Clostridium difficile Toxins: Mechanism of Action and Role in Disease (original) (raw)
Abstract
As the leading cause of hospital-acquired diarrhea, Clostridium difficile colonizes the large bowel of patients undergoing antibiotic therapy and produces two toxins, which cause notable disease pathologies. These two toxins, TcdA and TcdB, are encoded on a pathogenicity locus along with negative and positive regulators of their expression. Following expression and release from the bacterium, TcdA and TcdB translocate to the cytosol of target cells and inactivate small GTP-binding proteins, which include Rho, Rac, and Cdc42. Inactivation of these substrates occurs through monoglucosylation of a single reactive threonine, which lies within the effector-binding loop and coordinates a divalent cation critical to binding GTP. By glucosylating small GTPases, TcdA and TcdB cause actin condensation and cell rounding, which is followed by death of the cell. TcdA elicits effects primarily within the intestinal epithelium, while TcdB has a broader cell tropism. Important advances in the study of these toxins have been made in the past 15 years, and these are detailed in this review. The domains, subdomains, and residues of these toxins important for receptor binding and enzymatic activity have been elegantly studied and are highlighted herein. Furthermore, there have been major advances in defining the role of these toxins in modulating the inflammatory events involving the disruption of cell junctions, neuronal activation, cytokine production, and infiltration by polymorphonuclear cells. Collectively, the present review provides a comprehensive update on TcdA and TcdB's mechanism of action as well as the role of these toxins in disease.
INTRODUCTION
Originally named Bacillus difficilis, the organism now known as Clostridium difficile was first described in the mid-1930s (62). For the next 40 years, there were infrequent reports of C. difficile isolation, with few findings implying that this organism could cause disease. However, in 1978, C. difficile was identified as the primary cause of pseudomembranous colitis and shown to be a primary isolate from the feces of patients undergoing clindamycin treatment (10, 57). These seminal observations were followed by a series of reports which further showed a strong correlation between pseudomembranous colitis, antibiotic therapy, C. difficile colonization, and cytotoxin production (6, 11, 16, 36, 56, 116, 166, 183). Collectively, these original studies and observations revealed C. difficile as an emerging pathogen capable of causing severe gastrointestinal disease in individuals undergoing antibiotic therapy.
C. difficile is the leading cause of hospital-acquired diarrhea, known as _C. difficile_-associated disease, in the United States. The estimated number of cases of _C. difficile_-associated disease exceeds 250,000 per year (182), with total additional health care costs approaching US$1 billion annually (98). There are several possible explanations for the increase in C. difficile disease during the past three decades. First, better detection methods have almost certainly contributed to the increase in reported cases of _C. difficile_-associated disease. Second, the high-frequency use of antibiotics and chemotherapeutics increases the likelihood of acquiring _C. difficile_-associated disease. Third, as the frequency of disease has increased, hospitals have become contaminated with spores of C. difficile, making infection of susceptible patients more probable.
Despite this lack of understanding of the epidemiology of C. difficile disease, there has been major progress in dissecting this organism's mechanism of virulence. Indeed, a great deal has been learned about the toxins produced by C. difficile. The two major toxins, TcdA and TcdB, have been studied intensively since their initial recognition as major C. difficile virulence factors. The results from a collection of studies strongly suggest that detailed analysis of TcdA and TcdB activity is providing one of the quickest inroads to understanding _C. difficile_-related disease. In addition to their contribution to disease, TcdA and TcdB are the primary markers for diagnosis of C. difficile disease and are detected in the stools of patients by antibody-based and cytoxicity assays. For an update on the current state of C. difficile diagnosis, we recommend a recent review by Wilkins and Lyerly (182).
In this review, we focus on the progress that has been made in the study of TcdA and TcdB during recent years. For more complete details on C. difficile and _C. difficile_-associated disease, we recommend several excellent reviews (13, 41, 76, 79, 168). In addition, a previous review in this journal, in 1988, highlights several of the early observations on C. difficile disease and the discoveries of TcdA and TcdB and will also be of interest (104). The present review provides a comprehensive update on the major advances made in the understanding of TcdA and TcdB within the past 15 years and includes important new findings on the genetics and mechanism of action of these toxins.
TcdA (308 kDa) and TcdB (270 kDa) are glucosyltransferases, which inactivate Rho, Rac, and Cdc42 within target cells. TcdA and TcdB are among the largest bacterial toxins reported to date and are joined by Clostridium sordellii lethal toxin (TcsL) and hemorrhagic toxin (TcsH) and Clostridium novyi alpha toxin (Tcnα) to make up the group of large clostridial toxins (Table 1). TcdA and TcdB are encoded on a pathogenicity region within the chromosome of C. difficile and are expressed efficiently during the late log and stationary phases of growth in response to a variety of environmental stimuli. As expanded on in subsequent sections, their glycosylating activity allows TcdA and TcdB to modulate numerous physiological events in the cell and contribute directly to disease.
TABLE 1.
Bacterial host and substrate targets of large clostridial toxins
| Toxin | Organism | Size (kDa) | Intracellular targets |
|---|---|---|---|
| TcdA | C. difficile | 308 | Rho, Rac, Cdc42 |
| TcdB | C. difficile | 270 | Rho, Rac, Cdc42 |
| TcsH | C. sordellii | 300 | Rho, Rac, Cdc42 |
| TcsL | C. sordellii | 270 | Ras, Rac, Rap, Ral, (Cdc42)b |
| Tcnα | C. novyi | 250 | Rho, Rac, Cdc42 |
| TcdB-1470a | C. difficile 1470 | 270 | Ras, Rac, Rap, Ral, Cdc42 |
C. difficile is not amenable to genetic manipulation, making it difficult to generate isogenic strains deficient in toxin production. Thus, implication of C. difficile toxins in disease has taken more indirect and surrogate approaches, yet the current evidence strongly implicates these toxins in disease. For example, early work by Burdon and colleagues demonstrated a direct relationship between toxin levels and development of pseudomembranous colitis and duration of diarrhea (16). Furthermore, levels of immunoglobulin G against TcdA correlate directly with protection from disease following colonization, indicating that a robust antibody response to this toxin is sufficient for protection from _C. difficile_-associated disease (99). More direct evidence for the role of TcdA in _C. difficile_-associated disease comes from studies showing protection against disease in gnotobiotic mice with monoclonal antibodies to TcdA (33). Kurtz et al. have also recently shown that neutralization of C. difficile toxins with an anionic high-molecular-weight polymer protected 80% of experimental hamsters exposed to the organism (97). Finally, the hallmarks of pseudomembranous colitis, including fluid accumulation, inflammation, and cell damage, can be invoked with TcdA in animal models.
The role of TcdB in disease is not as well understood as the contributions of TcdA. In fact, an early study found that TcdB was unable to initiate disease unless TcdA was present (105). Naturally occurring TcdA- TcdB+ strains are occasionally identified from clinical isolates (87, 96, 101, 130, 148, 149) and have been useful in characterizing the role of TcdB in _C. difficile_-associated disease. Interestingly, these strains are capable of causing disease, and in some cases of extensive pseudomembranous colitis, patients died from disease caused by a TcdA-deficient strain (5). These observations do not completely reconcile with the studies mentioned above indicating that monoclonal antibodies to TcdA block disease. Based on more recent data concerning TcdA− TcdB+ strains, one might predict that TcdB would possibly contribute or substitute for the neutralized TcdA in these experiments. Other factors, such as the expression levels of TcdB, in these various strains may account for the observed differences and should therefore be the focus of continued investigations. Collectively, these data indicate that TcdB can contribute to disease, and a detailed understanding of the molecular mechanisms of action for this toxin, along with TcdA, will advance our appreciation of _C. difficile_-associated disease.
Given their important role in disease, detailed investigations into the fundamental biology of these toxins have provided meaningful insight into C. difficile's mechanism of pathogenesis. Important advances have been made in understanding the genetics, enzymology, cellular translocation, and impacts on cell physiology of TcdA and TcdB. In the following sections, we detail the seminal findings concerning these important virulence factors from C. difficile.
GENETICS OF TcdA AND TcdB
The genes encoding TcdA and TcdB, tcdA and tcdB, respectively, have been sequenced and are found in single open reading frames located within a ≈19.6-kb pathogenicity locus (8, 38). As expected, both open reading frames are large, with tcdA found within an 8,133-nucleotide region and tcdB is 7,098 nucleotides in length.
Both tcdA and tcdB are low-G+C (<28%) genes, which is comparable to the G+C content (≈29%) of the C. difficile genome, and the toxins exhibit a high degree of overall similarity (66%). Given the proximal locations of tcdA and tcdB and the high sequence and functional homology between the two proteins, it has been proposed that the two genes may have arisen as the result of a gene duplication event (173). Furthermore, the similarity in the biochemical activity of TcdA and TcdB, wherein both toxins use a highly conserved N-terminal domain to modify identical substrates, supports the notion of gene duplication. The major regions of homology between TcdA and TcdB fall within the enzymatic and receptor-binding domains of the two toxins (172). The N-terminal domains of TcdA and TcdB show 74% homology, and this homology provides a basis for the similar substrate specificity of these two toxins.
The C termini of TcdA and TcdB show a number of short, homologous regions termed combined repetitive oligopeptides (CROPs) (173). TcdA encodes five groups of CROPs, which range in size from 21 to 50 residues and can be repeated throughout the C terminus of the protein. TcdB also encodes five groups of CROPs, four of which show homology to the CROPs of TcdA. Yet the CROPs found in TcdB are more divergent and less frequent than those found in TcdA. CROPs appear to play a putative role in initial target cell interaction and receptor binding, but the mechanism explaining the necessity for these repeats in cell binding remains unclear.
In addition to the homology between the two toxins, TcdA and TcdB exhibit a high degree of similarity to the other large clostridial toxins. TcdB shows the most homology (85% homology and 74% identity) with TcsL, the cytotoxin produced by C. sordellii which glucosylates Ras, Rac, Rap, and Ral. The homology between TcdB and TcsL explains earlier observations on the cross-reactivity of antiserum raised against crude supernatants of C. sordellii with TcdB (23). Subsequent purification of TcsL demonstrated cross-reactivity between TcsL-specific antiserum and TcdB (132). The major contrasting regions in these two proteins are found at the N termini and are responsible for the difference in substrate specificity (73). TcdA is thought to be most similar in function to TcsH, the enterotoxin produced by C. sordellii, although the sequence of TcsH is yet to be determined. As is found with TcdB and TcsL, antiserum to TcdA recognizes TcsH and vice versa (113), further demonstrating the close homology between these large proteins. The remaining member of the large clostridial toxin family, Tcnα, produced by C. novyi, shows the least homology with the other large clostridial toxins, and this may be due to significant phylogenetic differences between the organisms. However, similar to TcdB, TcsH, and TcdA, Tcnα glycosylates Rho, Rac, and Cdc42 and differs only in using UDP-_N_-acetylglucosamine instead of UDP-glucose as cosusbtrate.
The results from studies on C. difficile strains 1470 and 8864 suggest that genetic exchange and recombination has occurred between large clostridial toxin-producing strains of clostridia. For example, strain 1470 (toxinotype VIII) produces a hybrid of TcdB and TcsL which contains the cell entry portion of TcdB and the enzymatic domain of TcsL (174). Thus, the strain 1470 toxin targets cells with the efficiency of TcdB yet causes morphological changes and cell death similar to TcsL. A similar hybrid toxin is encoded by C. difficile strain 8864 (toxinotype X) and, like the toxin-encoding strain 1470, lacks functional TcdA (169). These hybrid-producing strains were discovered during analysis of clinical isolates, suggesting that these genetic alterations do not prevent C. difficile from causing disease.
Pathogenicity Locus
TcdA and TcdB are both encoded on the same ≈19.6-kb pathogenicity locus (Fig. 1) in C. difficile (63). The two toxin genes are closely situated, with a 1,350-nucleotide intervening sequence on this locus, and are transcribed in the same direction. In addition to tcdA and tcdB, three other open reading frames are located on this pathogenicity locus and are thought to be involved in regulation of toxin production or release of the toxins from the cell (63). tcdC lies downstream of tcdA and is transcribed in the opposite direction from the two toxin genes, and tcdC is highly expressed in early exponential phase but declines as growth moves into the stationary phase (75). This decline in TcdC expression corresponds to increases in TcdA and TcdB, suggesting that TcdC may function as a negative regulator of toxin production.
FIG. 1.
Genetic arrangement of the C. difficile pathogenicity locus and proposed protein domain structures of TcdA and TcdB. Both TcdA and TcdB are encoded on the 19.6-kb pathogenicity locus. In addition to the two toxin genes tcdA and tcdB, three additional regulatory open reading frames are located on this island. tcdD is a proposed positive regulator, tcdE is a putative holin protein, and tcdC is a proposed negative regulator of toxin gene expression. Through deletion mutagenesis, research combined from multiple research groups has revealed a three-domain structure of the large clostridial toxins. The glycosyltransferase activity is located at the N terminus of the protein, and the C terminus is involved in receptor binding. Located in the middle domain of the protein is a putative transmembrane segment that is thought to be involved in membrane translocation.
tcdD is found upstream of tcdB and is coordinately expressed with both of the toxin genes. TcdD is similar to DNA-binding proteins and has been shown experimentally to enhance expression of promoter reporter fusions containing the promoter-binding regions of tcdA and tcdB (119). TcdD is also homologous to TetR and BotR, which serve as positive regulators of tetanus and botulinum toxin synthesis, respectively (114, 115). In addition, TcdD shows homology with UviA, which regulates a UV-inducible bacteriocin gene from Clostridium perfringens (52). Thus, TcdD may serve as a positive regulator of toxin expression. In line with this prediction, Mani and Dupuy (108) recently demonstrated that TcdD functions as an alternative sigma factor for RNA polymerase but does not directly bind to the promoter regions of tcdA and tcdB. Expression of TcdD is responsive to environmental conditions and repressed by glucose and is significantly increased in stationary phase (75). TcdD has also been reported to autoregulate its own expression in response to various environmental cues, such as the phase of cell growth and the immediate environmental makeup (109). Thus, TcdD appears to be a major positive regulator of tcdA and tcdB expression.
The gene encoding TcdE is positioned between tcdB and tcdA and shows homology with holin proteins and thus has been speculated to facilitate the release of TcdA and TcdB through permeabilization of the C. difficile cell wall (165). Thus, while still somewhat speculative, toxin expression appears to be dependent on decreases in TcdC, TcdD-enhanced expression, and TcdE-mediated release from the cell.
The organization of the toxin locus is not suggestive of polycistronic expression, but a series of studies indicate that transcriptional readthrough may occur between particular genes. In an analysis with a reverse transcription-PCR-based approach, it was reported that bicistronic elements were present between tcdD/tcdB, tcdB/tcdE, and tcdE/tcdA (75). These investigators reported a significant level of readthrough transcripts, while subsequent studies by another group using primer extensions and RNase protection assays indicated that bicistronic mRNAs were present but at substantially reduced levels (39). The explanation for these differences is unclear, but both instances suggest that multiple forms of mRNA may contribute to expression of the toxins.
Toxinotypes of C. difficile Isolates
While the primary work on TcdA and TcdB has been carried out on toxins from the reference strain VPI 10463, it is clear that several genetic variants of these toxins exist in clinical isolates. Some of these toxin variants include hybrids which have occurred due to genetic exchange between large clostridial toxin-producing clostridia. More commonly, subtle sequence variations, deletions, and duplications within the pathogenicity locus account for various toxinotypes of C. difficile. With these sequence variations, Rupnik and colleagues have developed an elegant typing system which distinguishes the various toxinotypes of C. difficile (146, 148). There are now at least 22 toxinotypes of C. difficile (59) containing small sequence variations in the case of toxinotype III, for example, or large deletions in tcdA, as is seen in toxinotypes X and XVII. Serogroup typing, of which there are at least 14 groups, has been useful in distinguishing strains of C. difficile, and the toxinotyping method may prove particularly useful as it categorizes new strains based on the major virulence factors of C. difficile. Whether toxinotyping will also be useful in predicting the degree of virulence of particular isolates is not clear. However, as we learn more about the contributions of each large clostridial toxin as well as the domains and subdomains of these proteins to virulence, it may be possible to anticipate the severity of disease based on the toxinotype.
Environmental Signals Regulating Toxin Expression
The precise environmental signals modulating toxin expression remain unclear, but in vitro studies indicate that toxin expression may be enhanced by stress (including antibiotics) and catabolite repression (39). Given the role of antibiotic therapy in initiating pseudomembranous colitis, early studies focused on the influence of various antimicrobials on toxin production and found that subinhibitory levels of penicillin and vancomycin enhanced toxin production in continuous cultures of C. difficile (127). While it is appealing to consider antibiotics specific inducers of toxin expression, this has not been borne out in several studies, and thus, subinhibitory levels of antibiotics may be one of many ways to provide stress and induce toxin expression.
Limiting biotin in defined medium enhances toxin production (188), and this phenomenon is striking, with a 64-fold increase in TcdB production and a 35-fold increase in TcdA when C. difficile was grown in the presence of 0.05 nM biotin. Under these biotin-limiting conditions, growth of C. difficile was reduced, and it is possible that this stress, like other stresses, triggered toxin production. Yet, in contrast to the observations on biotin, limiting amino acids, and thereby growth of C. difficile, does not enhance toxin production (187). Others have proposed links between purine biosynthesis and toxin production, while even further studies have argued against stress and catabolite repression as a mechanism of regulating the expression of TcdA and TcdB (86, 107). Indeed, a significant amount is still not understood about the factors that regulate toxin expression, and this is an area of C. difficile biology in critical need of further research.
MECHANISM OF ACTION AND FUNCTIONAL DOMAINS OF TcdA AND TcdB
TcdA and TcdB utilize a well-defined mechanism of action in order to modulate cell physiology and, consequently, alter the host environment. As mentioned above, these two toxins, along with the other members of the large clostridial toxin family of toxins, target the Ras superfamily of small GTPases for modification via glycosylation. This irreversible modification inactivates these small regulatory proteins, leading to disruption of vital signaling pathways in the cell. However, in addition to enzymatic modification of targets, there are several important steps in receptor binding and cell entry which are important for intoxication.
Cell Surface Receptors for TcdA and TcdB
In order to elicit cytotoxic effects, TcdA and TcdB must be internalized into the host cell via endocytosis and require an acidic endosome for access to the cytosol (46, 68, 117). Receptor binding is the first essential step in the process of cell entry. The receptors for the large clostridial toxins are thought to be nonproteinaceous and, for TcdA, show the disaccharide Galβ1-4GlcNac. This disaccharide is found on the I, X, and Y blood antigens present on a variety of cells, and these antigens have been shown to act as receptors for TcdA (171). Various animal species susceptible to TcdA may not necessarily show identical receptors. For example, TcdA is reported to bind to Galα1-3Galβ1-4GlcNAc (95), yet this receptor does not appear to be present on human cells.
In line with these studies, treatment of cells or tissue with galactosidase reduces binding by TcdA (31, 32, 95, 159). A possible protein receptor, sucrase-isomaltase, was found on cells in the ileum of rabbits, but again, this protein is not found on cells targeted within the human colon (135). The receptor for TcdB has been more elusive than that for TcdA. In our experiments and those of others, TcdB has been shown to intoxicate a broad range of cell types, indicating that the receptor for this toxin, while undefined, is ubiquitous.
Receptor-Mediated Endocytosis and Membrane Translocation
Both TcdA and TcdB enter the cell through receptor-mediated endocytosis and require an acidified endosome for translocation. The cytotoxic effects of large clostridial toxins can be directly inhibited by a variety of lysosomotropic inhibitors, such as bafilomycin A and ammonium chloride (46). Furthermore, conditions that prevent lysosome fusion with the endosome attenuate TcdB activity (47).
The requirement for low pH appears to be due to important structural changes which occur in the toxins, leading to exposure of hydrophobic domains prior to insertion into the target membrane (140). Indeed, Barth and colleagues have observed the formation of channels in lipid bilayers by TcdB through an acid pH-dependent process (9).
Until recently, it was not known whether the full toxin translocated to the cytoplasm or only the enzymatic domain. However, recent work by Pfeifer and colleagues showed that, following proteolytic cleavage, only the N-terminal domain of TcdB exits the endosome and gains access to substrates (129). While a putative membrane insertion-translocation domain, located within the middle of the protein, has been proposed for TcdA and TcdB, this region of the protein has not been definitively shown to promote translocation of the N-terminal enzymatic fragment.
Modification of Intracellular Targets
Following receptor binding and internalization into the target cell cytosol, TcdA and TcdB elicit specific effects that modulate host cell physiology. Discoveries from initial studies indicated that cells exposed to TcdB exhibited marked changes in the organization of their actin cytoskeleton (117, 167, 178). This adverse effect on the actin cytoskeleton has since become a hallmark of the intoxication of cells by TcdA and TcdB and is thought to be a prelude to cell death in a variety of cell types.
In line with these early observations, Just and colleagues discovered that Rho from TcdB-treated cell lysates was no longer subject to ADP-ribosylation by C. botulinum C3 exoenzyme (82). C3 ADP-ribosylates Asn-41 in Rho (156), yet TcdB was not found to have a similar activity. These observations did suggest, however, that TcdB was somehow altering Rho within the cell. Other experiments found that overexpression of Rho protected cells from TcdB-induced rounding, further implicating this protein as a target for TcdB. TcdA was subsequently found to exert a similar effect on Rho (83).
These initial studies allowed investigators to focus on Rho as a putative target of TcdB and TcdA. In a seminal report, Just and colleagues demonstrated the ability of TcdB to glucosylate RhoA via transfer of a sugar moiety to Thr-37 of the GTPase with UDP-glucose as a cosubstrate (84). In the same study, two other GTPases, Rac and Cdc42, were also found to be glucosylated by TcdB. Additionally, this group observed the ability of TcdA to modify RhoA with the same UDP-glucose-dependent mechanism as TcdB (85). In line with these observations, cells with reduced levels of UDP-glucose are less sensitive to the toxins (25). Collectively, these studies defined TcdA and TcdB as glucosyltransferases, capable of inactivating small GTPases within the cell.
Both TcdA and TcdB target isoforms of Rho (RhoA, -B, and -C), Rac, and Cdc42, resulting in actin condensation and consequent rounding of the cells, membrane blebbing, and eventual apoptosis and death of the target cell. While both of these large clostridial toxins exert their activities on a wide range of cell types, TcdB exhibits a higher rate of enzymatic activity than TcdA, leading to a quickened rate of cytopathic effects in some cell types (27). Additionally, Ciesla and Bobak demonstrated that UDP-glucose hydrolysis by TcdB occurs at a rate ≈5-fold greater than that by TcdA (30).
Small GTPase Proteins as Targets of TcdA and TcdB
The studies highlighted in the previous section clearly defined TcdA and TcdB as glucosyltransferases that inactivate small GTPases. Thus, to appreciate the role of TcdA and TcdB in cellular intoxication, it is important to understand Rho proteins and their activities. Rho, which includes mammalian isoforms A, B, and C, is a primary regulator of the actin cytoskeleton and is found ubiquitously in eukaryotic cells (61). Following expression, Rho is subject to posttranslational modification by prenylation and carboxymethylation, which aid in localization to the cytoplasmic side of the plasma membrane, where effective exchange of GTP and GDP occurs (2, 112).
Small GTPases, such as Rho, bind and hydrolyze GTP to GDP, and it is the alternation between the GTP-bound (active) and GDP-bound (inactive) states that regulates activity. The exchange of GDP for GTP is regulated by guanine exchange factors within the cell (189). Activated Rho intrinsically hydrolyzes GTP, removing the γ-phosphate group and returning to the “off” GDP-bound state through a process enhanced by GTPase-activating proteins (53, 54). Further regulation comes from GDP dissociation inhibitors, which block nucleotide exchange and maintain GTPases in the GDP-bound form within the cytoplasm, preventing association of Rho with the membrane and interactions with guanine exchange factors (51, 71, 118).
Activation of Rho signaling is initiated by factors that promote the dissociation of GDP dissociation inhibitors from GDP-bound Rho and release the protein for localization to the cell membrane. Thus, in a model of Rho activation, GDP dissociation inhibitors maintain inactive Rho within the cytosol, and following dissociation from these inhibitors, Rho localizes to the membrane, where guanine exchange factors enhance the dissociation of GDP. As GTP is in excess compared to GDP in the cell, Rho subsequently binds GTP and becomes activated, promoting downstream signaling events. Due to inherent GTPase activity and the action of GTPase-activating proteins, Rho subsequently returns to the off state, returning to the GDP-bound form. Rac and Cdc42 show similar mechanisms of regulation, and, while subtle differences are apparent, for the purposes of this review, their regulation can be considered relatively identical to that of Rho.
It is important to appreciate the downstream effects of Rho activation, since these can be impacted by TcdA and TcdB. Rho has several effectors that are activated by the GTP-bound form of this small GTPase. However, some targets, such as mitogen-activated protein kinase kinase kinase, can interact directly with the GDP-bound form of Rho, and GTP activation does not seem to be required (40). Rho plays a major role in regulation of stress fiber formation within the cell, and this is accomplished by interacting with and activating signaling proteins such as Rho-kinase (50), citron K (106), and phosphotidylinositol 4-phosphate 5-kinase (29), following activation from extracellular signals and integrin binding. Inhibiting Rho's ability to interact with these effectors leads to several changes in the actin cytoskeleton. Important to the role of TcdA and TcdB in disease is the fact that Rho regulates the localization of stress fibers at focal adhesion sites (142). Furthermore, Rho controls the formation of perijunctional rings at the apical side of epithelial cells (125). As discussed in later sections of this review, inactivation of Rho and subsequent loss of perijunctional rings and focal adhesions may lead to increased permeability of the epithelial layer of the intestines.
While Rho regulates stress fiber formation, motility, and focal adhesions, Rac and Cdc42 are more specifically involved with lamellipodium and filopodium formation (124). Lamellipodia and filopodia are important structural extensions at the leading edge of migrating cells and are essential to movement and sensing the environment during cell migration. It is worth noting TcdA and TcdB have been implicated in cellular chemotactic responses during pseudomembranous colitis inflammation (136, 161). Thus, paradoxically, TcdA and TcdB seem to promote cell migration while inactivating the primary cellular factors, Rho, Rac, and Cdc42, that are necessary for this process.
Predictably, the inactivation of Rho proteins by TcdA and TcdB could occur at various steps, including blocking membrane localization, guanine exchange factor interaction, or contact with downstream effectors. The elucidation of the crystal structures of RhoA/GDP (179) and RhoA/GTPγS (77) have provided the information necessary for making predictions regarding the effects of glucosylation on these small GTPases.
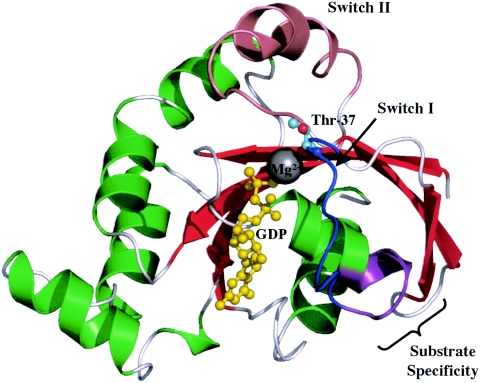
The RhoA/GDP structure (as shown in Fig. 2) has been determined to 2.1 Å, while the RhoA/GTPγS structure has been determined to 2.4 Å. Upon examination of the RhoA crystal structure, the impact of glucosylation would appear to prevent Thr-37 from forming a complex at the γ phosphate of the bound GTP molecule. In the GTP-bound form of Rho, Thr-37 contributes to Mg2+ interactions through the reactive hydroxyl group (77), which is also subject to glucosylation. Examination of the Rho three-dimensional structure in the GTP-bound form indicates that this hydroxyl group is not readily accessible, as it is complexed with Mg2+ and is directed away from the solvent. However, in the GDP-bound form, the hydroxyl group of Thr-37 aids in the stabilization of a water molecule that coordinates the Mg2+ ion but is susceptible to glucosylation.
FIG. 2.
Crystal structure of GDP-bound RhoA. In the GDP-bound form of Rho, the Mg2+ ion is coordinated by the β phosphate group of GDP and a water molecule that interacts with T37 for stabilization. The OH group of T37 is glucosylated by the large clostridial toxins, inactivating this regulatory protein. The region involved in substrate specificity is in purple and includes residues 24 to 29 (22 to 27 in Rac and Cdc42). This figure was generated and rendered with PyMOL (34).
In fact, the ability of TcdA and TcdB to more easily modify GDP-bound Rho than the GTP-bound form has been shown quite convincingly in previous studies (84). Interestingly, Herrmann et al. have shown that, while GTP blocks glucosylation of Ras by TcsL, glucosylation does not appear to reduce GTP binding (69). Based on the structure of GTP-bound Ras, it is difficult to appreciate how this protein is capable of stably binding GTP without the contributions from the hydroxyl group of Thr-35 (Thr-37 in Rho). This may suggest that other, more subtle, yet poorly understood mechanisms are involved in the process of glucosylating GTPases.
New structural insights have been gained regarding the mechanism of glucosylation of Rho, Rac, and Cdc42. How does the addition of a single sugar molecule lead to the inactivation of Rho, Rac, and Cdc42? A straightforward explanation is that glucosylation of Thr-37 in Rho or Thr-35 in Rac and Cdc42 prevents proper binding of GTP and blocks activation. Based on the structure of GTP-bound Rho and results from studies on the structure of glucosylated Ras, elimination of GTP binding appears reasonable; however, it is plausible that glucosylation could block interaction with downstream effectors or regulators such as guanine exchange factors or GTPase-activating proteins. Blocking interaction with GTPase-activating proteins would enhance Rho activity and, for this reason, can be eliminated as a possible outcome of glucosylation. The interruption of binding to guanine exchange factors would have the effect of maintaining Rho in the GDP-bound form and therefore could have the effect of inactivating Rho.
The Thr-37 and Thr-35 residues are highly conserved among small GTPases, yet only Rho, Rac, and Cdc42 are targeted by TcdA and TcdB. As shown in Fig. 2, the reason for this substrate specificity has been shown to be residues 24 to 29 in Rho (corresponding to residues 22 to 27 in Rac and Cdc42) that lie just outside the effector loop region and constitute part of the transition between the α1 helix and the switch I region (122). Through the use of Rho-Rac chimeric protein fusions and substitution mutagenesis, the importance of specific residues within this region has been defined, and the results indicate that the lysine residue at position 27 (25 in Rac and Cdc42) plays a major part in substrate distinction. The Rho mutant K27T allowed glucosylation of Rho by TcsL, while native Rho is not targeted by TcsL. These mutagenesis results have provided a strong basis for the substrate specificity observed among the large clostridial toxins.
Several interesting questions remain unanswered regarding the mechanism of Rho modification by TcdA and TcdB within the cell. For example, Rho proteins are complexed with GDP dissociation inhibitors within the cytoplasm and are in the conformation optimal for glucosylation, yet Rho-GDP dissociation inhibitor complexes cannot be modified by large clostridial toxins (55). This hindrance is due to the direct interaction between the OH group of Thr-37 with Asp-45 of Rho-GDP dissociation inhibitor (72). Due to this interference with Thr-37, the underlying implication of the absence of Rho-GDP dissociation inhibitor glucosylation is that the toxins must be accessing substrate at the plasma membrane. Yet, as pointed out above, GTP-bound Rho is not a good substrate for glucosylation. This further suggests that, of Rho's possible states, only the membrane-localized, GDP-bound form is a target of glucosylation. This raises additional questions regarding how TcdA and TcdB might be involved in posttranslocation trafficking to the cytoplasmic side of the inner membrane. Furthermore, it has still not been shown definitively if loss of interaction with downstream effectors is due simply to the fact that glucosylated Rho cannot bind GTP, and thus be activated, or if the glucose molecule directly blocks effector interaction. The biological outcome of both scenarios would be similar, yet continued studies dissecting this event may provide new insights into Rho proteins as well as TcdA and TcdB.
Protein Domain Structure of the Large Clostridial Toxins
Based on the known activities of TcdA and TcdB, the proteins were predicted to have enzymatic, translocation, and receptor-binding domains, each serving a specific function during cellular intoxication (Fig. 1). The N-terminal enzymatic and C-terminal receptor-binding domains are the best understood regions of these proteins, while the membrane-inserting translocation domain remains undefined.
Organization of the Enzymatic Domain
The activities necessary for glucosylation are found within a 546-residue N-terminal region of TcdB (74). This region can effectively complete glucosylation of substrates in vitro but lacks any detectable cytotoxic activity. Furthermore, our group has shown that delivery of this enzymatic domain, with surrogate cell entry systems, results in cytotoxic effects similar to that observed with full-length TcdB (163). It is worth noting 546 residues is still as large as or larger than most full-length intracellular bacterial toxins, yet even minor deletions (e.g., 30 residues) from the C-terminal end of this fragment dramatically reduce activity. Conflicting with these studies, Wagenknecht-Wiesner et al. reduced the enzymatically active portion of TcdB to residues 1 to 467, a region that retained the ability to glucosylate Rho, Rac, and Cdc42 in vitro (175). In our hands, deletions down to residues 1 to 500 are completely devoid of enzymatic activity (162).
The substrate recognition region of TcdB has been narrowed to residues 364 to 516 by using chimeric hybrids between this toxin and TcsL, which targets a different subset of small GTPases (73). By swapping this region between the two toxins, it was possible to change the substrate specificity between TcsL and TcdB and identify the region important for substrate modification. In addition to delineating the region responsible for substrate specificity, Busch and colleagues found that the glucosyltransferase activity of these large clostridial toxins is dependent on the presence of a DXD motif in the enzymatic domain (18). Mutations in this vital motif rendered forms of the toxin deficient in the ability to modify small GTPases via glucosylation in vitro. Although this research was conducted with TcsL, the DXD motif is conserved among all of the large clostridial toxins, indicating its importance in this toxin family. Continuing the investigation of critical regions involved in TcdB-induced glucosylation, Busch et al. determined that Trp-102 is involved in UDP-glucose binding, and mutants lacking this residue were no longer toxic to cells (17). The combined work of these groups has provided a strict basis for the regions of TcdA and TcdB involved in substrate specificity and enzymatic activity.
Receptor-Binding Domain
The C-terminal receptor-binding domain of TcdA makes up roughly one-third of the toxin and contains up to 38 repeating CROP modules. However, as noted by Rupnik et al., the CROP region of TcdA has isolate-specific repeats and deletions, suggesting that this region of the toxin may be derived from multiple duplication and recombination events within the gene (146). The CROP sequences vary in length by up to 50 amino acids but contain a consensus triad (YYF) and an overall hydrophilic stretch of residues indicative of a solvent-exposed region of the protein (186). This high degree of repetition may explain the agglutinating activity of TcdA. Furthermore, the CROP regions are similar to those of other proteins, including those from Streptococcus downei and Streptococcus mutans, which function as saccharide-binding proteins (185).
The experimental evidence for receptor binding by the C terminus of TcdA comes from a series of studies showing the neutralizing capacity of monoclonal antibodies to this region of the protein (48) and the ability of recombinant fragments from this region to protect against the toxin. A fragment of the C terminus containing the repeat moieties in TcdA was constructed by Sauerborn et al. and shown to block TcdA-induced cytotoxicity in cell culture by competitive inhibition of receptor binding (152). The same protein fragment was also found to immunize mice against TcdA. In recent studies, the entire C-terminal receptor-binding domain has been shown to be necessary for both receptor binding and effective endocytosis of the holotoxin (49).
INFLUENCE OF TcdA AND TcdB ON CELL PHYSIOLOGY
TcdA and TcdB have a dramatic influence on mammalian cell physiology, altering events ranging from cell signaling to ultrastructure maintenance. In many cases, cells do not survive intoxication by TcdA and TcdB, even at relatively low doses in comparison with other cytotoxins. Thus, cell death is one of the most obvious impacts of these toxins on cell physiology. Early studies demonstrated TcdA and TcdB's broad cell tropism and found TcdB to be a more effective cytotoxin. In an initial study by Donta et al., the researchers indicated that TcdB could intoxicate mouse adrenal cells, hamster ovary cells, rat hepatic cells, and human cervical epithelial cells (37). Depending on the cell type, TcdB ranged from 4-fold to 200-fold more cytotoxic than TcdA in these studies. Subsequent studies found that mouse teratocarcinoma cells were more sensitive than CHO cells to TcdA, possibly due to increased levels of TcdA-specific carbohydrate receptors (170).
Impact on Cell Morphology
The first and most obvious impact on cell physiology is the loss of structural integrity, which results from the decline in F-actin in cells exposed to TcdA and TcdB. Chang et al. were among the first investigators to examine ultrastructural changes in intoxicated cells and reported alterations in cell surface projections and rearranged microvilli (24). Upon closer examination, it was found that TcdA altered the ultrastructure of CHO cells, and distinct actinomorphic changes were identified by electron microscopy (44). TcdA-intoxicated cells also demonstrate a distinct cell retraction phenotype, and this is linked to changes in the microfilament system (44). In the same study, marginalization of the nucleus was observed, and again, this was likely a reflection of changes in the cytoskeleton. Similar studies have been performed with TcdB, and the cytopathic effects observed are like those induced by TcdA. Mechanistically, the loss of structural integrity in TcdA- and TcdB-treated cells is expected, since Rho, Rac, and Cdc42 each regulate structural processes dependent on actin polymerization.
Results from our group indicate that cell rounding and cell death are temporally distinct events (139). TcdB is capable of inducing cell rounding in less than 2 h, but cell death, as assayed by a variety of methods, does not occur until almost 24 h following treatment. Thus, as these events relate to disease, cytopathic effects may be more relevant than actual cell death, since cells with considerable loss of actin cytoskeleton integrity are unlikely to carry out their roles within the host.
Impact on GTPase Signaling Pathways
The substrate targets of TcdA and TcdB, Rho, Rac, and Cdc42, also regulate cellular events outside the actin cytoskeleton (Fig. 3A). For example, Rho, Rac, and Cdc42 are involved in regulation of the cell cycle and signaling through mitogen-activated protein kinase kinases. It is reasonable to assume that these toxins exert effects on cell physiology that may not involve the actin cytoskeleton. Furthermore, the temporal differences between cell rounding and cell death indicate that other events downstream of actin condensation can contribute to cell death. Indeed, TcdB alters levels of phospholipase A2 activity in toxin-treated cells, and the data indicate that this process is modulated independently of the events linked to the cytoskeleton (158).
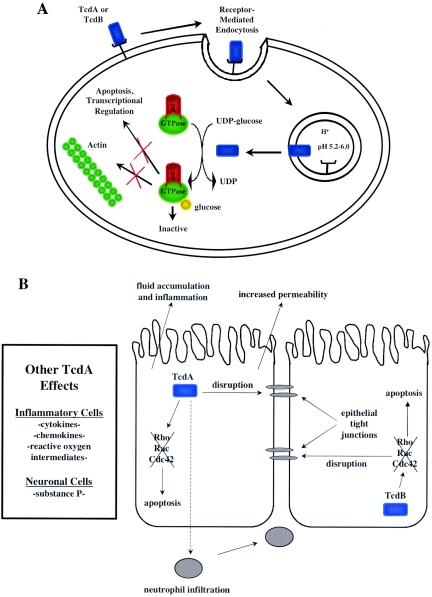
FIG. 3.
(A) Overview of intracellular modifications by TcdA and TcdB. Both TcdA and TcdB act intracellularly as glycosyltransferases. Each toxin modifies and inactivates Rho, Rac, and Cdc42 via transfer of a sugar moiety, with UDP-glucose as a cosubstrate. The effects of these modifications include actin condensation, transcriptional activation, and apoptosis. (B) Downstream effects of TcdA and TcdB in intestinal cells during disease. Exposure of intestinal epithelial cells to TcdA leads to neutrophil infiltration, substance P production, chemokine production, reactive oxygen intermediate production, disruption of tight junctions, and apoptosis. TcdB activity leads to disruption of tight junctions and apoptosis. A combination of one or more of these activities leads to fluid accumulation in the host and inflammatory responses.
Along these lines, TcdB has been found to inhibit receptor-coupled signaling through phospholipase D via inactivation of Rho proteins (155). It is unclear how modulation of these phospholipases may contribute to overall events during intoxication. TcdA is capable of increasing the permeability of colonic epithelial layers by inactivating RhoA through a process involving protein kinase C (28). This increase in permeability may further enhance the inflammatory events observed in pseudomembranous colitis by increasing access of polymorphonuclear cells to the colonic epithelium. TcdA activates differential expression of chemokines in human intestinal epithelial cells, and this may also serve as a mechanism to increase the inflammatory response (90). Furthermore, TcdA has been shown to activate mitogen-activated protein kinases in human THP-1 monocytes, in a process that appear to be independent of Rho inactivation and linked to production of interleukin-8 (176).
Apoptosis likely accounts for the mechanism of death in cells exposed to TcdA and TcdB. Rho inactivation in an endothelial cell line resulted in activation of caspase-3 and caspase-9, key components of the apoptotic cell death pathway (70). Brito and colleagues observed that TcdA induced the apoptotic pathway in T84 intestinal cells via caspases-3, -6, -8, and -9 and Bid activation (15) and further demonstrated the disruption of mitochondrial membrane potential and release of cytochrome c. However, there are both temporal and spatial reasons to suggest that TcdA can induce apoptosis outside of inactivating Rho proteins. Treatment of cells with TcdA results in accumulation of the toxin at the mitochondria within 5 min following exposure, and this localization event occurs before detectable glucosylation of Rho proteins (64). Thus, TcdA may induce apoptosis by disrupting mitochondria, which promotes proapoptotic events.
TcdB is also capable of triggering apoptosis. Our group has shown that HeLa cells intoxicated by TcdB undergo caspase-3-dependent apoptosis with a concurrent loss in host cell vimentin (139). The same study also found that TcdB was able to not only to induce caspase-dependent cell death, but also to induce caspase-independent apoptosis specifically due to substrate inactivation. The proteome of TcdB-treated cells contains fragments of intermediate filaments that result from cleavage by caspase-3. Since intermediate filaments, such as vimentin, are involved in maintaining the cell's structural integrity, the loss of these proteins may also account for changes in cell morphology. Together, these results demonstrate some of the potential effects of GTPase inactivation on the host cell due to intoxication by the large clostridial toxins and implicate apoptosis as a major method by which the toxins induce cell death.
ROLE OF TcdA AND TcdB IN DISEASE
The signs of C. difficile disease range from mild diarrhea to fulminant colitis in patients undergoing antibiotic treatment. The disease occurs almost exclusively in the large bowel and shows distinguishing microscopic and gross lesions. Microscopic pathology reveals the “volcanic eruption” characteristics of the pseudomembranous lesion observed in pseudomembranous colitis, although this is not a defining pathology of all _C. difficile_-associated disease (76). Gross pathology shows hallmark raised plaques within the intestines, which correspond to the nodules observed by endoscopy. These pathologies may explain the malabsorption and resulting diarrhea in patients with pseudomembranous colitis. Most of these pathologies can be ascribed to inflammatory events, which are likely modulated by TcdA and TcdB.
Both TcdA and TcdB are capable of mimicking the physiological events occurring in _C. difficile_-related pseudomembranous colitis (100, 153). With the rabbit or rat ileal loop model, the inflammatory response that occurs in pseudomembranous colitis can be triggered by isolated toxin; however, most of these studies have focused on TcdA. As shown in Fig. 3, inflammation, including increased epithelial permeability (42), cytokine and chemokine production (21, 65), neutrophil infiltration (89), activation of submucosal neurons (123), production of reactive oxygen intermediates (65), mast cell activation (180), substance P production (111), and direct damage to the intestinal mucosa, seems to contribute to the pathologies observed in pseudomembranous colitis. It should also be noted that, while pseudomembranous colitis-associated inflammation can be induced by toxin alone, isogenic strains lacking both toxins have not been investigated in these models. Furthermore, direct links between inactivation of Rho proteins and induction of inflammation have not been reported. For this reason, it should be acknowledged that other toxin activities (e.g., cell surface interactions) could contribute to inflammation.
One of the most direct events attributed to TcdA and TcdB during pseudomembranous colitis is the toxins' ability to disrupt tight junctions of epithelial barriers (42). This is likely due to inactivation of Rho proteins, since these small GTPases are known to be important in maintaining tight junctions (125). Initial studies by Hecht and colleagues found that TcdA reduced epithelial monolayer resistance within 8 h following exposure to this toxin (67). This appeared to occur prior to loss of confluency, suggesting that disruption of cell-cell contact occurs before contraction of the cell. Similar studies also found that TcdB was capable of disrupting epithelial integrity, which occurs due to a decline in F-actin and disruption of the perijunctional actomyosin ring (66). Subsequent studies have further defined the loss of epithelial tight junctions and demonstrated the loss of apical and basal F-actin, which is associated with loss of intact occludin and ZO-1 in the tight junction membrane (126). Thus, it seems reasonable to suggest that the loss of epithelial barrier integrity during pseudomembranous colitis inflammation may be due to the toxins' ability to act directly on these cells.
In addition to directly altering the integrity of the epithelium, loss of tight junctions may provide a convenient conduit for migration of neutrophils into the intestines (Fig. 3). Neutrophil accumulation is a hallmark of pseudomembranous colitis, and these cells contribute significantly to pseudomembrane formation (161). Recruitment of neutrophils to the site of infection during pseudomembranous colitis is proposed to occur in response to direct interaction with TcdA and as a result of secondary events mediated by inflammatory molecules (89). Studies in a rabbit model found that TcdA-induced inflammation involved direct binding of TcdA to neutrophils and this most likely involved a G protein-linked receptor (89). Blocking CD18 (a leukocyte adhesion molecule) prevented TcdA-triggered migration of neutrophils in rabbit ileal loops (89). TcdA also indirectly recruits neutrophils to the site of infection by increasing the expression of macrophage inflammatory protein 2 (MIP-2) (21). This is in line with other studies showing that CC chemokine receptor-1, a receptor for MIP-2, contributes to inflammation (121).
In addition to increases in the permeability of the epithelial barrier and recruitment of neutrophils, there is clear evidence that primary sensory neurons are also modulated by TcdA. Initial studies by Pothoulakis and colleagues found that a substance P antagonist attenuated the rat intestinal response to TcdA (111). Substance P is a small peptide associated with sensory neurons that line the submucosal regions of the intestinal epithelium and has been shown to be involved with inflammatory diarrhea (134). The mechanism by which TcdA modulates substance P production is not completely clear, but treatment of rat ileal loops with this toxin leads to increased substance P production (110). Furthering the inflammatory event, lamina propria macrophages are activated by substance P and release tumor necrosis factor alpha (20). Consequently, there is increased recruitment of neutrophils and inflammation via this substance P-dependent mechanism. In addition to substance P, dorsal root ganglia have also been found to express the neuropeptide calcitonin gene-regulated peptide in response to TcdA (88). Further studies have now shown that deletion of neural endopeptidase, a protein important for turnover of substance P, exacerbates the TcdA-induced inflammatory response (92). Collectively, these studies clearly indicate neuronal contributions to inflammatory pseudomembranous colitis.
TcdA has been shown to stimulate cytokine production, and this may contribute to early stages of inflammation in pseudomembranous colitis. TcdA triggers interleukin-8 secretion and the production of reactive oxygen intermediates by human colonocytes (65). Activation of interleukin-8 is dependent on TcdA's ability to enter the target cell and activate NF-κB and AP-1 (78). In line with these observations is the fact that interleukin-11 can inhibit TcdA-related damage by blocking the release of inflammatory mediators such as tumor necrosis factor alpha and MIP-2 (22). Intestinal secretory factor has also been found to be released by intestinal macrophages following exposure to TcdA through an interleukin-1β-dependent mechanism, and this is likely to cause further inflammation (145). During TcdA-induced enteritis, responding neutrophils release reactive oxygen metabolites and exacerbate inflammation (141). In addition to neutrophil infiltration, macrophages and mast cells respond to TcdA, which furthers the inflammatory response through production of factors such as tumor necrosis factor alpha (19). Indeed, mast cell-deficient mice are attenuated in neutrophil recruitment and show reduced levels of fluid accumulation following treatment with TcdA (180).
TcdA-Negative Strains Causing _C. difficile_-Associated Disease
Despite the consistent evidence described above that TcdA plays an important, if not the major, role in _C. difficile_-associated disease, there has been a substantial increase in the number of reported TcdA− clinical isolates (4, 5, 7, 12, 14, 81, 87, 93, 103, 120, 130, 131, 148, 151, 177, 181). Each of these TcdA− isolates produce TcdB but contain deletions in tcdA and encode truncated forms of the protein. C. difficile strain 8864 was the first TcdA− isolate to be characterized and was shown to have a ≈5.9-kb deletion in the 3′ end of the tcdA region (160). This strain now makes up the toxinotype X form of C. difficile, which also contains an insertion in tcdE (146). However, toxinotype X appears to be rare, and a majority of TcdA− strains occur due to a ≈1.8-kb deletion in the 3′ CROP region of tcdA (146, 148). To date, among the 22 toxinotypes of C. difficile, nine have been found to contain deletions in the 3′ end of the tcdA gene (146-148). The absence of the CROP region not only reduces cytopathic activity but prevents recognition of TcdA by most detection systems which use antibody against this region of the protein.
TcdA− mutants are isolated with different frequencies throughout the world and have been found in a variety of different patients, ranging from infants to the elderly (80, 148). An analysis by Sambol et al. found only seven TcdA− isolates out of 5,000 screened, suggesting that TcdA− strains occur at a very low frequency (149). In contrast, a survey by Pituch and colleagues reported that TcdA− strains made up 11% of their clinical isolates (130). This is consistent with the report of Geric and colleagues, which also found that 11% of isolates lacked functional TcdA (59). In addition to these findings, Samra et al. showed a high occurrence of TcdA− strains (56.5%) in their study of 530 stool specimes taken from individuals with severe disease (151). It is also clear that these TcdA− strains are being identified more frequently, as almost all of the publications describing these isolates have appeared within the past 4 years. Thus, TcdA− strains represent a substantial number of C. difficile isolates.
The occurrence of TcdA− strains is a conundrum, since, as previously described, TcdA has been shown to mimic many of the gastrointestinal signs of _C. difficile_-associated disease. TcdA neutralizing antibodies have also been found to protect against _C. difficile_-associated disease (91). Furthermore, earlier work by Lyerly et al. found that TcdB was ineffective unless TcdA was administered first, indicating that TcdB was not only lacking the ability to cause intestinal damage but also required TcdA to gain access to target cells (105). Curiously, TcdA is the potent enterotoxin from C. difficile, while there are only limited reports of TcdB's having any impact on cells of the gastrointestinal tract (43, 143, 153). Examination of the clinical course of disease for the TcdA− strains compared to the normal TcdA strains provides very little insight into this enigma. Indeed, the clinical disease is remarkably similar between TcdA− and TcdA+ strains of C. difficile (80). Therefore, the detection of TcdA− strains at such a high frequency is contradictory to the proposed mechanisms of C. difficile pathogenesis reported during the past two decades.
There are several possible explanations for the detection of TcdA− strains as clinical isolates of C. difficile. First, TcdB may play a more prominent role in _C. difficile_-associated disease than previously suspected, or TcdB could substitute in the absence of TcdA. In one very recent report, TcdB has been shown to function as a potent enterotoxin (153). Second, TcdB may function more effectively in the presence of other C. difficile factors. Third, the detection of TcdA− strains may represent a selection event within the host.
It is interesting that most of the tcdA deletions occur within the CROP region, which, due to the repeat sequences, would be susceptible to deletions by recombination. Thus, the disease could be initiated by a TcdA+ strain, but there is a selection for strains that have lost the ability to produce TcdA. Arguing against this possibility are statements in reports on the TcdA− strains (12, 149, 150) which indicate that these isolates are able to cause disease in hamster models, although delayed death is observed compared to infection with TcdA+ strains. Unfortunately, the possibility of resolving this paradox is not likely without the advancement of genetic tools for C. difficile. Without the ability to directly generate isogenic strains lacking TcdA or select deletions within tcdA, it will continue to be difficult to address this issue in a hypothesis-driven manner.
OTHER TOXINS PRODUCED BY C. DIFFICILE
In addition to TcdA and TcdB, a limited number of isolates have also been found to produce binary toxins that exhibit ADP-ribosyltransferase activity. Originally, C. difficile strain CD196, a strain isolated from a pseudomembranous colitis patient, was shown to produce a ≈43-kDa protein that was capable of modifying actin via its ADP-ribosyltransferase activity (133), similar to that of C. perfringens iota toxin and C. spiroforme toxin. This toxin, however, was not found to be cytotoxic. The toxin responsible for this activity was subsequently identified and found to be a two-component ADP-ribosyltransferase toxin encoded by the genes cdtA and cdtB (128). The proteins, termed CDTa and CDTb, show 81% and 84% identity, respectively, with the same two components from iota toxin.
Analysis of several strains of C. difficile found CDTa and CDTb only occasionally in particular strains of the organism. An examination of 24 clinical C. difficile isolates for the presence of cdtA and cdtB found the genes present in only three strains (128). A more exhaustive analysis found cdtA and cdtB in ≈6.4% of the 170 isolates taken from hospitals in the United Kingdom (164). Other studies have suggested that the ADP-ribosyltransferase is found in about 15% of C. difficile isolates (58). Interestingly, Florin and Thelestam identified a clinical isolate of C. difficile that did not produce either TcdA or TcdB yet appeared to produce the ADP-ribosyltransferase (45).
It appears that cdtA and cdtB are present due to genetic exchange among several clostridial species, and CDTa can bind to the cell entry component of iota toxin from C. perfringens due to these similarities (60). Strains encoding TcdA and TcdB as well as CDT do not appear to be more virulent, nor does the binary toxin seem to have any notable effects in animal models. Thus, while the roles of TcdA and TcdB seem to be well established and essential to C. difficile virulence, the possible contribution of CDT is not well understood.
In addition to the binary-toxin-producing strains of C. difficile, strains of C. difficile that contain variant toxin genes and thus produce hybrid toxins have been identified. One well-characterized example of these hybrid toxins is produced by C. difficile strain 1470 (26, 174). This toxigenic strain produces a toxin (TcdB-1470) that is closely related to TcdB produced by normal, nonhybrid strains in that it binds to the same receptor as TcdB and shows 99% homology in the C terminus and middle domain of the toxin. The major difference between TcdB-1470 and TcdB is that TcdB-1470 also has the ability to modify Ras, Rac, Rap, and Ral, which are the unique targets of TcsL from C. sordellii, making it a unique hybrid between TcsL and TcdB. This difference is accompanied by only 79% homology between the enzymatic domains of TcdB and TcdB-1470. Additionally, TcdB-1470 induces cytopathic effects in Swiss 3T3 fibroblasts similar to TcsL, with cell rounding being the main effect of toxin treatment. However, TcdB-1470 was found to be a more potent cytotoxin than TcsL, suggesting that this hybrid toxin could play a versatile role in disease.
Many other reports have investigated numerous strains of C. difficile that produce either TcdA and no TcdB or vice versa (5, 101). The impact of these strains on disease, as in the case of the ADP-ribosyltransferase toxins, is still unclear; however, the ability of these strains to vary their toxin expression may allow differential effects in disease that have not yet been identified.
TcdA AND TcdB AS TOOLS IN CELL BIOLOGY
A fortuitous outcome of dissecting the mechanism of action of large clostridial toxins is that these proteins are now important tools in cell biology. TcdA and TcdB are sold commercially for the purpose of inactivating Rho, Rac, and Cdc42 in a variety of experiments. The ability of large clostridial toxins to exert their effects on most cells in tissue culture makes them attractive candidates for use as a tool in this respect. Several cellular processes, including phagocytosis, cell proliferation, actin cytoskeleton regulation, cell mobility, and exocytosis, have been studied with large clostridial toxins. Most if not all of these studies utilized these toxins at a subtoxic dose to achieve inactivation of the GTPase of interest while leaving the cell in a viable, functioning state.
Schmidt et al. used TcdB to demonstrate a vital role for Rho in receptor signaling to phospholipases D and C, which are important signaling events in many cell types (154, 155). Prepens et al. demonstrated the importance of Rho family proteins in secretion from rat leukemia cells with TcdB (138). This secretion was later found, through the use of TcdB, to be regulated via a mechanism independent of the actin cytoskeleton (137). TcdA was also used to demonstrate the involvement of the Rho proteins in cytoskeletal rearrangement in endothelial cells (3). The involvement of Rho proteins in exocytosis and Ca2+ mobilization was also studied with TcdB (35). Most recently, these large clostridial toxins have been used to demonstrate the importance of the Rho family of proteins in neuron survival, cell adhesion, and regulation of nitric oxide synthase expression (102, 144, 184). The results from these studies and others indicate that understanding the mechanism of action of TcdA and TcdB will have impacts beyond the field of infectious disease.
CONCLUDING REMARKS AND FUTURE DIRECTIONS
_C. difficile_-associated disease is an interesting example of the impact that modern medicine has on a pathogen poised to cause new disease. Although significant advances have been made in the understanding of C. difficile biology and _C. difficile_-associated disease, this organism is still among the most important emerging pathogens. Antibiotic therapy has brought C. difficile to the forefront of bacterial pathogenesis but is unlikely to be the selective pressure responsible for the evolution of this organism's virulence. Interestingly, Shim et al. have shown in one study that over 25% of patients admitted to the hospital were colonized with C. difficile, and these individuals were less likely to develop _C. difficile_-associated disease while hospitalized (157). Yet there was no correlation between the propensity to get _C. difficile_-associated disease and whether these individuals were colonized with toxigenic or nontoxigenic strains. It is likely that a significant portion of the population is colonized with C. difficile yet will never develop disease unless they encounter a combination of conditions, including hospitalization, antibiotic therapy, and primary exposure to C. difficile.
As antibiotic therapy is the primary cause of pseudomembranous colitis, it seems reasonable to expect that the most successful treatments against this organism will be those outside the traditional realm of antimicrobials. Indeed, approaches such as vaccination may hold the most promise for the long-term management of _C. difficile_-associated disease. To this end, there has been at least one reported study on the phase 1 efficacy of a formalin-inactivated toxoid of TcdA and TcdB (94). Initial findings suggest that this vaccine is safe and elicits a toxin-specific antibody response which can be detected in the serum of treated subjects. While a comprehensive study on the protective effects of this vaccine in humans has not been completed, a recent report indicates that serum levels of anti-TcdA exceed that found in asymptomatic carriers of C. difficile (1). Thus, vaccination against the toxins of C. difficile is a future direction for prevention of _C. difficile_-associated disease that is worth continued consideration.
The primary focus of this review has been TcdA and TcdB, and the intention has been to emphasize the major advances in the understanding of these toxins. Research focused on these toxins has provided insight into the genetics, expression, cytotoxic mechanisms, and role in disease of both TcdA and TcdB, yet much remains to be learned about these toxins. One does not have to look far in toxin research to notice that major advances are often made following elucidation of high-resolution structures. Such a structure for TcdA or TcdB would likely reveal a great deal about the functional domains of these toxins and lead to new testable hypotheses, yet given the remarkably large size of these toxins, it will be interesting to see how easily such a structure can be solved.
In addition to the structure of the toxin, our understanding of _C. difficile_-associated disease will be enhanced by delineating the factors regulating C. difficile toxin production within the bowel. High levels of toxin from stationary-phase growth are obtained from in vitro cultures, but whether such levels are present under similar conditions in the host is not clear. Finally, there are numerous aspects of intracellular activity, including translocation, localization, and substrate interactions, that remain largely undefined. While understanding these events at the molecular and atomic level will improve our appreciation of TcdA and TcdB's mechanism of action, such observations may also lead to new therapies against C. difficile disease.
REFERENCES
- 1.Aboudola, S., K. L. Kotloff, L. Kyne, M. Warny, E. C. Kelly, S. Sougioultzis, P. J. Giannasca, T. P. Monath, and C. P. Kelly. 2003. Clostridium difficile vaccine and serum immunoglobulin G antibody response to toxin A. Infect. Immun. 71**:**1608-1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adamson, P., C. J. Marshall, A. Hall, and P. A. Tilbrook. 1992. Post-translational modifications of p21rho proteins. J. Biol. Chem. 267**:**20033-20038. [PubMed] [Google Scholar]
- 3.Aepfelbacher, M., M. Essler, E. Huber, M. Sugai, and P. C. Weber. 1997. Bacterial toxins block endothelial wound repair. Evidence that Rho GTPases control cytoskeletal rearrangements in migrating endothelial cells. Arterioscler. Thromb. Vasc. Biol. 17**:**1623-1629. [DOI] [PubMed] [Google Scholar]
- 4.al-Barrak, A., J. Embil, B. Dyck, K. Olekson, D. Nicoll, M. Alfa, and A. Kabani. 1999. An outbreak of toxin A negative, toxin B positive _Clostridium difficile_-associated diarrhea in a Canadian tertiary-care hospital. Can. Commun. Dis. Rep. 25**:**65-69. [PubMed] [Google Scholar]
- 5.Alfa, M. J., A. Kabani, D. Lyerly, S. Moncrief, L. M. Neville, A. Al-Barrak, G. K. Harding, B. Dyck, K. Olekson, and J. M. Embil. 2000. Characterization of a toxin A-negative, toxin B-positive strain of Clostridium difficile responsible for a nosocomial outbreak of _Clostridium difficile_-associated diarrhea. J. Clin. Microbiol. 38**:**2706-2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aronsson, B., R. Mollby, and C. E. Nord. 1981. Occurrence of toxin-producing Clostridium difficile in antibiotic-associated diarrhea in Sweden. Med. Microbiol. Immunol. (Berlin) 170**:**27-35. [DOI] [PubMed] [Google Scholar]
- 7.Barbut, F., V. Lalande, B. Burghoffer, H. V. Thien, E. Grimprel, and J. C. Petit. 2002. Prevalence and genetic characterization of toxin A variant strains of Clostridium difficile among adults and children with diarrhea in France. J. Clin. Microbiol. 40**:**2079-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barroso, L. A., S. Z. Wang, C. J. Phelps, J. L. Johnson, and T. D. Wilkins. 1990. Nucleotide sequence of Clostridium difficile toxin B gene. Nucleic Acids Res. 18**:**4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barth, H., G. Pfeifer, F. Hofmann, E. Maier, R. Benz, and K. Aktories. 2001. Low pH-induced formation of ion channels by Clostridium difficile toxin B in target cells. J. Biol. Chem. 276**:**10670-10676. [DOI] [PubMed] [Google Scholar]
- 10.Bartlett, J. G., N. Moon, T. W. Chang, N. Taylor, and A. B. Onderdonk. 1978. Role of Clostridium difficile in antibiotic-associated pseudomembranous colitis. Gastroenterology 75**:**778-782. [PubMed] [Google Scholar]
- 11.Bartlett, J. G., F. J. Tedesco, S. Shull, B. Lowe, and T. Chang. 1980. Symptomatic relapse after oral vancomycin therapy of antibiotic-associated pseudomembranous colitis. Gastroenterology 78**:**431-434. [PubMed] [Google Scholar]
- 12.Borriello, S. P., B. W. Wren, S. Hyde, S. V. Seddon, P. Sibbons, M. M. Krishna, S. Tabaqchali, S. Manek, and A. B. Price. 1992. Molecular, immunological, and biological characterization of a toxin A-negative, toxin B-positive strain of Clostridium difficile. Infect. Immun. 60**:**4192-4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brazier, J. S., and S. P. Borriello. 2000. Microbiology, epidemiology and diagnosis of Clostridium difficile infection. Curr. Top. Microbiol. Immunol. 250**:**1-33. [DOI] [PubMed] [Google Scholar]
- 14.Brazier, J. S., S. L. Stubbs, and B. I. Duerden. 1999. Prevalence of toxin A negative/B positive Clostridium difficile strains. J. Hosp. Infect. 42**:**248-249. [PubMed] [Google Scholar]
- 15.Brito, G. A., J. Fujji, B. A. Carneiro-Filho, A. A. Lima, T. Obrig, and R. L. Guerrant. 2002. Mechanism of Clostridium difficile toxin A-induced apoptosis in T84 cells. J. Infect. Dis. 186**:**1438-1447. [DOI] [PubMed] [Google Scholar]
- 16.Burdon, D. W., R. H. George, G. A. Mogg, Y. Arabi, H. Thompson, M. Johnson, J. Alexander-Williams, and M. R. Keighley. 1981. Faecal toxin and severity of antibiotic-associated pseudomembranous colitis. J. Clin. Pathol. 34**:**548-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Busch, C., F. Hofmann, R. Gerhard, and K. Aktories. 2000. Involvement of a conserved tryptophan residue in the UDP-glucose binding of large clostridial cytotoxin glycosyltransferases. J. Biol. Chem. 275**:**13228-13234. [DOI] [PubMed] [Google Scholar]
- 18.Busch, C., F. Hofmann, J. Selzer, S. Munro, D. Jeckel, and K. Aktories. 1998. A common motif of eukaryotic glycosyltransferases is essential for the enzyme activity of large clostridial cytotoxins. J. Biol. Chem. 273**:**19566-19572. [DOI] [PubMed] [Google Scholar]
- 19.Calderon, G. M., J. Torres-Lopez, T. J. Lin, B. Chavez, M. Hernandez, O. Munoz, A. D. Befus, and J. A. Enciso. 1998. Effects of toxin A from Clostridium difficile on mast cell activation and survival. Infect. Immun. 66**:**2755-2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castagliuolo, I., A. C. Keates, B. Qiu, C. P. Kelly, S. Nikulasson, S. E. Leeman, and C. Pothoulakis. 1997. Increased substance P responses in dorsal root ganglia and intestinal macrophages during Clostridium difficile toxin A enteritis in rats. Proc. Natl. Acad. Sci. USA 94**:**4788-4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castagliuolo, I., A. C. Keates, C. C. Wang, A. Pasha, L. Valenick, C. P. Kelly, S. T. Nikulasson, J. T. LaMont, and C. Pothoulakis. 1998. Clostridium difficile toxin A stimulates macrophage-inflammatory protein-2 production in rat intestinal epithelial cells. J. Immunol. 160**:**6039-6045. [PubMed] [Google Scholar]
- 22.Castagliuolo, I., C. P. Kelly, B. S. Qiu, S. T. Nikulasson, J. T. LaMont, and C. Pothoulakis. 1997. IL-11 inhibits Clostridium difficile toxin A enterotoxicity in rat ileum. Am. J. Physiol. 273**:**G333-341. [DOI] [PubMed] [Google Scholar]
- 23.Chang, T. W., S. L. Gorbach, and J. B. Bartlett. 1978. Neutralization of Clostridium difficile toxin by Clostridium sordellii antitoxins. Infect. Immun. 22**:**418-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang, T. W., P. S. Lin, S. L. Gorbach, and J. G. Bartlett. 1979. Ultrastructural changes of cultured human amnion cells by Clostridium difficile toxin. Infect. Immun. 23**:**795-798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaves-Olarte, E., I. Florin, P. Boquet, M. Popoff, C. von Eichel-Streiber, and M. Thelestam. 1996. UDP-glucose deficiency in a mutant cell line protects against glucosyltransferase toxins from Clostridium difficile and Clostridium sordellii. J. Biol. Chem. 271**:**6925-6932. [DOI] [PubMed] [Google Scholar]
- 26.Chaves-Olarte, E., P. Low, E. Freer, T. Norlin, M. Weidmann, C. von Eichel-Streiber, and M. Thelestam. 1999. A novel cytotoxin from Clostridium difficile serogroup F is a functional hybrid between two other large clostridial cytotoxins. J. Biol. Chem. 274**:**11046-11052. [DOI] [PubMed] [Google Scholar]
- 27.Chaves-Olarte, E., M. Weidmann, C. Eichel-Streiber, and M. Thelestam. 1997. Toxins A and B from Clostridium difficile differ with respect to enzymatic potencies, cellular substrate specificities, and surface binding to cultured cells. J. Clin. Investig. 100**:**1734-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen, M. L., C. Pothoulakis, and J. T. LaMont. 2002. Protein kinase C signaling regulates ZO-1 translocation and increased paracellular flux of T84 colonocytes exposed to Clostridium difficile toxin A. J. Biol. Chem. 277**:**4247-4254. [DOI] [PubMed] [Google Scholar]
- 29.Chong, L. D., A. Traynor-Kaplan, G. M. Bokoch, and M. A. Schwartz. 1994. The small GTP-binding protein Rho regulates a phosphatidylinositol 4-phosphate 5-kinase in mammalian cells. Cell 79**:**507-513. [DOI] [PubMed] [Google Scholar]
- 30.Ciesla, W. P., Jr., and D. A. Bobak. 1998. Clostridium difficile toxins A and B are cation-dependent UDP-glucose hydrolases with differing catalytic activities. J. Biol. Chem. 273**:**16021-16026. [DOI] [PubMed] [Google Scholar]
- 31.Clark, G. F., H. C. Krivan, T. D. Wilkins, and D. F. Smith. 1987. Toxin A from Clostridium difficile binds to rabbit erythrocyte glycolipids with terminal Galα1-3Galβ1-4GlcNAc sequences. Arch. Biochem. Biophys. 257**:**217-229. [DOI] [PubMed] [Google Scholar]
- 32.Cooke, D. L., and S. P. Borriello. 1998. Nonspecific binding of Clostridium difficile toxin A to murine immunoglobulins occurs via the fab component. Infect. Immun. 66**:**1981-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Corthier, G., M. C. Muller, T. D. Wilkins, D. Lyerly, and R. L'Haridon. 1991. Protection against experimental pseudomembranous colitis in gnotobiotic mice by use of monoclonal antibodies against Clostridium difficile toxin A. Infect. Immun. 59**:**1192-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeLano, W. L. 2002, posting date. The PyMOL Molecular Graphics Syst. [Online.] http://www.pymol.org.
- 35.Djouder, N., U. Prepens, K. Aktories, and A. Cavalie. 2000. Inhibition of calcium release-activated calcium current by Rac/Cdc42-inactivating clostridial cytotoxins in RBL cells. J. Biol. Chem. 275**:**18732-18738. [DOI] [PubMed] [Google Scholar]
- 36.Don, G. J., and A. E. Davis. 1981. The association between antibiotic- associated diarrhoea and C. difficile toxin in children. Aust. N. Z. J. Med. 11**:**433-434. [DOI] [PubMed] [Google Scholar]
- 37.Donta, S. T., N. Sullivan, and T. D. Wilkins. 1982. Differential effects of Clostridium difficile toxins on tissue-cultured cells. J. Clin. Microbiol. 15**:**1157-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dove, C. H., S. Z. Wang, S. B. Price, C. J. Phelps, D. M. Lyerly, T. D. Wilkins, and J. L. Johnson. 1990. Molecular characterization of the Clostridium difficile toxin A gene. Infect. Immun. 58**:**480-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dupuy, B., and A. L. Sonenshein. 1998. Regulated transcription of Clostridium difficile toxin genes. Mol. Microbiol. 27**:**107-120. [DOI] [PubMed] [Google Scholar]
- 40.Fanger, G. R., N. L. Johnson, and G. L. Johnson. 1997. MEK kinases are regulated by EGF and selectively interact with Rac/Cdc42. EMBO J. 16**:**4961-4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Farrell, R. J., and J. T. LaMont. 2000. Pathogenesis and clinical manifestations of Clostridium difficile diarrhea and colitis. Curr. Top. Microbiol. Immunol. 250**:**109-125. [DOI] [PubMed] [Google Scholar]
- 42.Feltis, B. A., S. M. Wiesner, A. S. Kim, S. L. Erlandsen, D. L. Lyerly, T. D. Wilkins, and C. L. Wells. 2000. Clostridium difficile toxins A and B can alter epithelial permeability and promote bacterial paracellular migration through HT-29 enterocytes. Shock 14**:**629-634. [DOI] [PubMed] [Google Scholar]
- 43.Fiorentini, C., A. Fabbri, L. Falzano, A. Fattorossi, P. Matarrese, R. Rivabene, and G. Donelli. 1998. Clostridium difficile toxin B induces apoptosis in intestinal cultured cells. Infect. Immun. 66**:**2660-2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fiorentini, C., W. Malorni, S. Paradisi, M. Giuliano, P. Mastrantonio, and G. Donelli. 1990. Interaction of Clostridium difficile toxin A with cultured cells: cytoskeletal changes and nuclear polarization. Infect. Immun. 58**:**2329-2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Florin, I., and M. Thelestam. 1991. ADP-ribosylation in Clostridium difficile toxin-treated cells is not related to cytopathogenicity of toxin B. Biochim. Biophys. Acta 1091**:**51-54. [DOI] [PubMed] [Google Scholar]
- 46.Florin, I., and M. Thelestam. 1983. Internalization of Clostridium difficile cytotoxin into cultured human lung fibroblasts. Biochim. Biophys. Acta 763**:**383-392. [DOI] [PubMed] [Google Scholar]
- 47.Florin, I., and M. Thelestam. 1986. Lysosomal involvement in cellular intoxication with Clostridium difficile toxin B. Microb. Pathog. 1**:**373-385. [DOI] [PubMed] [Google Scholar]
- 48.Frey, S. M., and T. D. Wilkins. 1992. Localization of two epitopes recognized by monoclonal antibody PCG-4 on Clostridium difficile toxin A. Infect. Immun. 60**:**2488-2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frisch, C., R. Gerhard, K. Aktories, F. Hofmann, and I. Just. 2003. The complete receptor-binding domain of Clostridium difficile toxin A is required for endocytosis. Biochem. Biophys. Res. Commun. 300**:**706-711. [DOI] [PubMed] [Google Scholar]
- 50.Fujisawa, K., P. Madaule, T. Ishizaki, G. Watanabe, H. Bito, Y. Saito, A. Hall, and S. Narumiya. 1998. Different regions of Rho determine Rho-selective binding of different classes of Rho target molecules. J. Biol. Chem. 273**:**18943-18949. [DOI] [PubMed] [Google Scholar]
- 51.Fukumoto, Y., K. Kaibuchi, Y. Hori, H. Fujioka, S. Araki, T. Ueda, A. Kikuchi, and Y. Takai. 1990. Molecular cloning and characterization of a novel type of regulatory protein (GDI) for the rho proteins, ras p21-like small GTP-binding proteins. Oncogene 5**:**1321-1328. [PubMed] [Google Scholar]
- 52.Garnier, T., and S. T. Cole. 1988. Studies of UV-inducible promoters from Clostridium perfringens in vivo and in vitro. Mol. Microbiol. 2**:**607-614. [DOI] [PubMed] [Google Scholar]
- 53.Garrett, M. D., G. N. Major, N. Totty, and A. Hall. 1991. Purification and N-terminal sequence of the p21rho GTPase-activating protein, rho GAP. Biochem. J. 276**:**833-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Garrett, M. D., A. J. Self, C. van Oers, and A. Hall. 1989. Identification of distinct cytoplasmic targets for ras/R-ras and rho regulatory proteins. J. Biol. Chem. 264**:**10-13. [PubMed] [Google Scholar]
- 55.Genth, H., K. Aktories, and I. Just. 1999. Monoglucosylation of RhoA at threonine 37 blocks cytosol-membrane cycling. J. Biol. Chem. 274**:**29050-29056. [DOI] [PubMed] [Google Scholar]
- 56.George, W. L., R. D. Rolfe, G. K. Harding, R. Klein, C. W. Putnam, and S. M. Finegold. 1982. Clostridium difficile and cytotoxin in feces of patients with antimicrobial agent-associated pseudomembranous colitis. Infection 10**:**205-208. [DOI] [PubMed] [Google Scholar]
- 57.George, W. L., V. L. Sutter, E. J. Goldstein, S. L. Ludwig, and S. M. Finegold. 1978. Aetiology of antimicrobial-agent-associated colitis. Lancet **i:**802-803. [DOI] [PubMed] [Google Scholar]
- 58.Geric, B., S. Johnson, D. N. Gerding, M. Grabnar, and M. Rupnik. 2003. Frequency of binary toxin genes among Clostridium difficile strains that do not produce large clostridial toxins. J. Clin. Microbiol. 41**:**5227-5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Geric, B., M. Rupnik, D. N. Gerding, M. Grabnar, and S. Johnson. 2004. Distribution of Clostridium difficile variant toxinotypes and strains with binary toxin genes among clinical isolates in an American hospital. J. Med. Microbiol. 53**:**887-894. [DOI] [PubMed] [Google Scholar]
- 60.Gulke, I., G. Pfeifer, J. Liese, M. Fritz, F. Hofmann, K. Aktories, and H. Barth. 2001. Characterization of the enzymatic component of the ADP-ribosyltransferase toxin CDTa from Clostridium difficile. Infect. Immun. 69**:**6004-6011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hall, A. 1990. The cellular functions of small GTP-binding proteins. Science 249**:**635-640. [DOI] [PubMed] [Google Scholar]
- 62.Hall, I. C., and E. O'Toole. 1935. Intestinal flora in newborn infants with a description of a new pathogenic anaerobe, Bacillus difficilis. Amer. J. Dis. Child. 49**:**390-402. [Google Scholar]
- 63.Hammond, G. A., and J. L. Johnson. 1995. The toxigenic element of Clostridium difficile strain VPI 10463. Microb. Pathog. 19**:**203-213. [DOI] [PubMed] [Google Scholar]
- 64.He, D., S. J. Hagen, C. Pothoulakis, M. Chen, N. D. Medina, M. Warny, and J. T. LaMont. 2000. Clostridium difficile toxin A causes early damage to mitochondria in cultured cells. Gastroenterology 119**:**139-150. [DOI] [PubMed] [Google Scholar]
- 65.He, D., S. Sougioultzis, S. Hagen, J. Liu, S. Keates, A. C. Keates, C. Pothoulakis, and J. T. Lamont. 2002. Clostridium difficile toxin A triggers human colonocyte IL-8 release via mitochondrial oxygen radical generation. Gastroenterology 122**:**1048-1057. [DOI] [PubMed] [Google Scholar]
- 66.Hecht, G., A. Koutsouris, C. Pothoulakis, J. T. LaMont, and J. L. Madara. 1992. Clostridium difficile toxin B disrupts the barrier function of T84 monolayers. Gastroenterology 102**:**416-423. [DOI] [PubMed] [Google Scholar]
- 67.Hecht, G., C. Pothoulakis, J. T. LaMont, and J. L. Madara. 1988. Clostridium difficile toxin A perturbs cytoskeletal structure and tight junction permeability of cultured human intestinal epithelial monolayers. J. Clin. Investig. 82**:**1516-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Henriques, B., I. Florin, and M. Thelestam. 1987. Cellular internalisation of Clostridium difficile toxin A. Microb. Pathog. 2**:**455-463. [DOI] [PubMed] [Google Scholar]
- 69.Herrmann, C., M. R. Ahmadian, F. Hofmann, and I. Just. 1998. Functional consequences of monoglucosylation of Ha-Ras at effector domain amino acid threonine 35. J. Biol. Chem. 273**:**16134-16139. [DOI] [PubMed] [Google Scholar]
- 70.Hippenstiel, S., B. Schmeck, P. D. N′Guessan, J. Seybold, M. Krull, K. Preissner, C. V. Eichel-Streiber, and N. Suttorp. 2002. Rho protein inactivation induced apoptosis of cultured human endothelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 283**:**L830-838. [DOI] [PubMed] [Google Scholar]
- 71.Hiraoka, K., K. Kaibuchi, S. Ando, T. Musha, K. Takaishi, T. Mizuno, M. Asada, L. Menard, E. Tomhave, J. Didsbury, et al. 1992. Both stimulatory and inhibitory GDP/GTP exchange proteins, smg GDS and rho GDI, are active on multiple small GTP-binding proteins. Biochem. Biophys. Res. Commun. 182**:**921-930. [DOI] [PubMed] [Google Scholar]
- 72.Hoffman, G. R., N. Nassar, and R. A. Cerione. 2000. Structure of the Rho family GTP-binding protein Cdc42 in complex with the multifunctional regulator Rho-GDI. Cell 100**:**345-356. [DOI] [PubMed] [Google Scholar]
- 73.Hofmann, F., C. Busch, and K. Aktories. 1998. Chimeric clostridial cytotoxins: identification of the N-terminal region involved in protein substrate recognition. Infect. Immun. 66**:**1076-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hofmann, F., C. Busch, U. Prepens, I. Just, and K. Aktories. 1997. Localization of the glucosyltransferase activity of Clostridium difficile toxin B to the N-terminal part of the holotoxin. J. Biol. Chem. 272**:**11074-11078. [DOI] [PubMed] [Google Scholar]
- 75.Hundsberger, T., V. Braun, M. Weidmann, P. Leukel, M. Sauerborn, and C. von Eichel-Streiber. 1997. Transcription analysis of the genes tcdA-E of the pathogenicity locus of Clostridium difficile. Eur. J. Biochem. 244**:**735-742. [DOI] [PubMed] [Google Scholar]
- 76.Hurley, B. W., and C. C. Nguyen. 2002. The spectrum of pseudomembranous enterocolitis and antibiotic-associated diarrhea. Arch. Intern. Med. 162**:**2177-2184. [DOI] [PubMed] [Google Scholar]
- 77.Ihara, K., S. Muraguchi, M. Kato, T. Shimizu, M. Shirakawa, S. Kuroda, K. Kaibuchi, and T. Hakoshima. 1998. Crystal structure of human RhoA in a dominantly active form complexed with a GTP analogue. J. Biol. Chem. 273**:**9656-9666. [DOI] [PubMed] [Google Scholar]
- 78.Jefferson, K. K., M. F. Smith, Jr., and D. A. Bobak. 1999. Roles of intracellular calcium and NF-kappa B in the Clostridium difficile toxin A-induced up-regulation and secretion of IL-8 from human monocytes. J. Immunol. 163**:**5183-5191. [PubMed] [Google Scholar]
- 79.Johnson, S., and D. N. Gerding. 1998. _Clostridium difficile_-associated diarrhea. Clin. Infect. Dis. 26**:**1027-1034. [DOI] [PubMed] [Google Scholar]
- 80.Johnson, S., S. A. Kent, K. J. O'Leary, M. M. Merrigan, S. P. Sambol, L. R. Peterson, and D. N. Gerding. 2001. Fatal pseudomembranous colitis associated with a variant Clostridium difficile strain not detected by toxin A immunoassay. Ann. Intern. Med. 135**:**434-438. [DOI] [PubMed] [Google Scholar]
- 81.Johnson, S., S. P. Sambol, J. S. Brazier, M. Delmee, V. Avesani, M. M. Merrigan, and D. N. Gerding. 2003. International typing study of toxin A-negative, toxin B-positive Clostridium difficile variants. J. Clin. Microbiol. 41**:**1543-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Just, I., G. Fritz, K. Aktories, M. Giry, M. R. Popoff, P. Boquet, S. Hegenbarth, and C. von Eichel-Streiber. 1994. Clostridium difficile toxin B acts on the GTP-binding protein Rho. J. Biol. Chem. 269**:**10706-10712. [PubMed] [Google Scholar]
- 83.Just, I., J. Selzer, C. von Eichel-Streiber, and K. Aktories. 1995. The low molecular mass GTP-binding protein Rho is affected by toxin A from Clostridium difficile. J. Clin. Investig. 95**:**1026-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Just, I., J. Selzer, M. Wilm, C. von Eichel-Streiber, M. Mann, and K. Aktories. 1995. Glucosylation of Rho proteins by Clostridium difficile toxin B. Nature 375**:**500-503. [DOI] [PubMed] [Google Scholar]
- 85.Just, I., M. Wilm, J. Selzer, G. Rex, C. von Eichel-Streiber, M. Mann, and K. Aktories. 1995. The enterotoxin from Clostridium difficile (ToxA) monoglucosylates the Rho proteins. J. Biol. Chem. 270**:**13932-13936. [DOI] [PubMed] [Google Scholar]
- 86.Karlsson, S., L. G. Burman, and T. Akerlund. 1999. Suppression of toxin production in Clostridium difficile VPI 10463 by amino acids. Microbiology 145 **(Pt 7):**1683-1693. [DOI] [PubMed] [Google Scholar]
- 87.Kato, H., N. Kato, K. Watanabe, N. Iwai, H. Nakamura, T. Yamamoto, K. Suzuki, S. M. Kim, Y. Chong, and E. B. Wasito. 1998. Identification of toxin A-negative, toxin B-positive Clostridium difficile by PCR. J. Clin. Microbiol. 36**:**2178-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Keates, A. C., I. Castagliuolo, B. Qiu, S. Nikulasson, A. Sengupta, and C. Pothoulakis. 1998. CGRP upregulation in dorsal root ganglia and ileal mucosa during Clostridium difficile toxin A-induced enteritis. Am. J. Physiol. 274**:**G196-202. [DOI] [PubMed] [Google Scholar]
- 89.Kelly, C. P., S. Becker, J. K. Linevsky, M. A. Joshi, J. C. O'Keane, B. F. Dickey, J. T. LaMont, and C. Pothoulakis. 1994. Neutrophil recruitment in Clostridium difficile toxin A enteritis in the rabbit. J. Clin. Investig. 93**:**1257-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim, J. M., J. S. Kim, H. C. Jun, Y. K. Oh, I. S. Song, and C. Y. Kim. 2002. Differential expression and polarized secretion of CXC and CC chemokines by human intestinal epithelial cancer cell lines in response to Clostridium difficile toxin A. Microbiol. Immunol. 46**:**333-342. [DOI] [PubMed] [Google Scholar]
- 91.Kim, P. H., J. P. Iaconis, and R. D. Rolfe. 1987. Immunization of adult hamsters against _Clostridium difficile_-associated ileocecitis and transfer of protection to infant hamsters. Infect. Immun. 55**:**2984-2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kirkwood, K. S., N. W. Bunnett, J. Maa, I. Castagliolo, B. Liu, N. Gerard, J. Zacks, C. Pothoulakis, and E. F. Grady. 2001. Deletion of neutral endopeptidase exacerbates intestinal inflammation induced by Clostridium difficile toxin A. Am. J. Physiol. Gastrointest. Liver Physiol. 281**:**G544-551. [DOI] [PubMed] [Google Scholar]
- 93.Komatsu, M., H. Kato, M. Aihara, K. Shimakawa, M. Iwasaki, Y. Nagasaka, S. Fukuda, S. Matsuo, Y. Arakawa, M. Watanabe, and Y. Iwatani. 2003. High frequency of antibiotic-associated diarrhea due to toxin A-negative, toxin B-positive Clostridium difficile in a hospital in Japan and risk factors for infection. Eur. J. Clin. Microbiol. Infect. Dis. 22**:**525-529. [DOI] [PubMed] [Google Scholar]
- 94.Kotloff, K. L., S. S. Wasserman, G. A. Losonsky, W. Thomas, Jr., R. Nichols, R. Edelman, M. Bridwell, and T. P. Monath. 2001. Safety and immunogenicity of increasing doses of a Clostridium difficile toxoid vaccine administered to healthy adults. Infect. Immun. 69**:**988-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Krivan, H. C., G. F. Clark, D. F. Smith, and T. D. Wilkins. 1986. Cell surface binding site for Clostridium difficile enterotoxin: evidence for a glycoconjugate containing the sequence Galα1-3Galβ1-4GlcNAc. Infect. Immun. 53**:**573-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kuijper, E. J., J. de Weerdt, H. Kato, N. Kato, A. P. van Dam, E. R. van der Vorm, J. Weel, C. van Rheenen, and J. Dankert. 2001. Nosocomial outbreak of _Clostridium difficile_-associated diarrhoea due to a clindamycin-resistant enterotoxin A-negative strain. Eur. J. Clin. Microbiol. Infect. Dis. 20**:**528-534. [DOI] [PubMed] [Google Scholar]
- 97.Kurtz, C. B., E. P. Cannon, A. Brezzani, M. Pitruzzello, C. Dinardo, E. Rinard, D. W. Acheson, R. Fitzpatrick, P. Kelly, K. Shackett, A. T. Papoulis, P. J. Goddard, R. H. Barker, Jr., G. P. Palace, and J. D. Klinger. 2001. GT160-246, a toxin binding polymer for treatment of Clostridium difficile colitis. Antimicrob. Agents Chemother. 45**:**2340-2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kyne, L., M. B. Hamel, R. Polavaram, and C. P. Kelly. 2002. Health care costs and mortality associated with nosocomial diarrhea due to Clostridium difficile. Clin. Infect. Dis. 34**:**346-353. [DOI] [PubMed] [Google Scholar]
- 99.Kyne, L., M. Warny, A. Qamar, and C. P. Kelly. 2001. Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet 357**:**189-193. [DOI] [PubMed] [Google Scholar]
- 100.Lima, A. A., D. M. Lyerly, T. D. Wilkins, D. J. Innes, and R. L. Guerrant. 1988. Effects of Clostridium difficile toxins A and B in rabbit small and large intestine in vivo and on cultured cells in vitro. Infect. Immun. 56**:**582-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Limaye, A. P., D. K. Turgeon, B. T. Cookson, and T. R. Fritsche. 2000. Pseudomembranous colitis caused by a toxin A− B+ strain of Clostridium difficile. J. Clin. Microbiol. 38**:**1696-1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Linseman, D. A., T. Laessig, M. K. Meintzer, M. McClure, H. Barth, K. Aktories, and K. A. Heidenreich. 2001. An essential role for Rac/Cdc42 GTPases in cerebellar granule neuron survival. J. Biol. Chem. 276**:**39123-39131. [DOI] [PubMed] [Google Scholar]
- 103.Lyerly, D. M., L. A. Barroso, T. D. Wilkins, C. Depitre, and G. Corthier. 1992. Characterization of a toxin A-negative, toxin B-positive strain of Clostridium difficile. Infect. Immun. 60**:**4633-4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lyerly, D. M., H. C. Krivan, and T. D. Wilkins. 1988. Clostridium difficile: its disease and toxins. Clin. Microbiol. Rev. 1**:**1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lyerly, D. M., K. E. Saum, D. K. MacDonald, and T. D. Wilkins. 1985. Effects of Clostridium difficile toxins given intragastrically to animals. Infect. Immun. 47**:**349-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Madaule, P., M. Eda, N. Watanabe, K. Fujisawa, T. Matsuoka, H. Bito, T. Ishizaki, and S. Narumiya. 1998. Role of citron kinase as a target of the small GTPase Rho in cytokinesis. Nature 394**:**491-494. [DOI] [PubMed] [Google Scholar]
- 107.Maegawa, T., T. Karasawa, T. Ohta, X. Wang, H. Kato, H. Hayashi, and S. Nakamura. 2002. Linkage between toxin production and purine biosynthesis in Clostridium difficile. J. Med. Microbiol. 51**:**34-41. [DOI] [PubMed] [Google Scholar]
- 108.Mani, N., and B. Dupuy. 2001. Regulation of toxin synthesis in Clostridium difficile by an alternative RNA polymerase sigma factor. Proc. Natl. Acad. Sci. USA 98**:**5844-5849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mani, N., D. Lyras, L. Barroso, P. Howarth, T. Wilkins, J. I. Rood, A. L. Sonenshein, and B. Dupuy. 2002. Environmental response and autoregulation of Clostridium difficile TxeR, a sigma factor for toxin gene expression. J. Bacteriol. 184**:**5971-5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mantyh, C. R., J. E. Maggio, P. W. Mantyh, S. R. Vigna, and T. N. Pappas. 1996. Increased substance P receptor expression by blood vessels and lymphoid aggregates in _Clostridium difficile_-induced pseudomembranous colitis. Dig. Dis. Sci. 41**:**614-620. [DOI] [PubMed] [Google Scholar]
- 111.Mantyh, C. R., T. N. Pappas, J. A. Lapp, M. K. Washington, L. M. Neville, J. R. Ghilardi, S. D. Rogers, P. W. Mantyh, and S. R. Vigna. 1996. Substance P activation of enteric neurons in response to intraluminal Clostridium difficile toxin A in the rat ileum. Gastroenterology 111**:**1272-1280. [DOI] [PubMed] [Google Scholar]
- 112.Marshall, C. J. 1993. Protein prenylation: a mediator of protein-protein interactions. Science 259**:**1865-1866. [DOI] [PubMed] [Google Scholar]
- 113.Martinez, R. D., and T. D. Wilkins. 1988. Purification and characterization of Clostridium sordellii hemorrhagic toxin and cross-reactivity with Clostridium difficile toxin A (enterotoxin). Infect. Immun. 56**:**1215-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Marvaud, J. C., U. Eisel, T. Binz, H. Niemann, and M. R. Popoff. 1998. TetR is a positive regulator of the tetanus toxin gene in Clostridium tetani and is homologous to botR. Infect. Immun. 66**:**5698-5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Marvaud, J. C., M. Gibert, K. Inoue, Y. Fujinaga, K. Oguma, and M. R. Popoff. 1998. botR/A is a positive regulator of botulinum neurotoxin and associated non-toxin protein genes in Clostridium botulinum A. Mol. Microbiol. 29**:**1009-1018. [DOI] [PubMed] [Google Scholar]
- 116.Meyers, S., L. Mayer, E. Bottone, E. Desmond, and H. D. Janowitz. 1981. Occurrence of Clostridium difficile toxin during the course of inflammatory bowel disease. Gastroenterology 80**:**697-670. [PubMed] [Google Scholar]
- 117.Mitchell, M. J., B. E. Laughon, and S. Lin. 1987. Biochemical studies on the effect of Clostridium difficile toxin B on actin in vivo and in vitro. Infect. Immun. 55**:**1610-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mizuno, T., K. Kaibuchi, T. Yamamoto, M. Kawamura, T. Sakoda, H. Fujioka, Y. Matsuura, and Y. Takai. 1991. A stimulatory GDP/GTP exchange protein for smg p21 is active on the post-translationally processed form of c-Ki-ras p21 and rhoA p21. Proc. Natl. Acad. Sci. USA 88**:**6442-6446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Moncrief, J. S., L. A. Barroso, and T. D. Wilkins. 1997. Positive regulation of Clostridium difficile toxins. Infect. Immun. 65**:**1105-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Moncrief, J. S., L. Zheng, L. M. Neville, and D. M. Lyerly. 2000. Genetic characterization of toxin A-negative, toxin B-positive Clostridium difficile isolates by PCR. J. Clin. Microbiol. 38**:**3072-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Morteau, O., I. Castagliuolo, A. Mykoniatis, J. Zacks, M. Wlk, B. Lu, C. Pothoulakis, N. P. Gerard, and C. Gerard. 2002. Genetic deficiency in the chemokine receptor CCR1 protects against acute Clostridium difficile toxin A enteritis in mice. Gastroenterology 122**:**725-733. [DOI] [PubMed] [Google Scholar]
- 122.Muller, S., C. von Eichel-Streiber, and M. Moos. 1999. Impact of amino acids 22-27 of Rho-subfamily GTPases on glucosylation by the large clostridial cytotoxins TcsL-1522, TcdB-1470 and TcdB-8864. Eur. J. Biochem. 266**:**1073-1080. [DOI] [PubMed] [Google Scholar]
- 123.Neunlist, M., J. Barouk, K. Michel, I. Just, T. Oreshkova, M. Schemann, and J. P. Galmiche. 2003. Toxin B of Clostridium difficile activates human VIP submucosal neurons, in part via an IL-1beta-dependent pathway. Am. J. Physiol. Gastrointest. Liver Physiol. 285**:**G1049-1055. [DOI] [PubMed] [Google Scholar]
- 124.Nobes, C. D., and A. Hall. 1995. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 81**:**53-62. [DOI] [PubMed] [Google Scholar]
- 125.Nusrat, A., M. Giry, J. R. Turner, S. P. Colgan, C. A. Parkos, D. Carnes, E. Lemichez, P. Boquet, and J. L. Madara. 1995. Rho protein regulates tight junctions and perijunctional actin organization in polarized epithelia. Proc. Natl. Acad. Sci. USA 92**:**10629-10633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nusrat, A., C. von Eichel-Streiber, J. R. Turner, P. Verkade, J. L. Madara, and C. A. Parkos. 2001. Clostridium difficile toxins disrupt epithelial barrier function by altering membrane microdomain localization of tight junction proteins. Infect. Immun. 69**:**1329-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Onderdonk, A. B., B. R. Lowe, and J. G. Bartlett. 1979. Effect of environmental stress on Clostridium difficile toxin levels during continuous cultivation. Appl. Environ. Microbiol. 38**:**637-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Perelle, S., M. Gibert, P. Bourlioux, G. Corthier, and M. R. Popoff. 1997. Production of a complete binary toxin (actin-specific ADP-ribosyltransferase) by Clostridium difficile CD196. Infect. Immun. 65**:**1402-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pfeifer, G., J. Schirmer, J. Leemhuis, C. Busch, D. K. Meyer, K. Aktories, and H. Barth. 2003. Cellular uptake of Clostridium difficile toxin B. Translocation of the N-terminal catalytic domain into the cytosol of eukaryotic cells. J. Biol. Chem. 278**:**44535-44541. [DOI] [PubMed] [Google Scholar]
- 130.Pituch, H., N. van den Braak, W. van Leeuwen, A. van Belkum, G. Martirosian, P. Obuch-Woszczatynski, M. Luczak, and F. Meisel-Mikolajczyk. 2001. Clonal dissemination of a toxin-A-negative/toxin-B-positive Clostridium difficile strain from patients with antibiotic-associated diarrhea in Poland. Clin. Microbiol. Infect. 7**:**442-446. [DOI] [PubMed] [Google Scholar]
- 131.Pituch, H., A. vavn Belkum, N. van den Braak, P. Obuch-Woszczatynski, A. Sawicka-Grzelak, H. Verbrugh, F. Meisel-Mikolajczyk, and M. Luczak. 2003. Clindamycin-resistant, toxin A-negative, toxin B-positive Clostridium difficile strains cause antibiotic-associated diarrhea among children hospitalized in a hematology unit. Clin. Microbiol. Infect. 9**:**903-904. [DOI] [PubMed] [Google Scholar]
- 132.Popoff, M. R. 1987. Purification and characterization of Clostridium sordellii lethal toxin and cross-reactivity with Clostridium difficile cytotoxin. Infect. Immun. 55**:**35-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Popoff, M. R., E. J. Rubin, D. M. Gill, and P. Boquet. 1988. Actin-specific ADP-ribosyltransferase produced by a Clostridium difficile strain. Infect. Immun. 56**:**2299-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pothoulakis, C., I. Castagliuolo, J. T. LaMont, A. Jaffer, J. C. O'Keane, R. M. Snider, and S. E. Leeman. 1994. CP-96,345, a substance P antagonist, inhibits rat intestinal responses to Clostridium difficile toxin A but not cholera toxin. Proc. Natl. Acad. Sci. USA 91**:**947-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pothoulakis, C., R. J. Gilbert, C. Cladaras, I. Castagliuolo, G. Semenza, Y. Hitti, J. S. Montcrief, J. Linevsky, C. P. Kelly, S. Nikulasson, H. P. Desai, T. D. Wilkins, and J. T. LaMont. 1996. Rabbit sucrase-isomaltase contains a functional intestinal receptor for Clostridium difficile toxin A. J. Clin. Investig. 98**:**641-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pothoulakis, C., R. Sullivan, D. A. Melnick, G. Triadafilopoulos, A. S. Gadenne, T. Meshulam, and J. T. LaMont. 1988. Clostridium difficile toxin A stimulates intracellular calcium release and chemotactic response in human granulocytes. J. Clin. Investig. 81**:**1741-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Prepens, U., I. Just, F. Hofmann, and K. Aktories. 1997. ADP-ribosylating and glucosylating toxins as tools to study secretion in RBL cells. Adv. Exp. Med. Biol. 419**:**349-353. [DOI] [PubMed] [Google Scholar]
- 138.Prepens, U., I. Just, C. von Eichel-Streiber, and K. Aktories. 1996. Inhibition of Fc epsilon-RI-mediated activation of rat basophilic leukemia cells by Clostridium difficile toxin B (monoglucosyltransferase). J. Biol. Chem. 271**:**7324-7329. [DOI] [PubMed] [Google Scholar]
- 139.Qa'Dan, M., M. Ramsey, J. Daniel, L. M. Spyres, B. Safiejko-Mroczka, W. Ortiz-Leduc, and J. D. Ballard. 2002. Clostridium difficile toxin B activates dual caspase-dependent and caspase-independent apoptosis in intoxicated cells. Cell. Microbiol. 4**:**425-434. [DOI] [PubMed] [Google Scholar]
- 140.Qa'Dan, M., L. M. Spyres, and J. D. Ballard. 2000. pH-induced conformational changes in Clostridium difficile toxin B. Infect. Immun. 68**:**2470-2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Qiu, B., C. Pothoulakis, I. Castagliuolo, S. Nikulasson, and J. T. LaMont. 1999. Participation of reactive oxygen metabolites in Clostridium difficile toxin A-induced enteritis in rats. Am. J. Physiol. 276**:**G485-490. [DOI] [PubMed] [Google Scholar]
- 142.Ridley, A. J., and A. Hall. 1992. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 70**:**389-399. [DOI] [PubMed] [Google Scholar]
- 143.Riegler, M., R. Sedivy, C. Pothoulakis, G. Hamilton, J. Zacherl, G. Bischof, E. Cosentini, W. Feil, R. Schiessel, J. T. LaMont, et al. 1995. Clostridium difficile toxin B is more potent than toxin A in damaging human colonic epithelium in vitro. J. Clin. Investig. 95**:**2004-2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Roberts, L. A., H. Glenn, C. S. Hahn, and B. S. Jacobson. 2003. Cdc42 and RhoA are differentially regulated during arachidonate-mediated HeLa cell adhesion. J. Cell. Physiol. 196**:**196-205. [DOI] [PubMed] [Google Scholar]
- 145.Rocha, M. F., A. M. Soares, C. A. Flores, T. S. Steiner, D. M. Lyerly, R. L. Guerrant, R. A. Ribeiro, and A. A. Lima. 1998. Intestinal secretory factor released by macrophages stimulated with Clostridium difficile toxin A: role of interleukin 1β. Infect. Immun. 66**:**4910-4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rupnik, M., V. Avesani, M. Janc, C. von Eichel-Streiber, and M. Delmee. 1998. A novel toxinotyping scheme and correlation of toxinotypes with serogroups of Clostridium difficile isolates. J. Clin. Microbiol. 36**:**2240-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rupnik, M., J. S. Brazier, B. I. Duerden, M. Grabnar, and S. L. Stubbs. 2001. Comparison of toxinotyping and PCR ribotyping of Clostridium difficile strains and description of novel toxinotypes. Microbiology 147**:**439-447. [DOI] [PubMed] [Google Scholar]
- 148.Rupnik, M., N. Kato, M. Grabnar, and H. Kato. 2003. New types of toxin A-negative, toxin B-positive strains among Clostridium difficile isolates from Asia. J. Clin. Microbiol. 41**:**1118-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sambol, S. P., M. M. Merrigan, D. Lyerly, D. N. Gerding, and S. Johnson. 2000. Toxin gene analysis of a variant strain of Clostridium difficile that causes human clinical disease. Infect. Immun. 68**:**5480-5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sambol, S. P., J. K. Tang, M. M. Merrigan, S. Johnson, and D. N. Gerding. 2001. Infection of hamsters with epidemiologically important strains of Clostridium difficile. J. Infect. Dis. 183**:**1760-1766. [DOI] [PubMed] [Google Scholar]
- 151.Samra, Z., S. Talmor, and J. Bahar. 2002. High prevalence of toxin A-negative toxin B-positive Clostridium difficile in hospitalized patients with gastrointestinal disease. Diagn. Microbiol. Infect. Dis. 43**:**189-192. [DOI] [PubMed] [Google Scholar]
- 152.Sauerborn, M., P. Leukel, and C. von Eichel-Streiber. 1997. The C-terminal ligand-binding domain of Clostridium difficile toxin A (TcdA) abrogates TcdA-specific binding to cells and prevents mouse lethality. FEMS Microbiol. Lett. 155**:**45-54. [DOI] [PubMed] [Google Scholar]
- 153.Savidge, T. C., W. H. Pan, P. Newman, M. O'Brien, P. M. Anton, and C. Pothoulakis. 2003. Clostridium difficile toxin B is an inflammatory enterotoxin in human intestine. Gastroenterology 125**:**413-420. [DOI] [PubMed] [Google Scholar]
- 154.Schmidt, M., C. Bienek, U. Rumenapp, C. Zhang, G. Lummen, K. H. Jakobs, I. Just, K. Aktories, M. Moos, and C. von Eichel-Streiber. 1996. A role for Rho in receptor- and G protein-stimulated phospholipase C. Reduction in phosphatidylinositol 4,5-bisphosphate by Clostridium difficile toxin B. Naunyn. Schmiedebergs. Arch. Pharmacol. 354**:**87-94. [DOI] [PubMed] [Google Scholar]
- 155.Schmidt, M., U. Rumenapp, C. Bienek, J. Keller, C. von Eichel-Streiber, and K. H. Jakobs. 1996. Inhibition of receptor signaling to phospholipase D by Clostridium difficile toxin B. Role of Rho proteins. J. Biol. Chem. 271**:**2422-2426. [DOI] [PubMed] [Google Scholar]
- 156.Sekine, A., M. Fujiwara, and S. Narumiya. 1989. Asparagine residue in the rho gene product is the modification site for botulinum ADP-ribosyltransferase. J. Biol. Chem. 264**:**8602-8605. [PubMed] [Google Scholar]
- 157.Shim, J. K., S. Johnson, M. H. Samore, D. Z. Bliss, and D. N. Gerding. 1998. Primary symptomless colonisation by Clostridium difficile and decreased risk of subsequent diarrhoea. Lancet 351**:**633-636. [DOI] [PubMed] [Google Scholar]
- 158.Shoshan, M. C., I. Florin, and M. Thelestam. 1993. Activation of cellular phospholipase A2 by Clostridium difficile toxin B. J. Cell. Biochem. 52**:**116-124. [DOI] [PubMed] [Google Scholar]
- 159.Smith, J. A., D. L. Cooke, S. Hyde, S. P. Borriello, and R. G. Long. 1997. Clostridium difficile toxin A binding to human intestinal epithelial cells. J. Med. Microbiol. 46**:**953-958. [DOI] [PubMed] [Google Scholar]
- 160.Soehn, F., A. Wagenknecht-Wiesner, P. Leukel, M. Kohl, M. Weidmann, C. von Eichel-Streiber, and V. Braun. 1998. Genetic rearrangements in the pathogenicity locus of Clostridium difficile strain 8864-implications for transcription, expression and enzymatic activity of toxins A and B. Mol. Gen. Genet. 258**:**222-232. [DOI] [PubMed] [Google Scholar]
- 161.Souza, M. H., A. A. Melo-Filho, M. F. Rocha, D. M. Lyerly, F. Q. Cunha, A. A. Lima, and R. A. Ribeiro. 1997. The involvement of macrophage-derived tumour necrosis factor and lipoxygenase products on the neutrophil recruitment induced by Clostridium difficile toxin B. Immunology 91**:**281-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Spyres, L. M., J. Daniel, A. Hensley, M. Qa'Dan, W. Ortiz-Leduc, and J. D. Ballard. 2003. Mutational analysis of the enzymatic domain of Clostridium difficile toxin B reveals novel inhibitors of the wild-type toxin. Infect. Immun. 71**:**3294-3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Spyres, L. M., M. Qa'Dan, A. Meader, J. J. Tomasek, E. W. Howard, and J. D. Ballard. 2001. Cytosolic delivery and characterization of the TcdB glucosylating domain by using a heterologous protein fusion. Infect. Immun. 69**:**599-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Stubbs, S., M. Rupnik, M. Gibert, J. Brazier, B. Duerden, and M. Popoff. 2000. Production of actin-specific ADP-ribosyltransferase (binary toxin) by strains of Clostridium difficile. FEMS Microbiol. Lett. 186**:**307-312. [DOI] [PubMed] [Google Scholar]
- 165.Tan, K. S., B. Y. Wee, and K. P. Song. 2001. Evidence for holin function of tcdE gene in the pathogenicity of Clostridium difficile. J. Med. Microbiol. 50**:**613-619. [DOI] [PubMed] [Google Scholar]
- 166.Tedesco, F. J. 1981. Antibiotic-associated colitis-an abating enigma. J. Clin. Gastroenterol. 3**:**221-224. [DOI] [PubMed] [Google Scholar]
- 167.Thelestam, M., and M. Bronnegard. 1980. Interaction of cytopathogenic toxin from Clostridium difficile with cells in tissue culture. Scand. J. Infect. Dis. Suppl. 22**:**16-29. [PubMed] [Google Scholar]
- 168.Thomas, C., M. Stevenson, and T. V. Riley. 2003. Antibiotics and hospital-acquired _Clostridium difficile_-associated diarrhoea: a systematic review. J. Antimicrob. Chemother. 51**:**1339-1350. [DOI] [PubMed] [Google Scholar]
- 169.Torres, J. F. 1991. Purification and characterisation of toxin B from a strain of Clostridium difficile that does not produce toxin A. J. Med. Microbiol. 35**:**40-44. [DOI] [PubMed] [Google Scholar]
- 170.Tucker, K. D., P. E. Carrig, and T. D. Wilkins. 1990. Toxin A of Clostridium difficile is a potent cytotoxin. J. Clin. Microbiol. 28**:**869-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Tucker, K. D., and T. D. Wilkins. 1991. Toxin A of Clostridium difficile binds to the human carbohydrate antigens I, X, and Y. Infect. Immun. 59**:**73-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.von Eichel-Streiber, C., R. Laufenberg-Feldmann, S. Sartingen, J. Schulze, and M. Sauerborn. 1990. Cloning of Clostridium difficile toxin B gene and demonstration of high N-terminal homology between toxin A and B. Med. Microbiol. Immunol. (Berlin) 179**:**271-279. [DOI] [PubMed] [Google Scholar]
- 173.von Eichel-Streiber, C., R. Laufenberg-Feldmann, S. Sartingen, J. Schulze, and M. Sauerborn. 1992. Comparative sequence analysis of the Clostridium difficile toxins A and B. Mol. Gen. Genet. 233**:**260-268. [DOI] [PubMed] [Google Scholar]
- 174.von Eichel-Streiber, C., D. Meyer zu Heringdorf, E. Habermann, and S. Sartingen. 1995. Closing in on the toxic domain through analysis of a variant Clostridium difficile cytotoxin B. Mol. Microbiol. 17**:**313-321. [DOI] [PubMed] [Google Scholar]
- 175.Wagenknecht-Wiesner, A., M. Weidmann, V. Braun, P. Leukel, M. Moos, and C. von Eichel-Streiber. 1997. Delineation of the catalytic domain of Clostridium difficile toxin B-10463 to an enzymatically active N-terminal 467 amino acid fragment. FEMS Microbiol. Lett. 152**:**109-116. [DOI] [PubMed] [Google Scholar]
- 176.Warny, M., A. C. Keates, S. Keates, I. Castagliuolo, J. K. Zacks, S. Aboudola, A. Qamar, C. Pothoulakis, J. T. LaMont, and C. P. Kelly. 2000. p38 MAP kinase activation by Clostridium difficile toxin A mediates monocyte necrosis, IL-8 production, and enteritis. J. Clin. Investig. 105**:**1147-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Webb, K. H. 2000. Prevalence of toxin A negative/B positive Clostridium difficile strains. J. Hosp. Infect. 45**:**331-332. [DOI] [PubMed] [Google Scholar]
- 178.Wedel, N., P. Toselli, C. Pothoulakis, B. Faris, P. Oliver, C. Franzblau, and T. LaMont. 1983. Ultrastructural effects of Clostridium difficile toxin B on smooth muscle cells and fibroblasts. Exp. Cell Res. 148**:**413-422. [DOI] [PubMed] [Google Scholar]
- 179.Wei, Y., Y. Zhang, U. Derewenda, X. Liu, W. Minor, R. K. Nakamoto, A. V. Somlyo, A. P. Somlyo, and Z. S. Derewenda. 1997. Crystal structure of RhoA-GDP and its functional implications. Nat. Struct. Biol. 4**:**699-703. [DOI] [PubMed] [Google Scholar]
- 180.Wershil, B. K., I. Castagliuolo, and C. Pothoulakis. 1998. Direct evidence of mast cell involvement in Clostridium difficile toxin A-induced enteritis in mice. Gastroenterology 114**:**956-964. [DOI] [PubMed] [Google Scholar]
- 181.Wilcox, M. H., and W. N. Fawley. 2001. Virulence of Clostridium difficile toxin A negative strains. J. Hosp. Infect. 48**:**81. [DOI] [PubMed] [Google Scholar]
- 182.Wilkins, T. D., and D. M. Lyerly. 2003. Clostridium difficile testing: after 20 years, still challenging. J. Clin. Microbiol. 41**:**531-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Willey, S. H., and J. G. Bartlett. 1979. Cultures for Clostridium difficile in stools containing a cytotoxin neutralized by Clostridium sordellii antitoxin. J. Clin. Microbiol. 10**:**880-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Witteck, A., Y. Yao, M. Fechir, U. Forstermann, and H. Kleinert. 2003. Rho protein-mediated changes in the structure of the actin cytoskeleton regulate human inducible NO synthase gene expression. Exp. Cell Res. 287**:**106-115. [DOI] [PubMed] [Google Scholar]
- 185.Wren, B. W. 1991. A family of clostridial and streptococcal ligand-binding proteins with conserved C-terminal repeat sequences. Mol. Microbiol. 5**:**797-803. [DOI] [PubMed] [Google Scholar]
- 186.Wren, B. W., R. R. Russell, and S. Tabaqchali. 1991. Antigenic cross-reactivity and functional inhibition by antibodies to Clostridium difficile toxin A, Streptococcus mutans glucan-binding protein, and a synthetic peptide. Infect. Immun. 59**:**3151-3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Yamakawa, K., S. Kamiya, X. Q. Meng, T. Karasawa, and S. Nakamura. 1994. Toxin production by Clostridium difficile in a defined medium with limited amino acids. J. Med. Microbiol. 41**:**319-323. [DOI] [PubMed] [Google Scholar]
- 188.Yamakawa, K., T. Karasawa, S. Ikoma, and S. Nakamura. 1996. Enhancement of Clostridium difficile toxin production in biotin-limited conditions. J. Med. Microbiol. 44**:**111-114. [DOI] [PubMed] [Google Scholar]
- 189.Zhou, K., Y. Wang, J. L. Gorski, N. Nomura, J. Collard, and G. M. Bokoch. 1998. Guanine nucleotide exchange factors regulate specificity of downstream signaling from Rac and Cdc42. J. Biol. Chem. 273**:**16782-16786. [DOI] [PubMed] [Google Scholar]