Cytochrome bd Oxidase, Oxidative Stress, and Dioxygen Tolerance of the Strictly Anaerobic Bacterium Moorella thermoacetica (original) (raw)
Abstract
The gram-positive, thermophilic, acetogenic bacterium Moorella thermoacetica can reduce CO2 to acetate via the Wood-Ljungdahl (acetyl coenzyme A synthesis) pathway. This report demonstrates that, despite its classification as a strict anaerobe, M. thermoacetica contains a membrane-bound cytochrome bd oxidase that can catalyze reduction of low levels of dioxygen. Whole-cell suspensions of M. thermoacetica had significant endogenous O2 uptake activity, and this activity was increased in the presence of methanol or CO, which are substrates in the Wood-Ljungdahl pathway. Cyanide and azide strongly (∼70%) inhibited both the endogenous and CO/methanol-dependent O2 uptake. UV-visible light absorption and electron paramagnetic resonance spectra of _n_-dodecyl-β-maltoside extracts of M. thermoacetica membranes showed the presence of a cytochrome bd oxidase complex containing cytochrome _b_561, cytochrome _b_595, and cytochrome d (chlorin). Subunits I and II of the bd oxidase were identified by N-terminal amino acid sequencing. The M. thermoacetica cytochrome bd oxidase exhibited cyanide-sensitive quinol oxidase activity. The M. thermoacetica cytochrome bd (cyd) operon consists of four genes, encoding subunits I and II along with two ABC-type transporter proteins, homologs of which in other bacteria are required for assembly of the bd complex. The level of this cyd operon transcript was significantly increased when M. thermoacetica was grown in the absence of added reducing agent (cysteine + H2S). Expression of a 35-kDa cytosolic protein, identified as a cysteine synthase (CysK), was also induced by the nonreducing growth conditions. The combined evidence indicates that cytochrome bd oxidase and cysteine synthase protect against oxidative stress and contribute to the limited dioxygen tolerance of M. thermoacetica.
Dioxygen is the preferred electron sink in the respiratory chain of most aerobic bacteria (58). Cytochrome oxidases are the membrane-bound, terminal components of the dioxygen-dependent respiratory chain, which reduces dioxygen to water with formation of pH and potential gradients (6, 45). Two main types of respiratory cytochrome oxidases are known in bacteria: the heme/copper oxidases (cytochrome _aa_3, cytochrome _c_aa3 oxidase, and cytochrome bo oxidase), and the heme-only cytochrome bd quinol oxidase, which is associated with microaerobic dioxygen respiration (24, 56, 59, 62). Cytochrome bd oxidases purified from aerobic bacteria have been characterized as 1:1 heterodimers of two integral membrane proteins referred to as subunits I and II (35, 55). The heterodimers contain three heme components: a low-spin heme, _b_558, and two high-spin hemes, _b_595 and d. In the genomes of Escherichia coli (31) and Azotobacter vinelandii (41), subunits I and II are encoded by cydA and cydB, respectively, of the cytochrome bd (cyd) operon. Two ABC-type transporter proteins, CydD and CydC, are typically required for assembly of the cytochrome bd complex in both gram-positive and gram-negative aerobic bacteria (14, 60). Aerobic respiration not only conserves energy but also generates reactive oxygen species, such as superoxide (O2−), hydrogen peroxide (H2O2), and hydroxyl radical, which are known to damage membrane lipids, proteins, DNA, and iron-sulfur clusters (3, 4, 28, 34, 43).
Strictly anaerobic bacteria would seem to have no need of respiratory cytochrome oxidases. Nevertheless, functional cytochrome _bd_-type oxidases have recently been discovered in bacteria classified as strict anaerobes: e.g., Bacteroides fragilis (2) and Desulfovibrio gigas (50). The gram-positive, thermophilic bacterium Moorella thermoacetica (formerly Clostridium thermoaceticum) is an obligately anaerobic acetogen that can grow autotrophically on CO2/H2 or CO via the Wood-Ljungdahl (acetyl coenzyme A [CoA]) pathway (21). This pathway conserves energy via chemiosmosis from a membrane-bound electron transport chain consisting of two _b_-type cytochromes (_b_559 and _b_554), and a menaquinone, MK7 (16, 19). Although classified as strict anaerobes, several acetogenic bacteria, including M. thermoacetica, have been reported to tolerate traces of dioxygen (7, 8, 39, 48). Karnholz et al. showed that M. thermoacetica can survive exposure to and consume low levels (0.5 to 2%) of headspace dioxygen in liquid culture and also reported NADH oxidase activity in cell extracts (39). Subsequently, we discovered a five-gene cluster in M. thermoacetica encoding oxidative and nitrosative stress protection proteins (20, 66). During the course of these studies, we found that the consumption of dioxygen by M. thermoacetica is inhibited by cyanide, a strong inhibitor of cytochrome oxidases. Accordingly, we have identified a cytochrome _bd_-type oxidase exhibiting menaquinol oxidase activity in membranes of M. thermoacetica. Subsequent genome sequence data and Northern blotting confirmed the presence of a cytochrome bd oxidase operon in M. thermoacetica.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
M. thermoacetica strain ATCC 39073 was grown on 200 mM methanol as the carbon source at 58°C under 100% CO2 gas in semidefined Drake's minimal medium (65) in 125-ml bottles, 4-liter flasks, or a 100-liter fermentor in either the presence or absence of reducing agent (Na2S, 0.5 g/liter; cysteine HCl, 0.5 g/liter) as previously described (18, 20). E. coli strain DH5α was grown on glucose (20 mM) at 37°C in 4-liter flasks in MZ9 salt medium (17, 20). Cultures were harvested at mid- to late log phase (optical density at 600 nm [OD600] of ∼1.0) by centrifugation at 6,000 × g and stored at −80°C until used. Cells used in O2 uptake experiments were harvested under anoxic conditions as described below.
Membrane preparation.
Membranes of M. thermoacetica were prepared from whole cells by a combination of lysozyme and French press methods, and E. coli membranes were prepared after breaking cells with a French press as described previously (17).
Membrane extracts and partial purification of cytochrome bd oxidase.
Crude membranes were washed in 50 mM Tris-HCl, pH 8.0, and then suspended (1.0 mg/ml) in 10 mM Tris (Tris)-HCl, pH 8.0, 2 mM EDTA, and 1 mM phenylmethylsulfonyl fluoride (PMSF) and incubated at 4°C overnight to remove F1-ATPase, which otherwise coextracted with cytochrome bd oxidase. Solubilization and partial purification of cytochrome bd oxidase from washed membranes were carried out according to Junemann and Wrigglesworth (36) with some modifications. F1-ATPase-depleted membranes were suspended in buffer A (potassium phosphate, pH 7.5, 1 mM EDTA, pH 8.0, 1 mM PMSF) to a protein concentration of 5 mg/ml. Sodium cholate was added to a final concentration of 1.5% (wt/vol), and the suspension was stirred at 4°C overnight. After centrifugation at 100,000 × g (1 h), the supernatant, containing loosely bound membrane protein and a small amount of cytochrome bd oxidase, was discarded. The pellet containing the majority of the cytochrome bd oxidase was suspended in buffer A at a protein concentration of 5 mg/ml, and _n_-dodecyl-β-maltoside (DM) (Anatrace, Maumee, Ohio) was added to a final concentration of 1% (wt/vol). The suspension was stirred at 4°C for 2 h and then centrifuged as described above. The greenish-yellow supernatant containing cytochrome bd oxidase was stored at −80°C until further use. This fraction, referred to as DM extracts, was concentrated, if necessary, by Amicon ultrafiltration and used in all spectroscopic studies. To further purify the enzyme, DM extracts (15 to 20 ml and 4 to 5 mg of protein per ml) were dialyzed against 1 liter of buffer A without EDTA but containing 0.02% DM and then loaded onto a 5-ml hydroxyapatite column (CHT column; Bio-Rad, Hercules, Calif.). The column was washed with the same buffer. Bound cytochrome bd oxidase was eluted from the column first with 0.4 M and then with 0.8 M potassium phosphate containing 0.02% DM. Most of the cytochrome bd oxidase was eluted by 0.8 M potassium phosphate, as confirmed by spectroscopic analysis.
DNA and RNA sources, PCR, and Northern hybridization.
M. thermoacetica genomic DNA was isolated with the Puregene DNA purification system (Gentra, Minneapolis, Minn.). Total RNA was isolated with the RNeasy mini kit (QIAGEN, Inc., Valencia, Calif.). Prior to gel electrophoresis and hybridization experiments, total RNA was treated with RNase-free DNase I (Roche Applied Sciences, Indianapolis, Ind.). For dot blot hybridization experiments RNA was denatured with formaldehyde (15% [vol/vol]) in 5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) at 60°C for 1 h prior to application onto nylon membranes (ICN, Costa Mesa, Calif.). Hybridization (Northern or dot blot) experiments were carried out with the Genius system (Roche Applied Sciences) as described previously (15, 20). Digoxigenin (DIG)-labeled PCR products used as probes in hybridization experiments were amplified with M. thermoacetica genomic DNA by using the FailSafe PCR system (EPICENTRE, Madison, Wis.). The following PCR products were used as probes for hybridization experiments: a 336-bp fragment belonging to cydA amplified with 5′-GATTTCTTCGCCCTACTA-3′ (forward primer; Fp) and 5′-TGTTTTGACTGGTTTTCCT-3′ (reverse primer; Rp); a 320-bp fragment belonging to the β-subunit-gene of the CODH operon (accession no. M62727) amplified with 5′-ATTATAGCGTCAAGGACGA-3′ (Fp) and 5′-ACAGGGTCGTTATCCATA-3′ (Rp); a 355-bp fragment belonging to cysK (cysteine synthase K) encoding a 35-kDa protein (see Results); and a 390-bp fragment belonging to the β-subunit gene (atpD) of the F1-ATP synthase operon amplified with primers as described previously (15).
Native PAGE and heme staining.
Native polyacrylamide gel electrophoresis (PAGE) (without sodium dodecyl sulfate [SDS]) of DM extracts of membranes was carried out according to Laemmli (49) in the presence of 0.1% DM in the resolving gel. The gels were stained for heme with 3,3′,5,5′-tetramethylbenzidine (TMBZ) according to the method described by Thomas et al. (70). After staining for heme, the same gel was destained and then restained for the detection of protein with Coomassie brilliant blue. The Coomassie-stained protein band corresponding to the heme complex was excised from the native gel and subjected to SDS-PAGE (64).
Antibodies and Western blotting.
Polyclonal antibodies against a peptide designed from the highly conserved carboxyl-terminal amino acid sequence (Q302-V318) of E. coli CydD (14) were a gift from Robert K. Poole of the University of Sheffield, Sheffield, United Kingdom. Western blotting experiments were carried out according to Bio-Rad (17).
N-terminal peptide sequences.
Partially purified M. thermoacetica cytochrome bd oxidase was subjected to SDS-PAGE and then trans-blotted onto polyvinylidene difluoride (PVDF) membranes (Bio-Rad). Resolved proteins were briefly stained with Coomassie brilliant blue and then excised from the blots. The N-terminal amino acid sequences were determined either in the Integrated Biotechnology Laboratories at the University of Georgia or in the Microchemical and Proteomics Facility at the Emory University School of Medicine.
Spectroscopic measurements.
UV-visible absorption spectra were recorded with a dual-wavelength Shimadzu, Inc., model 2051PC spectrophotometer. Cytochromes were reduced chemically with a few grains of sodium dithionite or enzymatically with reduced duroquinone (DQH2) or reduced ubibiquinone-1 (QH2). Quinols were prepared from duroquinone or ubiquinone-1 (from Sigma Chemical Co.) by reduction with dithionite as described previously (63). The reductions of cytochromes by quinols were carried out anoxically in a 1-cm-path-length quartz cuvette, under 100% argon. Electron paramagnetic resonance (EPR) spectra were recorded with a Bruker ESP-300E spectrometer equipped with an ER-4116 dual-mode cavity and an Oxford Instruments ESR-flow cryostat (20) under conditions given in the figure legend.
Assays and activity measurements.
All operations using whole cells were carried out under anoxic conditions in a Coy chamber (Vacuum Atmospheres Company, Hawthorne, Calif.) containing an atmosphere of 5% H2, 10% CO2, and 85% N2 by volume; those using membranes or DM extracts were carried out under oxic (atmospheric dioxygen) conditions. For O2 uptake measurements, washed membranes or cells were suspended to 50 to 100 μg of protein/ml in 2 ml of 50 mM morpholinepropanesulfonic acid (MOPS; pH 7.0) for membranes and 20 mM potassium phosphate pH 7.0 (sparged with N2 gas) for cell suspensions. Dioxygen uptake experiments were carried out at room temperature (∼25°C) in the Coy anoxic chamber, using a Clarke-type oxygen electrode calibrated with air-saturated and N2-saturated buffer as described previously (39). The initial dioxygen concentrations used for the measurement of O2 uptake by whole-cell suspensions were adjusted to 15 to 20 μM by injecting appropriate volumes of air into the headspace of the culture bottles. Anoxic stock solutions of all substrates and inhibitors were prepared under 100% N2 gas prior to injection into assay mixtures. Quinol oxidase activity of cytochrome bd oxidase was determined in 100 mM potassium phosphate buffer (pH 7.0) by measuring O2 uptake according to Junemann and Wrigglesworth (36). The reaction mixture (2.0 ml) contained 100 μM quinols as substrate (DQH2 or QH2 prepared as described above). The reaction was initiated by adding 50 to 100 μg of protein from DM extracts or partially purified cytochrome bd oxidase that had been preincubated on ice for 5 min with 1 mM phospholipids, l-α-phosphatidylcholine type XV1-E (Sigma). The consumption of dioxygen was recorded at room temperature with the oxygen electrode as described above. For inhibition studies, cyanide was added to the reaction mixture along with the substrate (quinol) prior to the addition of the protein.
M. thermoacetica genome sequence.
A draft annotated nucleotide sequence of the M. thermoacetica (ATCC 39073) genome has been completed at the Joint Genome Institute, Department of Energy, and can be viewed at http://genome.jgi-psf.org/draft_microbes/mooth/mooth.draft.html.
RESULTS
The M. thermoacetica cytochrome bd oxidase operon.
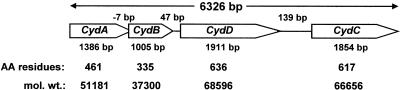
Although we had isolated the M. thermoacetica cytochrome bd oxidase prior to any knowledge of the genome sequence, the results are more logically presented by first establishing the existence and transcription of a cytochrome bd oxidase (cyd) operon. Figure 1 diagrams the composition of the 6,326-bp four-gene M. thermoacetica cyd operon, annotated as cydABCD (genes 1660 to 1663 on contig 300 of the M. thermoacetica genome sequence). The deduced amino acid sequences of the two genes annotated as cydA and cydB are homologous to those of CydA (subunit I) and CydB (subunit II), respectively, of the cytochrome _bd_-type quinol oxidases from E. coli (43 and 40% identities, respectively) and B. subtilis (53 and 46% identities, respectively). A hydropathy plot of the predicted amino acid sequence of M. thermoacetica CydA (not shown) indicates nine membrane-spanning regions, the locations of which are very similar to those of CydA from E. coli (77), with the exception of some minor differences at the carboxyl-terminal end, due mostly to the absence of 56 residues from the M. thermoacetica sequence (Gly-322 to Met-377 of E. coli CydA) (cf. Fig. 2); these residues are also missing in CydAs of other gram-positive bacteria (e.g., Bacillus subtilis; NCBI accession no. A69611). Three of the four axial ligands to the irons of the three hemes have been identified as His-19, His-186, and Met-393 of CydA (subunit I) in the E. coli cytochrome bd oxidase (25, 40, 68, 69). The corresponding residues are conserved as His-18, His-183, and Met-333 in M. thermoacetica CydA. A highly conserved sequence, GWXXXEXGRQPW, near the quinol-binding site of E. coli CydA (76) is also conserved in M. thermoacetica CydA, except that Q is replaced by Y. Hydropathy plots of the E. coli and M. thermoacetica CydB amino acid sequences (data not shown) are also very similar to each other.
FIG. 1.
Composition of the M. thermoacetica cytochrome bd oxidase operon (cydABDC) and the relationship between the genes and their products. The direction of transcription is from left to right. AA, amino acid; mol. wt., molecular weight.
FIG. 2.
Alignment of the deduced amino acid sequences at the COOH-terminal end of CydAs from M. thermoacetica (MtCydA), B. subtilis (BsCydA; accession no. A69611), M. tuberculosis (MyCydA; accession no. E70558), E. coli (EcCydA; accession no. S17958), A. vinelandii (AvCydA; accession no. A38170), and V. cholerae (VcCydA; accession no. E82149).
The amino acid sequences of the proteins encoded by cydD and cydC of the M. thermoacetica cyd operon are homologous to ABC-type membrane transporters, CydD and CydC, respectively, from E. coli (35.8 and 36.2% identities, respectively) and B. subtilis (32.4 and 32.4% identities, respectively). Both CydD and CydC are required for the biosynthesis and assembly of the cytochrome bd complex in E. coli (60). Four-gene cydABDC operons similar to that of M. thermoacetica are found in Mycobacterium tuberculosis (13), Lactobacillus lactis (NCBI accession no. NC_002662), B. subtilis (75), and Listeria innocua (NCBI accession no. NC_003212), whereas cydD and cydC are found in separate loci from cydAB in the E. coli and A. vinelandii genomes (31, 41).
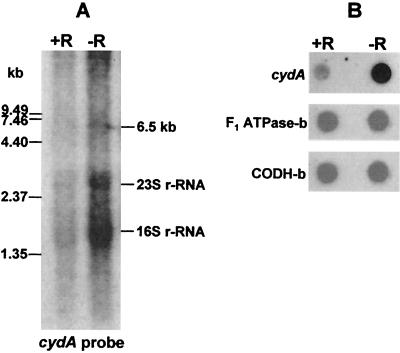
The structural genes encoding subunit I (CydA) and subunit II (CydB) of M. thermoacetica cytochrome bd oxidase overlap by 7 bp (cf. Fig. 1), a feature often referred to as translational coupling of functionally related genes (47). A putative promoter sequence, 5′-CTTGAAA-N23-TATACT-3′, resembling the E. coli consensus σ70 promoter (5′-cCTTGACa-N15-21-TATAaT-3′; lowercase type indicates nucleotides that are not strictly conserved) (33) is present 46 bp upstream of the cydA start codon. No secondary promoter was apparent in the two intergenic regions, between cydB and cydD or between cydD and cydC, suggesting that the M. thermoacetica cyd operon is transcribed as a single polycistronic message. To verify this presumption, total RNA from M. thermoacetica was subjected to Northern hybridization and probed with a 336-bp DIG-labeled DNA fragment amplified from cydA. Northern blots containing RNA from cells grown in nonreducing medium (i.e., omitting addition of cysteine and H2S to the growth medium) showed a weak signal corresponding to an approximate size of 6.5 kb (lane −R, Fig. 3A). The 6.5-kb band is close to the predicted size of the M. thermoacetica cyd operon, 6,326 kb. A smaller-size smear starting at ∼3.5 kb indicates degradation of the cyd transcripts. In addition to _cyd_-specific transcripts, the cydA probe also hybridized nonspecifically to 16S and 23S rRNA; this nonspecific hybridization may have been aided by the overlaps of the rRNA bands with the degraded cydA transcripts. These features, including degradative smearing and nonspecific hybridization to rRNA, were also observed on Northern blots of B. subtilis cyd transcripts (75). A similar hybridization signal was obtained in Northern blots of M. thermoacetica RNA probed with a PCR-amplified, DIG-labeled DNA fragment from cydC (data not shown), indicating that all four genes of the M. thermoacetica cyd operon are transcribed into a single polycistronic transcript, also as reported for B. subtilis (75). The Northern hybridization intensities corresponding to cyd transcripts were relatively stronger with RNA from cells grown in nonreducing (−R) than in reducing (+R) medium. Consistent with the Northern blot results, RNA dot blots (Fig. 3B) probed with the cydA fragment showed higher intensities from cells grown in nonreducing medium, whereas dot blot s probed for two “housekeeping” genes, encoding F1-ATP synthase and CO dehydrogenase, each exhibited comparable +R and −R hybridization intensities.
FIG. 3.
(A) Northern blot of total RNA (5 μg in each lane) isolated from M. thermoacetica grown on methanol in the presence (+R) or absence (−R) of reducing agent after hybridization with a DIG-labeled DNA fragments amplified from cydA (A) of the M. thermoacetica cyd operon. The positions of RNA size standards are indicated on the left. The probe also hybridized nonspecifically to 23S (2.928 kb) and 16S (1.553 kb) rRNA. (B) RNA dot blots of M. thermoacetica total RNA (each spot loaded with 5 μg of DNase-treated RNA) after hybridization with the DIG-labeled DNA fragment amplified from cydA, the F1-ATPase β-subunit gene (atpD), and the CO dehydrogenase β-subunit gene.
Heme staining and identification of M. thermoacetica cytochrome bd oxidase subunits.
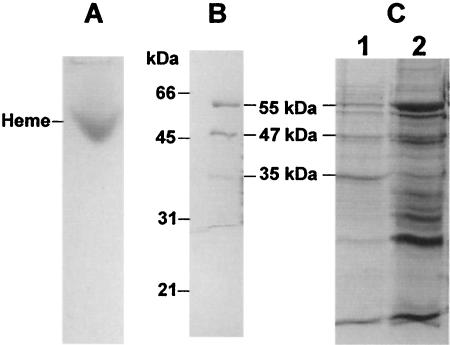
DM extracts of M. thermoacetica membranes were subjected to native PAGE and heme staining. A single heme-positive band was found (Fig. 4A), which was resolved by SDS-PAGE into three major protein bands with approximate molecular masses of 35, 47, and 55 kDa (Fig. 4B). These sizes matched closely those of three of the four proteins from a partially purified cytochrome bd complex (Fig. 4C). The N-terminal amino acid sequences of the three proteins excised from the heme-stained gel were 35 kDa, MDL(I)XILWFILV; 47 kDa, XGQQKSGAEQ; and 55 kDa, A/MDAL/GLLARWQ(G)F (where “X” denotes an unidentified residue and the letter in parentheses indicates a tentatively assigned residue). The experimentally determined N-terminal amino acid sequences of the 35- and the 55-kDa proteins matched the predicted N-terminal sequences of the cydA and cydB gene products, respectively, of the M. thermoacetica cyd operon (cf. Fig. 1). The N-terminal sequence of the 47-kDa protein matched that annotated as a branched-amino acid ABC transporter, the substrate-binding protein in the M. thermoacetica genome. This 47-kDa protein is not part of the cyd operon (cf. Fig. 1), and the role that this copurifying protein may play in M. thermoacetica cytochrome bd oxidase was not further investigated.
FIG. 4.
PAGE and SDS-PAGE of M. thermoacetica cytochrome bd oxidase. (A) Native PAGE (8% acrylamide in resolving gel) of DM extracts (40 μg of protein) after heme staining with TMBZ. (B) SDS-PAGE of the heme-positive band excised from the native gel shown in panel A after staining with Coomassie brilliant blue. (C) SDS-PAGE of DM extracts (lane 2, 40 μg of protein) and partially purified cytochrome bd oxidase (lane 1, 20 μg of protein).
In order to verify CydD expression, DM extracts from M. thermoacetica cells grown in the absence of reducing agent were subjected to Western blotting with antibodies against a synthetic peptide, 302QPLRDLGTFYHAKAQAV318, designed from the carboxyl-terminal end of E. coli CydD (14). The amino acid sequence of this peptide shares 53% identical residues with the corresponding region, 288LPLRLLGSRYHAGLAGV304, of the M. thermoacetica CydD. The E. coli CydD peptide antibodies were found to react with a single protein of molar mass ∼65 kDa in M. thermoacetica DM extracts (not shown). This mass is close to that predicted (68,596 Da, cf. Fig. 1) from the deduced amino acid sequence of M. thermoacetica cydD.
Spectroscopic analysis of M. thermoacetica cytochrome bd oxidase.
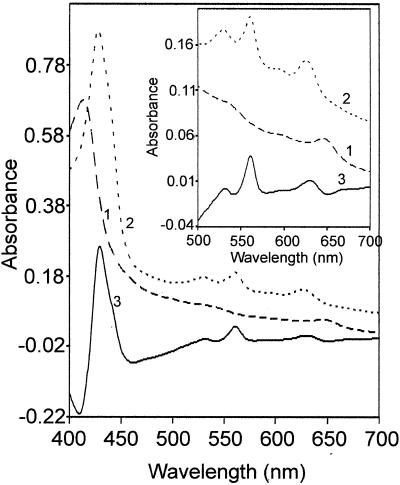
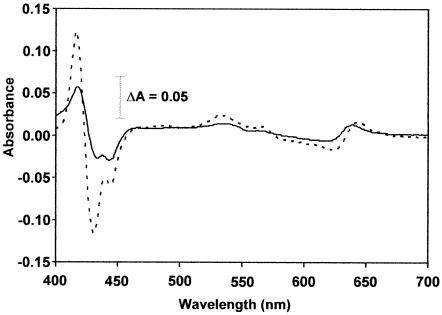
Figure 5 shows UV-visible absorption spectra of DM extracts of M. thermoacetica membranes, both air oxidized (as-isolated) and dithionite reduced, as well as the dithionite-reduced − air-oxidized difference spectrum. The main features of these spectra included absorption peaks at 649 and 411 nm of oxidized samples; shifting of these peaks to 630 and 431 nm, respectively, upon reduction by dithionite; and the appearance of a prominent peak at 561 nm in dithionite-reduced samples. These spectra are very similar to corresponding spectra of purified cytochrome bd oxidases from several other bacteria (30, 36, 55). The absorption peaks at 561 and 630 nm from dithionite-reduced membranes correspond to heme _b_561 and heme d (chlorin), respectively, of cytochrome bd oxidase (36, 46). The reduced − oxidized absorption difference spectra of cytochrome bd oxidases from E. coli and A. vinelandii exhibit an absorption peak at 595 nm, corresponding to the third heme, _b_595, of the enzyme complex (36, 46); the 595-nm absorption was rather weak in the difference spectrum of M. thermoacetica DM membrane extracts.
FIG. 5.
UV-visible absorption spectra of M. thermoacetica cytochrome bd oxidase. The spectra were recorded on DM extracts (4.5 mg of protein/ml) of cholate-washed M. thermoacetica membranes in 0.1 M potassium phosphate (pH 7.5) and 1 mM EDTA. Trace 1 is air oxidized, trace 2 is dithionite reduced, and trace 3 is dithionite reduced minus air oxidized.
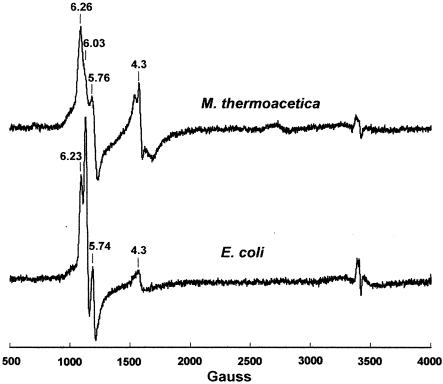
EPR spectra can also identify these cytochromes, and Fig. 6 shows that the EPR spectra of DM extracts from M. thermoacetica and E. coli are very similar to each other. The axial signal component at g = 6.03 (better resolved in the E. coli spectrum) corresponds to the high-spin ferric heme d (chlorin) and the rhombic signal components at g = 6.26 and 5.76 in the M. thermoacetica spectrum (or 6.23 and 5.74 in the E. coli spectrum) correspond to the high-spin ferric heme _b_595 (36, 53). The additional signals at g = 4.3 have been assigned to nonspecifically bound iron (54).
FIG. 6.
EPR spectra of as-isolated cytochrome bd oxidases from M. thermoacetica and E. coli. DM extracts of M. thermoacetica or E. coli membranes (20 mg of protein/ml) in 50 mM Tris-HCl (pH 8.0) and 100 mM EDTA. EPR conditions were as follows: temperature, 4.0 K; microwave frequency, 9.602 GHz; microwave power, 2 mW; and modulation amplitude, 6.34 G.
For simplicity, these DM extracts are, hereafter, referred to as cytochrome bd oxidase. Figure 7 compares the UV-visible absorption spectral changes of reduced M. thermoacetica versus E. coli cytochrome bd oxidases after treatment with CO. CO binds to reduced cytochrome d and induces characteristic spectral changes (30, 36, 38). The dithionite-reduced + CO − dithionite-reduced difference absorption spectrum of M. thermoacetica cytochrome bd oxidase revealed peaks at 417, 534, and 639 nm and troughs at 433, 443, and 629 nm, which closely resemble peaks at 417, 540, and 642 nm and troughs at 432, 445, and 624 nm in the spectrum of E. coli cytochrome bd oxidase. Similar spectra have been obtained for CO-treated cytochrome bd oxidases from other bacteria (30, 36, 38, 42).
FIG. 7.
Dithionite-reduced + CO − dithionite-reduced UV-visible absorption difference spectra of cytochrome bd oxidase in DM extracts (5.5 mg of protein/ml) of cholate-washed membranes from M. thermoacetica (solid trace) or E. coli (dashed trace).
Dioxygen uptake by whole cells and membranes.
Several bacteria classified as strict anaerobes, including some acetogens, have been shown to tolerate and consume dioxygen (7, 8, 10, 11, 26, 27). M. thermoacetica cell suspensions showed significant consumption of O2 in the absence of any exogenous electron donor (see footnote to Table 1), as has been observed in many other bacteria, including acetogens (7, 10, 26, 27). The rates of endogenous dioxygen consumption by M. thermoacetica cell suspensions grown in the presence (14.2 ± 3.6 nmol · min−1 · mg of protein−1) or absence (29.8 ± 5.7 nmol · min−1 · mg protein−1) of reducing agent were similar to those reported for other acetogenic bacteria (6). Exogenous methanol and CO, which are substrates in the Wood-Ljungdahl pathway, supported an increased O2 uptake rate (Table 1), but these rates were much lower than those reported for other acetogens, e.g., Sporomusa termitida, Acetonema longum, or Acetobacterium woodii (7). These differences could be due to the wide variation of their origin and other physiological parameters (21). Both the endogenous and substrate-dependent O2 uptake rates of M. thermoacetica cell suspensions were significantly higher for cells grown in the absence of reducing agent than those grown in its presence. Cyanide (1 mM) and azide (2 mM) inhibited both endogenous and substrate-dependent O2 uptake up to 70% in whole-cell suspensions, indicating that most of the endogenous and exogenous substrate-dependent consumption of dioxygen follows an azide- and cyanide-sensitive pathway. Cyanide inhibition of O2 consumption by whole cells has been reported in other bacteria, including acetogens (7, 37, 61).
TABLE 1.
Methanol- and CO-dependent O2 uptake rates by M. thermoacetica cell suspensionsa
| Reducing agentb | Uptake rate (nmol · min−1 · mg of protein−1) | |||||
|---|---|---|---|---|---|---|
| CO (saturated) | Methanol (200 mM) | |||||
| None | Azide | Cyanide | None | Azide | Cyanide | |
| +Red | 16.7 | 5.7 | 5.4 | 24.4 | 7.9 | 7.3 |
| −Red | 28.8 | 9.1 | 8.6 | 40.0 | 13.0 | 11.9 |
NADH oxidase activity has been reported in cell extracts of several acetogens, including M. thermoacetica (39, 48), and Ivey (35) had reported NADH dehydrogenase activity and NADH-dependent cytochrome reduction in membranes of M. thermoacetica. The M. thermoacetica genome also encodes a putative NADH dehydrogenase complex. Accordingly, we found NADH-driven O2 uptake activities in M. thermoacetica membranes: 7 nmol · min−1 · mg of protein−1 from cells grown in the presence of reducing agent and 13 nmol · min−1 · mg of protein−1 from cells grown in the absence of reducing agent. These membrane O2 uptake activities were 50 to 55% inhibited by rotenone (60 μM), HQNO (50 μM), and cyanide (1 mM) and 37 to 40% inhibited by antimycin (5 μM), indicating that NADH-driven O2 uptake by M. thermoacetica is coupled to a membrane electron transport chain.
Quinol oxidase activity.
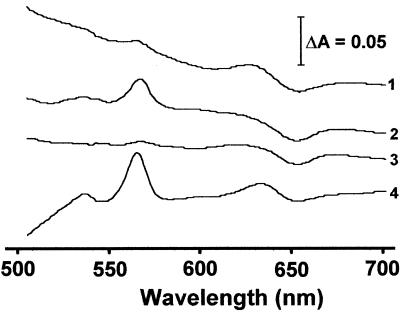
Quinols are the typical physiological electron donor for cytochrome bd oxidases. As shown in Fig. 8, traces 1 and 2, DQH2 was able to reduce cytochrome d (detected as increased absorbance at 630 nm) in untreated M. thermoacetica cytochrome bd oxidase preparations but failed to do so in cyanide-pretreated samples (lower absorbance at 630 nm), consistent with the cyanide inhibition of O2 uptake by whole cells and membranes presented above. Similar spectral changes were found with reduced ubiquinone-1 in place of DQH2 (not shown). Comparison of the absorption peaks of _b_561 reduced by dithionite and quinols in Fig. 8 indicate incomplete reduction of cytochrome _b_561 by quinols. On the other hand, the levels of reduction of cytochrome d (absorption at 629 nm) by dithionite and quinol were comparable. These results suggest preferential reduction of M. thermoacetica cytochrome d over cytochrome _b_561 by quinols. Partial reduction of cytochrome _b_561 by quinols could be due to excess of the latter in the reaction mixture. Two _b_-type cytochromes, _b_554 and _b_559, have previously been characterized from M. thermoacetica (16, 19). The M. thermoacetica genome also encodes two recognizable _b_-type cytochromes, _b_556 and _b_561. Cytochrome _b_556 (encoded by gene 2271 of contig 306) belongs to a putative operon annotated as anaerobic-type dimethyl sulfoxide (DMSO) reductase, while cytochrome _b_561, encoded by cydA, belongs to the cyd operon (cf. Fig. 1). The previously described cytochrome _b_554 thus apparently belongs to the DMSO reductase complex, while the cytochrome _b_559 should correspond to cytochrome _b_561 of cytochrome bd oxidase. We had shown previously that destruction of menaquinone (by UV irradiation) in M. thermoacetica membranes had little effect on the reduction of cytochrome _b_559 (equivalent to _b_561 in the present study) by CO (a physiological electron donor) and that the addition of oxidized menaquinone rapidly oxidized the reduced cytochrome _b_559 (19). In the E. coli cytochrome bd oxidase, the reduction of cytochrome d is coupled to _b_561, which mediates transfer of electrons between quinol and cytochrome d (32). In M. thermoacetica membranes, the present results suggest that either electrons are transferred directly from quinols to cytochrome d, bypassing cytochrome _b_561, or cytochrome d rapidly reoxidizes the quinol-reduced _b_561.
FIG. 8.
UV-visible absorption difference spectra of M. thermoacetica cytochrome bd oxidase in DM extracts (6 mg of protein/ml). Traces: 1, DQH2 reduced − air oxidized; 2, air oxidized + cyanide + DQH2 − air oxidized + cyanide; 3, air oxidized + cyanide − air oxidized; 4, dithionite reduced − air oxidized.
Quinol oxidase activity of M. thermoacetica cytochrome bd oxidase was determined by measuring O2 uptake by membranes, DM extracts, and an enriched cytochrome bd oxidase fraction partially purified from a hydroxyapatite column with DQH2 or QH2 as the electron donor. The data in Table 2 show that the rates of O2 uptake by membranes, DM extracts, and the partially purified cytochrome bd oxidase were 31, 25, and 37% higher, respectively, with DQH2 than with QH2. Significantly higher O2 uptake activity of cytochrome bd oxidase with DQH2 than QH2 as a substrate was also reported for the D. gigas cytochrome bd oxidase (50). The quinol-dependent O2 uptake in all three preparations was strongly inhibited (up to 75%) by cyanide (1 mM), as also reported in other bacteria (30, 38, 46).
TABLE 2.
Quinol-dependent O2 uptake rates by M. thermoacetica cytochrome bd oxidase preparationsa
| Preparation | Uptake rate (nmol · min−1 · mg of protein−1) | |||
|---|---|---|---|---|
| Duroquinolb | Ubiquinol-1b | |||
| None | CNc | None | CN−c | |
| Membranes | 7.2 | 2.2 | 5.5 | 1.4 |
| DM extracts | 15.0 | 3.7 | 12.0 | 3.0 |
| Cytochrome bd oxidased | 25.0 | 3.7 | 18.2 | 2.8 |
Oxidative stress protection by cytochrome bd oxidase and cysteine.
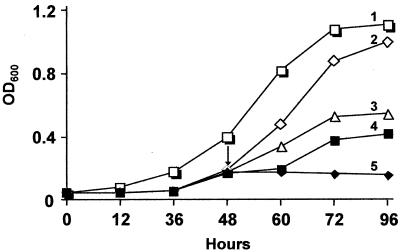
Growth of M. thermoacetica in the absence or presence of cysteine, dioxygen, and azide (a cytochrome bd oxidase inhibitor) was investigated. Cultures were grown in anoxic bottles with the indicator dye resazurin (0.001% [wt/vol]), which turns pink in the presence of traces of dioxygen. Figure 9 shows that the maximum growth (OD600) of the cultures nearly doubled in the presence compared to the absence of reducing agent (Fig. 9). After 48 h of growth, air was injected into the headspace of the culture bottles so as to reach an initial dioxygen concentration of 1.5 vol% (unreduced cultures failed to grow at headspace dioxygen concentrations above 1.5%). This addition of dioxygen had no effect on cultures grown in the presence of reducing agent (not shown), but those grown in the absence of reducing agent grew only after a lag of 12 h. During this lag period, the resazurin gradually changed from pink to colorless, indicating consumption of dioxygen. Similar results have been reported for other acetogenic bacteria (7). Unreduced cultures treated with cysteine prior to the exposure to dioxygen grew without any lag (and the resazurin quickly changed from pink to colorless) and had a maximum growth (OD600) comparable to that of reduced cultures (Fig. 9), indicating that cysteine protected the cultures from growth inhibition by oxidative stress. On the other hand, addition of sodium azide (2 mM) to unreduced cultures prior to dioxygen addition inhibited growth (and the resazurin color remained pink throughout), indicating that the inhibition of cytochrome bd oxidase by azide diminished the ability of the cultures to reduce dioxygen. These results suggest protective roles of both cysteine and cytochrome bd oxidase against dioxygen and oxidative stress in M. thermoacetica.
FIG. 9.
Effect of oxidative stress on growth of M. thermoacetica. Cultures were grown on methanol (200 mM) in Drake's medium (65) (80 ml) under 100% CO2 in anoxic bottles (125-ml capacity) in the presence (trace 1) or absence (traces 2 through 5) of reducing agent (cysteine + H2S). After 48 h, either cysteine (2.8 mM) or azide (2 mM) was injected (shown by arrow) into replicate cultures grown in the absence of reducing agent followed immediately by injection of air into the headspace to give a final dioxygen concentration of 1.5 vol% (based on 21 vol% dioxygen in air). The cultures were shaken vigorously for 20 s (to equilibrate dioxygen) and then incubated at 58°C. Traces: 2, cysteine + dioxygen; 3, no treatment; 4, dioxygen only; 5, azide (2 mM) + dioxygen. At the times indicated, 1-ml aliquots of the cultures were withdrawn via syringe and centrifuged, and the cell pellets were suspended in equal volumes of 50 mM Tris-HCl, pH 8.0, prior to measurement of the OD600. The results are average of three replicate experiments with standard deviations between 0 and 8%.
Induction of a cysteine synthase in M. thermoacetica.
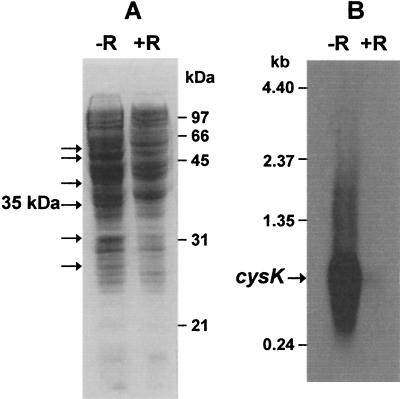
The protein profile of cell extracts of M. thermoacetica cultures grown in either the presence or absence of reducing agent is shown in Fig. 10. Several proteins (marked by arrows) were expressed at higher levels in unreduced cultures (−R) than in reduced cultures (+R), including a 35-kDa protein (Fig. 10A). The N-terminal sequence of this 35-kDa protein was determined to be A/MKIARDVTQLIGQT, which is identical to the corresponding amino acid sequence of a predicted 306-residue protein encoded by an open reading frame of 918 bp in the nucleotide sequence of a small contig (contig 132) in the M. thermoacetica genome sequence. The calculated molar mass of this putative protein, 32,619 Da, is in close agreement with the SDS-PAGE-estimated value of 35 kDa, and its predicted amino acid sequence has high homologies (between 68 and 55% identities) to cysteine synthase K from B. subtilis (accession no. S66103), M. tuberculosis (accession no. G70660), and Clostridium acetobutylicum (accession no. E97175). We, therefore, refer to this 35-kDa M. thermoacetica protein as CysK.
FIG. 10.
(A) SDS-PAGE (12% acrylamide in resolving gel) of cytosolic extracts (40 μg of protein) of M. thermoacetica grown in the presence (+R) or absence (−R) of reducing agent. Proteins overexpressed in the absence of reducing agent were indicated by arrows. (B) Northern blot of total RNA (5 μg in each lane) isolated from M. thermoacetica grown on methanol in the presence (+R) or absence (−R) of reducing agent after hybridization with DIG-labeled DNA fragments amplified from cysK.
In order to investigate the transcription of this cysK, Northern blots containing total RNA from M. thermoacetica were hybridized with DIG-labeled PCR products amplified from the cysK of contig 132. A strong hybridization band corresponding to an approximate size of 1 kb (Fig. 10B) was found on blots containing RNA from cells grown in medium lacking reducing agent (−Red), whereas little or no such band was found in the lane from cells grown in medium containing reducing agent (+Red). The size of the band is in close agreement with the size of the putative cysK, 918 bp. A putative promoter sequence, 5′-CTTGACA-N26-AATAAT-N23-AGGAG-3′, resembling the E. coli consensus s70 promoter (5′-CTTGACa-N15-21-TATAaT-AGGAG-3′) (33) was found upstream of the cysK start codon. C_ysK_ transcription was, thus, apparently strongly repressed by the exogenous reducing agent.
DISCUSSION
Cytochrome bd oxidase has not been previously reported from an acetogenic or, to our knowledge, any gram-positive strictly anaerobic bacterium. Based on subunit composition and spectroscopic and biochemical properties, the M. thermoacetica cytochrome bd oxidase closely resembles the corresponding enzyme from other bacteria, including both aerobes and anaerobes (30, 36, 55). The M. thermoacetica cyd operon consists of four genes encoding CydA, CydB, CydD and CydC, which we verified are transcribed in a single polycistronic message. Several membrane-spanning conserved domains, including nine transmembrane helices of CydA and the ligands to the iron of the three different hemes were found to be conserved in the M. thermoacetica cytochrome bd oxidase, which reinforces the universal lineage of this enzyme among the bacteria (2).
Recently, hydrogen (a substrate in the Wood-Ljungdahl pathway) was shown to support dioxygen uptake in several acetogens (7). Results described herein show that dioxygen uptake by M. thermoacetica cell suspensions at room temperature were stimulated by CO or methanol and strongly inhibited by azide and cyanide, which are specific inhibitors of cytochrome bd oxidase. Apparently, the majority of the O2 uptake (∼70% based on cyanide inhibition) by M. thermoacetica cell suspensions is via cytochrome bd oxidase. Based on inhibition studies, dioxygen uptake (presumably reduction) by M. thermoacetica cytochrome bd oxidase is coupled to the membrane electron transport chain, as reported in other bacteria (59). Rates of dioxygen uptake and the level of cyd transcript were both higher in M. thermoacetica cells grown in nonreducing medium, further supporting the involvement of cytochrome bd oxidase in O2 uptake and tolerance. Finally, growth of M. thermoacetica under nonreducing conditions with trace dioxygen was inhibited by azide, a known inhibitor of cytochrome bd oxidase.
Homologs of several proteins implicated in oxidative stress protection are encoded in the M. thermoacetica genome (http://genome.jgi-psf.org/draft_microbes/mooth/mooth.draft.html), including “anaerobic type” superoxide reductase, rubrerythrin, type A flavoprotein and rubredoxin, some of which have been characterized (20, 66). The M. thermoacetica genome also encodes “aerobic type” Fe/Mn superoxide dismutase (SOD) and Mn catalase, which have not been previously reported in M. thermoacetica. Curiously, while Karnholz et al. detected NADH oxidase activity, they did not detect SOD or catalase activities (at either 50°C or room temperature) in extracts of M. thermoacetica cells that had been exposed to low levels of dioxygen in growth medium lacking reducing agent (39).
There is now increasing evidence that cytochrome bd oxidase plays a crucial role in the survival and oxidative stress protection of a wide variety of facultative and anaerobic bacteria, at least under microaerobic growth conditions (2, 23, 51, 74). Mutation or deletion of the genes encoding cytochrome bd oxidase has been shown to increase sensitivity to oxidative stress in E. coli (51, 74) and A. vinelandii (23). The ability of cytochrome bd oxidases to reduce dioxygen directly to water may minimize the generation of toxic reactive oxygen species and also protect crucial metabolic enzymes in their dioxygen-sensitive reduced states (67). Amino acid sequence comparisons of CydAs from bacteria and archaea indicated that cytochrome bd oxidase is present in some of the most ancient bacteria and, therefore, that sufficient atmospheric dioxygen may have been present to support dioxygenic respiration even prior to the emergence of organic photosynthesis (2). It is unclear whether cytochrome bd oxidase can generate energy from the reduction of dioxygen in M. thermoacetica. However, the CO- and methanol-dependent reduction of O2 by cytochrome bd oxidase in M. thermoacetica demonstrated in this work suggests coupling of the bd oxidase dioxygen reduction pathway to the membrane electron transport chain of the Wood-Ljungdahl pathway.
The growth of M. thermoacetica in nonreducing medium also led to increased levels of several other proteins, including a 35-kDa protein that we identified as CysK, a cysteine synthase. Expression of cysK has been reported to be a general response to stress in bacteria and plants (5, 57, 71, 73). In bacteria, cysteine is synthesized from _O_-acetylserine and H2S catalyzed by _O_-acetylserine sulfhydrolase (CysK) while _O_-acetylserine is produced from serine and acetyl-CoA catalyzed by serine acetyltransferase (CysE) (12, 29). In the M. thermoacetica genome, a CysE homolog is encoded by gene 521 on contig 282. The M. thermoacetica genome also encodes a second CysK homolog (32,722 Da), encoded by gene 55 on contig 256, which shares 66% identical residues with the CysK found to be induced under nonreducing growth conditions in this work. The presence of two CysK homologues has also been reported in the gram-positive bacterium Lactococcus lactis (29). Stimulation of growth of unreduced M. thermoacetica cultures by exogenous cysteine and induction of cysK transcription and expression in the absence of added cysteine and H2S in the growth medium suggest that CysK could also play an important role in oxidative stress protection in this bacterium. In many microorganisms, cysteine-containing molecules, (e.g., glutathione and thioredoxin) play major roles in maintaining an intracellular reducing environment and in protection against oxidative and other stress conditions (9, 22, 52). Cell extracts of M. thermoacetica can produce cysteine from cystine by enzymatic reduction with molecular hydrogen as electron donor (44). Results described herein thus provide strong evidence that both cytochrome bd oxidase and cysteine (and, by inference, CysK) play significant roles in dioxygen tolerance and oxidative stress protection of M. thermoacetica by scavenging dioxygen and/or maintaining a low intracellular reduction potential, both of which are known to be essential for growth of many anaerobic bacteria (1, 72).
Acknowledgments
This work was funded by grant DE-FG02-93ER20127 from the Department of Energy (to L.G.L.) and grant GM40388 from the National Institutes of Health (to D.M.K.).
R.S.-D. thanks the Chemistry Department of Babesh-Bolyai University, Cluj-Napoca, Romania, for a leave of absence.
REFERENCES
- 1.Barnes, E. M., and M. Ingram. 1956. The effect of redox potential on the growth of Clostridium welchii strains isolated from horse muscle. J. Appl. Bacteriol. 19**:**117-122. [Google Scholar]
- 2.Baughn, A. D., and M. N. Malamy. 2004. The strict anaerobe Bacteroides fragilis grows in and benefits from nanomolar concentrations of oxygen. Nature 427**:**441-444. [DOI] [PubMed] [Google Scholar]
- 3.Beinert, H. 2000. Iron-sulfur proteins: ancient structures, still full of surprises. J. Biol. Inorg. Chem. 5**:**2-15. [DOI] [PubMed] [Google Scholar]
- 4.Beinert, H., and P. J. Kiley. 1999. Fe-S proteins in sensing and regulatory functions. Curr. Opin. Chem. Biol. 3**:**152-157. [DOI] [PubMed] [Google Scholar]
- 5.Benov, L., N. M. Kredich, and I. Fridovich. 1996. The mechanism of the auxotrophy for sulfur-containing amino acids imposed upon Escherichia coli by superoxide. J. Biol. Chem. 271**:**21037-21040. [DOI] [PubMed] [Google Scholar]
- 6.Bertsova, Y. V., A. V. Bogachev, and V. P. Skulachev. 1997. Generation of protonic potential by the _bd_-type quinol oxidase of Azotobacter vinelandii. FEBS Lett. 414**:**369-372. [DOI] [PubMed] [Google Scholar]
- 7.Boga, H. I., and A. Brune. 2003. Hydrogen-dependent oxygen reduction by homoacetogenic bacteria isolated from termite guts. Appl. Environ. Microbiol. 69**:**779-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boga, H. I., W. Ludwig, and A. Brune. 2003. Sporomusa aerivorans sp. nov., an oxygen-reducing homoacetogenic bacterium from the gut of a soil-feeding termite. Int. J. Syst. Evol. Microbiol. 53**:**1397-1404. [DOI] [PubMed] [Google Scholar]
- 9.Carmel-Harel, O., and G. Storz. 2000. Roles of the glutathione- and thioredoxin-dependent reduction systems in the Escherichia coli and Saccharomyces cerevisiae responses to oxidative stress. Annu. Rev. Microbiol. 54**:**439-461. [DOI] [PubMed] [Google Scholar]
- 10.Carneiro de Melo, A. M. S., G. M. Cook, R. J. Miles, and R. K. Poole. 1996. Nisin stimulates oxygen consumption by Staphylococcus aureus and Escherichia coli. Appl. Environ. Microbiol. 62**:**1831-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang, H. T., S. W. Marcelli, A. A. Davison, P. A. Chalk, R. K. Poole, and R. J. Miles. 1995. Kinetics of substrate oxidation by whole cells and cell membranes of Helicobacter pylori. FEMS Microbiol. Lett. 129**:**33-38. [DOI] [PubMed] [Google Scholar]
- 12.Chang, Z., and L. C. Vining. 2002. Biosynthesis of sulfur-containing amino acids in Streptomyces venezuelae ISP5230: roles for cystathionine beta-synthase and transsulfuration. Microbiology 148**:**2135-2147. [DOI] [PubMed] [Google Scholar]
- 13.Cole, S. T., K. Eiglmeier, J. Parkhill, J. D. James, N. R. Thomson, P. R. Wheeler, N. Honore, T. Garnier, C. Churcher, D. Harris, K. Mungall, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. M. Davies, K. Devlin, S. Duthoy, T. Feltwell, A. Fraser, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, C. Lacroix, J. Maclean, S. Moule, L. Murphy, K. Oliver, M. A. Quail, M. A. Rajandream, K. M. Rutherford, S. Rutter, K. Seeger, S. Simon, M. Simmonds, J. Skelton, R. Squares, S. Squares, K. Stevens, K. Taylor, S. Whitehead, J. R. Woodward, and B. G. Barrell. 2001. Massive gene decay in the leprosy bacillus. Nature 409**:**1007-1011. [DOI] [PubMed] [Google Scholar]
- 14.Cook, G. M., H. Cruz-Ramos, A. J. Moir, and R. K. Poole. 2002. A novel haem compound accumulated in Escherichia coli overexpressing the cydDC operon, encoding an ABC-type transporter required for cytochrome assembly. Arch. Microbiol. 178**:**358-369. [DOI] [PubMed] [Google Scholar]
- 15.Das, A., and L. G. Ljungdahl. 1997. Composition and primary structure of the F1F0 ATP synthase from the obligately anaerobic bacterium Clostridium thermoaceticum. J. Bacteriol. 179**:**3746-3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Das, A., and L. G. Ljungdahl. 2002. Electron-transport system in acetogens, p. 191-204. In L. G. Ljungdahl, M. M. Adams, L. Barton, J. G. Ferry, and M. Johnson (ed.), Biochemistry and physiology of anaerobic bacteria. Springer Verlag, New York, N.Y.
- 17.Das, A., and L. G. Ljungdahl. 2003. Clostridium pasteurianum F1F0 ATP synthase: operon, composition, and some properties. J. Bacteriol. 185**:**5527-5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Das, A., D. M. Ivey, and L. G. Ljungdahl. 1997. Purification and reconstitution into proteoliposomes of the F1F0 ATP synthase from the obligately anaerobic gram-positive bacterium Clostridium thermoautotrophicum. J. Bacteriol. 179**:**1714-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Das, A., J. Hugenholtz, H. van Halbeek, and L. G. Ljungdahl. 1989. Structure and function of a menaquinone involved in electron transport in membranes of Clostridium thermoautotrophicum and Clostridium thermoaceticum. J. Bacteriol. 171**:**5823-5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Das, A., E. D. Coulter, D. M. Kurtz, Jr., and L. G. Ljungdahl. 2001. Five-gene cluster in Clostridium thermoaceticum consisting of two divergent operons encoding rubredoxin oxidoreductase-rubredoxin and rubrerythrin-type A flavoprotein-high-molecular-weight rubredoxin. J. Bacteriol. 183**:**1560-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drake, H. L. 1994. Acetogenesis, acetogenic bacteria, and the acetyl-CoA “Wood/Ljungdahl pathway”: past and current perspectives, p. 3-60. In H. L. Drake (ed.), Acetogenesis. Chapman & Hall, New York, N.Y.
- 22.Dzink, J. L., C. M. Smith, and S. S. Socransky. 1987. Development of a broth medium for Bacteroides forsythus. J. Clin. Microbiol. 25**:**925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edwards, S. E., C. S. Loder, G. Wu, H. Corker, B. W. Bainbridge, S. Hill, and R. K. Poole. 2000. Mutation of cytochrome bd quinol oxidase results in reduced stationary phase survival, iron deprivation, metal toxicity and oxidative stress in Azotobacter vinelandii. FEMS Microbiol. Lett. 185**:**71-77. [DOI] [PubMed] [Google Scholar]
- 24.Efiok, B. J., and D. A. Webster. 1990. A cytochrome that can pump sodium ion. Biochem. Biophys. Res. Commun. 173**:**370-375. [DOI] [PubMed] [Google Scholar]
- 25.Fang, H., R. J. Lin, and R. B. Gennis. 1989. Location of heme axial ligands in the cytochrome d terminal oxidase complex of Escherichia coli determined by site-directed mutagenesis. J. Biol. Chem. 264**:**8026-8032. [PubMed] [Google Scholar]
- 26.Fareleira, P., J. LeGall, A. V. Xavier, and H. Santos. 1997. Pathways for utilization of carbon reserves in Desulfovibrio gigas under fermentative and respiratory conditions. J. Bacteriol. 179**:**3972-3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fareleira, P., B. S. Santos, C. Antonio, P. Moradas-Ferreira, J. LeGall, A. V. Xavier, and H. Santos. 2003. Response of a strict anaerobe to oxygen: survival strategies in Desulfovibrio gigas. Microbiology 149**:**1513-1522. [DOI] [PubMed] [Google Scholar]
- 28.Farr, S. B., and T. Kogoma. 1991. Oxidative stress responses in Escherichia coli and Salmonella typhimurium. Microbiol. Rev. 55**:**561-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fernández, M., M. Kleerebezem, O. P. Kuipers, R. J. Siezen, and R. van Kranenburg. 2002. Regulation of the _metC_-cysK operon, involved in sulfur metabolism in Lactococcus lactis. J. Bacteriol. 184**:**82-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gilmour, R., and T. A. Krulwich. 1997. Construction and characterization of a mutant of alkaliphilic Bacillus firmus OF4 with a disrupted cta operon and purification of a novel cytochrome bd. J. Bacteriol. 179**:**863-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Green, G. N., H. Fang, R. J. Lin, G. Newton, M. Mather, C. D. Georgiou, and R. B. Gennis. 1988. The nucleotide sequence of the cyd locus encoding the two subunits of the cytochrome d terminal oxidase complex of Escherichia coli. J. Biol. Chem. 263**:**13138-13143. [PubMed] [Google Scholar]
- 32.Hata-Tanka, A., K. Matsuura, S. Itoh, and Y. Aranku. 1987. Electron flow and heme-heme interaction between cytochromes _b_558, _b_595 and d in a terminal oxidase of Escherichia coli. Biochim. Biophys. Acta 893**:**289-295. [DOI] [PubMed] [Google Scholar]
- 33.Hawley, D. K., and W. R. McClure. 1983. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 25**:**2237-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Imlay, J. A., and S. Linn. 1988. DNA damage and oxygen radical toxicity. Science 240**:**1302-1309. [DOI] [PubMed] [Google Scholar]
- 35.Ivey, D. M. 1987. Generation of energy during CO2-fixation in acetogenic bacteria. Ph.D. dissertation. Department of Biochemistry, University of Georgia, Athens.
- 36.Junemann, S., and J. M. Wrigglesworth. 1995. Cytochrome bd oxidase from Azotobacter vinelandii. J. Biol. Chem. 270**:**16213-16220. [DOI] [PubMed] [Google Scholar]
- 37.Kalnenieks, U., N. Galinina, M. M. Toma, and R. K. Poole. 2000. Cyanide inhibits respiration yet stimulates aerobic growth of Zymomonas mobilis. Microbiology 146**:**1259-1266. [DOI] [PubMed] [Google Scholar]
- 38.Kamimura, K., S. Fujii, and T. Sugio. 2001. Purification and some properties of ubiquinol oxidase from obligately chemolithotrophic iron-oxidizing bacterium, Thiobacillus ferrooxidans NASF-1. Biosci. Biotechnol. Biochem. 65**:**63-71. [DOI] [PubMed] [Google Scholar]
- 39.Karnholz, A., K. Küsel, A. Gößner, A. Schramm, and H. L. Drake. 2002. Tolerance and metabolic response of acetogenic bacteria toward oxygen. Appl. Environ. Microbiol. 68**:**1005-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaysser, T. M., J. B. Ghaim, C. Georgiou, and R. B. Gennis. 1995. Methionine-393 is an axial ligand of the heme _b_558 component of the cytochrome bd ubiquinol oxidase from Escherichia coli. Biochemistry 34**:**13491-13501. [DOI] [PubMed] [Google Scholar]
- 41.Kelly, M. J. S., R. K. Poole, M. G. Yates, and C. Kennedy. 1990. Cloning and mutagenesis of genes encoding the cytochrome bd terminal oxidase complex in Azotobacter vinelandii: mutants deficient in the cytochrome d complex are unable to fix nitrogen in air. J. Bacteriol. 172**:**6010-6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keyhani, E., and D. Minai-Tehrani. 2001. The binding of cyanide to cytochrome d in intact cells, spheroplasts, membrane fragments and solubilized enzyme from Salmonella typhimurium. Biochim. Biophys. Acta 1506**:**1-11. [DOI] [PubMed] [Google Scholar]
- 43.Kiley, P. J., and H. Beinert. 1998. Oxygen sensing by the global regulator, FNR: the role of the iron-sulfur cluster. FEMS Microbiol. Rev. 22**:**341-352. [DOI] [PubMed] [Google Scholar]
- 44.Koesnandar, N. N., A. Yamamoto, and S. Nagai. 1991. Enzymatic reduction of cystine into cysteine by cell-free extract of Clostridium thermoaceticum. J. Ferm. Bioeng. 72**:**11-14. [Google Scholar]
- 45.Kolonay, J. F., Jr., and R. J. Maier. 1997. Formation of pH and potential gradients by the reconstituted Azotobacter vinelandii cytochrome bd respiratory protection oxidase. J. Bacteriol. 179**:**3813-3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Konishi, K., M. Ouchi, K. Kita, and I. Horikoshi. 1986. Purification and properties of a cytochrome _b_560-d complex, a terminal oxidase of the aerobic respiratory chain of Photobacterium phosphoreum. J. Biochem. 99**:**1227-1236. [DOI] [PubMed] [Google Scholar]
- 47.Krakauer, D. C. 2000. Stability and evolution of overlapping genes. Evol. Int. J. Org. Evol. 54**:**731-739. [DOI] [PubMed] [Google Scholar]
- 48.Küsel, K., A. Karnholz, T. Trinkwalter, R. Devereux, G. Acker, and H. L. Drake. 2001. Physiological ecology of Clostridium glycolicum RD-1, an aerotolerant acetogen isolated from sea grass roots. Appl. Environ. Microbiol. 67**:**4734-4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227**:**680-685. [DOI] [PubMed] [Google Scholar]
- 50.Lemos, R. S., C. M. Gomes, M. Santana, J. LeGall, A. V. Xavier, and M. Teixeira. 2001. The ‘strict’ anaerobe Desulfovibrio gigas contains a membrane-bound oxygen-reducing respiratory chain. FEBS Lett. 496**:**40-43. [DOI] [PubMed] [Google Scholar]
- 51.Lindqvist, A., J. Membrillo-Hernandez, R. K. Poole, and G. M. Cook. 2000. Roles of respiratory oxidases in protecting Escherichia coli K12 from oxidative stress. Antonie Leeuwenhoek 78**:**23-31. [DOI] [PubMed] [Google Scholar]
- 52.Lithgow, J. K., E. J. Hayhurst, G. Cohen, Y. Aharonowitz, and S. J. Foster. 2004. Role of a cysteine synthase in Staphylococcus aureus. J. Bacteriol. 186**:**1579-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lorence, R. M., and R. B. Gennis. 1989. Spectroscopic and quantitative analysis of the oxygenated and peroxy states of the purified cytochrome d complex of Escherichia coli. J. Biol. Chem. 264**:**7135-7140. [PubMed] [Google Scholar]
- 54.Meinhardt, S. W., R. B. Gennis, and T. Ohnishi. 1989. EPR studies of the cytochrome-d complex of Escherichia coli. Biochim. Biophys. Acta 975**:**175-184. [DOI] [PubMed] [Google Scholar]
- 55.Miller, M. J., and R. B. Gennis. 1983. The purification and characterization of the cytochrome d terminal oxidase complex of the Escherichia coli aerobic respiratory chain. J. Biol. Chem. 258**:**9159-9165. [PubMed] [Google Scholar]
- 56.Minagawa, J., R. B. Gennis, and Y. Aranku. 1992. Identification of heme and copper ligands in subunit I of the cytochrome bo complex of Escherichia coli. J. Biol. Chem. 267**:**2096-2104. [PubMed] [Google Scholar]
- 57.Noji, M., M. Saito, M. Nakamura, M. Aono, H. Saji, and K. Saito. 2001. Cysteine synthase overexpression in tobacco confers tolerance to sulfur-containing environmental pollutants. Plant Physiol. 126**:**973-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Poole, R. K. 1983. Bacterial cytochrome oxidases: a structurally and functionally diverse group of electron transfer proteins. Biochim. Biophys, Acta 726**:**205-243. [DOI] [PubMed] [Google Scholar]
- 59.Poole, R. K., and G. M. Cook. 2000. Redundancy of aerobic respiratory chains in bacteria: routes, reasons and regulation. Adv. Microb. Physiol. 43**:**165-224. [DOI] [PubMed] [Google Scholar]
- 60.Poole, R. K., L. Hatch, M. W. Cleeter, F. Gibson, G. B. Cox, and G. Wu. 1993. Cytochrome bd biosynthesis in Escherichia coli: the sequences of the cydC and cydD genes suggest that they encode the components of an ABC membrane transporter. Mol. Microbiol. 10**:**421-430. [PubMed] [Google Scholar]
- 61.Porter, N., J. W. Drozd, and J. D. Linton. 1983. The effects of cyanide on the growth and respiration of Enterobacter aerogenes in continuous culture. J. Gen. Microbiol. 129**:**7-16. [DOI] [PubMed] [Google Scholar]
- 62.Richter, O. M., and B. Ludwig. 2003. Cytochrome c oxidase: structure, function, and physiology of a redox-driven molecular machine. Rev. Physiol. Biochem. Pharamacol. 147**:**47-74. [DOI] [PubMed] [Google Scholar]
- 63.Rieske, J. S. 1967. Preparation and properties of reduced coenzyme Q-cytochrome c reductase (complex III of the respiratory chain). Methods Enzymol. 10**:**239-245. [Google Scholar]
- 64.Schägger, H., W. A. Cramer, and G. von Jagow. 1994. Analysis of molecular masses and oligomeric states of protein complexes by blue native electrophoresis and isolation of membrane protein complexes by two-dimensional native electrophoresis. Anal. Biochem. 217**:**220-230. [DOI] [PubMed] [Google Scholar]
- 65.Seifritz, C., S. L. Daniel, A. Gößner, and H. L. Drake. 1993. Nitrate as a preferred electron sink for the acetogen Clostridium thermoaceticum. J. Bacteriol. 175**:**8008-8013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Silaghi-Dumitrescu, R., E. D. Coulter, A. Das, L. G. Ljungdahl, G. N. L. Jameson, B. H. Huynh, and D. M. Kurtz, Jr. 2003. A flavo-diiron protein (FprA) and high-molecular weight rubredoxin (Hrb) from Moorella thermoacetica with nitric oxide reductase activity. Biochemistry 42**:**2806-2815. [DOI] [PubMed] [Google Scholar]
- 67.Skulachev, V. P. 1994. Decrease in the intracellular concentration of O2 as a special function of the cellular respiratory system. Biokhimia 59**:**1910-1912. [PubMed] [Google Scholar]
- 68.Spinne, F., M. R. Cheesman, A. J. Thomson, T. Kaysser, R. B. Gennis, Q. Peng, and J. Peterson. 1995. The haem _b_558 component of the cytochrome bd quinol oxidase complex from Escherichia coli has histidine-methionine axial ligation. Biochem. J. 308**:**641-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sun, J., M. A. Kahlow, T. M. Kaysser, J. P. Osborne, J. J. Hill, R. J. Rohlfs, R. Hille, R. B. Gennis, and T. M. Loehr. 1996. Resonance Raman spectroscopic identification of a histidine ligand of _b_595 and the nature of the ligation of chlorin d in the fully reduced Escherichia coli cytochrome bd oxidase. Biochemistry 35**:**2403-2412. [DOI] [PubMed] [Google Scholar]
- 70.Thomas, P. E., D. Ryan, and W. Levin. 1976. An improved staining procedure for the detection of the peroxidase activity of cytochrome P-450 on sodium dodecyl sulfate polyacrylamide gels. Anal. Biochem. 75**:**168-176. [DOI] [PubMed] [Google Scholar]
- 71.Vasquez, C. C., C. P. Saavedra, C. A. Loyola, M. A. Araya, and S. Pichuantes. 2001. The product of the cysK gene of Bacillus stearothermophilus V mediates potassium tellurite resistance in Escherichia coli. Curr. Microbiol. 43**:**418-423. [DOI] [PubMed] [Google Scholar]
- 72.Vennesland, B., and M. E. Hanke. 1940. The oxidation-reduction potential requirements of a non-spore-forming, obligate anaerobe. J. Bacteriol. 39**:**139-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vorobjeva, L., P. Leverrier, A. Zinchenko, P. Boyaval, E. Khodjaev, S. Varioukhina, G. Ponomareva, E. Gordeeva, and G. Jan. 2004. Anti-stress activity of Propionibacterium freudenreichii: identification of a reactivative protein. Antonie Leeuwenhoek 85**:**53-62. [DOI] [PubMed] [Google Scholar]
- 74.Wall, D., J. M. Delaney, O. Fayet, B. Lipinska, T. Yamamoto, and C. Georgopoulos. 1992. _arc_-dependent thermal regulation and extragenic suppression of the Escherichia coli cytochrome d operon. J. Bacteriol. 174**:**6554-6562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Winstedt, L., K. Yoshida, Y. Fujita, and C. von Wachenfeldt. 1998. Cytochrome bd biosynthesis in Bacillus subtilis: characterization of the cydABCD operon. J. Bacteriol. 180**:**6571-6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang, J., P. Hellwig, J. P. Osborne, H. W. Huang, P. Moenne-Loccoz, A. A. Konstantinov, and R. B. Gennis. 2001. Site-directed mutation of the highly conserved region near the Q-loop of the cytochrome bd quinol oxidase from Escherichia coli specifically perturbs heme _b_595. Biochemistry 40**:**8548-8556. [DOI] [PubMed] [Google Scholar]
- 77.Zhang, J., B. Barquera, and R. B. Gennis. 2004. Gene fusions with beta-lactamase show that subunit I of the cytochrome bd quinol oxidase from E. coli has nine transmembrane helices with the O2 reactive site near the periplasmic surface. FEBS Lett. 561**:**58-62. [DOI] [PubMed] [Google Scholar]