Inscuteable-independent apicobasally oriented asymmetric divisions in the Drosophila embryonic CNS (original) (raw)
Abstract
Inscuteable is the founding member of a protein complex localised to the apical cortex of Drosophila neural progenitors that controls their asymmetric division. Aspects of asymmetric divisions of all identified apicobasally oriented neural progenitors characterised to date, in both the central and peripheral nervous systems, require inscuteable. Here we examine the generality of this requirement. We show that many identified neuroblast lineages, in fact, do not require inscuteable for normal morphological development. To elucidate the requirements for apicobasal asymmetric divisions in a context where inscuteable is not essential, we focused on the MP2 > dMP2 + vMP2 division. We show that for MP2 divisions, asymmetric localisation and segregation of Numb and the specification of distinct dMP2 and vMP2 identities require bazooka but not inscuteable. We conclude that inscuteable is not required for all apicobasally oriented asymmetric divisions and that, in some cellular contexts, bazooka can mediate apicobasal asymmetric divisions without inscuteable.
INTRODUCTION
Neural progenitors of the Drosophila embryonic central nervous system (CNS) divide asymmetrically with their mitotic spindles aligned along the apical/basal (A/B) axis and cell-fate determinants localised to the basal cell cortex. An apically localised protein complex that includes Inscuteable (Insc) (Kraut and Campos-Ortega, 1996; Kraut et al., 1996) and Bazooka (Baz) (Schober et al., 1999; Wodarz et al., 1999) mediates these divisions. However, the proposed role of apical complex molecules like Insc in specifying CNS cell fate is based on studies involving a small number of identified early born neurons from a few well-characterised lineages (e.g. Buescher et al., 1998; Yu et al., 2000). Here we use cell transplantations to examine the requirement for insc during the development of complete neuroblast lineages; we show that the morphology of several characterised ventral neuroblast lineages (Bossing et al., 1996; Schmid et al., 1999) does not require insc. We focused on the MP2 > dMP2 + vMP2 asymmetric division, in which the resolution of distinct daughter fates requires Notch and numb (Spana et al., 1995; Spana and Doe, 1996). We show that for the A/B-oriented MP2 divisions, asymmetric localisation and segregation of Numb and the specification of distinct dMP2 and vMP2 identities occur normally in the absence of insc. Although Insc is dispensable, there is a requirement for Baz for the MP2 asymmetric division; when baz is limiting, Numb is cortical in dividing MP2, leading to the duplication of dMP2 at the expense of vMP2. We conclude that insc is not required for all A/B-oriented asymmetric divisions; in some cellular contexts (Lu et al., 2001), baz can mediate both planar (Bellaiche et al., 2001) and A/B asymmetric divisions (MP2) in the absence of insc.
RESULTS AND DISCUSSION
Cell transplantations and immunohistochemistry were performed to uncover the role of insc in the development of complete neuroblast (NB) lineages. Cells removed from the ventral neuroectoderm of insc 22 (an amorphic allele of insc) mutant embryos were isotopically and isochronically implanted into wild-type (WT) hosts (Figure 1; Table I). The resulting NB lineages were analysed and benchmarked against corresponding WT lineages with respect to the composition of their neurons and/or glia, cell positions and axonal morphologies. We obtained a total of 112 mutant ventral nervous system clones from donors homozygous for insc 22. The 112 mutant lineages represented 15 out of the 17 described neuroblast lineages from the ventral neuroectoderm (Bossing et al., 1996). We limit our discussion to nine out of the 15 ventral insc mutant lineages, each of which was represented by at least three independent mutant clones (Table I).
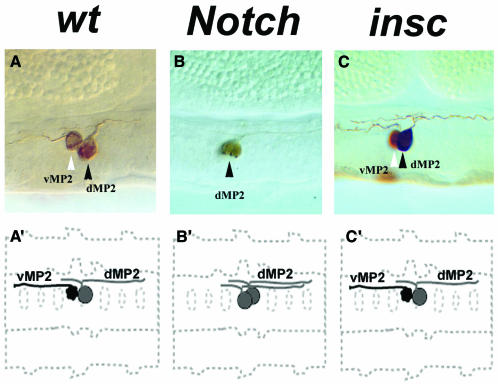
Fig. 1. The resolution of distinct fates for the progeny of the MP2 asymmetric division does not require insc function. Representative horseradish peroxidase (HRP)-labelled MP2 clones obtained by transplanting WT and mutant donor cells into WT hosts are shown. (A–C) HRP lineages in a lateral view. (A′–C′) Summary of morphologies of typical clones from the corresponding experiments in a horizontal orientation. Anterior is to the left. (A) The WT MP2 lineage consists of two cells. The dMP2 neuron (black arrowhead) projects its axon posteriorly, while its sibling neuron vMP2 (white arrowhead) sends projections in the anterior direction. (B) MP2 clone obtained from heterogenetic transplantations of homozygous N 55e11 (an amorphic allele of N) donor cells into WT hosts. The vMP2 neuron is transformed into the dMP2 neuron, as seen by the duplication of the dMP2 fate and the absence of the vMP2 projection. (C) MP2 clone obtained from heterogenetic transplantations of insc 22 donor cells into WT hosts. The clone is indistinguishable from its WT counterpart.
Table I. Clonal types from the ventral neuroectoderm (0–20% VD) obtained by transplanting insc mutant neuroectodermal cells.
| Clone | No. | Phenotype (with respect to wt and Notch mutants) |
|---|---|---|
| NB1-1 | 9 | Partially transformed |
| NB4-2 | 6 | Partially transformed |
| NB7-1 | 15 | Partially transformed |
| MP2 | 11 | Wild type |
| NB1-2 | 8 | Wild type |
| NB3-2 | 8 | Wild type |
| NB4-1 | 3 | Wild type |
| NB5-2 | 13 | Wild type |
| NB7-2 | 7 | Wild type |
Three insc mutant lineages, NB1-1, NB4-2 and NB7-1, showed deviations from the WT morphology (Table I; data not shown). In all three mutant lineages, insc appears to be required specifically for the cell-fate resolution of the earliest born neurons, later born components of these three lineages appear not to require or can partially bypass insc for WT morphological development. A detailed description of the roles of insc, Notch, Delta and kuzbanian in neuroblast lineage development will be presented elsewhere. Here we are concerned with NB lineages that do not require insc. All of the insc clones associated with six of the NB lineages (NB1-2, NB3-2, NB4-1, NB5-2, NB7-2 and MP2) were morphologically WT in terms of cell numbers, positions and axonal projections (Table I). We focused on the simple MP2 lineage in an attempt to understand the nature of at least some of these _insc_-independent asymmetric divisions. The MP2 precursor forms adjacent to the ventral midline in each segment and undergoes a single A/B-oriented asymmetric division to produce two daughter cells, vMP2 and dMP2 (Spana et al., 1995). The vMP2 neuron projects a single axon anteriorly, while dMP2 sends out a single axon into the ipsilateral connective where it bifurcates into an anterior and posterior branch (Figure 1A and A′). Mutations in N cause a transformation of vMP2 into dMP2 (Spana and Doe, 1996). The same phenotype was seen in transplanted N –/– MP2s (Figure 1B and B′). Loss-of-function mutations in the cell-fate determinant Numb cause the reverse transformation dMP2>vMP2, as Numb apparently antagonises N signalling directly by protein–protein interactions (Spana and Doe, 1996). Thus, one might expect mutations in N and insc to be similar with respect to terminal phenotypes of sibling neurons, if insc were to be responsible for the asymmetric localisation and segregation of Numb.
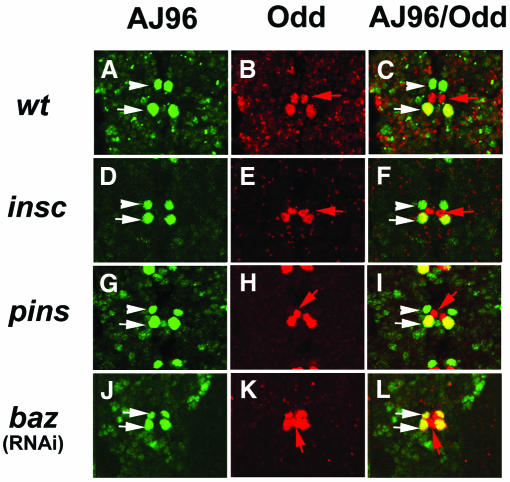
We analysed 11 MP2 clones, which were derived from insc mutant neuroectodermal cells, and all the clones were WT with respect to cell positioning and axonal projections (Figure 1C and C′). This finding was further substantiated using molecular markers. Odd-skipped (Odd) is expressed in the MP2 precursor and the newly born dMP2 and vMP2 neurons. Expression is extinguished in vMP2 at stage 11, but maintained in dMP2. Odd is also expressed in the midline lineage, MP1. The axonal marker 22C10 also labels both MP2 progeny, but not MP1. The enhancer trap line AJ96 expresses β-Gal strongly throughout the MP2 lineage (Menne and Klambt, 1994). In stage 13–14 insc 22, AJ96 embryos, we found an essentially WT staining pattern of one Odd+ and β-Gal– cell (MP1) and one Odd and β-Gal double-positive cell (dMP2) in all the analysed hemisegments (n = 500; Figure 2D–F). Using mAb22C10 (data not shown), we observed the WT pattern of anterior dMP2 projections and posterior vMP2 projections in all analysed insc 22 hemisegments (n = 110). We confirmed these findings with another allele, insc P49. In addition, we removed possible maternal (and zygotic) component(s) of insc using the FLP-FRT/ovoD technique (Chou and Perrimon, 1996). All hemisegments (n = 260) analysed showed a WT phenotype (data not shown), ruling out the involvement of an insc maternal component. These data show that the resolution of distinct dMP2 and vMP2 fates does not require insc.
Fig. 2. baz is required for dMP2 and vMP2 cell-fate determination. Confocal images of WT and mutant embryos (stage 14) in AJ96 background double labelled with anti-β-Gal (green) and anti-Odd (red), and superimposed images. In WT (A–C), insc (D–F) and pins (G–I) embryos, both vMP2 (arrowhead) and dMP2 (arrow) are β-Gal positive (green); anti-Odd also labels dMP2 (red) and MP1 (red arrow). In baz RNAi (J–L) embryos, the anti-β-Gal pattern remains unchanged but both progeny cells of MP2 are Odd positive (red), suggesting a vMP2>dMP2 cell-fate transformation. There are additional Odd-positive cells, but these are AJ96 negative and are probably supernumery MP1s. One segment of embryos is shown in all panels. Anterior is towards the top.
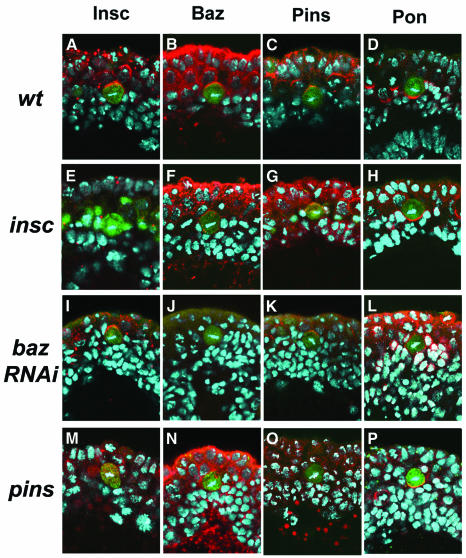
As the MP2 asymmetric divisions are unaffected by insc loss of function, we asked whether Insc is expressed in the MP2 progenitor in WT embryos. We stained homozygous AJ96 embryos with anti-Insc and anti-β-Gal, and detected apically localised crescents of Insc at mitosis (n = 20; Figure 3A). The mitotic MP2 precursor was identified by diffused cytoplasmic AJ96 β-Gal staining and DNA stain. In addition, we checked the expression patterns of several proteins known to be asymmetrically localised during NB mitosis. Baz and Pins are expressed as apical crescents in the dividing MP2 in a manner similar to Insc (Figure 3B and C). Numb (Uemura et al., 1989; Rhyu et al., 1994) and Partner of Numb (Pon) (Lu et al., 1998) are asymmetrically localised to the basal cortex of the MP2 precursor (data not shown; Figure 3D). Prospero (Pros) protein is nuclear in the MP2 precursor and equally distributed to both the progeny after division (Skeath and Doe, 1996). This is in contrast to typical NBs, in which Pros localisation is basal; only in the ganglion mother cell (GMC) is Pros released from the cortex to assume a nuclear localisation (Hirata et al., 1995; Knoblich et al., 1995). Lastly, we checked the localisation of Miranda, which is required for the basal localisation of Pros in NBs (Ikeshima-Kataoka et al., 1997; Shen et al., 1997; Schuldt et al., 1998). We could not detect Miranda immunoreactivity at any stage in MP2 (data not shown).
Fig. 3. Localisation of proteins asymmetrically localised in NBs in dividing MP2. Superimposed confocal images showing Insc (red), Baz (red), Pins (red) and Pon (red) in dividing MP2 (green); DNA is blue. In WT embryos, Insc (A), Baz (B) and Pins (C) are localised to the apical cortex of MP2, while Pon forms a basal crescent (D). In insc embryos, although the signal intensity is weaker than WT, Baz (F) and Pins (G) apical localisation is not affected. Localisation of both Insc and Pins in metaphase MP2 of baz (RNAi) embryos remains apical (I and K). Asymmetric localisation of Pon in MP2 is defective and becomes cortical in baz (RNAi) embryos (L). In pins embryos, Insc is cytoplasmic (M), as seen in other neuroblasts; Baz apical crescent is rather weak, sometimes undetectable (N). In insc and pins mutants, the basal localisation of Pon is not affected (H and P).
We next examined the localisation of Pon/Numb in dividing MP2s of insc mutant embryos. We found that Pon and Numb remain asymmetrically localised to the basal cortex of dividing insc mutant MP2s (n = 19; Figure 3H; data not shown). Moreover, the spindle is oriented largely within 45° of the A/B axis, as in WT (33/37). These findings, together with the transplantation results, indicate that although Insc is expressed and asymmetrically localised in MP2, it is not required either for Pon/Numb asymmetric localisation or for the resolution of distinct dMP2/vMP2 fates.
Two obvious candidate molecules that might be responsible for mediating the MP2 asymmetric division are Baz and Partner of Inscuteable (Pins) (Parmentier et al., 2000; Schaefer et al., 2000; Yu et al., 2000). pins appears not to be a major player (Figure 2G–I) since, in embryos lacking both maternal and zygotic pins, only 5.7% of the hemisegments show dMP2 duplication (n = 140), as demonstrated by three odd-positive cells (data not shown). Assessing the role of baz was problematic since loss of zygotic function had no effect, and removing both the maternal and zygotic baz resulted in embryos with severe morphological defects that prevent scoring of dMP2 and vMP2 fates in older embryos. We circumvented these problems by performing RNAi (Kennerdell and Carthew, 1998) with baz double-stranded RNA on AJ96 embryos (see Figure 2 legend), which yielded ∼25% embryos with reduced Baz protein but without the severe morphological defects that prevented scoring of vMP2 and dMP2 identities. In such embryos, vMP2>dMP2 transformations are observed in the great majority of hemisegments (57/60), as demonstrated by the presence of two cells double positive for β-Gal and Odd (Figure 2J–L). Moreover, localisation of Pon (Figure 3L) and Numb (data not shown) becomes cortical in dividing MP2 (38/40). However, there does not appear to be a dramatic defect on the orientation of the cell division, with almost all of the MP2 divisions oriented within 45° of the A/B axis (32/34). These defects associated with baz RNAi cannot merely be due to secondary effects associated with a disruption of the epithelium since, in crumbs loss of function, Baz (12/12) and Pon (10/10) remain correctly localised to the MP2 apical and basal cortex, respectively. These results indicate that Baz is required for the asymmetric localisation of Pon and Numb in the MP2 asymmetric division.
Although apical complex members, like Baz, Insc and Pins, are expressed and apically localised in both MP2 and NBs, their behaviour appears to differ somewhat in the two cellular contexts. For example, when baz function is attenuated, Insc is in the cytosol of NBs whilst Insc remains localised as an apical crescent in most dividing MP2 cells (24/32; Figure 3I); similar results are seen in dividing MP2s of embryos derived from baz Xi106 germline clones, which lack both maternal and zygotic function (data not shown). Moreover, it is interesting to note that in the absence of insc function, Baz (22/22) and Pins (20/20) can be localised to the apical cortex of metaphase MP2s (Figure 3F and G), although the intensity of staining is always reduced compared with WT MP2s, and in some cases the weak apical crescent of Baz can be difficult to detect. These observations suggest that even a small amount of apically localised Baz is sufficient to mediate basal localisation of Pon and direct the MP2 asymmetric division. Strikingly, Baz (Figure 3L), but not Insc (Figure 3H) and Pins (Figure 3P), seems to play a dominant role in mediating Pon/Numb basal localisation in MP2. When baz function is attenuated, Pon/Numb become cortically localised (Figure 3L; data not shown) even though Insc and Pins can remain apically localised (Figure 3I and K). These observations indicate that the precise requirements for asymmetric protein localisation differ between MP2s and NBs.
MP2 appears to be the only known A/B-oriented asymmetric division that does not require insc. Although MP2 delaminates from the neuroectoderm and divides in an apico-basal fashion like NBs, there are unique features that set MP2 apart from other neuroblasts. Unlike NBs that divide in a stem-cell-like mode, MP2 undergoes one differentiative division, making it more like a GMC or a pIIb division. Insc is present in both GMC/SOP lineages. A/B-oriented asymmetric GMC divisons, like those of GMC4-2a, require insc (Buescher et al., 1998). While the first SOP division (the anterior–posterior pI > pIIa + pIIb) does not require insc, recent work has shown that the spindle orientation of the strikingly GMC-like A/B division of the pIIb cell is dependent on Insc (Roegiers et al., 2001b). Finally, unlike NBs, Pros shows nuclear localisation in MP2 (Skeath and Doe, 1996). There is evidence supporting the view that Pros acts to terminate cell proliferation during Drosophila neurogenesis (Li and Vaessin, 2000). It is plausible that both GMCs and the MP2 precursor use nuclear Pros as a cue to reduce their mitotic potential and undergo a single differentiative division. Two recent reports have shown that planar asymmetric divisions undertaken by pI in the peripheral nervous system (Bellaiche et al., 2001; Roegiers et al., 2001a) and epithelial cells with disrupted adherens junctions (Lu et al., 2001) both require baz and not insc. We demonstrate here that insc is not required for all A/B-oriented asymmetric divisions. Our findings further support the view that baz is a more general mediator of asymmetric divisions than insc, and can act to promote both A/B and planar asymmetric divisions in the absence of insc.
METHODS
Transplantations. The transplantation procedure and subsequent handling of the recipient embryos were performed as described previously (Prokop and Technau, 1993). The genotype of the donor embryos was determined by staining each donor embryo for the presence/absence of a Blue-balancer chromosome.
RNAi experiments. RNAi experiments were carried out essentially as described (Kennerdell and Carthew, 1998). A 0.7 kb _Pst_I fragment spanning nucleotides 3618–4343 of baz cDNA was used as the template for double-stranded RNA synthesis. Approximately 50–100 pl of dsRNA solution (∼1 mg/ml) were injected into each embryo. For the purposes of determining resolution of dMP2 and vMP2 cell fates, injected embryos were aged to stages 13–14 prior to the fixation and immunostaining; for the purposes of determining protein localisation in dividing MP2, injected embryos were aged at 25°C for 6.5 h before fixation and immunostaining.
Immunohistochemistry and image collection. Primary rabbit antibodies used for immunostaining included anti-Insc (1:1000), anti-Pins (1:1000), anti-Baz (1:1000, a gift from F. Matsuzaki), anti-Pon (1:1000, a gift from Y.N. Jan) and rabbit anti-Odd (1:1000, a gift from Jim Skeath). Mouse anti-β-Gal (1:200) was obtained from Promega, and Cy3 and FITC-conjugated secondary antibodies were obtained from Jackson Laboratories. The images were collected with a Bio-Rad laser confocal microscope (MRC 1024) and processed with Adobe Photoshop.
Acknowledgments
ACKNOWLEDGEMENTS
We thank Chris Doe, Yuh-Nung Jan, Juergen Knoblich, Eli Knust, Fumio Matsuzaki, Jim Skeath, Gerd Technau, Andreas Wodarz, Kathy Matthews and the Bloomington Stock Centre for fly stocks, help and reagents. X.Y. is an adjunct staff, Department of Anatomy, National University of Singapore. W.C. is a Wellcome Trust Principal Research Fellow. This study was supported by the IMCB, Singapore, and the Wellcome Trust.
REFERENCES
- Bellaiche Y., Radovic, A., Woods, D.F., Hough, C.D., Parmentier, M.L., O’Kane, C.J., Bryant, P.J. and Schweisguth, F. (2001) The Partner of Inscuteable/Discs-large complex is required to establish planar polarity during asymmetric cell division in Drosophila. Cell, 106, 355–366. [DOI] [PubMed] [Google Scholar]
- Bossing T., Udolph, G., Doe, C.Q. and Technau, G.M. (1996) The embryonic central nervous system lineages of Drosophila melanogaster. I. Neuroblast lineages derived from the ventral half of the neuroectoderm. Dev. Biol., 179, 41–64. [DOI] [PubMed] [Google Scholar]
- Buescher M., Yeo, S.L., Udolph, G., Zavortink, M., Yang, X., Tear, G. and Chia, W. (1998) Binary sibling neuronal cell fate decisions in the Drosophila embryonic central nervous system are nonstochastic and require inscuteable-mediated asymmetry of ganglion mother cells. Genes Dev., 12, 1858–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou T.B. and Perrimon, N. (1996) The autosomal FLP-DFS technique for generating germline mosaics in Drosophila melanogaster. Genetics, 144, 1673–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata J., Nakagoshi, H., Nabeshima, Y. and Matsuzaki, F. (1995) Asymmetric segregation of the homeodomain protein Prospero during Drosophila development. Nature, 377, 627–630. [DOI] [PubMed] [Google Scholar]
- Ikeshima-Kataoka H., Skeath, J.B., Nabeshima, Y., Doe, C.Q. and Matsuzaki, F. (1997) Miranda directs Prospero to a daughter cell during Drosophila asymmetric divisions. Nature, 390, 625–629. [DOI] [PubMed] [Google Scholar]
- Kennerdell J.R. and Carthew, R.W. (1998) Use of dsRNA-mediated genetic interference to demonstrate that frizzled and frizzled 2 act in the wingless pathway. Cell, 95, 1017–1026. [DOI] [PubMed] [Google Scholar]
- Knoblich J.A., Jan, L.Y. and Jan, Y.N. (1995) Asymmetric segregation of Numb and Prospero during cell division. Nature, 377, 624–627. [DOI] [PubMed] [Google Scholar]
- Kraut R. and Campos-Ortega, J.A. (1996) inscuteable, a neural precursor gene of Drosophila, encodes a candidate for a cytoskeleton adaptor protein. Dev. Biol., 174, 65–81. [DOI] [PubMed] [Google Scholar]
- Kraut R., Chia, W., Jan, L.Y., Jan, Y.N. and Knoblich, J.A. (1996) Role of inscuteable in orienting asymmetric cell divisions in Drosophila. Nature, 383, 50–55. [DOI] [PubMed] [Google Scholar]
- Li L. and Vaessin, H. (2000) Pan-neural Prospero terminates cell proliferation during Drosophila neurogenesis. Genes Dev., 14, 147–151. [PMC free article] [PubMed] [Google Scholar]
- Lu B., Rothenberg, M., Jan, L.Y. and Jan, Y.N. (1998) Partner of Numb colocalizes with Numb during mitosis and directs Numb asymmetric localization in Drosophila neural and muscle progenitors. Cell, 95, 225–235. [DOI] [PubMed] [Google Scholar]
- Lu B., Roegiers, F., Jan, L.Y. and Jan, Y.N. (2001) Adherens junctions inhibit asymmetric division in the Drosophila epithelium. Nature, 409, 522–525. [DOI] [PubMed] [Google Scholar]
- Menne T.V. and Klambt, C. (1994) The formation of commissures in the Drosophila CNS depends on the midline cells and on the Notch gene. Development, 120, 123–133. [DOI] [PubMed] [Google Scholar]
- Parmentier M.L., Woods, D., Greig, S., Phan, P.G., Radovic, A., Bryant, P. and O’Kane, C.J. (2000) Rapsynoid/partner of inscuteable controls asymmetric division of larval neuroblasts in Drosophila. J. Neurosci., 20, RC84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokop A. and Technau, G. (1993) Cell transplantation. In Hartley, E.D. (ed.), Cellular Interactions in Development: A Practical Approach. Oxford University Press, Oxford, UK, pp. 33–57.
- Rhyu M.S., Jan, L.Y. and Jan, Y.N. (1994) Asymmetric distribution of numb protein during division of the sensory organ precursor cell confers distinct fates to daughter cells. Cell, 76, 477–491. [DOI] [PubMed] [Google Scholar]
- Roegiers F., Younger-Shepherd, S., Jan, L.Y. and Jan, Y.N. (2001a) Bazooka is required for localization of determinants and controlling proliferation in the sensory organ precursor cell lineage in Drosophila. Proc. Natl Acad. Sci. USA, 98, 14469–14474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roegiers F., Younger-Shepherd, S., Jan, L.Y. and Jan, Y.N. (2001b) Two types of asymmetric divisions in the Drosophila sensory organ precursor cell lineage. Nat. Cell Biol., 3, 58–67. [DOI] [PubMed] [Google Scholar]
- Schaefer M., Shevchenko, A. and Knoblich, J.A. (2000) A protein complex containing Inscuteable and the Gα-binding protein Pins orients asymmetric cell divisions in Drosophila. Curr. Biol., 10, 353–362. [DOI] [PubMed] [Google Scholar]
- Schmid A., Chiba, A. and Doe, C.Q. (1999) Clonal analysis of Drosophila embryonic neuroblasts: neural cell types, axon projections and muscle targets. Development, 126, 4653–4689. [DOI] [PubMed] [Google Scholar]
- Schober M., Schaefer, M. and Knoblich, J.A. (1999) Bazooka recruits Inscuteable to orient asymmetric cell divisions in Drosophila neuroblasts. Nature, 402, 548–551. [DOI] [PubMed] [Google Scholar]
- Schuldt A.J., Adams, J.H., Davidson, C.M., Micklem, D.R., Haseloff, J., St Johnston, D. and Brand, A.H. (1998) Miranda mediates asymmetric protein and RNA localization in the developing nervous system. Genes Dev., 12, 1847–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C.P., Jan, L.Y. and Jan, Y.N. (1997) Miranda is required for the asymmetric localization of Prospero during mitosis in Drosophila. Cell, 90, 449–458. [DOI] [PubMed] [Google Scholar]
- Skeath J.B. and Doe, C.Q. (1996) The achaete–scute complex proneural genes contribute to neural precursor specification in the Drosophila CNS. Curr. Biol., 6, 1146–1152. [DOI] [PubMed] [Google Scholar]
- Spana E.P. and Doe, C.Q. (1996) Numb antagonizes Notch signaling to specify sibling neuron cell fates. Neuron, 17, 21–26. [DOI] [PubMed] [Google Scholar]
- Spana E.P., Kopczynski, C., Goodman, C.S. and Doe, C.Q. (1995) Asymmetric localization of numb autonomously determines sibling neuron identity in the Drosophila CNS. Development, 121, 3489–3494. [DOI] [PubMed] [Google Scholar]
- Uemura T., Shepherd, S., Ackerman, L., Jan, L.Y. and Jan, Y.N. (1989) numb, a gene required in determination of cell fate during sensory organ formation in Drosophila embryos. Cell, 58, 349–360. [DOI] [PubMed] [Google Scholar]
- Wodarz A., Ramrath, A., Kuchinke, U. and Knust, E. (1999) Bazooka provides an apical cue for Inscuteable localization in Drosophila neuroblasts. Nature, 402, 544–547. [DOI] [PubMed] [Google Scholar]
- Yu F., Morin, X., Cai, Y., Yang, X. and Chia, W. (2000) Analysis of partner of inscuteable, a novel player of Drosophila asymmetric divisions, reveals two distinct steps in inscuteable apical localization. Cell, 100, 399–409. [DOI] [PubMed] [Google Scholar]