A γ-secretase inhibitor blocks Notch signaling in vivo and causes a severe neurogenic phenotype in zebrafish (original) (raw)
Abstract
Inhibition of amyloid β-peptide (Aβ) production by blocking γ-secretase activity is at present one of the most promising therapeutic strategies to slow progression of Alzheimer’s disease pathology. γ-secretase inhibitors apparently block Aβ generation via interference with presenilin (PS) function. Besides being an essential component of the γ-secretase complex, PS itself may be an aspartyl protease with γ-secretase activity, which is not only required for Aβ production but also for a similar proteolytic process involved in Notch signaling. Here we demonstrate that treatment of zebrafish embryos with a known γ-secretase inhibitor affects embryonic development in a manner indistinguishable from Notch signaling deficiencies at morphological, molecular and biochemical levels. This indicates severe side-effects of γ-secretase inhibitors in any Notch-dependent cell fate decision and demonstrates that the zebrafish is an ideal vertebrate system to validate compounds that selectively affect Aβ production, but not Notch signaling, under in vivo conditions.
INTRODUCTION
Accumulation of amyloid β-peptide (Aβ) is an invariant feature associated with Alzheimer’s disease (AD) pathology. Aβ is generated by the consecutive cuts of two proteases, known as β- and γ-secretase (Esler and Wolfe, 2001), which liberate the amyloidogenic peptide from its precursor, the β-amyloid precursor protein (APP). Subsequent aggregation is thought to result in the formation of neurotoxic protofibrils (Walsh et al., 1997; Nilsberth et al., 2001) and the deposition of amyloid plaques. While β-secretase has been identified (for a review, see Vassar and Citron, 2000), the nature of γ-secretase is still unclear (De Strooper and Annaert, 2001). The two homologous presenilin (PS) proteins, PS1 and PS2, which are critically required for the intramembranous γ-secretase cut, may be aspartyl proteases with γ-secretase activity (for a review, see Esler and Wolfe, 2001). This is supported by the identification of critical aspartates within the putative transmembrane domains 6 and 7 of PSs (Wolfe et al., 1999c), which may be part of a catalytically active center of an intrinsic aspartyl protease activity (Wolfe et al., 1999a). Moreover, γ-secretase inhibitors, which block Aβ generation, have been found to bind to PS1 (Esler et al., 2000; Li et al., 2000b) and PSs share considerable homology around the critical aspartate in transmembrane domain 7 with bacterial aspartyl proteases (Steiner et al., 2000). However, additional co-factors are required to allow formation of the biologically active PS complex (Capell et al., 1998; Li et al., 2000a), and the cellular distribution of PS does not necessarily reflect the location of γ-secretase activity (Cupers et al., 2001). Thus, it is currently unclear whether PSs are identical to the γ-secretase or just support its targeting to its cellular sites of action (Cupers et al., 2001).
PSs are not only essential for the γ-secretase cut of the Aβ domain, but also for the highly similar site 3 (S3) cleavage of Notch (De Strooper et al., 1999; for a review, see Mumm and Kopan, 2000). This cut produces the Notch intracellular domain (NICD), which translocates to the nucleus to regulate target gene transcription (Mumm and Kopan, 2000). Ablation of the PS1 and PS2 genes therefore results in a phenotype indistinguishable from that caused by a Notch knockout (Donoviel et al., 1999), and totally blocks Aβ and NICD production (Herreman et al., 2000; Zhang et al., 2000). Moreover, mutagenesis of the critical aspartates also blocks the function of human PS in Notch signaling (Steiner et al., 1999). Therefore, inhibition of PS activity not only blocks Aβ production, but also interferes with NICD generation and the Notch pathway. Indeed, pharmacological inhibition of PS1 activity blocks Notch signaling in cultured cells (De Strooper et al., 1999; Berezovska et al., 2000; Martys-Zage et al., 2000; Doerfler et al., 2001; Hadland et al., 2001). From a therapeutic point of view, this suggests that drugs developed to lower Aβ production by interfering with γ-secretase activity might affect PS function and therefore also block Notch signaling in vertebrates. To prove whether a known γ-secretase inhibitor (DAPT; Dovey et al., 2001) produces phenotypic side-effects in a living vertebrate, we used zebrafish (Danio rerio) as a model system. Our data not only demonstrate that a γ-secretase inhibitor fully blocks Notch signaling in a living vertebrate, but also suggest zebrafish as a suitable system to evaluate the effects of Aβ-lowering drugs on Notch signaling in vivo.
RESULTS
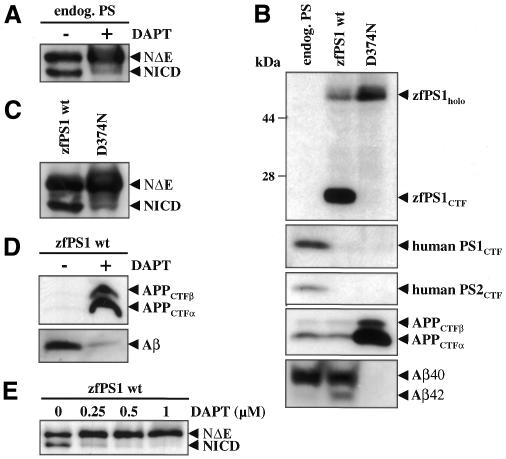
As a prototype γ-secretase inhibitor, we investigated the highly specific γ-secretase inhibitor DAPT (Dovey et al., 2001) for its capacity to block Notch endoproteolysis. With this aim, HEK293 cells expressing endogenous PSs were stably transfected with the NotchΔE cDNA [encoding a tagged version of the transmembrane and intracellular domains of Notch (Schroeter et al., 1998)] and treated with or without DAPT. As shown in Figure 1A, the NICD fragment is readily visible in untreated cells, but its generation is inhibited by DAPT treatment. Next, before testing the effects of DAPT in the zebrafish in vivo, we verified that DAPT was also active on a zebrafish PS1 (zfPS1)-controlled γ-secretase activity. We stably transfected HEK293 cells expressing Swedish mutant APP (HEK293/sw) (Citron et al., 1992) with cDNA encoding wild-type (wt) zfPS1 or the non-functional zfPS1 D374N mutant (Leimer et al., 1999). As expected (Leimer et al., 1999), zfPS1 was endoproteolytically processed, while endoproteolysis of zfPS1 D374N was blocked and the full-length protein accumulated (Figure 1B). Endogenous human PS1 and PS2 were replaced (Thinakaran et al., 1997) by wt and mutant zfPS1, demonstrating that zfPS1 is incorporated into the PS complex, the formation of which is required for γ-secretase activity (Li et al., 2000a) (Figure 1B). Expression of zfPS1 D374N caused a dramatic accumulation of the substrates of γ-secretase, the APP C-terminal fragments (CTFs), which was accompanied by an almost complete inhibition of total Aβ (Aβ40 and Aβ42) generation (Figure 1B). In contrast, expression of wt zfPS1 did not cause APP CTF accumulation and allowed normal total Aβ production (Figure 1B). We next analyzed Notch endoproteolysis in HEK293/sw cells stably co-expressing NotchΔE and wt or D374N mutant zfPS1. Expression of wt zfPS1 allowed robust NICD production, which was strongly inhibited by the zfPS1 D374N mutant (Figure 1C). Taken together, these results demonstrate that zfPS1 is capable of controlling γ-secretase and S3 protease activity in human cells. We therefore next investigated the effects of DAPT on APP and Notch endoproteolysis in the presence of zfPS1-controlled γ-secretase and S3 protease activity. DAPT caused a strong accumulation of APP CTFs with concomitant inhibition of Aβ generation (Figure 1D). DAPT also inhibited S3 cleavage of Notch in the presence of zfPS1. As shown in Figure 1E, NICD production was inhibited by DAPT in a dose-dependent manner. Thus, these experiments demonstrate that DAPT efficiently blocks a zfPS1-dependent γ-secretase and S3 protease activity.
Fig. 1. DAPT blocks Notch endoproteolysis and inhibits a zfPS1-dependent γ-secretase and S3 protease activity in HEK293 cells. (A) HEK293 cells stably transfected with the NotchΔE cDNA (Schroeter et al., 1998) were treated with or without 1 µM DAPT for 4 h. Cell lysates were analyzed for NotchΔE (NΔE; uncleaved form of Notch) and NICD (cleaved form of Notch) by immunoblotting using antibody 9E10 to the myc tag at the C-terminus of these Notch variants. (B) Panels 1–3: cell lysates from HEK293/sw cells stably transfected with the indicated zfPS1 cDNAs were analyzed by immunoblotting with antibody zfPS1loop, 3027 to human PS1 or 3711 to human PS2. Note that endogenous human PS1 and PS2 are replaced by zfPS1 variants. Replacement of endogenous PSs by overexpressed PS variants is an important indication for incorporation of the ectopic PS into a biologically functional PS complex (Thinakaran et al., 1997). Panel 4: cell lysates were analyzed for APP CTFs by immunoblotting with antibody 6687. Panel 5: conditioned media were analyzed for total Aβ (Aβ40 and Aβ42) species by immunoprecipitation/immunoblotting with antibodies 3926/6E10. Immunoprecipitates were separated on a Tris–bicine–urea gel that allows the identification of Aβ40 and Aβ42 (Wiltfang et al., 1997). Note that expression of wt zfPS1 not only allows robust Aβ (Aβ40 and Aβ42) production, but also leads to increased production of Aβ42 (Leimer et al., 1999). (C) HEK293/sw cells expressing either wt zfPS1 or mutant zfPS1 D374N were stably transfected with the NotchΔE cDNA (Schroeter et al., 1998) and cell lysates were analyzed for NotchΔE (NΔE) and NICD as in (A). (D) HEK293/sw cells stably expressing wt zfPS1 were treated with or without 1 µM DAPT for 4 h and analyzed for APP CTFs as in (B) and for Aβ by direct immunoblotting with antibody 3926. (E) HEK293/sw cells stably expressing wt zfPS1 were treated with increasing amounts of DAPT for 4 h and cell lysates were analyzed for NotchΔE (NΔE) and NICD as in (A).
We subsequently used the zebrafish system to assess the effect of DAPT on Notch signaling at the morphological, molecular and biochemical levels in vivo. A number of zebrafish mutants affecting Notch signaling have been identified and display characteristic early phenotypes, such as impaired segmentation of the somites (van Eeden et al., 1996), and exacerbated primary neurogenesis (Jiang et al., 1996). Their phenotypes constitute an experimental counterpart with which the in vivo effects of DAPT can be compared.
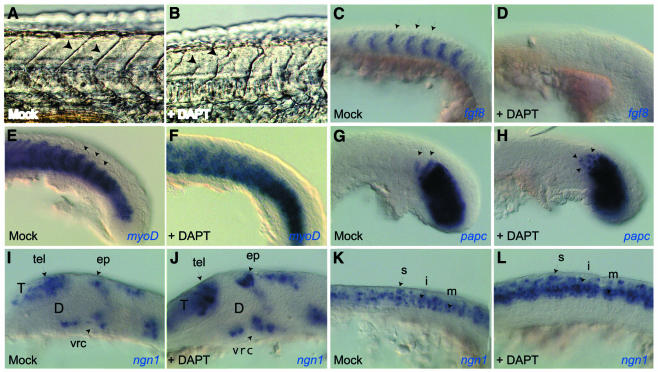
Treating zebrafish embryos with DAPT during the first 24 h of development did not trigger gross morphological abnormalities, but reproducibly impaired somite formation (91%, n = 54). At 24 h, somitic boundaries were misshapen, and delimited somites of irregular size (Figure 2A and B). Morphological observations at earlier stages indicated that, upon DAPT treatment, the first 4–8 somites formed normally while most posterior somites did not (data not shown). This phenotype strikingly resembles zebrafish Notch pathway mutants such as beamter (bea), deadly-seven (des), after-eight (aei) and white-tail (wit) (Jiang et al., 1996; van Eeden et al., 1996). At the molecular level, Notch activity normally controls somite anteroposterior (AP) polarity, as well as the cycling expression of somite prepatterning genes in the presomitic mesoderm (Pourquie, 2000). To confirm our morphological analysis, we assessed the effect of DAPT on these two Notch-dependent processes. Probing DAPT-treated embryos for fgf8 and myoD expression, which respectively label the anterior and posterior halves of each presumptive somite in the unsegmented mesoderm (Figure 2C and E), confirmed that DAPT, like Notch deficiencies, affected somite AP polarity: fgf8 expression was abolished upon DAPT treatment, while myoD expression became ubiquitous (100%, n = 18 and 23) (Figure 2D and F). The latter phenotype is similar to that reported in bea, des, aei and wit mutants [see figure 7 in van Eeden et al. (1996), figure 5 in Jiang et al. (1996) and figure 5 in Holley et al. (2000)]. In the region of nascent somites, Notch-dependent synchronized gene cycling normally lays down a banded pattern of paraxial protocadherin (papc) expression, which highlights the cells most recently arrested in a somitic state (Yamamoto et al., 1998; Jiang et al., 2000) (Figure 2G). papc expression is modified to a randomized pattern in Notch signaling mutants [figure 3 in Jiang et al. (2000)]. Similarly, we observed a random mixture of _papc_-positive and -negative cells in the anterior presomitic mesoderm of DAPT-treated embryos (Figure 2H) (100%, n = 12). Thus, DAPT treatment affects somitogenesis in vivo in a manner similar to deficiencies in Notch signaling.
Fig. 2. DAPT affects somitogenesis (A–H) and neurogenesis (I–L) in vivo in a manner indistinguishable from Notch signaling deficiencies. Mock-treated (A, C, E, G, I, K) and DAPT-treated (B, D, F, H, J, L) embryos visualized at 24 h [(A) and (B), live views] or at 15 somites (C–L), flat mounts following whole-mount in situ hybridization with the probes indicated, anterior to the left and dorsal up except in (E) and (F) (dorsal views). (A–H), (K) and (L) are close-up views of the trunk and tail; (I) and (J) are close-up views of the brain. DAPT treatment alters somitic borders [arrows in (A) and (B)]. It affects AP polarization of the somitic mesoderm, normally revealed by the complementary expression of fgf8 and myoD [arrows in (C) and (E)], and randomizes expression of cycling-dependent genes such as papc in nascent somites [arrows in (G) and (H)]. In the embryonic nervous system, DAPT triggers a neurogenic phenotype, with an increased number of _ngn1_-positive neuroblasts in every proneural cluster [compare the clusters identified by arrows in (I) and (J), (K) and (L)]. D, presumptive diencephalon; ep, epiphyseal cluster; i, intermediate neurons; m, motoneurons; s, sensory neurons; T, presumptive telencephalon; tel, telencephalic cluster; vrc, ventro-rostral cluster.
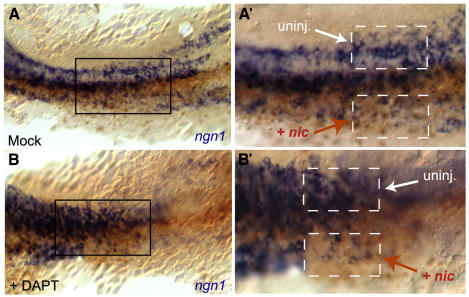
Fig. 3. Expression of the soluble cytoplasmic domain of Notch prevents DAPT-mediated inhibition of Notch signaling. Flat-mounted, mock-treated (A and A′) and DAPT-treated (B and B′) embryos visualized at 10 somites following in situ hybridization for ngn1 expression (blue staining) (dorsal view, anterior to the left). All embryos were injected into one blastomere at 4 cells with the NICD-encoding nic RNA and nlslacZ RNA as lineage tracer (brown nuclei identify the progeny of the injected blastomere). (A′) and (B′) (same magnification) are enlarged views of the boxed areas in (A) and (B), respectively. Injected (+ nic) and non-injected (uninj.) territories are indicated on either side of the embryo midline and boxed with a dotted line in (A′) and (B′). DAPT strongly increases the number of primary neurons within the neural plate (Figure 2L and non-injected territory in Figure 3B′ compared with 3A′), while nic has the opposite effect (compare injected and non-injected territories in A′). Note that NICD activity overrides the neurogenic effect of DAPT, as a similarly reduced number of neurons follows nic expression with or without DAPT [compare injected sides in (A′) and (B′)].
We subsequently tested whether DAPT treatment also mimics Notch signaling impairments during neurogenesis. Primary neurogenesis in lower vertebrates involves the selection of individual neuroblasts from proneural clusters by a Notch-dependent lateral inhibition process (Lewis, 1998; Chitnis, 1999; Blader and Strahle, 2000). Specifically, analysis of zebrafish mutants (Jiang et al., 1996) and in vivo misexpression experiments (Dornseifer et al., 1997; Haddon et al., 1998; Takke et al., 1999) demonstrated that Notch signaling maintains its expressing cells in an undifferentiated state, while neighboring Delta-positive cells express the neuronal specification factor neurogenin (Ngn1) (Blader et al., 1997) and generate neuroblasts. We observed that DAPT treatment strongly and reproducibly increased the number of _ngn1_-positive cells within each proneural cluster at all levels of the body axis during primary neurogenesis (Figure 3I–L) (100%, n = 13), triggering a neurogenic phenotype similar to zebrafish mutants deficient in Notch signaling, such as wit (Jiang et al., 1996), and to zebrafish embryos rendered insensitive to Notch by misexpression of the extracellular form of Delta (Haddon et al., 1998). Thus, like an absence of Notch signaling, DAPT prevents the lateral inhibition process of neuroblast selection in vivo.
The neurogenic phenotype resulting from Notch signaling deficiencies can be reverted in vivo by overproduction of the NICD fragment (see Haddon et al., 1998; Takke et al., 1999). To further confirm that the neurogenic phenotype triggered by DAPT in vivo resulted from impaired Notch processing, we therefore tested whether it could be reverted by injection of nic, an mRNA encoding the NICD fragment of zebrafish Notch1 (Takke et al., 1999). As concluded previously (Haddon et al., 1998; Takke et al., 1999), local misexpression of NICD reduced the number of _ngn1_-positive primary neurons in the embryonic neural plate (Figure 3A and A′). This effect is epistatic to the action of DAPT, as the number of primary neurons in _nic_-injected areas was similarly reduced in DAPT-treated and control embryos (compare Figure 3B and B′ and A and A′). Elsewhere in the neural plate, and as documented above (Figure 2I–L), neurogenesis was prominently enhanced by DAPT (Figure 3B and B′). Thus, the neurogenic effect of DAPT can be reverted by NICD, confirming that DAPT acts by interfering with Notch signaling and upstream of NICD activity. Taken together, our results demonstrate that DAPT affects zebrafish embryonic development in a manner indistinguishable from Notch signaling deficiencies, both at the morphological and molecular levels.
DISCUSSION
Our results demonstrate that DAPT, a known and carefully characterized γ-secretase inhibitor (Dovey et al., 2001; Sastre et al., 2001), severely interferes with Notch signaling in zebrafish embryos. DAPT and other γ-secretase inhibitors were developed as Aβ-lowering drugs thought to be used for long-term treatment in human patients (Wolfe et al., 1999b; Dovey et al., 2001). However, concerns about such a strategy were raised because it could apparently interfere with the biological function of PS. Based on numerous previous findings, PS clearly plays a role in Notch signaling by facilitating NICD generation (for a review, see Mumm and Kopan, 2000; Steiner and Haass, 2000). One may therefore expect that such inhibitors not only have beneficial effects with regard to Aβ production, but also unwanted side-effects on the control of cellular differentiation via interference with the Notch signaling pathway. Along this line, Hadland et al. (2001) recently demonstrated that a distinct γ-secretase inhibitor (Cpd.11) (Wolfe et al., 1999b) added to fetal organ cultures represses thymocyte development, probably by reducing Notch signaling. However, in this study, direct evidence that proteolytic generation of NICD generation was indeed affected in the CD4–/CD8– precursor cells was lacking. We now demonstrate for the first time that a γ-secretase inhibitor interferes with Notch signaling in vertebrates in vivo, directly suggesting that rather significant putative side-effects are to be expected from such drugs during long-term treatment in humans.
The detrimental effects of DAPT were observed during embryogenesis of zebrafish. However, they are likely to occur in adults as well, as Notch signaling is active at all stages and in multiple tissues. For example, hematopoiesis is required throughout life and thymocyte differentiation requires Notch signaling (Hadland et al., 2001). All Notch factors are also expressed in the adult brain (Weinmaster et al., 1992; Higuchi et al., 1995; Berezovska et al., 1998; Irvin et al., 2001), where they are likely to play pivotal roles in terminally differentiated neurons, as well as in the control of gliogenesis and neural stem cell differentiation. However, a conditional knockout of the PS1 gene had no obvious effects on Notch signaling in mice (Yu et al., 2001). The lack of effects on Notch signaling is likely to be due to the abundant expression of PS2 (Yu et al., 2001), which supports Notch signaling like PS1 (Steiner et al., 1999). In contrast to the conditional PS1 depletion, γ-secretase inhibitors will affect both PS1 and PS2 function (Esler et al., 2000; Li et al., 2000b). Besides putative side-effects on Notch signaling, PSs bind β-catenin, thus independently interacting with yet another signaling pathway potentially controlling cell proliferation in adults. Indeed, loss of PS1 in mice also results in enhanced β-catenin signaling, which causes skin tumors in adult mice (Xia et al., 2001).
In summary, our data not only demonstrate that a γ-secretase inhibitor blocks Notch signaling in a living vertebrate, but also provide a novel model system for the validation of drugs that differentially affect Aβ production and NICD formation (Petit et al., 2001). After only 24 h, numerous zebrafish embryos can be investigated for deficits in somitogenesis or neurogenesis, which provide a precise and reliable read-out of Notch signaling activity. Therefore, our results identify the zebrafish as a valuable test system for the validation of Aβ-lowering drugs that do not interfere with other physiologically important signaling pathways.
METHODS
Cell lines and cell culture. HEK293 cell lines were generated and cultured as described previously (Steiner et al., 2000).
Antibodies. The polyclonal antibodies against PS1 (3027) and PS2 (3711), against zfPS1 (zfP1loop) (Leimer et al., 1999), against the C-terminus of APP (6687) and against Aβ1–42 (3926) have been described previously (Steiner et al., 2000). Monoclonal antibodies against Aβ1–17 (6E10) and the c-myc epitope (9E10) were obtained from Senetek (6E10) and from the Developmental Studies Hybridoma Bank, University of Iowa (9E10).
PS, APP and Notch endoproteolysis. Analysis of human and zebrafish PS expression was as described previously (Leimer et al., 1999; Steiner et al., 2000). APP CTFs were analyzed as described before (Steiner et al., 2000) and Aβ production was analyzed by combined immunoprecipitation/immunoblotting of conditioned media with antibodies 3926/6E10 as described previously (Sastre et al., 2001) or by direct immunoblotting of aliquots of conditioned media with antibody 6E10. Notch endoproteolysis was analyzed by immunoblotting of cell lysates with antibody 9E10.
Fish strains. Embryos were obtained from natural spawning of wild-type (AB strain) adults; they were raised and staged according to Kimmel et al. (1995).
DAPT treatments. A 10 mM stock of DAPT in DMSO was diluted in embryo medium and applied to dechorionated zebrafish embryos at 28°C from the sphere stage (late blastula) until the stage of analysis (see figure legends). Control embryos were mock treated with embryo medium containing the same concentration of DMSO carrier only. We first performed a dose–response analysis and established that a minimal concentration of 50 µM DAPT was required to affect somitogenesis at the morphological and molecular levels (30 and 60% of cases, respectively, n = 34; not shown). All the results reported here were obtained using a dose of 100 µM DAPT. HEK293 cells were treated with 1 µM DAPT for 4 h.
Capped mRNA injections in zebrafish embryos. nic capped RNA (encoding the NICD of zebrafish Notch1) was synthesized as described by Takke et al. (1999) using the mMessage mMachine kit (Ambion), and 5 pg were injected (together with 4 pg of nlslacZ RNA as lineage tracer) into a single blastomere of 4-celled embryos. Nucleus-localized β-galactosidase was revealed using rabbit anti-β-galactosidase (1/4000) followed by goat anti-rabbit–HRP (1/200; Jackson Laboratories) antibodies and DAB revelation.
In situ hybridizations and immunocytochemistry. In situ hybridizations were carried out according to standard protocols (Thisse et al., 1993) using the following probes: fgf8 (Reifers et al., 1998), myoD (Weinberg et al., 1996), papc (Yamamoto et al., 1998) and ngn1 (Blader et al., 1997).
Acknowledgments
ACKNOWLEDGEMENTS
We thank the Boehringer Ingelheim Pharma KG for the gift of DAPT and S. Amacher, P. Blader, J.A. Campos-Ortega, D. Edbauer, R. Kopan, U. Leimer, C. Thisse and E. Weinberg for providing cDNAs, antibodies and cell lines, and G. Basset for technical assistance. We thank R. Baumeister, C. Goridis and W. Wurst for critically reading this manuscript, and H. Takeda for communicating unpublished data. This work was supported by grants from the Deutsche Forschungsgemeinschaft (to C.H. and H.S.), the European Community (to C.H.) and the VolkswagenStiftung (to A.G. and L.B.-C.).
REFERENCES
- Berezovska O., Xia, M.Q. and Hyman, B.T. (1998) Notch is expressed in adult brain, is coexpressed with presenilin-1, and is altered in Alzheimer disease. J. Neuropathol. Exp. Neurol., 57, 738–745. [DOI] [PubMed] [Google Scholar]
- Berezovska O. et al. (2000) Aspartate mutations in presenilin and γ-secretase inhibitors both impair Notch1 proteolysis and nuclear translocation with relative preservation of Notch1 signaling. J. Neurochem., 75, 583–593. [DOI] [PubMed] [Google Scholar]
- Blader P. and Strahle, U. (2000) Zebrafish developmental genetics and central nervous system development. Hum. Mol. Genet., 9, 945–951. [DOI] [PubMed] [Google Scholar]
- Blader P., Fischer, N., Gradwohl, G., Guillemont, F. and Strahle, U. (1997) The activity of neurogenin1 is controlled by local cues in the zebrafish embryo. Development, 124, 4557–4569. [DOI] [PubMed] [Google Scholar]
- Capell A. et al. (1998) The proteolytic fragments of the Alzheimer’s disease-associated presenilin-1 form heterodimers and occur as a 100–150-kDa molecular mass complex. J. Biol. Chem., 273, 3205–3211. [DOI] [PubMed] [Google Scholar]
- Chitnis A.B. (1999) Control of neurogenesis—lessons from frogs, fish and flies. Curr. Opin. Neurobiol., 9, 18–25. [DOI] [PubMed] [Google Scholar]
- Citron M. et al. (1992) Mutation of the β-amyloid precursor protein in familial Alzheimer’s disease increases β-protein production. Nature, 360, 672–674. [DOI] [PubMed] [Google Scholar]
- Cupers P., Bentahir, M., Craessaerts, K., Orlans, I., Vanderstichele, H., Saftig, P., De Strooper, B. and Annaert, W. (2001) The discrepancy between presenilin subcellular localization and γ-secretase processing of amyloid precursor protein. J. Cell Biol., 154, 731–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Strooper B. and Annaert, W. (2001) Presenilins and the intramembrane proteolysis of proteins: facts and fiction. Nat. Cell Biol., 3, E221–E225. [DOI] [PubMed] [Google Scholar]
- De Strooper B. et al. (1999) A presenilin-1-dependent γ-secretase-like protease mediates release of Notch intracellular domain. Nature, 398, 518–522. [DOI] [PubMed] [Google Scholar]
- Doerfler P., Shearman, M.S. and Perlmutter, R.M. (2001) Presenilin-dependent γ-secretase activity modulates thymocyte development. Proc. Natl Acad. Sci. USA, 98, 9312–9317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoviel D.B., Hadjantonakis, A.K., Ikeda, M., Zheng, H., Hyslop, P.S. and Bernstein, A. (1999) Mice lacking both presenilin genes exhibit early embryonic patterning defects. Genes Dev., 13, 2801–2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dornseifer P., Takke, C. and Campos-Ortega, J.A. (1997) Overexpression of a zebrafish homologue of the Drosophila neurogenic gene Delta perturbs differentiation of primary neurons and somite development. Mech. Dev., 63, 159–171. [DOI] [PubMed] [Google Scholar]
- Dovey H.F. et al. (2001) Functional γ-secretase inhibitors reduce β-amyloid peptide levels in brain. J. Neurochem., 76, 173–181. [DOI] [PubMed] [Google Scholar]
- Esler W.P. and Wolfe, M.S. (2001) A portrait of Alzheimer secretases—new features and familiar faces. Science, 293, 1449–1454. [DOI] [PubMed] [Google Scholar]
- Esler W.P. et al. (2000) Transition-state analogue inhibitors of γ-secretase bind directly to presenilin-1. Nat. Cell Biol., 2, 428–433. [DOI] [PubMed] [Google Scholar]
- Haddon C., Smithers, L., Schneider-Maunoury, S., Coche, T., Henrique, D. and Lewis, J. (1998) Multiple delta genes and lateral inhibition in zebrafish primary neurogenesis. Development, 125, 359–370. [DOI] [PubMed] [Google Scholar]
- Hadland B.K., Manley, N.R., Su, D., Longmore, G.D., Moore, C.L., Wolfe, M.S., Schroeter, E.H. and Kopan, R. (2001) γ-secretase inhibitors repress thymocyte development. Proc. Natl Acad. Sci. USA, 98, 7487–7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herreman A., Serneels, L., Annaert, W., Collen, D., Schoonjans, L. and De Strooper, B. (2000) Total inactivation of γ-secretase activity in presenilin-deficient embryonic stem cells. Nat. Cell Biol., 2, 461–462. [DOI] [PubMed] [Google Scholar]
- Higuchi M., Kiyama, H., Hayakawa, T., Hamada, Y. and Tsujimoto, Y. (1995) Differential expression of Notch1 and Notch2 in developing and adult mouse brain. Brain Res. Mol. Brain Res., 29, 263–272. [DOI] [PubMed] [Google Scholar]
- Holley S.A., Geisler, R. and Nusslein-Volhard, C. (2000) Control of her1 expression during zebrafish somitogenesis by a delta-dependent oscillator and an independent wave-front activity. Genes Dev., 14, 1678–1690. [PMC free article] [PubMed] [Google Scholar]
- Irvin D.K., Zurcher, S.D., Nguyen, T., Weinmaster, G. and Kornblum, H.I. (2001) Expression patterns of Notch1, Notch2, and Notch3 suggest multiple functional roles for the Notch-DSL signaling system during brain development. J. Comp. Neurol., 436, 167–181. [PubMed] [Google Scholar]
- Jiang Y.J. et al. (1996) Mutations affecting neurogenesis and brain morphology in the zebrafish, Danio rerio. Development, 123, 205–216. [DOI] [PubMed] [Google Scholar]
- Jiang Y.J., Aerne, B.L., Smithers, L., Haddon, C., Ish-Horowicz, D. and Lewis, J. (2000) Notch signalling and the synchronization of the somite segmentation clock. Nature, 408, 475–479. [DOI] [PubMed] [Google Scholar]
- Kimmel C.B., Ballard, W.W., Kimmel, S.R., Ullmann, B. and Schilling, T.F. (1995) Stages of embryonic development of the zebrafish. Dev. Dyn., 203, 253–310. [DOI] [PubMed] [Google Scholar]
- Leimer U., Lun, K., Romig, H., Walter, J., Grunberg, J., Brand, M. and Haass, C. (1999) Zebrafish (Danio rerio) presenilin promotes aberrant amyloid β-peptide production and requires a critical aspartate residue for its function in amyloidogenesis. Biochemistry, 38, 13602–13609. [DOI] [PubMed] [Google Scholar]
- Lewis J. (1998) Notch signalling and the control of cell fate choices in vertebrates. Semin. Cell Dev. Biol., 9, 583–589. [DOI] [PubMed] [Google Scholar]
- Li Y.-M. et al. (2000a) Presenilin 1 is linked with γ-secretase activity in the detergent solubilized state. Proc. Natl Acad. Sci. USA, 97, 6138–6143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.-M. et al. (2000b) Photoactivated γ-secretase inhibitors directed to the active site covalently label presenilin 1. Nature, 405, 689–694. [DOI] [PubMed] [Google Scholar]
- Martys-Zage J.L., Kim, S.H., Berechid, B., Bingham, S.J., Chu, S., Sklar, J., Nye, J. and Sisodia, S.S. (2000) Requirement for presenilin 1 in facilitating Jagged 2-mediated endoproteolysis and signaling of Notch 1. J. Mol. Neurosci., 15, 189–204. [DOI] [PubMed] [Google Scholar]
- Mumm J.S. and Kopan, R. (2000) Notch signaling: from the outside in. Dev. Biol., 228, 151–165. [DOI] [PubMed] [Google Scholar]
- Nilsberth C. et al. (2001) The ‘Arctic’ APP mutation (E693G) causes Alzheimer’s disease by enhanced Aβ protofibril formation. Nat. Neurosci., 4, 887–893. [DOI] [PubMed] [Google Scholar]
- Petit A., Bihel, F., da Costa, C.A., Pourquie, O., Checler, F. and Kraus, J.L. (2001) New protease inhibitors prevent γ-secretase-mediated production of Aβ40/42 without affecting Notch cleavage. Nat. Cell Biol., 3, 507–511. [DOI] [PubMed] [Google Scholar]
- Pourquie O. (2000) Vertebrate segmentation: is cycling the rule? Curr. Opin. Cell Biol., 12, 747–751. [DOI] [PubMed] [Google Scholar]
- Reifers F., Bohli, H., Walsh, E.C., Crossley, P.H., Stainier, D.Y. and Brand, M. (1998) Fgf8 is mutated in zebrafish acerebellar (ace) mutants and is required for maintenance of midbrain–hindbrain boundary development and somitogenesis. Development, 125, 2381–2395. [DOI] [PubMed] [Google Scholar]
- Sastre M., Steiner, H., Fuchs, K., Capell, A., Multhaup, G., Condron, M.M., Teplow, D.B. and Haass, C. (2001) Presenilin dependent γ-secretase processing of β-amyloid precursor protein at a site corresponding to the S3 cleavage of Notch. EMBO rep., 2, 835–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter E.H., Kisslinger, J.A. and Kopan, R. (1998) Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature, 393, 382–386. [DOI] [PubMed] [Google Scholar]
- Steiner H. and Haass, C. (2000) Intramembrane proteolysis by presenilins. Nat. Rev. Mol. Cell Biol., 1, 217–224. [DOI] [PubMed] [Google Scholar]
- Steiner H. et al. (1999) A loss of function mutation of presenilin-2 interferes with amyloid β-peptide production and Notch signaling. J. Biol. Chem., 274, 28669–28673. [DOI] [PubMed] [Google Scholar]
- Steiner H. et al. (2000) Glycine 384 is required for presenilin-1 function and is conserved in polytopic bacterial aspartyl proteases. Nat. Cell Biol., 2, 848–851. [DOI] [PubMed] [Google Scholar]
- Takke C., Dornseifer, P., v Weizsacker, E. and Campos-Ortega, J.A. (1999) her4, a zebrafish homologue of the Drosophila neurogenic gene E(spl), is a target of NOTCH signalling. Development, 126, 1811–1821. [DOI] [PubMed] [Google Scholar]
- Thinakaran G., Harris, C.L., Ratovitski, T., Davenport, F., Slunt, H.H., Price, D.L., Borchelt, D.R. and Sisodia, S.S. (1997) Evidence that levels of presenilins (PS1 and PS2) are coordinately regulated by competition for limiting cellular factors. J. Biol. Chem., 272, 28415–28422. [DOI] [PubMed] [Google Scholar]
- Thisse C., Thisse, B., Schilling, T.F. and Postlethwait, J.H. (1993) Structure of the zebrafish snail1 gene and its expression in wild-type, spadetail and no tail mutant embryos. Development, 119, 1203–1215. [DOI] [PubMed] [Google Scholar]
- van Eeden F.J. et al. (1996) Mutations affecting somite formation and patterning in the zebrafish, Danio rerio. Development, 123, 153–164. [DOI] [PubMed] [Google Scholar]
- Vassar R. and Citron, M. (2000) Aβ-generating enzymes: recent advances in β- and γ-secretase research. Neuron, 27, 419–422. [DOI] [PubMed] [Google Scholar]
- Walsh D.M., Lomakin, A., Benedek, G.B., Condron, M.M. and Teplow, D.B. (1997) Amyloid β-protein fibrillogenesis. Detection of a protofibrillar intermediate. J. Biol. Chem., 272, 22364–22372. [DOI] [PubMed] [Google Scholar]
- Weinberg E.S. et al. (1996) Developmental regulation of zebrafish MyoD in wild-type, no tail and spadetail embryos. Development, 122, 271–280. [DOI] [PubMed] [Google Scholar]
- Weinmaster G., Roberts, V.J. and Lemke, G. (1992) Notch2: a second mammalian Notch gene. Development, 116, 931–941. [DOI] [PubMed] [Google Scholar]
- Wiltfang J. et al. (1997) Improved electrophoretic separation and immunoblotting of β-amyloid (Aβ) peptides 1–40, 1–42, and 1–43. Electrophoresis, 18, 527–532. [DOI] [PubMed] [Google Scholar]
- Wolfe M.S., De Los Angeles, J., Miller, D.D., Xia, W. and Selkoe, D.J. (1999a) Are presenilins intramembrane-cleaving proteases? Implications for the molecular mechanism of Alzheimer’s disease. Biochemistry, 38, 11223–11230. [DOI] [PubMed] [Google Scholar]
- Wolfe M.S., Xia, W., Moore, C.L., Leatherwood, D.D., Ostaszewski, B., Rahmati, T., Donkor, I.O. and Selkoe, D.J. (1999b) Peptidomimetic probes and molecular modeling suggest that Alzheimer’s γ-secretase is an intramembrane-cleaving aspartyl protease. Biochemistry, 38, 4720–4727. [DOI] [PubMed] [Google Scholar]
- Wolfe M.S., Xia, W., Ostaszewski, B.L., Diehl, T.S., Kimberly, W.T. and Selkoe, D.J. (1999c) Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and γ-secretase activity. Nature, 398, 513–517. [DOI] [PubMed] [Google Scholar]
- Xia X. et al. (2001) Loss of presenilin 1 is associated with enhanced β-catenin signaling and skin tumorigenesis. Proc. Natl Acad. Sci. USA, 98, 10863–10868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto A., Amacher, S.L., Kim, S.H., Geissert, D., Kimmel, C.B. and De Robertis, E.M. (1998) Zebrafish paraxial protocadherin is a downstream target of spadetail involved in morphogenesis of gastrula mesoderm. Development, 125, 3389–3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H. et al. (2001) APP processing and synaptic plasticity in presenilin-1 conditional knockout mice. Neuron, 31, 713–726. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Nadeau, P., Song, W., Donoviel, D., Yuan, M., Bernstein, A. and Yankner, B.A. (2000) Presenilins are required for γ-secretase cleavage of β-APP and transmembrane cleavage of Notch-1. Nat. Cell Biol., 2, 463–465. [DOI] [PubMed] [Google Scholar]