Argonaute protein PIWI controls mobilization of retrotransposons in the Drosophila male germline (original) (raw)
Abstract
Proteins of the Argonaute family have been identified as key components of RNA interference (RNAi) pathway. RNAi-related mechanisms are implicated in the regulation of gene expression and repression of transposable elements in eukaryotes. The piwi gene encoding protein of the Drosophila Argonaute family was shown to be required for the germ stem cells maintenance. Here, we show that piwi is involved in silencing of LTR retrotransposons in testes. piwi mutations led to derepression of endogenous retrotransposon copia as well as to upregulation of the reporter gene driven by copia LTR. piwi mutation causes accumulation of retrotransposon mdg1 transcripts at the apical tip of testes, including germinal proliferative center where PIWI protein was shown to be expressed. We applied inverse PCR approach to detect the newly arisen insertions of the mdg1 retrotransposon in the progeny of individual piwi mutant males. Owing to piwi mutation a high rate of mdg1 transpositions was revealed. Thus, piwi is involved in the silencing of retrotransposons in the precursors of male gametes. Our results provide the first evidence that protein of the Argonaute family prevents retrotranspositions. It is supposed that the disturbance of RNA silencing system in germinal cells might cause transposition burst.
INTRODUCTION
Gene silencing by homologous double-stranded RNA (dsRNA) is termed RNA interference (RNAi) (1). Long dsRNA is processed by Dicer enzymes into small interfering RNAs (siRNAs) that target repression of homologous sequences (2–4). siRNAs guide sequence-specific cleavage activity of the RNA-induced silencing complex (RISC) to complementary mRNA (4–6) or induce chromatin-based events that result in transcriptional silencing (7–10). RNAi plays a role in defense against transposable elements and viruses, which can generate dsRNA (11–13). Antisense transcription and generation of hairpin structures in nascent transcripts are considered as a source of dsRNA of transposable elements in cells (14,15). Some RNAi-deficient mutants show a relief of transposon silencing in Caenorhabditis elegans (13,15–17). Mutations in genes that impaired RNAi cause an increase of retrotransposon transcript abundance in Trypanosoma (18), Drosophila (19–22) and mouse (23). Mobilization of retrotransposon was demonstrated in Chlamydomonas as a result of mutation in RNA-helicase encoding gene involved in the post-transcriptional silencing of transgenes (24). Silencing mediated by natural dsRNA has been implicated in the essential biological processes, including functioning of centromers in Schizosaccharomyces pombe (8), development of macronucleus in Tetrahymena (25) and male spermatogenesis in Drosophila melanogaster (20,26,27). In these cases, dsRNA formation is provided by the transcription of the vestiges of transposable elements indicating that mechanisms of mobile elements silencing may be extended to the regulation of host genome.
Contribution of RNAi-based regulation to the retrotransposon-derived transcripts abundance in the Drosophila germline has been demonstrated (19–22), but no direct evidence in favor of the role of RNAi machinery in the control of their transposition rate has been obtained. Genetic control of retrotransposon mobilization in Drosophila is far from being understood. Spontaneous transposition rates of retroelements are usually low (28). However, a high level of retrotransposon mobility was detected in some stocks under particular genetic conditions. Mobility of gypsy, ZAM and Idefix retrotransposons was shown to be controlled by the yet uncharacterized X-linked flamenco locus (29,30). Non-LTR retrotransposon I is mobilized in the female germline as a result of definite outcrosses. This phenomenon is known as I–R hybrid dysgenesis (31). In this case, the mechanism of retrotransposon activation is also unclear. We revealed the effect of the RNAi genes spn-E, aub and armi on the I element expression in the female germline (22). We propose that hybrid dysgenesis associated with I element transposition burst may be caused by the impair of RNAi (22).
We investigated a role of the piwi gene in the control of retrotransposon mobilization in the male germline. piwi encodes a nucleoplasmic protein known to be required for self-renewal of stem cells in the male and female germline and the regulation of their division (32,33). Control of stem cell maintenance is the conserved developmental function of piwi and its orthologs in plants and mammals (34–38). PIWI is related to the highly conserved proteins of the Argonaute family. Argonautes are implicated in RNAi and related silencing pathways, including those that affect heterochromatin assembly and translational regulation via microRNA (miRNA)-directed pathway in different organisms [reviewed in (39–41)]. These proteins including PIWI are defined by two characteristic domains, PAZ and PIWI. PAZ domain recognizes the unique terminal structures of siRNAs (42,43) and, therefore, is essential for small RNA binding. The PIWI domain of Argonaute from the archaebacterium Pyrococcus furiosis is similar to a ribonuclease H domain (44), suggesting the role of Argonautes in the nucleolytic activity of RISC. However, only one of the four mammalian Argonaute 1 subfamily members (Ago2) was associated with the cleavage of target mRNA, suggesting that the other Argonautes might operate in different forms of RNA silencing (45). Drosophila Ago1 and Ago2 regulate the assembly of miRNAs and siRNAs, respectively, into RISC (46). piwi mutation relieves the transcriptional and post-transcriptional gene silencing of the multiple-copy transgenes (47) and impedes the repression of retrotransposon gypsy in the D.melanogaster genome (48).
In this study, we demonstrate that piwi mutations cause overexpression of retrotransposons mdg1 and copia in the male germline, alteration of their expression pattern and mobilization of mdg1 retrotransposon. Our results provide the first evidence that Argonaute family protein prevents mobilization of a retrotransposon. copia and mdg1 are related to two major families of LTR retrotransposons, Ty1/copia and Ty3/gypsy groups, respectively. These groups are structurally differed by the location of integrase domain (49). The Ty1/copia group is termed the Pseudoviridae, reflecting structural similarity to retroviruses. Number of full-length copies of copia is 26 and mdg1 is 13 according to the analysis of euchromatic part of the Drosophila genome (50). Spontaneous rate of transpositions is ∼10−4 per element per generation for copia (51), while no mdg1 transpositions has been revealed in related stocks (28). It has been reported that conventional inbred crosses may generate spontaneous copia transpositions (52). We show that transposition burst of retrotransposon mdg1 in the Drosophila germline is a result of mutation in the piwi gene involved in the stem cell development and RNA-mediated silencing phenomena. It is supposed that RNAi-related system of silencing controls transposition rate in the germline and its disturbance might cause transposition burst.
MATERIALS AND METHODS
Drosophila strains
Strain bearing spindle-E (spn-E) mutation was ru1 st1 spn-E1 e1 ca1/TM3, Sb1 es. piwi mutants were piwi3 (PZ insertional mutation) and piwi2 (P-ry11 transposon insertion) (32,53). piwi2 mutation was balanced with the CyO, P{w+m = hsp70: GAL4} P{w+m = UAS: GFP}. Su(var)2-501, Su(var)3-903 and E(z)61 alleles were used. P-element transformed flies carrying _copia_LTR-lacZ construct on the X and second chromosomes were kindly provided by E. G. Pasyukova (54).
In situ RNA analysis
In situ RNA analysis was carried out according to the procedure described previously (21). DIG-labeled riboprobes were synthesized according to the manufacturer's recommendations. PCR products carrying T7 promoter or plasmids containing the cloned fragments of retrotransposons were used as transcription templates. mdg1: 0.35 kb EcoRV–NsiI fragment from plasmid Dm688 (55) was subcloned into pBS SK− and used to obtain sense or antisense riboprobe. 1731: PCR-amplified fragment using primers (5′-ACGAATTCAGCGAACTGTCGTCG-3′) and (5′-ATGGATCCATGTGACTGGTAGC-3′) corresponding to 1138–1271 nt (GenBank accession no. X07656) was cloned into pTZ19R and used to detect sense 1731 transcripts. copia: 2.3 kb fragment from the 5′ copia LTR to the EcoRI site into pBS SK− was used to obtain a probe to detect sense or antisense copia RNA. GATE: PCR product carrying T7 promoter corresponding to 3055–3237 nt (GenBank accession no. AJ010298) was used for the detection of GATE sense RNA. PCR product carrying T7 promoter for piwi transcripts detection was amplified using primers 5′-GGCCGCGGACTTATGTGAGAGCAATGGATG-3′ and 5′-GCCCCGGCCACCGTCTCGATAAAATACG-3′ corresponding to 7886–8519 nt (GenBank accession no. NG000285).
RT–PCR analysis
RT–PCR analysis was performed according to the protocol described previously (20). First strand of cDNA was synthesized using SuperScript II reverse transcriptase (Gibco BRL) and oligo(dT) primer according to the manufacturer's instructions. For PCR, the following primers were used: 5′-AAACTGGCCCCCATTACCG-3′ and 5′-CAAGTCCAGTTTCCAGATG-3′ corresponding to 180 752–180 967 nt fragment of constitutively expressed Adh gene (GenBank accession no. AE003410.1); 5′-GCATGAGAGGTTTGGCCATATAAGC-3′ and 5′-GGCCCACAGACATCTGAGTGTACTACA-3′ corresponding to 1685–1903 nt for copia (GenBank accession no. M11240); 5′-CCTAAGGAATTAGGGTGGTCCTAAGTTTAC-3′ and 5′-TTCAAAAGGAGGGAGATGTAGTATATACG-3′ corresponding to 7023–7166 nt for mdg1 (GenBank accession no. X59545). Results of RT–PCR analysis was evaluated using ImageQuant5.2.
X-gal staining
X-gal staining of testes was performed according to the protocol described previously (20).
Inverse PCR and detection of the mdg1 transpositions
DNA samples isolated from the progeny of individual crosses of female piwi3/+ to piwi3/piwi3 or piwi3/+ males were analyzed. DNA was isolated according to the standard method (56) from individual F1 and parental flies after gonad removal. DNA (equivalent to one-fourth of a fly, ∼0.2 μg) was digested by HhaI restriction enzyme recognizing 4-bases site in the 5′-untranslated region (5′-UTR) of mdg1. After inactivation of enzyme, restriction fragments were ligated overnight in a 100 μl in the presence of 1 U of T4 ligase (Promega) and precipitated. DNA (1/30 of fly per reaction) was PCR amplified using _mdg1_-specific primers: 5′-CGATCTGAGTGAGTAGAGTGTCAGTGTG-3′ and 5′-CCTAAGGAATTAGGGTGGTCCTAAGTTTAC-3′ [according to _mdg1_ (GenBank accession no. X59545)]. At the annealing temperature of 60°C, 35 cycles were performed in the presence of 5 μCi [α-33P]dATP per reaction. Samples were loaded on a 5% denaturing acrylamide gel and products were visualized by using autoradiography. To detect the sites of the mdg1 transpositions, the new bands, pointing to new insertions, were cut from the gel, eluted and amplified using primers 5′-ATGGATCCGTGTCAGTGTGATGGGAAAAAACAG-3′ and 5′-ATGAATTCGGGTGGTCCTAAGTTTACTTATTAGTTGAC-3′ [according to _mdg1_ (GenBank accession no. X59545)]. Cloning and sequencing of three products allowed us to identify the region of insertions on the X, second and third chromosomes (GenBank accession nos AC104515, AC007291 and AC010011, respectively). Three primers corresponding to the region of insertions (5′-AAGCACAAATTCCTCTCCATAACTTTG-3′; 5′-ACGCGTTGAAGTTTAAGTCAACATG-3′; 5′-GCACGAAATGGTATCATCAGTTTATC-3′) and primer 5′-CCTAAGGAATTAGGGTGGTCCTAAGTTTAC-3′ corresponding to mdg1 LTR were used to demonstrate the presence of insertions in F1 DNA samples and their absence in parental DNAs.
RESULTS
The piwi gene is indispensable for silencing of mdg1 and copia LTR retrotransposons in testes
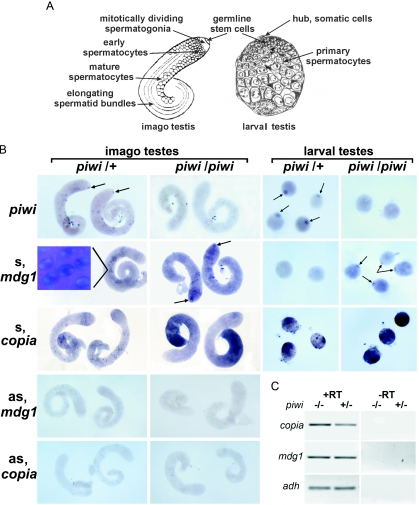
We investigated the effect of piwi mutations on the expression of LTR retrotransposons in testes by in situ RNA analysis using strand-specific riboprobe. piwi2 and piwi3 mutations are caused by the P-element insertions into the coding and 5′-UTRs of the piwi gene, respectively (32). The PIWI protein expression is known to be attributed to a germinal proliferative center at the apical tip of larval and adult testes containing somatic hub cells and mitotically dividing germ stem cells (32,33) (see Figure 1A). piwi transcripts were revealed by in situ RNA hybridization using piwi riboprobe in the region of germinal proliferative center of the piwi3/+ adult and piwi2/+ larval testes (Figure 1B, arrows) in accordance with the the observations reported previously (33). The absence of piwi transcripts in the larval piwi2/piwi2 and adult piwi3/piwi3 testes is closely correlated with the emergence of the mdg1 sense transcripts at the tip regions of testes (Figure 1B, arrows). At the same time, in the remaining part of testes, the level and pattern of the sense mdg1 transcripts is roughly similar in the piwi3/+ and piwi3/piwi3 males (Figure 1B and C). The mdg1 transcripts are localized in spermatocyte nuclei, near the DAPI stained compact regions of chromatin (Figure 1B, inset). Sense copia transcripts are detected in a limited number of spermatocytes in testes of piwi3/+ males (Figure 1B). copia sense transcript accumulation in testes of homozygous piwi3 males is detected in the basal part of testis where postmeiotic stages of spermatogenesis are known to have occurred (Figure 1B). No copia sense transcripts are detected at the apical tip of testes. A significant increase in copia transcript abundance was also observed in spermatocytes of larval testes of homozygous piwi2 males as compared with heterozygous piwi2/+ males (Figure 1B). Thus, the derepression of copia in piwi mutants was observed at the later stages of spermatogenesis, where PIWI protein has not been detected (33). mdg1 and copia antisense transcripts are not detected by in situ RNA hybridization (Figure 1B). RT–PCR analysis confirmed the derepression of copia in testes of piwi mutants (Figure 1C). Amount of copia and mdg1 transcripts, normalized against adh transcripts, increases in testes of piwi2/piwi2 males by 1.9 and 1.3 times, respectively. It is not surprising that the local accumulation of mdg1 transcripts in the region of stem cells exerts no noticeable effect on their total amount evaluated by RT–PCR (Figure 1C).
Figure 1.
piwi mutation relieves silencing of retrotransposons mdg1 and copia in the testes. (A) Diagrammatic representation of imago and larval testes (out of scale) [according to (66) and (67), respectively]. The apical tip of adult testis contains mitotically dividing stem cells and spermatogonial cells; spermatocytes and elongating spermatid bundles are indicated. Tip of larval testis contains the somatic hub cells and the germline cells where PIWI protein is expressed (33). Larger cells of larval testes are represented by mature spermatocytes. (B) In situ RNA hybridization to piwi3/+ and piwi3/piwi3 adult and piwi2/+ and piwi2/piwi2 larval testes of riboprobes detecting sense (s) and antisense (as) transcripts. Genotypes are indicated on the top of the panels; probes are indicated on the left. Arrows show piwi and mdg1 transcripts location in proliferative center. Absence of piwi transcripts in piwi3/piwi3 adult and piwi2/piwi2 larval testes is correlated with a relief of the mdg1 expression in the homozygous piwi mutants. Inset: mdg1 sense transcripts are detected in spermatocyte nuclei. DAPI staining visualizes compact regions of chromosomes in maturating spermatocytes; mdg1 transcripts are detected in non-DAPI stained region of spermatocyte nuclei. copia transcripts are not detected at apical tip. copia expression is enhanced in spermatocytes of piwi2/piwi2 larval testes and in mature spermatocytes and postmeiotic cells of the adult piwi3/piwi3 testes. No antisense (as) mdg1 and copia transcripts are revealed in piwi3/+ and piwi3/piwi3 adult testes. (C) RT–PCR analysis of copia and mdg1 expression in adult testes of piwi3/+ and piwi3/piwi3 males. Coamplification with alcohol dehydrogenase (adh) primers was used to control RNA quantity.
LTR retrotransposons 1731 and GATE have been shown to be transcribed in primary spermatocytes (21,57), but the level of transcripts of these elements detected by in situ RNA hybridization was not affected by piwi mutation (data not shown).
It has been reported that mutations of some Drosophila RNAi genes and piwi cause mislocalization of heterochromatic proteins HP1, HP2 and a loss of histone H3 methylation in nuclei of somatic cells (58), suggesting the role of RNAi machinery in heterochromatin assembly and transcriptional gene silencing. Thus, PIWI might be proposed to be a component of chromatin silencing complex that induces transcriptional repression of transposable elements. However, no effects of mutations in genes encoding HP1 [SU(VAR)02-05] and histone H3 methyltransferases SU(VAR)3-9 and E(Z) on the expression of copia and mdg1 were revealed in testes by in situ RNA analysis (data not shown).
piwi mutation leads to the derepression of a copia LTR–lacZ fusion construct in testes
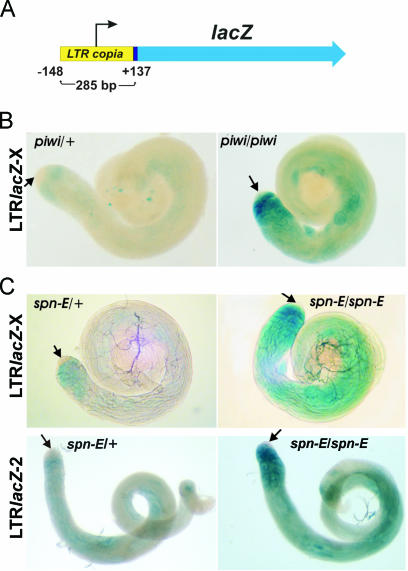
To study whether copia LTR is a target of silencing mediated by piwi, we used a construct containing full-size copia LTR fused to lacZ (_copia_LTR-lacZ). copia LTR comprises the known upstream regulatory region, including 137 bp of the transcribed fragment (54) (Figure 2A). We introduced theX-chromosome containing _copia_LTR-lacZ into the stock carrying piwi2 mutation. The level of reporter gene expression is significantly increased in piwi2/piwi2 testes. No staining at the apical tip of testes is revealed (Figure 2B, arrows). lacZ expression is detected in early spermatocytes as well as in the basal parts of piwi2 homozygous testes where the accumulation of endogenous copia transcripts was observed (Figures 1B and 2B). The difference in the expression patterns of endogenous copia and _copia_LTR-lacZ might be related to the absence of some copia regulatory regions in the transgene as compared with full-size endogenous copies.
Figure 2.
Overexpression of the _copia_LTR-lacZ construct in piwi2 and spn-E1 testes. (A) The _copia_LTR-lacZ construct comprises full-size copia LTR fused to the lacZ reporter. (B) X-gal staining of testes from piwi2 males carrying the _copia_LTR-lacZ construct on the X-chromosome. (C) X-gal staining of testes from spn-E1 males carrying the _copia_LTR-lacZ construct on the X (upper panels) and second chromosomes (lower panels). A level of lacZ expression is greatly increased in the homozygous piwi/piwi and spn-E/spn-E testes as compared with heterozygous piwi/+ and spn-E/+. Arrows indicate the apical tips of testes, where β-galactosidase activity is not detected.
To obtain additional arguments in favor of a relief of _copia_LTR-lacZ expression owing to disturbance of RNA silencing, we tested the effect of mutation of RNAi gene spn-E. spn-E encodes putative RNA-helicase, and was shown to be responsible for the production/stabilization of siRNA necessary to silence the Stellate repeats in testes (27). spn-E mutation causes accumulation of copia transcripts in testes (19). Expression of the _copia_LTR-lacZ reporter constructs located on the X or second chromosomes is significantly increased in testes of the homozygous spn-E1 males (Figure 2C). The patterns of the lacZ expression in testes of the spn-E1 homozygous males are similar to the observed distribution of lacZ expression in testes of piwi2 homozygous males. Thus, copia LTR contains regulatory sequences that confer spn-E and piwi dependence of retrotransposon repression.
mdg1 transposes in the piwi3 male germline
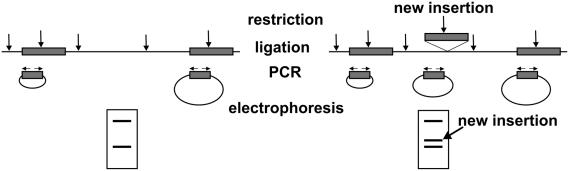
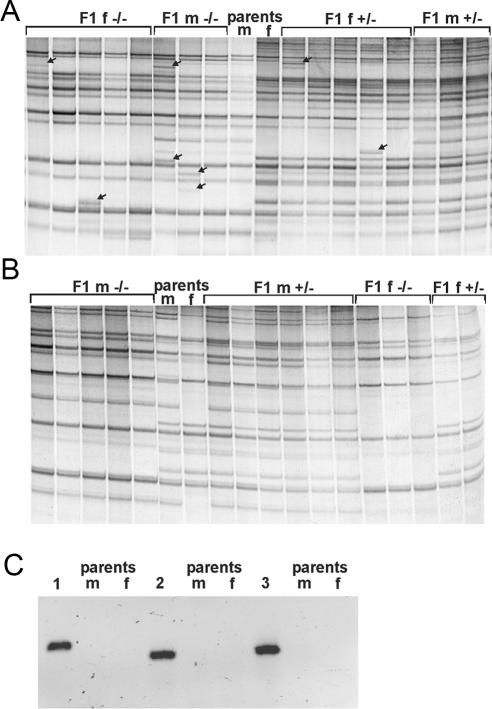
The mdg1 overexpression in piwi mutants is observed in the germinal stem cells, suggesting PIWI protein involvement in the repression of transpositions in the precursors of male gametes. To check the mdg1 transpositions, single piwi3 homozygous males were crossed to the individual piwi3/+ females. A comparative inverse PCR analysis was applied to the single F1 progeny flies and their parents. Inverse PCR approach allows us to estimate the copy number of transposable elements and to detect the newly arisen insertions. Figure 3 shows the rationale of the method. The number of full-length and partial euchromatic mdg1 copies per D.melanogaster genome amounts to 25 copies according to the Release 3 of euchromatic genome sequence (50). We detected ∼35 PCR bands per genome and this surplus number of copies may be contributed by the heterochromatic part of the genome. Our estimation of the number of mdg1 copies corresponds well with the results obtained by using Southern-blot analysis (59). Differences between the patterns of PCR bands detected in individual parental DNAs reflect the polymorphisms of mdg1 sites peculiar to the piwi and balancer chromosomes as well as to the presence of the Y-specific bands in male DNA (Figure 4A and B). The new bands in F1 DNA samples that are non-detected in parental DNAs demonstrate the occurrence of transposition events (Figure 4A). We failed to reveal newly appeared PCR bands in the progeny of piwi3/+ males (Figure 4B), while the number of new insertions in the progeny of the piwi3/piwi3 males amounts to 19 per 101 flies analyzed (Table 1). To confirm the emergence of the new bands as the result of transpositions, three new bands detected in three individuals were cloned and sequenced. The results of sequencing allowed us to attribute the mdg1 insertions in three F1 individuals to the regions of unique genomic DNA on the X, second and third chromosomes. PCR analysis of DNA from F1 and parental flies using primers specific to the insertion sites and the mdg1 LTR demonstrates the occurrence of insertions in F1 samples and ‘empty sites’ in the parental DNAs (Figure 4C). The average frequency of mdg1 transposition in testes of piwi3 mutants amounts to 0.19 transpositions per generation. However, this value may be underestimated as a result of low resolution of high-molecular weight PCR bands. The new PCR bands of the same size were observed in F1 individuals. At least some of these bands may correspond to identical mdg1 insertions, indicating the presence of the germ cell clusters carrying identical sites of insertions and suggesting that transpositions occur at the premeiotic stages of spermatogenesis.
Figure 3.
Detection of the retrotransposon insertions by inverse PCR analysis. Gray boxes represent transposable elements; restriction sites are indicated by vertical arrows and horizontal arrows designate primers. Restriction–ligation of DNA samples followed by PCR using retrotransposon-specific primers revealed PCR products corresponding to insertions of retrotransposon.
Figure 4.
Mobilization of retrotransposon mdg1 in testes of piwi mutants. Genomic DNA from individual flies was digested, ligated and PCR amplified using _mdg1_-specific primers. Genomic distribution of mdg1 copies in DNAs from individual F1 flies are shown to the left and to the right. Pattern of PCR bands of parents are in the middle (m, males; f, females; −/−, piwi3/piwi3; and +/−, piwi3/+). (A) Parents are piwi3/piwi3 males and piwi3/+ females. Mobilization of mdg1 is demonstrated by the new bands revealed in progenitor DNAs (indicated by arrows), which are not detected in parental DNAs. (B) Parents are piwi3/+ males and piwi3/+ females. No new bands differing from parental DNAs were detected in F1 DNA. (C) Detection of mdg1 insertion sites. PCR analysis of DNA from F1 (1, 2 and 3) and parental flies (m and f) using specific primers corresponding to the insertion sites in the X, second and third chromosomes, respectively, and the mdg1 LTR primer. Appearance of insertions in F1 samples (bands) and ‘empty sites’ in parental DNAs are shown.
Table 1.
Estimation of the mdg1 mobility in testes of piwi3/piwi3 (−/−) and piwi3/+ (+/−) males
| Male genotype | Number of analyzed F1 individuals | Number of new PCR bands |
|---|---|---|
| −/− | 50 | 9 |
| −/− | 51 | 10 |
| +/− | 35 | 0 |
| +/− | 16 | 0 |
DISCUSSION
We presented evidence that the piwi gene, coding a member of the Argonaute protein family, is required for the silencing of the retrotransposons in the Drosophila male germline. piwi mutation causes the derepression of retrotransposon mdg1 in the germinal proliferative center and, as a result, relieves transpositions of this mobile element. This observation is the first evidence that mutation in a single gene triggers retrotransposition in the Drosophila germline. We suggest that activation of retrotransposon caused by piwi mutation occurs as a result of the RNAi machinery disturbance. Strong evidence in favor of Drosophila PIWI as a component of RNAi is not obtained, but it has been reported that piwi mutation causes a relief of transgene repeats silencing and diminishing transgene-related siRNA (47). We failed to detect copia and mdg1 antisense transcripts by in situ RNA hybridization suggesting the low level of these transcripts in testes. Antisense transcripts corresponding to LTR sequence of copia were revealed in ovaries (M. S. Klenov, unpublished data). siRNAs corresponding to transposable elements including copia have been detected in Drosophila testes (60) supporting the participation of RNAi-related mechanism in retrotransposon silencing.
Here, we demonstrated that lacZ expression driven by the copia LTR is upregulated in testes of RNAi mutants. piwi and spn-E mutations have similar effect on a reporter gene expression, suggesting the participation of both genes in the same silencing pathway. Thus, LTR is considered as a target of silencing machinery. The role of LTRs in RNAi-mediated regulation of retrotransposons and adjacent host genes in yeast has been emphasized (9). The most abundant repeat-associated siRNAs were related to the LTR of multicopy retotransposon roo in D.melanogaster (60).
Presence of PIWI protein in embryos is restricted to the presumptive gonad. In adults, PIWI is required in somatic signaling cells to maintain germline stem cells (33). Our results show the role of piwi in silencing of the mdg1 retrotransposon in the germinal proliferative cells and copia at the late spermatocytes and postmeiotic stages where PIWI protein is not detected. Thus, PIWI presumably affects silencing mechanisms that are operated at the later stages of spermatogenesis. Effect of piwi may be indirect and mediated through the other targeted genes operating on chromatin level. Actually, the role of PIWI, in concert with HP1 and histone H3 methylation, in the epigenetically inherited silencing has been reported previously (58). However, we revealed no effect of HP1 and SU(VAR)3-9 and E(Z) histone H3 methylases mutations on the expression of mdg1 and copia retrotransposons in testes. Further experiments will elucidate the mechanism of PIWI-mediated retrotransposon silencing.
Transcript abundance of retrotransposons 1731 and GATE, which are active in primary spermatocytes (21,57) is not affected by piwi mutation. It is significant that retrotransposon transcript abundance may not correlate with retrotransposition frequency that is dependent on the DNA copy production and immediate insertion. It has been shown that mutations of RNAi genes in C.elegans, including putative RNA-helicase gene, have resulted only in the activation of the selected DNA transposons (15,61). The similar effects of RNAi silencing affecting selective mobile elements in plant genome have been also reported previously (62). All these results argue against universal RNAi-dependent silencing of retrotransposons. Specific pattern of dsRNA formation, which triggers the RNAi pathway, might be responsible for these differences. Moreover, distinct members of Argonaute family may act in distinct silencing pathways at different developmental stages. Ago2 is essential for siRNA-directed RNA cleavage in Drosophila embryos (46). The regulatory effect of the piwi gene may be realized upstream of the ago2 in the respective silencing mechanism in the germline. Alternatively, both piwi and ago2 products may be indispensable to organize specific RISC complex directed to retrotransposon silencing. In this case, piwi mutation may compromise the functioning of this complex.
We revealed the effect of piwi mutation on the mdg1 mobilization and the derepression of copia in the male germline. Involvement of the other RNAi genes (spn-E, aub and armi) have been revealed in the control of expression of different families of transposable elements in the Drosophila female germline, including the non-LTR retrotransposons HeT-A and I (22). At the same time, a growing body of evidence demonstrates a profound contribution of the RNAi mechanism to the regulation of host development. RNAi genes, including spn-E, aub and armi, were shown to be responsible for specifying the axes of developing oocyte and embryonic patterning in Drosophila (63–65). RNAi genes are proposed to prevent premature translation of the key embryonic determinant oscar via miRNA pathway (65). Thus, a dual function of RNAi-related genes implicated both in the developmental control and in the retrotransposon silencing is unveiled. We suggest that the same RNAi-related mechanism mediated by naturally occurring dsRNA via PIWI protein assistance suppresses mobile element transpositions and regulates host genes ensuring stem cell renewal in testes. Further refining studies are needed to reveal the mechanism ensuring this dual function of RNAi protein complexes.
Acknowledgments
We thank E. Pasyukova and Y. Shevelyov for critical comments, and Y. Abramov, T. Morozova and N. Roshina for technical assistance. The authors are thankful to anonymous reviewers for helpful comments. This work was supported by grants from the Russian Foundation for Basic Researches (N 04-04-48087), INTAS (N 01-0369), the Russian Foundation for Science School (N 2074, 2003.4), RAS programme for Molecular and Cell Biology. The Open Access publication charges for this article were waived by Oxford University Press.
Conflict of interest statement. None declared.
REFERENCES
- 1.Fire A., Xu S., Montgomery M.K., Kostas S.A., Driver S.E., Mello C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 2.Hamilton A.J., Baulcombe D.C. A species of small antisense RNA in posttranscriptional gene silencing in plants. Science. 1999;286:950–952. doi: 10.1126/science.286.5441.950. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein E., Caudy A.A., Hammond S.M., Hannon G.J. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature. 2001;409:363–366. doi: 10.1038/35053110. [DOI] [PubMed] [Google Scholar]
- 4.Elbashir S.M., Lendeckel W., Tuschl T. RNA interference is mediated by 21- and 22-nucleotide RNAs. Genes Dev. 2001;15:188–200. doi: 10.1101/gad.862301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zamore P.D., Tuschl T., Sharp P.A., Bartel D.P. RNAi: double-stranded RNA directs the ATP-dependent cleavage of mRNA at 21 to 23 nucleotide intervals. Cell. 2000;101:25–33. doi: 10.1016/S0092-8674(00)80620-0. [DOI] [PubMed] [Google Scholar]
- 6.Hammond S.M., Bernstein E., Beach D., Hannon G.J. An RNA-directed nuclease mediates post-transcriptional gene silencing in Drosophila cells. Nature. 2000;404:293–296. doi: 10.1038/35005107. [DOI] [PubMed] [Google Scholar]
- 7.Mette M.F., Aufsatz W., van der Winden J., Matzke M.A., Matzke A.J. Transcriptional silencing and promoter methylation triggered by double-stranded RNA. EMBO J. 2000;19:5194–5201. doi: 10.1093/emboj/19.19.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Volpe T.A., Kidner C., Hall I.M., Teng G., Grewal S.I., Martienssen R.A. Regulation of heterochromatic silencing and histone H3 lysine-9 methylation by RNAi. Science. 2002;297:1833–1837. doi: 10.1126/science.1074973. [DOI] [PubMed] [Google Scholar]
- 9.Schramke V., Allshire R. Hairpin RNAs and retrotransposon LTRs effect RNAi and chromatin-based gene silencing. Science. 2003;301:1069–1074. doi: 10.1126/science.1086870. [DOI] [PubMed] [Google Scholar]
- 10.Verdel A., Jia S., Gerber S., Sugiyama T., Gygi S., Grewal S.I., Moazed D. RNAi-mediated targeting of heterochromatin by the RITS complex. Science. 2004;303:672–676. doi: 10.1126/science.1093686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Kaff N.S., Covey S.N., Kreike M.M., Page A.M., Pinder R., Dale P.J. Transcriptional and posttranscriptional plant gene silencing in response to a pathogen. Science. 1998;279:2113–2115. doi: 10.1126/science.279.5359.2113. [DOI] [PubMed] [Google Scholar]
- 12.Baulcombe D. RNA silencing in plants. Nature. 2004;431:356–363. doi: 10.1038/nature02874. [DOI] [PubMed] [Google Scholar]
- 13.Tabara H., Sarkissian M., Kelly W.G., Fleenor J., Grishok A., Timmons L., Fire A., Mello C.C. The rde-1 gene, RNA interference, and transposon silencing in C.elegans. Cell. 1999;99:123–132. doi: 10.1016/s0092-8674(00)81644-x. [DOI] [PubMed] [Google Scholar]
- 14.Lankenau S., Corces V.G., Lankenau D.H. The Drosophila micropia retrotransposon encodes a testis-specific antisense RNA complementary to reverse transcriptase. Mol. Cell. Biol. 1994;14:1764–1775. doi: 10.1128/mcb.14.3.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sijen T., Plasterk R.H. Transposon silencing in the Caenorhabditis elegans germ line by natural RNAi. Nature. 2003;426:310–314. doi: 10.1038/nature02107. [DOI] [PubMed] [Google Scholar]
- 16.Ketting R.F., Haverkamp T.H., van Luenen H.G., Plasterk R.H. Mut-7 of C.elegans, required for transposon silencing and RNA interference, is a homolog of Werner syndrome helicase and RNaseD. Cell. 1999;99:133–141. doi: 10.1016/s0092-8674(00)81645-1. [DOI] [PubMed] [Google Scholar]
- 17.Vastenhouw N.L., Fischer S.E., Robert V.J., Thijssen K.L., Fraser A.G., Kamath R.S., Ahringer J., Plasterk R.H. A genome-wide screen identifies 27 genes involved in transposon silencing in C.elegans. Curr. Biol. 2003;13:1311–1316. doi: 10.1016/s0960-9822(03)00539-6. [DOI] [PubMed] [Google Scholar]
- 18.Shi H., Djikeng A., Tschudi C., Ullu E. Argonaute protein in the early divergent eukaryote Trypanosoma brucei: control of small interfering RNA accumulation and retroposon transcript abundance. Mol. Cell. Biol. 2004;24:420–427. doi: 10.1128/MCB.24.1.420-427.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stapleton W., Das S., McKee B.D. A role of the Drosophila homeless gene in repression of Stellate in male meiosis. Chromosoma. 2001;110:228–240. doi: 10.1007/s004120100136. [DOI] [PubMed] [Google Scholar]
- 20.Aravin A.A., Naumova N.M., Tulin A.V., Vagin V.V., Rozovsky Y.M., Gvozdev V.A. Double-stranded RNA-mediated silencing of genomic tandem repeats and transposable elements in the D.melanogaster germline. Curr. Biol. 2001;11:1017–1027. doi: 10.1016/s0960-9822(01)00299-8. [DOI] [PubMed] [Google Scholar]
- 21.Kogan G.L., Tulin A.V., Aravin A.A., Abramov Y.A., Kalmykova A.I., Maisonhaute C., Gvozdev V.A. The GATE retrotransposon in Drosophila melanogaster: mobility in heterochromatin and aspects of its expression in germline tissues. Mol. Genet. Genomics. 2003;269:234–242. doi: 10.1007/s00438-003-0827-1. [DOI] [PubMed] [Google Scholar]
- 22.Vagin V.V., Klenov M.S., Kalmykova A.I., Stolyarenko A.D., Kotelnikov R.N., Gvozdev V.A. The RNA interference proteins and vasa locus are involved in the silencing of retrotransposons in the female germline of Drosophila melanogaster. RNA Biol. 2004;1:51–55. [PubMed] [Google Scholar]
- 23.Svoboda P., Stein P., Anger M., Bernstein E., Hannon G.J., Schultz R.M. RNAi and expression of retrotransposons MuERV-L and IAP in preimplantation mouse embryos. Dev. Biol. 2004;269:276–285. doi: 10.1016/j.ydbio.2004.01.028. [DOI] [PubMed] [Google Scholar]
- 24.Wu-Scharf D., Jeong B., Zhang C., Cerutti H. Transgene and transposon silencing in Chlamydomonas reinhardtii by a DEAH-Box RNA helicase. Science. 2000;290:1159–1163. doi: 10.1126/science.290.5494.1159. [DOI] [PubMed] [Google Scholar]
- 25.Mochizuki K., Fine N.A., Fujisawa T., Gorovsky M.A. Analysis of a piwi-related gene implicates small RNAs in genome rearrangement in tetrahymena. Cell. 2002;110:689–699. doi: 10.1016/s0092-8674(02)00909-1. [DOI] [PubMed] [Google Scholar]
- 26.Gvozdev V.A., Aravin A.A., Abramov Y.A., Klenov M.S., Kogan G.L., Lavrov S.A., Naumova N.M., Olenkina O.M., Tulin A.V., Vagin V.V. Stellate repeats: targets of silencing and modules causing cis-inactivation and trans-activation. Genetica. 2003;117:239–245. doi: 10.1023/a:1022952315467. [DOI] [PubMed] [Google Scholar]
- 27.Aravin A.A., Klenov M.S., Vagin V.V., Bantignies F., Cavalli G., Gvozdev V.A. Dissection of a natural RNA silencing process in the Drosophila melanogaster germ line. Mol. Cell. Biol. 2004;24:6742–6750. doi: 10.1128/MCB.24.15.6742-6750.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nuzhdin S.V., Mackay T.F. The genomic rate of transposable element movement in Drosophila melanogaster. Mol. Biol. Evol. 1995;12:180–181. doi: 10.1093/oxfordjournals.molbev.a040188. [DOI] [PubMed] [Google Scholar]
- 29.Robert V., Prud'homme N., Kim A., Bucheton A., Pelisson A. Characterization of the flamenco region of the Drosophila melanogaster genome. Genetics. 2001;158:701–713. doi: 10.1093/genetics/158.2.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Desset S., Meignin C., Dastugue B., Vaury C. COM, a heterochromatic locus governing the control of independent endogenous retroviruses from Drosophila melanogaster. Genetics. 2003;164:501–509. doi: 10.1093/genetics/164.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bucheton A., Busseau I., Teninges D. I Elements in Drosophila melanogaster. In: Craig N.L., Craigie R., Gellert M., Lambowitz A.M., editors. Mobile DNA II. Washington, DC: ASM Press; 2002. pp. 796–813. [Google Scholar]
- 32.Cox D.N., Chao A., Baker J., Chang L., Qiao D., Lin H. A novel class of evolutionarily conserved genes defined by piwi are essential for stem cell self-renewal. Genes Dev. 1998;12:3715–3727. doi: 10.1101/gad.12.23.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cox D.N., Chao A., Lin H. piwi encodes a nucleoplasmic factor whose activity modulates the number and division rate of germline stem cells. Development. 2000;127:503–514. doi: 10.1242/dev.127.3.503. [DOI] [PubMed] [Google Scholar]
- 34.Bohmert K., Camus I., Bellini C., Bouchez D., Caboche M., Benning C. AGO1 defines a novel locus of Arabidopsis controlling leaf development. EMBO J. 1998;17:170–180. doi: 10.1093/emboj/17.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moussian B., Schoof H., Haecker A., Jurgens G., Laux T. Role of the ZWILLE gene in the regulation of central shoot meristem cell fate during Arabidopsis embryogenesis. EMBO J. 1998;17:1799–1809. doi: 10.1093/emboj/17.6.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lynn K., Fernandez A., Aida M., Sedbrook J., Tasaka M., Masson P., Barton M.K. The PINHEAD/ZWILLE gene acts pleiotropically in Arabidopsis development and has overlapping functions with the ARGONAUTE1 gene. Development. 1999;126:469–481. doi: 10.1242/dev.126.3.469. [DOI] [PubMed] [Google Scholar]
- 37.Sharma A.K., Nelson M.C., Brandt J.E., Wessman M., Mahmud N., Weller K.P., Hoffman R. Human CD34+ stem cells express the hiwi gene, a human homologue of the Drosophila gene piwi. Blood. 2001;97:426–434. doi: 10.1182/blood.v97.2.426. [DOI] [PubMed] [Google Scholar]
- 38.Qiao D., Zeeman A.M., Deng W., Looijenga L.H., Lin H. Molecular characterization of hiwi, a human member of the piwi gene family whose overexpression is correlated to seminomas. Oncogene. 2002;21:3988–3999. doi: 10.1038/sj.onc.1205505. [DOI] [PubMed] [Google Scholar]
- 39.Carmell M.A., Xuan Z., Zhang M.Q., Hannon G.J. The Argonaute family: tentacles that reach into RNAi, developmental control, stem cell maintenance, and tumorigenesis. Genes Dev. 2002;16:2733–2742. doi: 10.1101/gad.1026102. [DOI] [PubMed] [Google Scholar]
- 40.Grewal S.I., Rice J.C. Regulation of heterochromatin by histone methylation and small RNAs. Curr. Opin. Cell Biol. 2004;16:230–238. doi: 10.1016/j.ceb.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 41.Bartel D.P. MicroRNAs: genomics, biogenesis, mechanism and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 42.Song J.J., Liu J., Tolia N.H., Schneiderman J., Smith S.K., Martienssen R.A., Hannon G.J., Joshua-Tor L. The crystal structure of the Argonaute2 PAZ domain reveals an RNA binding motif in RNAi effector complexes. Nature Struct. Biol. 2003;10:1026–1032. doi: 10.1038/nsb1016. [DOI] [PubMed] [Google Scholar]
- 43.Lingel A., Simon B., Izaurralde E., Sattler M. Nucleic acid 3′-end recognition by the Argonaute2 PAZ domain. Nature Struct. Mol. Biol. 2004;11:576–577. doi: 10.1038/nsmb777. [DOI] [PubMed] [Google Scholar]
- 44.Song J.J., Smith S.K., Hannon G.J., Joshua-Tor L. Crystal structure of Argonaute and its implications for RISC slicer activity. Science. 2004;305:1434–1437. doi: 10.1126/science.1102514. [DOI] [PubMed] [Google Scholar]
- 45.Liu J., Carmell M.A., Rivas F.V., Marsden C.G., Thomson J.M., Song J.J., Hammond S.M., Joshua-Tor L., Hannon G.J. Argonaute2 is the catalytic engine of mammalian RNAi. Science. 2004;305:1437–1441. doi: 10.1126/science.1102513. [DOI] [PubMed] [Google Scholar]
- 46.Okamura K., Ishizuka A., Siomi H., Siomi M.C. Distinct roles for Argonaute proteins in small RNA-directed RNA cleavage pathways. Genes Dev. 2004;18:1655–1666. doi: 10.1101/gad.1210204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pal-Bhadra M., Bhadra U., Birchler J.A. RNAi related mechanisms affect both transcriptional and posttranscriptional transgene silencing in Drosophila. Mol. Cell. 2002;9:315–327. doi: 10.1016/s1097-2765(02)00440-9. [DOI] [PubMed] [Google Scholar]
- 48.Sarot E., Payen-Groschene G., Bucheton A., Pelisson A. Evidence for a piwi-dependent RNA silencing of the gypsy endogenous retrovirus by the Drosophila melanogaster flamenco gene. Genetics. 2004;166:1313–1321. doi: 10.1534/genetics.166.3.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eickbush T.H., Malik H.S. Origins and evolution of retrotransposons. In: Craig N.L., Craigie R., Gellert M., Lambowitz A.M, editors. Mobile DNA II. Washington, DC: ASM Press; 2002. pp. 1111–1144. [Google Scholar]
- 50.Kaminker J.S., Bergman C.M., Kronmiller B., Carlson J., Svirskas R., Patel S., Frise E., Wheeler D.A., Lewis S.E., Rubin G.M., Ashburner M., Celniker S.E. The transposable elements of the Drosophila melanogaster euchromatin: a genomics perspective. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-12-research0084. RESEARCH0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nuzhdin S.V., Mackay T.F. Direct determination of retrotransposon transposition rates in Drosophila melanogaster. Genet. Res. 1994;63:139–144. doi: 10.1017/s0016672300032249. [DOI] [PubMed] [Google Scholar]
- 52.Garcia Guerreiro M.P., Biemont C. Changes in the chromosomal insertion pattern of the process of making chromosomes homozygous in Drosophila melanogaster. Mol. Genet. Genomics. 1995;264:206–211. doi: 10.1007/BF00294683. [DOI] [PubMed] [Google Scholar]
- 53.Lin H., Spradling A.C. A novel group of pumilio mutations affects the asymmetric division of germline stem cells in the Drosophila ovary. Development. 1997;124:2463–2476. doi: 10.1242/dev.124.12.2463. [DOI] [PubMed] [Google Scholar]
- 54.Morozova T.V., Tsybulko E.A., Filatov D.A., Pasyukova E.G. Impact of the regulatory regions of retrotransposon copia on the level of its expression in testes of Drosophila melanogaster. Genetika. 2004;40:173–179. [PubMed] [Google Scholar]
- 55.Ilyin Y., Chmeliauskaite V.G., Kulguskin V.V., Georgiev G.P. Mobile dispersed genetic element MDG1 of Drosophila melanogaster: transcription pattern. Nucleic Acids Res. 1980;8:5347–5361. doi: 10.1093/nar/8.22.5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ashburner M. Drosophila: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. pp. 106–107. [Google Scholar]
- 57.Haoudi A., Rachidi M., Kim M.H., Champion S., Best-Belpomme M., Maisonhaute C. Developmental expression analysis of the 1731 retrotransposon reveals an enhancement of Gag-Pol frameshifting in males of Drosophila melanogaster. Gene. 1997;196:83–93. doi: 10.1016/s0378-1119(97)00203-5. [DOI] [PubMed] [Google Scholar]
- 58.Pal-Bhadra M., Leibovitch B.A., Gandhi S.G., Rao M., Bhadra U., Birchler J.A., Elgin S.C. Heterochromatic silencing and HP1 localization in Drosophila are dependent on the RNAi machinery. Science. 2004;303:669–672. doi: 10.1126/science.1092653. [DOI] [PubMed] [Google Scholar]
- 59.Alonso-Gonzalez L., Dominguez A., Albornoz J. Structural heterogeneity and genomic distribution of Drosophila melanogaster LTR retrotransposons. Mol. Biol. Evol. 2003;20:401–409. doi: 10.1093/molbev/msg047. [DOI] [PubMed] [Google Scholar]
- 60.Aravin A.A., Lagos-Quintana M., Yalcin A., Zavolan M., Marks D., Snyder B., Gaasterland T., Meyer J., Tuschl T. The small RNA profile during Drosophila melanogaster development. Dev. Cell. 2003;5:337–350. doi: 10.1016/s1534-5807(03)00228-4. [DOI] [PubMed] [Google Scholar]
- 61.Tijsterman M., Ketting R.F., Okihara K.L., Sijen T., Plasterk R.H. RNA helicase MUT-14-dependent gene silencing triggered in C.elegans by short antisense RNAs. Science. 2002;295:694–697. doi: 10.1126/science.1067534. [DOI] [PubMed] [Google Scholar]
- 62.Zilberman D., Cao X., Jacobsen S.E. ARGONAUTE4 control of locus-specific siRNA accumulation and DNA and histone methylation. Science. 2003;299:716–719. doi: 10.1126/science.1079695. [DOI] [PubMed] [Google Scholar]
- 63.Gillespie D.E., Berg C.A. homeless is required for RNA localization in Drosophila oogenesis and encodes a new member of the DE-H family of RNA-dependent ATPases. Genes Dev. 1995;9:2495–2508. doi: 10.1101/gad.9.20.2495. [DOI] [PubMed] [Google Scholar]
- 64.Wilson J.E., Connell J.E., Macdonald P.M. aubergine enhances oskar translation in the Drosophila ovary. Development. 1996;122:1631–1639. doi: 10.1242/dev.122.5.1631. [DOI] [PubMed] [Google Scholar]
- 65.Cook H.A., Koppetsch B.S., Wu J., Theurkauf W.E. The Drosophila SDE3 homolog armitage is required for oskar mRNA silencing and embryonic axis specification. Cell. 2004;116:817–829. doi: 10.1016/s0092-8674(04)00250-8. [DOI] [PubMed] [Google Scholar]
- 66.Hiller M.A., Lin T.Y., Wood C., Fuller M.T. Developmental regulation of transcription by a tissue-specific TAF homolog. Genes Dev. 2001;15:1021–1030. doi: 10.1101/gad.869101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fuller M.T. Spermatogenesis. In: Bate M., Martinez Arias A., editors. Development of Drosophila melanogaster. NY: Cold Spring Harbor Laboratory Press; 1993. [Google Scholar]