Role of Intimin and Bundle-Forming Pili in Enteropathogenic Escherichia coli Adhesion to Pediatric Intestinal Tissue In Vitro (original) (raw)
Abstract
Attaching and effacing (A/E) lesion formation is central to enteropathogenic Escherichia coli (EPEC) pathogenesis. In vitro experiments with human epithelial cell lines have implicated virulence plasmid-encoded bundle-forming pili (BFP) in initial binding and intimin in intimate attachment and A/E lesion formation. This study investigated the role of BFP and intimin in EPEC interactions with pediatric small intestinal biopsy tissue in in vitro organ culture. Organ culture infections (2 to 8 h) were performed with E2348/69 (a wild-type EPEC O127:H6 clinical isolate) and E2348/69 derivatives including CVD206 (eae deficient), CVD206(pCVD438) (eae_-complemented CVD206), CVD206(pCVD438/01) (expressing intimin, which is nonfunctional due to a single amino acid substitution), JPN15 (spontaneous EPEC adherence factor virulence plasmid-cured E2348/69), and 31-6-1(1) (E2348/69 with a Tn_phoA insertion inactivation mutation in the virulence plasmid-encoded bfpA gene). Scanning and transmission electron microscopy revealed that after 8 h E2348/69 and CVD206(pCVD438) (both Int+ BFP+) adhered to all specimens, causing A/E lesions with surrounding microvillous elongation. JPN15 and 31-6-1(1) (both Int+ BFP−) adhered and caused A/E lesions although bacteria adhered in “flat,” two-dimensional groups. CVD206 and CVD206(pCVD438/01) (both Int− BFP+) did not adhere to any sample, and no pathological tissue changes were seen. Thus, in human intestinal organ culture, BFP do not appear to be involved in the initial stages of EPEC nonintimate adhesion but are implicated in the formation of complex, three-dimensional colonies via bacterium-bacterium interactions. Intimin appears to play an essential role in establishing colonization of EPEC on pediatric small intestinal tissue.
Enteropathogenic Escherichia coli (EPEC) is an important cause of severe diarrhea in developing countries, particularly among young infants (4, 17, 36). Proximal small intestinal biopsies taken from EPEC-infected children show bacteria adherent to the mucosal surface in discrete, localized colonies. Electron microscopic examination reveals bacteria adherent to cup-like pedestals with effacement of microvilli (44, 48), constituting what is known as the attaching and effacing (A/E) lesion (41), which is central to EPEC pathogenesis.
Observations from experiments using cultured human epithelial cell lines have implicated several genes and their protein products in A/E lesion formation, and a three-stage model of EPEC infection has been suggested (6). The first stage of the infection involves initial, nonintimate attachment of the bacillus to the enterocyte cell surface via bundle-forming pili (BFP) (16) encoded by the bfp gene cluster (46, 47) on the 60- to 70-kDa EPEC adherence factor (EAF) plasmid (1). The second stage of the infection is thought to involve a type III secretion apparatus that mediates the secretion of EPEC-secreted proteins (22). These proteins are implicated in triggering a host cell signal transduction cascade (10, 27) resulting in cytoskeletal component rearrangement and microvillous elongation and vesiculation (42). Recent data have provided evidence that EPEC transfers a protein into the host cell which is then phosphorylated and inserted in the membrane to create a cell surface binding epitope for intimin (a 94-kDa outer membrane adhesin encoded by the chromosomal eae gene [24]). The protein has been called the translocated intimin receptor or Tir (26). It was previously referred to as Hp90 and was thought to be of host cell origin (43). The third stage of infection according to this model is characterized by intimin-mediated intimate attachment and accumulation of polymerized actin in pedestals at sites of intimate bacterial attachment (24), a process which is also thought to be triggered by Tir (26). Deletion of the eae gene has been shown to reduce EPEC pathogenicity in human volunteer challenge studies (8). Experiments have shown that eae deletion mutants can adhere to cultured epithelial cells but do not adhere intimately or form A/E lesions (5). Conversely, Knutton et al. (30) showed that a wild-type EPEC strain spontaneously cured of the EAF plasmid, and therefore deficient in BFP expression, was still able to adhere to cultured adult intestinal mucosa in small microcolonies (although in reduced numbers) and form A/E lesions, suggesting that the plasmid is not required for later-stage A/E lesion formation. All the genes necessary for the second and third stages of A/E lesion formation are reported to be contained within a 35-kbp chromosomal pathogenicity island called the locus of enterocyte effacement region (39, 40). Plasmid-encoded regulatory genes (per genes) have been described which activate intimin expression (18). More recently, EPEC per genes have been shown to down-regulate intimin expression following A/E lesion formation (35).
In vitro organ culture (IVOC) with human intestinal tissue has been used to study EPEC pathogenesis (20, 30). Using this as a more appropriate model of the in vivo environment, Knutton and colleagues (30) showed that EPEC isolated from cases of infant diarrhea was able to form A/E lesions on adult duodenal mucosa which were indistinguishable from A/E lesions seen in vivo. EPEC pathogenesis was studied further in this work, and this report questions the role of BFP in initial nonintimate attachment to the mucosa and emphasizes the importance of intimin in establishing colonization of pediatric small intestinal tissue.
(Preliminary findings have been published previously in abstract form [21].)
MATERIALS AND METHODS
Bacterial strains.
The bacterial strains examined in this study were E2348/69 (a wild-type EPEC) (37) and five E2348/69 derivatives: CVD206 (5), CVD206(pCVD438) (24), CVD206(pCVD438/01) (11), JPN15 (23), and 31-6-1(1) (7). A summary of the strain characteristics is shown in Table 1. CVD206(pCVD438/01) (11) was included because although intimin is biologically inactive in this strain it is still inserted into the outer membrane and therefore there should be less disruption of the constitution of the outer membrane. All bacterial strains were stored routinely at −70°C in a Microbank system (Prolab Diagnostics, Neston, England). Prior to adhesion studies, bacterial strains were subcultured into brain heart infusion broth (containing chloramphenicol at a concentration of 12 μg/ml or kanamycin at a concentration of 20 μg/ml where appropriate) and incubated aerobically overnight at 37°C without agitation.
TABLE 1.
Bacterial strains
| Strain | Description | Reference | Presence of: | FAS test result | |
|---|---|---|---|---|---|
| Functional intimin | bfpA/BFP | ||||
| E2348/69 | Wild-type EPEC (O127:H6) | 36 | + | + | + |
| JPN15 | E2348/69 cured of EAF plasmid | 23 | + | − | + |
| 31-6-1(1) | E2348/69 mutated in bfpAa, Kanr | 7 | + | − | + |
| CVD206 | E2348/69 mutated in eae | 5 | − | + | − |
| CVD206(pCVD438) | CVD206 complemented with eae, Cmr | 24 | + | + | + |
| CVD206(pCVD438/01) | CVD206 complemented with eae mutated to produce Cys937 to Ala substitution, intimin expressed in an inactive form | 11 | − | + | − |
Immunostaining of HEp-2 cell-adherent EPEC.
To ensure that the EPEC strains were capable of expressing the appropriate surface antigens HEp-2 cell-adherent bacteria were examined by the following immunostaining techniques. A standard 3-h HEp-2 cell assay was performed (3) with cells grown overnight on 13-mm-diameter glass coverslips to a confluency of approximately 80%. After incubation with the bacterial strains, cells were washed thoroughly five times with sterile phosphate-buffered saline (PBS) and fixed with 4% buffered formalin for 90 min. After repeated washing with PBS, cells and adherent bacteria were incubated at room temperature for 30 min with nonimmune swine serum (diluted 1:10 in PBS) to block nonspecific binding sites. Cells were stained for 1 h with a rabbit polyclonal antibody raised against the cell binding domain of intimin from an O127:H6 isolate (35) (1:100) or with a polyclonal antibody raised against the bundlin subunit of BFP (1:500) (kindly donated by Jorge Girón). Negative controls included the omission of either primary antibody and the use of an unrelated polyclonal (anti-O114) antibody. Anti-E. coli antibody (Dako Ltd, High Wycombe, United Kingdom) was used as a positive control. Goat anti-rabbit immunoglobulin G-fluorescein isothiocyanate (1:100) was used, for 1 h at room temperature, as the secondary antibody. All coverslips were mounted by using Citifluor fluorescence mounting medium (Agar Scientific Ltd, Stansted, United Kingdom) and were stored in the dark at 4°C until being viewed with a Zeiss Universal microscope with appropriate fluorescein isothiocyanate filters.
FAS test.
Each of the six EPEC strains was examined by using the fluorescent-actin staining (FAS) test. This test was performed by the method described by Knutton and colleagues (31).
SEM of HEp-2 cell-adherent EPEC.
To control for the effects of bacterial growth conditions and scanning electron microscopy (SEM) processing, E2348/69 and CVD206 were cultured overnight as described above and incubated with HEp-2 cells (grown on 13-mm-diameter coverslips also as described above) for 1, 2, 3, and 6 h. At the end of the incubation the coverslips were washed thoroughly three times in sterile PBS and processed routinely for SEM.
Tissue samples.
Tissue samples were obtained from 14 children, with fully informed parental consent and ethical approval, from the distal duodenum or duodeno-jejunal junction with either a double-port pediatric Crosby capsule (28) or a grasp biopsy forceps during routine endoscopic (Olympus PCF pediatric endoscope) investigation of intestinal disorders. Terminal ileal tissue was taken from seven patients by the grasp biopsy method from areas showing no endoscopic abnormality or from the ileal margin of surgically resected tissue showing no disease involvement (one patient). The age range of patients (19 males and 3 females) was between 12 and 190 months (median, 102 months). All intestinal histologies were reported to be normal.
Organ culture adhesion assay.
IVOC was performed as described previously (20). The assay was terminated at 2 and 4 h on three occasions to determine “early” events. “Later” events were observed at 6 h on two occasions, and all other experiments were maintained for 8 h. Tissue culture medium was changed every 2 h, although these changes were not complete as some medium plus bacterial inoculum would remain within the foam insert supporting the tissue sample and in association with the tissue by surface tension. Each bacterial strain was examined in IVOC on at least three occasions with tissue from different children. Strain E2348/69 and an uninoculated specimen were included with each experimental culture to act as appropriate positive and negative controls, respectively.
Tissue processing.
After the culture period specimens were washed thoroughly three times to remove any nonadherent bacteria and then prepared for SEM as described previously (20). Following fixation and dehydration, specimens were placed in 100% ethanol and critical point dried in liquid CO2 with an Emitech K850 critical point drying apparatus. Samples were then mounted on aluminium stubs and sputter coated with gold-palladium by using a Polaron sputter coating apparatus. Specimens were observed with a JEOL JSM-5300 SEM at an accelerating voltage of 30 kV. For transmission electron microscopy (TEM) postfixed specimens were dehydrated in 2,2-dimethoxypropane with three changes over 9 min and embedded in TAAB resin (TAAB Laboratories, Reading, United Kingdom). Ultrathin sections (0.1 μm) were double stained with 2% uranyl acetate and lead citrate and examined in a JEOL JEM 1200-EX II TEM at an accelerating voltage of 80 kV.
RESULTS
Immunostaining of HEp-2 cell-adherent EPEC.
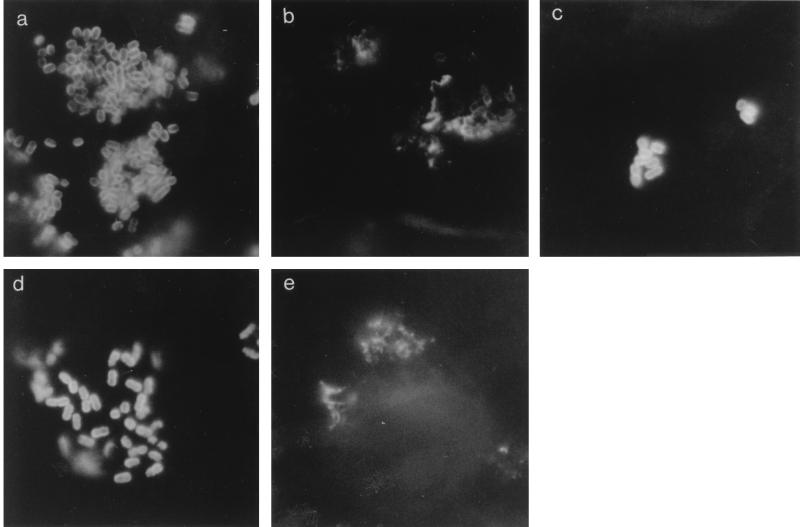
Prior to infection of IVOC we confirmed, by indirect immunofluorescence staining, that the E2348/69 derivatives were capable of expressing intimin and/or BFP. All six strains adhered to HEp-2 cells. Results from negative and positive controls were as expected: no fluorescing bacteria were seen on any coverslip stained either with anti-O114 or with the primary antibody stage omitted despite many bacteria being observed by light microscopy. All six EPEC strains were strongly fluorescent with anti-E. coli sera. Localized colonies were formed by E2348/69 and CVD206(pCVD438); small clusters of adhering bacteria were seen with strains 31-6-1(1), JPN15, CVD206, and CVD206(pCVD438/01). EPEC E2348/69 reacted positively with anti-intimin and with anti-BFP; a smooth fluorescent pattern was noted around bacteria stained with anti-intimin (Fig. 1a) and a more spiky pattern was noted around bacteria stained with anti-BFP (Fig. 1b). Similar staining patterns were noted with CVD206(pCVD438) (data not shown) and CVD206(pCVD438/01); the latter strain was positive for the anti-intimin antibody (Fig. 1c) despite expressing an inactive intimin molecule. EAF plasmid-cured strain JPN15 (data not shown) and _bfpA_-mutated 31-6-1(1) (Fig. 1d) reacted positively with anti-intimin but not with anti-BFP. EPEC CVD206 showed no fluorescing bacteria when stained with anti-intimin but had a positive fluorescing pattern with anti-BFP (Fig. 1e).
FIG. 1.
Panel of immunofluorescence micrographs of HEp-2 cell-adherent bacteria stained with anti-intimin or anti-BFP. (a) E2348/69 stained with anti-intimin, (b) E2348/69 stained with anti-BFP, (c) CVD206(pCVD438/01) stained with anti-intimin, (d) _bfpA_-mutated 31-6-1(1) stained with anti-intimin, (e) CVD206 stained with anti-BFP.
Strains expressing functional intimin were FAS test positive, and the other (two) strains were FAS test negative (Table 1).
SEM of adhesion of EPEC E2468/69 and derivative CVD206 to HEp-2 cells.
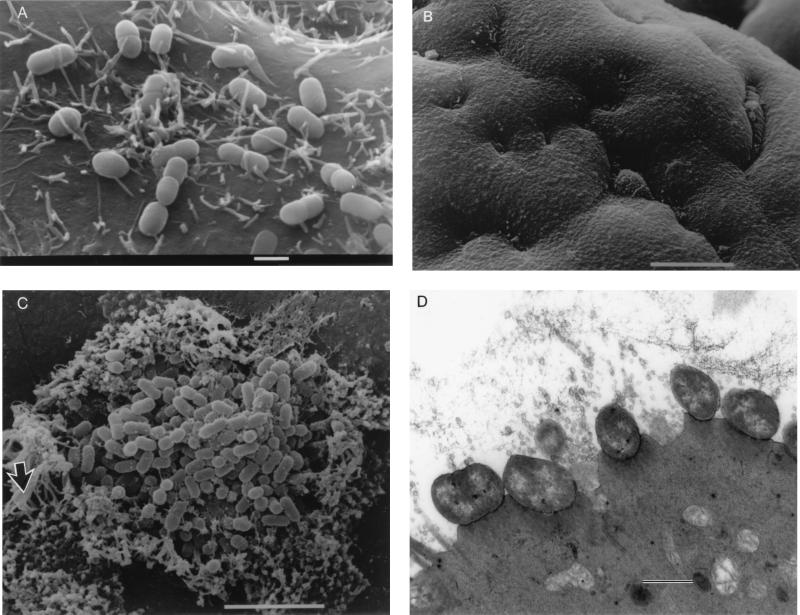
Bacteria were identified adhering to the cells at all time points. E2348/69 showed A/E lesion formation at 2 to 3 h and completely covered the cells at 6 h (data not shown). CVD206 did not show A/E lesion formation, and there was an increase in the number of adhering bacteria from 1 to 3 h (Fig. 2A) and a decrease at 6 h.
FIG. 2.
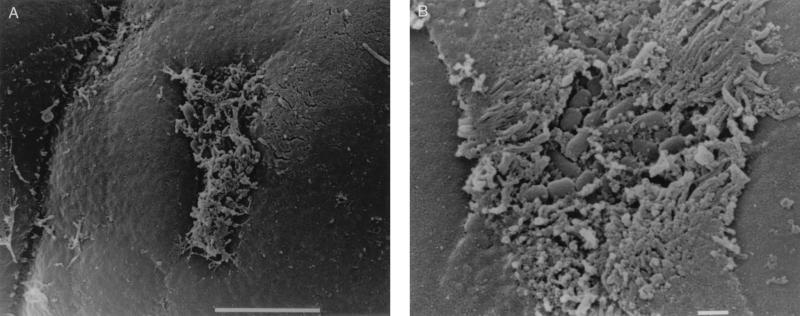
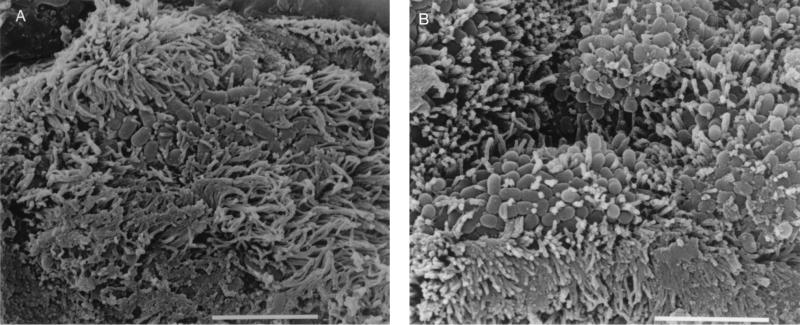
(A) Scanning electron micrograph of CVD206 adhering to HEp-2 cells after 3 h of incubation. Bar = 1 μm. (B) Scanning electron micrograph of uninoculated small intestinal tissue after 8 h in organ culture. Bar = 10 μm. (C) Pediatric ileal biopsy tissue incubated with E2348/69 for 8 h. Bacteria adhere in localized colonies causing extreme microvillous elongation (arrow) and vesiculation. Bar = 5 μm. (D) Transmission electron micrograph showing A/E lesions with microvillous vesiculation caused by E2348/69. Bar = 0.5 μm. (E) CVD206 causing no tissue damage or adhesion to small intestinal tissue. Bar = 5 μm. (F) CVD206(pCVD438) adhering in localized colonies to small intestinal mucosa with an appearance similar to that of E2348/69 shown in panel C. Bar = 5 μm. (G) CVD206(pCVD438/01) with an appearance similar to that of CVD206 shown in panel E. Bar = 5 μm.
Adhesion of EPEC E2348/69 and Int+ BFP+ and Int− BFP+ derivatives to cultured pediatric small intestinal mucosa.
At all time points uninoculated IVOC specimens appeared morphologically normal. These showed good preservation of villous architecture, an intact epithelial surface without loss of the glycocalyx, and no evidence of excess extrusion of enterocytes or mucus (Fig. 2B). No adherent bacteria were noted on the surface of these specimens.
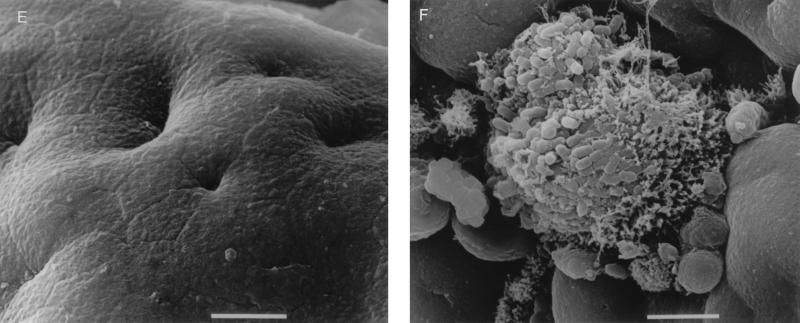
After 6 to 8 h of culture, specimens incubated with E2348/69 (Int+ BFP+) showed evidence of increased rounding up and extrusion of cells compared to uninoculated controls. E2348/69 adhered to all specimens in a clustered, localized manner similar to that seen on HEp-2 cells with surrounding microvillous elongation. This is indicative of A/E lesion formation as determined by SEM (Fig. 2C) and was confirmed by TEM (Fig. 2D). Microvilli appeared more elongated at the periphery of adherent bacterial groups, and this feature was particularly prominent with ileal tissue. Intimin-deficient CVD206 (Int− BFP+) did not adhere to any sample, and no pathological tissue changes were seen (Fig. 2E). However, specimens incubated with intimin-reconstituted strain CVD206(pCVD438) (Int+ BFP+) had an appearance similar to that of specimens incubated with E2348/69 in that bacteria adhered to the small intestinal mucosa in localized groups causing A/E lesion formation (Fig. 2F). Strain CVD206(pCVD438/01), expressing an inactive surface intimin (Int− BFP+), did not adhere to any sample and no pathological tissue changes were seen (Fig. 2G).
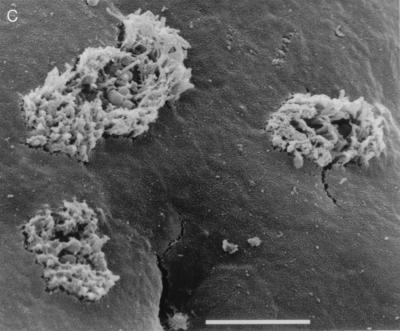
Despite evidence that CVD206 was able to adhere to HEp-2 cells in vitro (Fig. 2A) in the absence of intimin (5), there was no observed adhesion to pediatric small intestinal tissue after 8 h of culture. In order to determine whether early adhesion may occur with subsequent detachment of bacteria, further IVOC incubations were performed with 2- and 4-h end points. After 2 h of culture with each of the six EPEC strains there appeared to be a slight increase in rounded and extruding cells. However, no adherent bacteria or pathological damage was seen, except in one of the three incubations with E2348/69, where several individual adherent bacteria were seen in association with minor microvillous elongation (Fig. 3A). No other strain was observed to adhere. After 4 h in organ culture strains E2348/69 and CVD206(pCVD438) consistently showed small localized colonies associated with microvillous elongation in all experiments (Fig. 3B), while CVD206 and CVD206(pCVD438/01) did not adhere to any specimen.
FIG. 3.
Proximal small intestinal mucosa incubated with E2348/69. (A) Small group of adherent bacteria associated with minor microvillous elongation after 2 h of culture. Bar = 5 μm. (B) Larger bacterial group seen after 4 h of culture. Bar = 1 μm.
Adhesion of EPEC derivatives (Int+ BFP−) to cultured pediatric small intestinal mucosa.
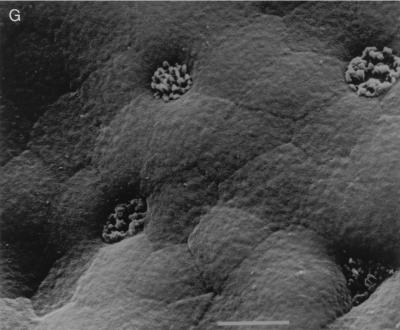
As no bacterial adhesion was seen with Int− BFP+ strains questions were raised as to the role of BFP in initial adhesion. After 6 to 8 h in organ culture two BFP-deficient strains, plasmid-cured JPN15 and Tn_phoA_-mutated 31-6-1(1) (both Int+ BFP−), adhered to all specimens and caused microvillous elongation with A/E lesion formation (Fig. 4A and B, respectively, and confirmed by TEM [data not shown]), although smaller numbers of bacteria were seen and microvilli were not as elongated as with E2348/69. Bacterial groups were more “two dimensional,” i.e., bacteria adhered in a single layer with spaces between them (Fig. 4A) rather than in the tight localized adhering (LA) clusters seen with E2348/69 and CVD206(pCVD438). Early events were again studied. After 2 h in IVOC no bacterial adhesion or pathological changes were noted. However, after 4 h, on two of three occasions, JPN15 formed small flat groups and 31-6-1(1) adhered as single bacteria, and all were associated with minor microvillous elongation (Fig. 4C) which was less exaggerated than at later time points.
FIG. 4.
(A) JPN15 after 8 h in IVOC showing two-dimensional flat group with surrounding microvillous elongation. Bar = 5 μm. (B) 31-6-1(1) showing a similar appearance to JPN15 in panel A. Bar = 5 μm. (C) JPN15 after 4 h in IVOC showing small clusters of adherent bacteria and minor microvillous elongation. Bar = 5 μm.
DISCUSSION
Experiments using human epithelial cells in culture and molecular biological manipulation of EPEC strains have given major insights into the pathogenetic mechanisms of these organisms (4, 6, 29, 31, 38). However, although HEp-2 cells, one of the more widely used in vitro models for studying E. coli pathogens, are easy to handle and manipulate, these cells are nonpolarized carcinoma cells derived from a nonintestinal source which have been maintained in continuous culture for many years. Here, we have demonstrated that EPEC strains interact differently with freshly harvested intestinal tissue from children, the age group most affected by these bacterial pathogens, than with cell lines in culture. IVOC has been well validated as a model for the study of E. coli pathogenesis including that of EPEC (30), enterotoxigenic E. coli (32), and enteroaggregative E. coli (20, 34).
EPEC strains expressing biologically active intimin, with or without BFP, were able to adhere to the small intestinal mucosal surface and cause A/E lesions indistinguishable from those seen in vivo. The strain CVD206, which lacks the eae gene and therefore did not express intimin but does possess BFP, did not adhere in IVOC. Complementation of CVD206 with the eae gene restored the surface expression of intimin (35) and resulted in adhesion and A/E lesion formation in IVOC. In addition, a strain in which a single amino acid substitution (Cys397→Ala) in the cell domain (12) abrogated intimin biological activity but did not prevent its surface expression showed no adhesion to human intestinal tissue in vitro and no pathological alterations were detected. This suggests that the ability of EPEC to form intimin-mediated intimate contact with the human intestinal mucosa is central to intestinal colonization.
According to the three-stage model of EPEC pathogenesis (6), it is thought that BFP are important for the initial attachment of EPEC to the host cell surface. However, Knutton et al. (30) showed that the plasmid-cured EPEC strain JPN15 was still able to adhere to human intestinal mucosa and cause A/E lesion formation, albeit less efficiently than wild-type EPEC E2348/69. Incubating JPN15 with pediatric IVOC confirmed the report of Knutton et al. (30) and showed A/E lesion formation, although there was less adhesion than with E2348/69 (in terms of the number of adhering organisms). However, it was also observed that bacteria were spaced further apart and that colonies appeared two, rather than three, dimensional, suggesting that plasmid-borne genes were involved in the formation of interbacterial links in the LA colony, allowing it to rise above the mucosal surface. The same appearance of flat, two-dimensional LA colonies with A/E lesion formation was seen when strain 31-6-1(1) (7) was used. In this strain a single gene, bfpA, has been mutated, which disrupts BFP production but leaves the rest of the EAF plasmid intact. This indicates that it is BFP which are responsible for complex three-dimensional colony formation, presumably via bacterium-bacterium interconnections. Steric effects of BFP binding may contribute to the three-dimensional appearance.
Thus, the evidence from pediatric intestinal IVOC suggests that BFP are not involved in the initial attachment of EPEC to the mucosal surface but that these fimbrial structures enhance three-dimensional microcolony formation after intimate attachment and A/E lesion formation have been achieved.
Although BFP-negative strains of _eae_-positive E. coli are isolated from cases of diarrheal disease (2, 33), there is some debate about the true pathogenic significance of such strains (25). The observations that EAF probe-negative E. coli strains isolated from pediatric cases of acute diarrheal disease are able to cause A/E lesions on adult intestinal tissue in vitro (33) and that O119 serogroup E. coli, strains, which do not express BFP and are EAF probe negative, are bfpA gene probe positive (19), indicating they have not simply lost the plasmid during passage through the gut, support the idea that these strains have a role in diarrhea. However, despite the results of case-controlled epidemiological studies in Chile (38) and Thailand (9) which did not find higher rates of isolation of such strains in children with diarrhea than in children without diarrhea, these strains have been identified in diarrheal cases where no other pathogens were detected (45) and an EAF-negative, _eae_-positive strain has been implicated in a diarrheal outbreak (49).
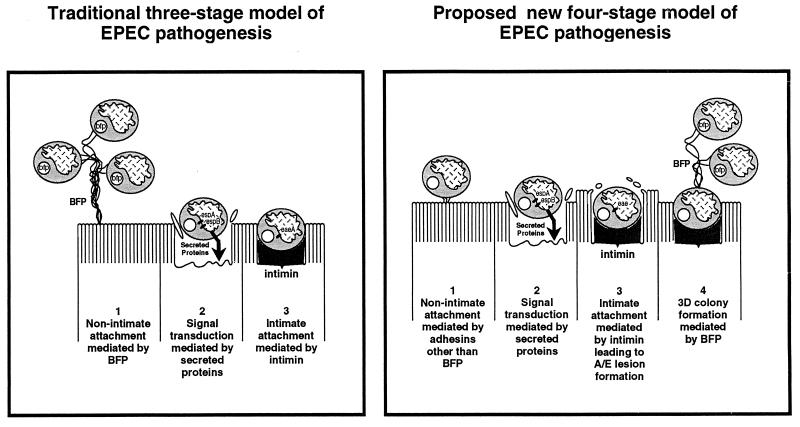
Based on our results we propose a modification to the three-stage model of EPEC infection (6) (Fig. 5). Stage 1 involves the nonintimate adhesion to the host cell surface via an adhesin(s) other than BFP. This leads to stage 2 in which cellular signal transduction events mediated by EPEC-secreted proteins induce cytoskeletal disruption and produce stage 3. Stage 3 involves intimate attachment to the enterocyte surface via intimin, producing A/E lesion formation. Typical EPEC strains expressing BFP proceed to stage 4, which involves three-dimensional expansion of the microcolony. The microcolonies are maintained and stabilized by BFP which connect bacteria to one another. Interbacterial connections mediated by BFP, in the absence of intimate mucosal adherence, may allow detachment and colonization of other mucosal sites, possibly contributing to the spread of infection along the gut. Atypical EPEC strains, lacking BFP expression, would not proceed to stage 4.
FIG. 5.
Proposed model of EPEC infection of pediatric small intestinal tissue in vitro in comparison to the model based primarily on studies using HEp-2 cells. The latter model is from Donnenberg and Kaper (6).
The fact that at early time points of observation individual bacteria or small groups are seen adhering to the mucosal surface suggests that EPEC microcolonies form from adhering bacteria multiplying in situ or from recruitment of individual bacteria from the surrounding medium and not that LA groups are ready formed in the bacterial culture prior to organ culture assay and act as infectious units (50).
Another factor which may be relevant to the differences observed between adhesion to HEp-2 cells in culture and to human intestinal tissue in IVOC is the difference in the apical membranes. Recently it has been shown that the presence of a thick glycocalyx coating the surface of intact intestinal epithelial tissue can act as a barrier to bacterial adhesion (14). This layer is not present on HEp-2 cells, and there is a much more complex microvillous brush border on intestinal epithelium. In these respects the two model systems are quite distinct and different and/or additional virulence factors may be required in the in vivo situation.
All strains used in this study adhered to HEp-2 cells. The importance of Cys937 for intimin activity (13) was confirmed because, although surface expression of intimin by strain CVD206(pCVD438/01) adhering to HEp-2 cells was demonstrated by immunofluorescence, no A/E lesion was detected by the FAS test. Although our findings indicate that BFP do not appear to be involved in the initial stages of adhesion of EPEC to intestinal mucosa in organ culture, there is evidence that BFP mediate the adhesion of EPEC to HEp-2 cells. Adhesion can be blocked by a polyclonal BFP antiserum, although this blockage was incomplete and only reduced adhesion by around 50% (16). Mutants deficient in BFP production do not form localized colonies (7), although if the HEp-2 cell assay is continued for 6 h bacteria begin to adhere (7). Despite these data uncertainty still exists concerning whether BFP mediate the initial adhesion of EPEC to HEp-2 cells (15). The findings in this paper based on studies with IVOC support the original suggestion of Girón et al. (16) that “BFP participate in the formation of the bacterial colony by forming bundles that link one bacterium to another.”
If BFP are not involved in initial adhesion then either intimin mediates initial and subsequent events or other adhesins await recognition. Gómez-Duarte and Kaper (18) showed that the addition of the cloned per region to JPN15 increased adherence and produced a poor localized adherence pattern; the increased adherence was specific for eae since inactivation of eae prevented the increased adhesion, indicating that intimin can promote adherence. Evidence has been put forward to suggest that the intimin receptor is the bacterial protein Tir (26) and that this protein must be transferred from bacillus to host and be phosphorylated to allow binding (26, 43). Adhesion of EPEC to the mucosa would be necessary for the efficient delivery of secreted molecules to the cell surface, and this necessity lends weight to the suggestion that further adhesins await discovery. However, no adhesion of intimin-deficient strains was seen in IVOC. It is possible that intimin-negative strains adhered so transiently that the time scale of these experiments missed the phenomenon or that the adhesion was so tenuous that washing prior to fixation removed the bacteria. These suggestions seem unlikely as only a few adhering bacteria were seen after 2 h in IVOC, CVD206 adheres well to HEp-2 cells over a similar time scale when identical growth and processing conditions are used, and colonization based on tenuous adhesion seems unlikely in vivo in the face of peristalsis and other nonspecific host defense mechanisms. Thus, the finding remains that intimin appears to be central to A/E lesion formation and EPEC colonization of pediatric intestinal mucosae. Possibly, additional intimin receptors await recognition. Future work is necessary to study the very early stages of EPEC adhesion to the mucosa and to investigate the suggested role of BFP in complex microcolony formation.
ACKNOWLEDGMENTS
We thank John Walker-Smith, Simon Murch, Mike Thomson, and David Casson (University Department of Paediatric Gastroenterology, Royal Free Hospital, London, United Kingdom) for providing intestinal biopsy tissue and Jorge Girón for providing anti-BFP antiserum.
J.B.K. is supported by grant AI21657 from the National Institutes of Health. G.D. is supported by a program grant from the Wellcome Trust.
REFERENCES
- 1.Baldini M M, Kaper J B, Levine M M, Candy D C A, Moon H W. Plasmid-mediated adhesion in enteropathogenic Escherichia coli. J Pediatr Gastroenterol Nutr. 1983;2:534–538. doi: 10.1097/00005176-198302030-00023. [DOI] [PubMed] [Google Scholar]
- 2.Bokete T N, Whittam T S, Wilson R A, Clausen C R, O’Callahan C M, Moseley S L, Fritsche T R, Tarr P I. Genetic and phenotypic analysis of Escherichia coli with enteropathogenic characteristics isolated from Seattle children. J Infect Dis. 1997;175:1382–1389. doi: 10.1086/516470. [DOI] [PubMed] [Google Scholar]
- 3.Cravioto A, Gross R J, Scotland S M, Rowe B. An adhesive factor found in strains of Escherichia coli belonging to traditional infantile enteropathogenic serotypes. Curr Microbiol. 1979;3:95–99. [Google Scholar]
- 4.Cravioto A, Tello A, Navarro A, Ruiz J, Villafán H, Uribe F, Eslava C. Association of Escherichia coli HEp-2 adherence patterns with type and duration of diarrhoea. Lancet. 1991;337:262–264. doi: 10.1016/0140-6736(91)90868-p. [DOI] [PubMed] [Google Scholar]
- 5.Donnenberg M S, Kaper J B. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection vector. Infect Immun. 1991;59:4310–4317. doi: 10.1128/iai.59.12.4310-4317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donnenberg M S, Kaper J B. Enteropathogenic Escherichia coli. Infect Immun. 1992;60:3953–3961. doi: 10.1128/iai.60.10.3953-3961.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donnenberg M S, Girón J A, Nataro J P, Kaper J B. A plasmid-encoded type IV fimbrial gene of enteropathogenic Escherichia coli associated with localised adherence. Mol Microbiol. 1992;6:3427–3437. doi: 10.1111/j.1365-2958.1992.tb02210.x. [DOI] [PubMed] [Google Scholar]
- 8.Donnenberg M S, Tacket C O, James S P, Losomsky G, Nataro J P, Wasserman S S, Kaper J B, Levine M M. Role of the eaeA gene in experimental enteropathogenic Escherichia coli infection. J Clin Invest. 1993;92:1412–1417. doi: 10.1172/JCI116717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Echeverria P, Orskov F, Orskov I, Knutton S, Scheutz F, Brown J E, Lexomboon U. Attaching and effacing enteropathogenic Escherichia coli as a cause of infantile diarrhoea in Bangkok. J Infect Dis. 1991;164:550–554. doi: 10.1093/infdis/164.3.550. [DOI] [PubMed] [Google Scholar]
- 10.Foubister V I, Rosenshine I, Donnenberg M S, Finlay B B. The eaeB gene of enteropathogenic Escherichia coli is necessary for signal transduction in epithelial cells. Infect Immun. 1994;62:3038–3040. doi: 10.1128/iai.62.7.3038-3040.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frankel, G., A. D. Phillips, M. Novakova, S. Hicks, and G. Dougan. Unpublished data.
- 12.Frankel G, Candy D C A, Fabiani E, Adu-Bobie J, Gil S, Novakova M, Phillips A D, Dougan G. Molecular characterization of a carboxy-terminal eukaryotic cell binding domain of intimin from enteropathogenic Escherichia coli. Infect Immun. 1995;63:4323–4328. doi: 10.1128/iai.63.11.4323-4328.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frankel, G., A. D. Phillips, J. Adu-Bobie, D. C. A. Candy, G. Douce, and G. Dougan. 1996. Intimin adherence to epithelial cells. Rev. Microbiol. 27(Suppl. 1)**:**99–103.
- 14.Frey A, Giannasca K T, Weltzin R, Giannasca P J, Reggio H, Lencer W I, Neutra M R. Role of the glycocalyx in regulating access of microparticles to apical plasma membranes of intestinal epithelial cells: implications for microbial attachment and oral vaccine targeting. J Exp Med. 1996;184:1045–1059. doi: 10.1084/jem.184.3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Girón, J. A. 1996. Fimbriae of enteropathogenic Escherichia coli. Rev. Microbiol. 27(Suppl. 1)**:**72–76.
- 16.Girón J A, Ho A S, Schoolnik G K. An inducible bundle-forming pilus of enteropathogenic Escherichia coli. Science. 1991;254:710–713. doi: 10.1126/science.1683004. [DOI] [PubMed] [Google Scholar]
- 17.Gomes T A T, Vieira M A M, Wachsmuth L K, Blake P A, Trabulsi L R. Serotype-specific prevalence of Escherichia coli strains with EPEC adherence factor genes in infants with and without diarrhoea in São Paulo, Brazil. J Infect Dis. 1989;160:131–135. doi: 10.1093/infdis/160.1.131. [DOI] [PubMed] [Google Scholar]
- 18.Gómez-Duarte O G, Kaper J B. A plasmid-encoded regulatory region activates chromosomal eaeA expression in enteropathogenic Escherichia coli. Infect Immun. 1995;63:1767–1776. doi: 10.1128/iai.63.5.1767-1776.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goncalves A G, Campos L C, Gomes T A T, Rodrigues J, Sperandio V, Whittam T S, Trabulsi T R. Virulence properties and clonal structure of strains of Escherichia coli O119 serotypes. Infect Immun. 1997;65:2034–2040. doi: 10.1128/iai.65.6.2034-2040.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hicks S, Candy D C A, Phillips A D. Adhesion of enteroaggregative Escherichia coli to pediatric intestinal mucosa in vitro. Infect Immun. 1996;64:4751–4760. doi: 10.1128/iai.64.11.4751-4760.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hicks S, Frankel G, Dougan G, Phillips A D. The role of BFP and intimin in enteropathogenic E. coli (EPEC) pathogenesis. J Pediatr Gastroenterol Nutr. 1997;24:468. [Google Scholar]
- 22.Jarvis K G, Giron J A, Jerse A E, McDaniel T K, Donnenberg M S, Kaper J B. Enteropathogenic Escherichia coli contains a putative type III secretion system necessary for the export of proteins involved in attaching and effacing lesion formation. Proc Natl Acad Sci USA. 1995;92:7996–8000. doi: 10.1073/pnas.92.17.7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jerse A E, Yu J, Tall B, Kaper J B. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc Natl Acad Sci USA. 1990;87:7839–7843. doi: 10.1073/pnas.87.20.7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jerse A E, Kaper J B. The eae gene of enteropathogenic Escherichia coli encodes a 94-kilodalton membrane protein, the expression of which is influenced by the EAF plasmid. Infect Immun. 1991;59:4302–4309. doi: 10.1128/iai.59.12.4302-4309.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaper, J. B. 1996. Defining EPEC. Rev. Microbiol. 27(Suppl. 1)**:**130–133.
- 26.Kenny B, DeVinney R, Stein M, Reinscheid D J, Frey E A, Finlay B B. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell. 1997;91:511–520. doi: 10.1016/s0092-8674(00)80437-7. [DOI] [PubMed] [Google Scholar]
- 27.Kenny B, Finlay B B. Protein secretion by enteropathogenic Escherichia coli is essential for transducing signals in epithelial cells. Proc Natl Acad Sci USA. 1995;92:7991–7995. doi: 10.1073/pnas.92.17.7991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kilby A. Paediatric small intestinal biopsy capsule with two ports. Gut. 1976;17:158–159. doi: 10.1136/gut.17.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knutton S, Baldini M M, Kaper J B, McNeish A S. Role of plasmid-encoded adherence factors in adhesion of enteropathogenic Escherichia coli to HEp-2 cells. Infect Immun. 1987;55:78–85. doi: 10.1128/iai.55.1.78-85.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knutton S, Lloyd D R, McNeish A S. Adhesion of enteropathogenic Escherichia coli to human intestinal enterocytes and cultured human intestinal mucosa. Infect Immun. 1987;55:69–77. doi: 10.1128/iai.55.1.69-77.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knutton S, Baldwin T, Williams P H, McNeish A S. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic and enterohemorrhagic Escherichia coli. Infect Immun. 1989;57:1290–1298. doi: 10.1128/iai.57.4.1290-1298.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knutton S, McConnell M M, Rowe B, McNeish A S. Adhesion and ultrastructural properties of human enterotoxigenic Escherichia coli colonization factor antigens III and IV. Infect Immun. 1989;57:3364–3371. doi: 10.1128/iai.57.11.3364-3371.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knutton S, Phillips A D, Smith H R, Gross R J, Shaw R, Watson P, Price E. Screening for enteropathogenic Escherichia coli in infants with diarrhea by the fluorescent-actin staining test. Infect Immun. 1991;59:365–371. doi: 10.1128/iai.59.1.365-371.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knutton S, Shaw R K, Bhan M K, Smith H R, McConnell M M, Cheasty T, Williams P H, Baldwin T J. Ability of enteroaggregative Escherichia coli strains to adhere in vitro to human intestinal mucosa. Infect Immun. 1992;60:2083–2091. doi: 10.1128/iai.60.5.2083-2091.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knutton S, Adu-Bobie J, Bain C, Phillips A D, Dougan G, Frankel G. Down regulation of intimin expression during attaching and effacing enteropathogenic Escherichia coli adhesion. Infect Immun. 1997;65:1644–1652. doi: 10.1128/iai.65.5.1644-1652.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levine M M. Escherichia coli that cause diarrhoea: enterotoxigenic, enteropathogenic, enteroinvasive, enterohaemorrhagic, and enteroadherent. J Infect Dis. 1987;155:377–389. doi: 10.1093/infdis/155.3.377. [DOI] [PubMed] [Google Scholar]
- 37.Levine M M, Berquist E J, Nalin D R, Waterman D H, Hornick R B, Young C R, Stoman S, Rowe B. Escherichia coli strains that cause diarrhoea but do not produce heat-labile or heat-stable enterotoxins and are non-invasive. Lancet. 1978;i:119–122. doi: 10.1016/s0140-6736(78)90299-4. [DOI] [PubMed] [Google Scholar]
- 38.Levine M M, Prado V, Robins-Browne R, Lior H, Kaper J B, Moseley S L, Gicquelais K, Nataro J P, Vial P, Tall B. Use of DNA probes and HEp-2 cell adherence assay to detect diarrhoeagenic Escherichia coli. J Infect Dis. 1988;158:224–228. doi: 10.1093/infdis/158.1.224. [DOI] [PubMed] [Google Scholar]
- 39.McDaniel T, Jarvis K G, Donnenberg M S, Kaper J B. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc Natl Acad Sci USA. 1995;92:1664–1668. doi: 10.1073/pnas.92.5.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McDaniel T K, Kaper J B. A cloned pathogenicity island from enteropathogenic Escherichia coli confers the attaching and effacing phenotype on E. coli K-12. Mol Microbiol. 1997;23:399–407. doi: 10.1046/j.1365-2958.1997.2311591.x. [DOI] [PubMed] [Google Scholar]
- 41.Moon H W, Whipp S C, Argenzio R A, Levine M M, Gianella R A. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect Immun. 1983;41:1340–1351. doi: 10.1128/iai.41.3.1340-1351.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosenshine I, Donnenberg M S, Kaper J B, Finlay B B. Signal transduction between enteropathogenic Escherichia coli (EPEC) and epithelial cells: EPEC induces tyrosine phosphorylation of host cell proteins to initiate cytoskeletal rearrangement and bacterial uptake. EMBO J. 1992;11:3551–3560. doi: 10.1002/j.1460-2075.1992.tb05438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosenshine I, Ruschkowski S, Stein M, Reinscheid D J, Mills S D, Finlay B B. A pathogenic bacterium triggers epithelial signals to form a functional bacterial receptor that mediates pseudopod formation. EMBO J. 1996;15:2613–2624. [PMC free article] [PubMed] [Google Scholar]
- 44.Rothbaum R, McAdams A J, Giannella R, Partin J C. A clinicopathologic study of enterocyte-adherent Escherichia coli: a cause of protracted diarrhoea in infants. Gastroenterology. 1982;83:441–454. [PubMed] [Google Scholar]
- 45.Smith, H. R., S. M. Scotland, T. Cheasty, G. A. Willshaw, and B. Rowe. 1996. Enteropathogenic Escherichia coli infections in the United Kingdom. Rev. Microbiol. 27(Suppl. 1)**:**45–49.
- 46.Sohel I, Puente J L, Ramer S W, Bieber D, Wu C-Y, Schoolnik G K. Enteropathogenic Escherichia coli: identification of a gene cluster coding for bundle-forming pilus morphogenesis. J Bacteriol. 1996;178:2613–2628. doi: 10.1128/jb.178.9.2613-2628.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stone K D, Zhang H-Z, Carlson L K, Donnenberg M S. A cluster of fourteen genes from enteropathogenic Escherichia coli is sufficient for the biogenesis of a type IV pilus. Mol Microbiol. 1996;20:325–337. doi: 10.1111/j.1365-2958.1996.tb02620.x. [DOI] [PubMed] [Google Scholar]
- 48.Ulshen M H, Rollo J L. Pathogenesis of Escherichia coli gastroenteritis in man—another mechanism. N Engl J Med. 1980;302:99–101. doi: 10.1056/NEJM198001103020207. [DOI] [PubMed] [Google Scholar]
- 49.Viljanen M K, Peltola T, Junnila S Y T, Olkkonen L, Jarvinen H, Kustila M, Huovinen P. Outbreak of diarrhoea due to Escherichia coli O111:B4 in school children and adults: association of Vi antigen-like reactivity. Lancet. 1990;336:831–834. doi: 10.1016/0140-6736(90)92337-h. [DOI] [PubMed] [Google Scholar]
- 50.Vuopio-Varkila J, Schoolnik G K. Localised adherence by enteropathogenic Escherichia coli is an inducible phenotype associated with the expression of new outer membrane proteins. J Exp Med. 1991;174:1167–1177. doi: 10.1084/jem.174.5.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]