray data for the exocellular β-lactamase of Bacillus licheniformis 749/C. J Mol Biol. 1985;181:145–146. doi: 10.1016/0022-2836(85)90333-x. [DOI] [PubMed] [Google Scholar]
- 14.Escobar W A, Tan A K, Fink A L. Site-directed mutagenesis of β-lactamase leading to accumulation of a catalytic intermediate. Biochemistry. 1991;30:10783. doi: 10.1021/bi00108a025. [DOI] [PubMed] [Google Scholar]
- 15.Georgopapadakou N H. Penicillin-binding proteins and bacterial resistance to β-lactams. Antimicrob Agents Chemother. 1993;37:2045–2053. doi: 10.1128/aac.37.10.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghuysen J M. Serine β-lactamases and penicillin-binding proteins. Annu Rev Microbiol. 1991;45:37–67. doi: 10.1146/annurev.mi.45.100191.000345. [DOI] [PubMed] [Google Scholar]
- 17.Ghuysen J M. Molecular structures of penicillin-binding proteins and β-lactamases. Trends Microbiol. 1994;2:372–380. doi: 10.1016/0966-842x(94)90614-9. [DOI] [PubMed] [Google Scholar]
- 18.Ghuysen J M. Penicillin-binding proteins. Wall peptidoglycan assembly and resistance to penicillin: facts, doubts and hopes. Int J Antimicrob Agents. 1997;8:45–60. doi: 10.1016/s0924-8579(96)00358-5. [DOI] [PubMed] [Google Scholar]
- 19.Ghuysen J M, Charlier P, Coyette J, Duez C, Fonzé E, Fraipont C, Goffin C, Joris B, Nguyen-Distèche M. Penicillin and beyond: evolution, protein fold, multimodular polypeptides, and multiprotein complexes. Microb Drug Resist. 1996;2:163–175. doi: 10.1089/mdr.1996.2.163. [DOI] [PubMed] [Google Scholar]
- 20.Grebe T, Hakenbeck R. Penicillin-binding proteins 2b and 2x of Streptococcus pneumoniae are primary resistance determinants for different classes of β-lactam antibiotics. Antimicrob Agents Chemother. 1996;40:829–834. doi: 10.1128/aac.40.4.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hardt K, Joris B, Lepage S, Brasseur R, Lampen J O, Frère J M, Fink A L, Ghuysen J M. The penicillin sensory transducer, BlaR, involved in the inducibility of β-lactamase synthesis in Bacillus licheniformis is embedded in the plasma membrane via a four-α-helix bundle. Mol Microbiol. 1997;23:935–944. doi: 10.1046/j.1365-2958.1997.2761642.x. [DOI] [PubMed] [Google Scholar]
- 22.Hardy L W, Kirsch J F. pH dependence and solvent deuterium oxide kinetic isotope effect on Bacillus cereus β-lactamase I catalyzed reactions. Biochemistry. 1984;23:1275. doi: 10.1021/bi00301a041. [DOI] [PubMed] [Google Scholar]
- 23.Herzberg O. Refined crystal structure of β-lactamase from Staphylococcus aureus PC1 at 2.0 Å resolution. J Mol Biol. 1991;217:701–719. doi: 10.1016/0022-2836(91)90527-d. [DOI] [PubMed] [Google Scholar]
- 24.Holland H D. Geochemistry-evidence for life on Earth more than 3850-million years ago. Science. 1997;275:38–39. doi: 10.1126/science.275.5296.38. [DOI] [PubMed] [Google Scholar]
- 25.Jasmin M, Wilkin J M, Frère J M. Bacterial dd-transpeptidases and penicillin. Essays Biochem. 1995;29:1–24. [PubMed] [Google Scholar]
- 26.Jelsch C, Lenfant F, Masson J M, Samama J P. β-Lactamase TEM1 of E. coli. Crystal structure determination at 2.5 Å resolution. FEBS Lett. 1992;299:135–142. doi: 10.1016/0014-5793(92)80232-6. [DOI] [PubMed] [Google Scholar]
- 27.Jelsch C, Mourey L, Masson J M, Samama J P. Crystal structure of Escherichia coli TEM1 β-lactamase at 1.8 Å resolution. Proteins. 1993;16:364–383. doi: 10.1002/prot.340160406. [DOI] [PubMed] [Google Scholar]
- 28.Kelly J A, Dideberg O, Charlier P, Wery J P, Libert M, Moews P C, Knox J R, Duez C, Fraipont C, Joris B, Dusart J, Frère J M, Ghuysen J M. On the origin of bacterial resistance to penicillin: comparison of a β-lactamase and a penicillin target. Science. 1986;231:1429–1431. doi: 10.1126/science.3082007. [DOI] [PubMed] [Google Scholar]
- 29.Kelly J A, Kuzin A P. The refined crystallographic structure of a dd-peptidase penicillin-target enzyme at 1.6 Å resolution. J Mol Biol. 1995;254:223–236. doi: 10.1006/jmbi.1995.0613. [DOI] [PubMed] [Google Scholar]
- 30.Knox J R, Moews P C. β-Lactamase of Bacillus licheniformis 749/C. Refinement at 2 Å resolution and analysis of hydration. J Mol Biol. 1991;220:435–455. doi: 10.1016/0022-2836(91)90023-y. [DOI] [PubMed] [Google Scholar]
- 31.Knox J R, Moews P C, Frère J M. Molecular evolution of bacterial β-lactam resistance. Chem Biol. 1996;3:937–947. doi: 10.1016/s1074-5521(96)90182-9. [DOI] [PubMed] [Google Scholar]
- 32.Kuwaharaarai K, Kondo N, Hori S, Tatedasuzuki E, Hiramatsu K. Suppression of methicillin resistance in a mecA-containing pre-methicillin-resistant Staphylococcus aureus strain is caused by the mecI-mediated repression of PBP 2′ production. Antimicrob Agents Chemother. 1996;40:2680–2685. doi: 10.1128/aac.40.12.2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuzin A P, Liu H, Kelly J A, Knox J R. Binding of cephalothin and cefotaxime to d-Ala-d-Ala-peptidase reveals a functional basis of a natural mutation in a low-affinity penicillin-binding protein and in extended-spectrum β-lactamases. Biochemistry. 1995;34:9532–9540. doi: 10.1021/bi00029a030. [DOI] [PubMed] [Google Scholar]
- 34.Lobkovsky E, Billings E M, Moews P C, Rahil J, Pratt R F, Knox J R. Crystallographic structure of a phosphonate derivative of the Enterobacter cloacae P99 cephalosporinase: mechanistic interpretation of a β-lactamase transition-state analog. Biochemistry. 1994;33:6762–6772. doi: 10.1021/bi00188a004. [DOI] [PubMed] [Google Scholar]
- 35.Lobkovsky E, Moews P C, Liu H, Zhao H, Frère J M, Knox J R. Evolution of an enzyme activity: crystallographic structure at 2-Å resolution of cephalosporinase from the ampC gene of Enterobacter cloacae P99 and comparison with a class A penicillinase. Proc Natl Acad Sci USA. 1993;90:11257–11261. doi: 10.1073/pnas.90.23.11257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manchester L C, Poeggeler B, Alvares F L, Ogden G B, Reiter R J. Melatonin immunoreactivity in the photosynthetic prokaryote Rhodospirillum rubrum: implications for an ancient antioxidant system. Cell Mol Biol Res. 1995;41:391–395. [PubMed] [Google Scholar]
- 37.Maveyraud L, Massova I, Brick C, Miyashita K, Samama J P, Mobashery S. Crystal-structure of 6α-(hydroxymethyl)penicillanate complexed to the TEM-1 β-lactamase from Escherichia coli: evidence on the mechanism of action of a novel inhibitor designed by a computer-aided process. J Am Chem Soc. 1996;118:7435–7440. [Google Scholar]
- 38.Medeiros, A. A. 1997. Evolution and dissemination of β-lactamases accelerated by generations of β-lactam antibiotics. Clin. Infect. Dis. 24(Suppl.)**:**S19–S45. [DOI] [PubMed]
- 39.Moews P C, Knox J R, Dideberg O, Charlier P, Frère J M. β-Lactamase of Bacillus licheniformis 749/C at 2 Å resolution. Proteins Struct Funct. 1990;7:156–171. doi: 10.1002/prot.340070205. [DOI] [PubMed] [Google Scholar]
- 40.Molecular Simulations Inc. Insight II package. Molecular Simulations, Inc., San Diego, Calif.
- 41.Needleman S B, Wunsch C D. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J Mol Biol. 1970;48:443–453. doi: 10.1016/0022-2836(70)90057-4. [DOI] [PubMed] [Google Scholar]
- 42.Oefner C, D’Arcy A, Daly J J, Gubernator K, Charnas R L, Heinze I, Hubschwerlen C, Winkler F K. Refined crystal structure of β-lactamase from Citrobacter freundii indicates a mechanism for β-lactam hydrolysis. Nature. 1990;343:284–288. doi: 10.1038/343284a0. [DOI] [PubMed] [Google Scholar]
- 43.Pares S, Mouz N, Petillot Y, Hakenbeck R, Dideberg O. X-ray structure of Streptococcus pneumoniae PBP2x, a primary penicillin target enzyme. Nature Struct Biol. 1996;3:284–289. doi: 10.1038/nsb0396-284. [DOI] [PubMed] [Google Scholar]
- 44.Piras G, Raze D, el Kharroubi A, Hastir D, Englebert S, Coyette J, Ghuysen J M. Cloning and sequencing of the low-affinity penicillin-binding protein 3r-encoding gene of Enterococcus hirae S185: modular design and structural organization of the protein. J Bacteriol. 1993;175:2844–2852. doi: 10.1128/jb.175.10.2844-2852.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44a.Samama, J.-P. Unpublished data by personal communication.
- 45.Samraoui B, Sutton B J, Todd R J, Artymiuk P J, Waley S G, Phillips D C. Tertiary structural similarity between a class A β-lactamase and a penicillin-sensitive d-alanyl carboxypeptidase-transpeptidase. Nature. 1986;320:378–380. doi: 10.1038/320378a0. [DOI] [PubMed] [Google Scholar]
- 46.Sirot D, Recule C, Chaibi E B, Bret L, Croize J, Chanal-Claris C, Labia R, Sorot J. A complex mutant of TEM-1 β-lactamase with mutations encountered in both IRT-4 and extended-spectrum TEM-15, produced by an Escherichia coli clinical isolate. Antimicrob Agents Chemother. 1997;41:1322–1325. doi: 10.1128/aac.41.6.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sneath P H A, Sokal R R. Sequential, agglomerative, nonoverlapping clustering methods. In: Kennedy D, Park R B, editors. Numerical taxonomy. 1973. pp. 230–234. The principles and practice of numerical classification. W. H. Freeman & Co., San Francisco, Calif. [Google Scholar]
- 47a.Sonnhammer E L L, Kahn D. The modular arrangement of proteins as inferred from analysis of homology. Protein Sci. 1994;3:482–492. doi: 10.1002/pro.5560030314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sougakoff W, Jarlier V, Delettre J, Colloc’h N, L’Hermite G, Nordmann P, Naas T. Purification, crystallization, and preliminary X-ray diffraction analysis of the carbapenem-hydrolyzing class A β-lactamase Sme-1 from Serratia marcescens. J Struct Biol. 1996;116:313–316. doi: 10.1006/jsbi.1996.0046. [DOI] [PubMed] [Google Scholar]
- 49.Strynadka N C, Adachi H, Jensen S E, Johns K, Sielecki A, Betzel C, Sutoh K, James M N. Molecular structure of the acyl-enzyme intermediate in β-lactam hydrolysis at 1.7 Å resolution. Nature. 1992;359:700–705. doi: 10.1038/359700a0. [DOI] [PubMed] [Google Scholar]
- 50.Taibi-Tronche P, Massova I, Vakulenko S B, Lerner S A, Mobashery S. Evidence for structural elasticity of class-A β-lactamases in the course of catalytic turnover of the novel cephalosporin cefepime. J Am Chem Soc. 1996;118:7441–7448. [Google Scholar]
- 51.Thunnissen A M, Dijkstra B W. Cure for a crisis? Nature Struct Biol. 1996;3:218–221. doi: 10.1038/nsb0396-218. [DOI] [PubMed] [Google Scholar]
- 52.Tripos, Inc. Sybyl molecular modeling software. Tripos, Inc., St. Louis, Mo.
- 53.World Health Organization. The world health report 1996. Fighting disease, fostering development. Geneva, Switzerland: World Health Organization; 1996. [PubMed] [Google Scholar]
- 54.Zorzi W, Zhou X Y, Dardenne O, Lamotte J, Raze D, Pierre J, Gutmann L, Coyette J. Structure of the low-affinity penicillin-binding protein 5 PBP5fm in wild-type and highly penicillin-resistant strains of Enterococcus faecium. J Bacteriol. 1996;178:4948–4957. doi: 10.1128/jb.178.16.4948-4957.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]