Proteins Encoded by the cag Pathogenicity Island of Helicobacter pylori Are Required for NF-κB Activation (original) (raw)
Abstract
Helicobacter pylori is the etiological agent in the development of chronic gastritis, duodenal ulceration, and gastric adenocarcinoma. The difference in virulence between individual strains is reflected in their ability to induce interleukin-8 (IL-8) secretion from gastric epithelial cells. It has been shown that virulence is associated with the presence of a bacterial gene cluster (a pathogenicity island). We have recently demonstrated that _H. pylori_-mediated IL-8 secretion requires activation of the transcription factor NF-κB. Here, we show that NF-κB induction requires six membrane proteins encoded within the pathogenicity island.
Helicobacter pylori infection can cause a wide variety of diseases in humans. While most individuals develop only superficial gastritis, in a small proportion of individuals infection progresses to duodenal ulceration and gastric adenocarcinoma (4, 17, 18). This variability in the clinical manifestations of H. pylori infection is potentially due to differences in the virulence of individual Helicobacter strains. Until recently, the presence of a cytotoxin-associated antigen (cagA) was the best predictor of strain virulence. While cagA is present in 50 to 60% of H. pylori isolates from patients with gastritis, it is found in 88 to 100% of strains from patients with duodenal ulceration (7). Because of the strong inflammatory response to Helicobacter infections, the role of inflammatory cytokines was investigated. It was shown that mucosal biopsies from patients with H. pylori infections contain significantly elevated levels of interleukin-1β (IL-1β), IL-6, tumor necrosis factor alpha (TNF-α), and IL-8 compared to those in specimens from uninfected individuals (9, 10, 12, 16). Moreover, among those infected, patients with active gastritis show higher levels of TNF-α and IL-8 than do patients with chronic gastritis (9, 12).
Exposure of gastric epithelial cell lines to H. pylori induces the secretion of IL-8 (8, 23). This model system was used to test various H. pylori strains for their ability to stimulate cytokine production. While cagA+ strains induce significantly higher IL-8 levels than do _cagA_-negative strains (8, 14, 21), it was recently shown that isogenic cagA mutants elicit IL-8 to the same degree as does the wild-type parent strain (11, 23). Therefore, although cagA is a marker of enhanced pathogenicity, it is not the molecular mediator of the inflammatory response. It was shown last year that the cagA gene resides within a pathogenicity island, a DNA segment which contains over 40 genes encoding bacterial virulence factors (6). Using isogenic mutants, Censini et al. (6) demonstrated that several genes located within the pathogenicity island are required for the ability of Helicobacter to elicit IL-8 secretion from gastric epithelial cells. Therefore, the presence of a pathogenicity island is associated with increased virulence of a Helicobacter strain.
Genes encoding IL-8, IL-1β, IL-6, and TNF-α are targets for the human transcription factor NF-κB (2). This protein plays an integral role in regulating the human immune response. It is present in an inactive, cytoplasmic form in almost all cell types (1). NF-κB is activated upon stimulation by a large variety of pathogenic agents (2). Activation occurs via phosphorylation, ubiquitinilation, and proteolytic degradation of IκB, the inhibitory subunit (3, 5, 13, 24–26). The released NF-κB dimer rapidly translocates to the nucleus, where it activates transcription of target genes including those encoding IL-1, IL-6, IL-8, and TNF-α (2). We have recently shown that exposure of gastric epithelial cell lines to H. pylori potently activates NF-κB (15). Transcription factor induction by various H. pylori strains correlates with their ability to elicit IL-8 production. Indeed, cytokine production requires NF-κB activation, since its prevention by the antioxidant curcumin completely suppresses IL-8 production. Unlike other gram-negative bacteria, which induce NF-κB via their lipopolysaccharide molecules, H. pylori does not use lipopolysaccharide to activate the transcription factor (15). Rather, a gene located within the pathogenicity island, cagE, is required, since its mutation abolishes NF-κB induction. In this study we investigate the role of additional pathogenicity island genes in NF-κB activation. We show that while two genes, cagF and cagN, are not required for transcription factor activation, six genes, cagE, cagG, cagH, cagI, cagL, and cagM, are absolutely necessary, since isogenic Helicobacter strains carrying mutations in these loci no longer induce NF-κB activity. We propose that the proteins encoded by these genes form a surface structure which acts as the NF-κB-inducing agent.
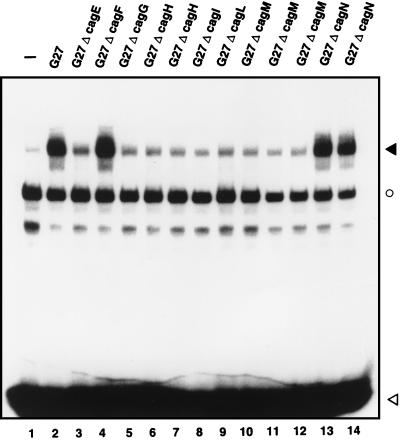
Proteins encoded by the cag pathogenicity island are required for NF-κB activation.
In order to investigate whether proteins encoded in the recently discovered cag pathogenicity island are required for NF-κB activation, KATO-III cells (ATCC HTB 103) were cocultured with various Helicobacter strains: a wild-type G27 strain as well as 12 isogenic strains, each with a mutation in a single gene encoded within the pathogenicity island (6). Coculture was performed as reported previously (15). Subsequently, the cells were harvested and total cell extracts were prepared as previously described (15, 19, 20). These were assayed for NF-κB DNA binding by an electrophoretic mobility shift assay (EMSA) as described previously (15). Coculture of KATO-III cells with the wild-type G27 strain induces a novel protein-DNA complex, which we have previously identified as NF-κB (Fig. 1, lane 2) (15). Strains with mutations in the genes encoding cagF and cagN also activate NF-κB (lanes 4, 13, and 14), while strains with mutations in six other genes, cagE, cagG, cagH, cagI, cagL, and cagM, no longer induce the transcription factor (lanes 3 and 5 to 12). We cannot exclude a polar effect in some of our mutants. Therefore, not all six Cag proteins may be required for NF-κB induction.
FIG. 1.
Proteins encoded by the cag pathogenicity island are required for NF-κB activation. KATO-III cells were cocultured with 1 ml of bacterial culture of H. pylori G27 (lane 2) or its isogenic mutants (lanes 3 to 14). Control cells were left untreated (lane 1). After 1 h of coculture, total cell extracts were prepared and assayed by EMSA with a high-affinity κB-binding site as a probe. The closed arrowhead indicates specific NF-κB complexes. The open circle denotes nonspecific binding to the probe, and the open arrowhead indicates unbound oligonucleotide.
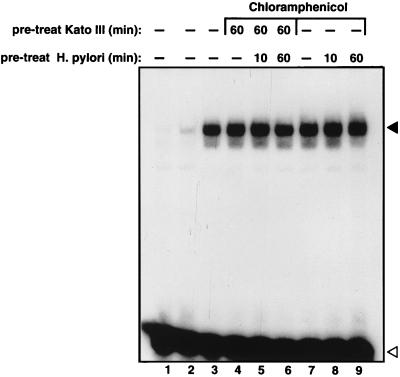
The NF-κB-activating product is preformed prior to cell contact.
We wished to determine whether the NF-κB-inducing H. pylori proteins are preformed in the bacteria or become expressed only after contact with gastric epithelial cells. We therefore preincubated KATO-III cells with 2 mg of chloramphenicol per liter for 60 min. In addition, liquid Helicobacter cultures were also pretreated with 2 mg of chloramphenicol per liter for 10 or 60 min at 37°C, after which they were nonviable as shown by culture experiments. Pretreated or untreated KATO-III cells were cocultured with chloramphenicol-treated or untreated bacteria for 1 h. Total cell extracts were prepared and analyzed for NF-κB DNA binding (Fig. 2). Chloramphenicol treatment neither of the bacteria nor of the epithelial cells had any effect on the ability of Helicobacter to induce NF-κB activation. Therefore, the NF-κB-inducing factor is preformed in the bacterium before exposure to the epithelial cells.
FIG. 2.
The NF-κB-activating product is preformed prior to cell contact. KATO-III cells were either left untreated (lanes 1 to 3 and 7 to 9) or pretreated for 60 min with 2 mg of chloramphenicol per liter (lanes 4 to 6). Similarly, liquid cultures of H. pylori G27 were either left untreated (lanes 1 to 4 and 7) or pretreated for 10 min (lanes 5 and 8) or 60 min (lanes 6 and 9) with chloramphenicol. Subsequently, the epithelial cells and the bacteria were cocultured for 1 h, after which total cell extracts were prepared and assayed for NF-κB DNA binding by EMSA. The closed arrowhead indicates specific NF-κB complexes, and the open arrowhead indicates unbound oligonucleotide.
We propose that proteins encoded in the cag pathogenicity island form a multimeric structure on the H. pylori surface. When bacteria are cocultured with gastric epithelial cells, this structure is capable of eliciting a signal transduction cascade which leads to activation of transcription factor NF-κB. Future research will aim at elucidating the molecular components of this signal transduction pathway. For example, it is not clear whether H. pylori uses a specific receptor on the epithelial cell surface. It has recently been suggested that tyrosine kinases are required for NF-κB activation by H. pylori; however, the enzymes involved remain unknown (22). Elucidation of this signal transduction pathway may point out novel therapeutic targets. Continuous and recurring NF-κB activation, which leads to production of IL-8 as well as other inflammatory cytokines such as IL-1, IL-6, and TNF-α, is a critical step in establishing the chronic inflammation seen in H. pylori gastritis. By inhibiting NF-κB activation, the secretion of inflammatory cytokines could be abolished. This inhibition may impede the establishment of a chronic H. pylori gastritis, perhaps rendering the infection asymptomatic.
Acknowledgments
We thank Brigitte Schneider for expert technical assistance.
This work was supported by grants from the Deutsche Forschungsgemeinschaft (SFB 364 and Pa 611/1-2) to H.L.P.
REFERENCES
- 1.Baeuerle P A, Baltimore D. I kappa B: a specific inhibitor of the NF-kappa B transcription factor. Science. 1988;242:540–546. doi: 10.1126/science.3140380. [DOI] [PubMed] [Google Scholar]
- 2.Baeuerle P A, Henkel T. Function and activation of NF-κB in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 3.Beg A A, Finco T S, Nantermet P V, Baldwin A S., Jr Tumor necrosis factor and interleukin-1 lead to phosphorylation and loss of IκB-α: a mechanism for NF-κB activation. Mol Cell Biol. 1993;13:3301–3310. doi: 10.1128/mcb.13.6.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blaser M J. Helicobacter pylori and the pathenogenesis of gastroduodenal inflammation. J Infect Dis. 1990;161:626–633. doi: 10.1093/infdis/161.4.626. [DOI] [PubMed] [Google Scholar]
- 5.Brown K, Gerstberger S, Carlson L, Franzoso G, Siebenlist U. Control of IκB-α proteolysis by site-specific, signal induced phosphorylation. Science. 1995;267:1485–1488. doi: 10.1126/science.7878466. [DOI] [PubMed] [Google Scholar]
- 6.Censini S, Lange C, Xiang Z, Crabtree J E, Ghiara P, Borodovsky M, Rappuoli R, Covacci A. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci USA. 1996;93:14648–14653. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Covacci A, Censini S, Bugnoli M, Petracca R, Burroni D, Macchia G, Massone A, Papini E, Xiang Z, Figura N, Rappuoli R. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc Natl Acad Sci USA. 1993;90:5791–5795. doi: 10.1073/pnas.90.12.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crabtree J E, Farmery S M, Lindley I J D, Figura N, Peichl P, Tompkins D S. CagA cytotoxic strains of Helicobacter pylori and interleukin-8 in gastric epithelial cell lines. J Clin Pathol. 1994;47:945–950. doi: 10.1136/jcp.47.10.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crabtree J E, Shallcross T M, Heatley R V, Wyatt J I. Mucosal tumor necrosis factor alpha and interleukin-6 in patients with Helicobacter pylori associated gastritis. Gut. 1991;32:1473–1477. doi: 10.1136/gut.32.12.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crabtree J E, Wyatt J I, Trejdosiewicz L K, Peichl P, Nichols P H, Ramsay N, Primrose J N, Lindley I J D. Interleukin-8 expression in Helicobacter pylori infected, normal, and neoplastic gastroduodenal mucosa. J Clin Pathol. 1994;47:61–66. doi: 10.1136/jcp.47.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crabtree J E, Xiang Z, Lindley I J D, Tompkins D S, Rappuoli R, Covacci A. Induction of interleukin-8 secretion from gastric epithelial cells by cagA negative isogenic mutant of Helicobacter pylori. J Clin Pathol. 1995;48:967–969. doi: 10.1136/jcp.48.10.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gionchetti P, Vaira D, Campieri M, Holton J, Menegatti M, Belluzzi A, Bertinelli E, Ferretti M, Brignola C, Migliolo M, Barbara L. Enhanced mucosal interleukin-6 and -8 in Helicobacter pylori-positive dyspeptic patients. Am J Gastroenterol. 1994;89:883–887. [PubMed] [Google Scholar]
- 13.Henkel T, Machleidt T, Alkalay I, Ben-Neriah K M Y, Baeuerle P A. Rapid proteolysis of IκB-α is necessary for activation of transcription factor NF-κB. Nature. 1993;365:182–184. doi: 10.1038/365182a0. [DOI] [PubMed] [Google Scholar]
- 14.Huang J, O’Toole P W, Doig P, Trust T J. Stimulation of interleukin-8 in epithelial cell lines by Helicobacter pylori. Infect Immun. 1995;63:1732–1738. doi: 10.1128/iai.63.5.1732-1738.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Münzenmaier, A., C. Lange, E. Glocker, A. Moran, A. Covacci, P. A. Baeuerle, M. Kist, and H. L. Pahl. A shed/secreted product of Helicobacter pylori is required for activation of the transcription factor NF-κB. J. Immunol. **159:**6140–6147. [PubMed]
- 16.Noach L A, Bosma N B, Jansen J, Hoek F J, van Deventer S J H, Tytgat G N J. Mucosal tumor necrosis factor-α, interleukin-1β, and interleukin-8 production in patients with Helicobacter pylori infection. Scand J Gastroenterol. 1994;29:425–429. doi: 10.3109/00365529409096833. [DOI] [PubMed] [Google Scholar]
- 17.Nomura A, Stemmermann G N, Chyou P, Kato I, Perez-Perez G I, Blaser M J. Helicobacter pylori infection and gastric carcinoma in a population of Japanese-Americans in Hawaii. N Engl J Med. 1991;325:1132–1136. doi: 10.1056/NEJM199110173251604. [DOI] [PubMed] [Google Scholar]
- 18.Nomura A, Stemmermann G N, Chyou P, Perez-Perez G I, Blaser M J. Helicobacter pylori infection and the risk for duodenal and gastric ulceration. Ann Intern Med. 1994;120:977–981. doi: 10.7326/0003-4819-120-12-199406150-00001. [DOI] [PubMed] [Google Scholar]
- 19.Pahl H L, Baeuerle P A. A novel signal transduction pathway from the endoplasmic reticulum to the nucleus is mediated by transcription factor NF-κB. EMBO J. 1995;14:2580–2588. doi: 10.1002/j.1460-2075.1995.tb07256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pahl H L, Sester M, Burgert H-G, Baeuerle P A. Activation of transcription factor NF-κB by adenovirus E3/19K requires its ER-retention. J Cell Biol. 1996;132:511–522. doi: 10.1083/jcb.132.4.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peek R M, Miller G G, Tham K T, Perez-Perez G I, Zhao X, Atherton J C, Blaser M J. Heightened inflammatory response and cytokine expression in vivo to cagA+ Helicobacter pylori strains. Lab Invest. 1995;71:760–770. [PubMed] [Google Scholar]
- 22.Segal E D, Lange C, Covacci A, Tompkins L S, Falkow S. Induction of host signal transduction pathways by Helicobacter pylori. Proc Natl Acad Sci USA. 1997;94:7595–7599. doi: 10.1073/pnas.94.14.7595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma S A, Tumuru M R, Miller G G, Blaser M J. Interleukin-8 response of gastric epithelial cell lines to Helicobacter pylori stimulation in vitro. Infect Immun. 1995;63:1681–1687. doi: 10.1128/iai.63.5.1681-1687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun S-C, Ganchi P A, Béraud C, Ballard D W, Greene W C. Autoregulation of the NF-kappa B transactivator RelA (p65) by multiple cytoplasmic inhibitors containing ankyrin motifs. Proc Natl Acad Sci USA. 1994;87:1346–1350. doi: 10.1073/pnas.91.4.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Traenckner E B-M, Pahl H L, Henkel T, Schmidt K N, Wilk S, Baeuerle P A. Phosphorylation of human IκB-α on serine 32 and 36 controls IκB-α proteolysis and NF-κB activation in response to diverse stimuli. EMBO J. 1995;14:2876–2883. doi: 10.1002/j.1460-2075.1995.tb07287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Traenckner E B-M, Wilk S, Baeuerle P A. A proteasome inhibitor prevents activation of NF-κB and stabilizes a newly phosphorylated form of IκB-α that is still bound to NF-κB. EMBO J. 1994;13:5433–5441. doi: 10.1002/j.1460-2075.1994.tb06878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]