Fas (CD95)-Dependent Cell-Mediated Immunity to Listeria monocytogenes (original) (raw)
Abstract
Two distinct and complementary pathways, one mediated by perforin and the other dependent upon CD95 (Fas), effect cell-mediated cytotoxicity. We examined the relative roles of these pathways in host defenses against the intracellular bacterial pathogen Listeria monocytogenes by using murine listeriosis as a model system. Mice which lacked both perforin and Fas (P0L0) were generated, and their responses to primary and secondary listeriosis were compared to those of wild-type (WT), Fas-deficient (L0), and perforin knockout (P0) mice. Relative to WT mice during primary listeriosis, P0 mice exhibited a reduced capacity to clear the infection from their spleens but not their livers whereas L0 mice had elevated bacterial titers in their livers and a modestly increased titer in their spleens. In contrast, bacterial titers in P0L0 mice were increased approximately 50- to 560-fold in their spleens and 230- to 1,000-fold in their livers; eventual clearance of listeriae from both organs was significantly delayed. Furthermore, the resistance of P0L0 mice to secondary listeriosis was significantly reduced in their spleens and livers compared to that of WT, P0, or L0 mice. In vitro experiments indicated that immune cytotoxic T lymphocytes (CTL) lysed _L. monocytogenes_-infected hepatocytes primarily via a Fas-dependent, perforin-independent mechanism. The absence of Fas severely abrogated the lysis of infected hepatocytes by immune CD8+ CTL. Taken together, these results provide the first evidence for Fas-dependent CTL-mediated lysis of _L. monocytogenes_-infected hepatocytes and demonstrate complementary roles for Fas and perforin in host defenses against an intracellular bacterial pathogen.
Listeria monocytogenes is a gram-positive, facultative intracellular bacterium that is widely used to investigate cell-mediated immunity against intracellular pathogens (28). The propensity to proliferate intracellularly shelters L. monocytogenes from the potential effects of antibodies and dictates a requirement for cell-mediated immune mechanisms during the control and clearance of listeriosis (36). Following intravenous (i.v.) inoculation of mice, L. monocytogenes accumulates rapidly in the liver and spleen, which serve as prominent sites of infection (14, 16, 36). Within these organs, L. monocytogenes is internalized by resident macrophages and parenchymal cells. In the liver, the parenchymal cells (i.e., hepatocytes) serve as the principal site of bacterial replication (14, 16, 47). Recent in vitro and in vivo experiments demonstrated that _L. monocytogenes_-infected hepatocytes are targets of cell-mediated immunity (15, 18, 21). A large variety of cytokines, including interleukin-12, gamma interferon (IFN-γ), and tumor necrosis factor alpha (TNF-α), and a wide range of cell types, such as neutrophils, NK cells, macrophages, and α/β and γ/δ T-cell receptor (TCR)-positive T cells, are involved in the immune response to L. monocytogenes (28).
The role of α/β TCR+ T cells in host defenses against L. monocytogenes has received considerable attention (28). Although both major histocompatibility complex (MHC) class II-restricted CD4+- and MHC class I-restricted CD8+-T-cell subsets play roles in protective immunity, CD8+ T cells appear to be particularly important. Mice depleted of CD8+ T cells but not CD4+ T cells by antibody treatment exhibit an increased susceptibility to both primary and secondary listerial infections (9, 39, 48). Similarly, mice rendered CD8+-T-cell deficient through genetic manipulation are more susceptible to listeriosis than are CD4-deficient mice (29, 30, 46). Conversely, mice adoptively immunized with CD8+ T cells are more resistant to challenge with L. monocytogenes than are mice immunized with CD4+ T cells; CD4+ T cells, however, do confer some degree of protection, which is dependent upon the production of IFN-γ (2, 38, 44).
It has been suggested that a major function of CD8+ T lymphocytes in host defenses against listeriosis is to produce IFN-γ, which promotes the resistance of uninfected cells and/or stimulates the antimicrobial activity of infected cells (5, 28). While the role of IFN-γ in host resistance to listeriosis is well documented, recent evidence indicates that it is not a critical mediator of CD8+-T-cell activity. Monoclonal anti-IFN-γ antibody does not negate the protective immunity conferred upon immunologically naive animals by immune CD8+ T lymphocytes (20). Moreover, mice given immune CD8+ T cells derived from IFN-γ gene knockout mice exhibited the same degree of resistance to listerial infection as did mice adoptively immunized with comparable CD8+-T-cell populations derived from wild-type (WT) animals (19).
Alternatively, it has been proposed that the principal function of CD8+ T lymphocytes in resistance to L. monocytogenes is to lyse infected host cells, which otherwise serve as a protected environment for growth of the organism (5, 10, 19–21). Studies of cell-mediated cytotoxicity (CMC) have defined two TCR-triggered lytic mechanisms at the molecular level (3, 6, 25, 27, 34). The first lytic pathway requires the action of perforin, a secreted protein that aggregates in target cell membranes to form pores, thus allowing effector proteases access to the target cell cytoplasm and resulting in programmed cell death. The second killing mechanism is a nonsecretory, perforin-independent process involving the interaction of Fas ligand (CD95L) expressed by cytolytic effector cells with Fas (CD95) present on the surface of the target cell which also results in programmed cell death. The availability of mice lacking either perforin (P0) (24, 55) or Fas (L0) (7) permits analysis of the roles played by these two lytic pathways in cell-mediated immune responses to intracellular pathogens (23, 24, 55).
Previous studies by other investigators (23) demonstrated impaired resistance of P0 mice to L. monocytogenes replicating in the spleen during primary infection and in the spleen and liver during secondary challenge; P0 mice, however, retained significant resistance to L. monocytogenes and recovered from listeriosis. These findings suggest that additional effector mechanisms could compensate for the absence of perforin-mediated cytotoxicity during the resolution of listerial infections. In the present study, we used P0 mice, L0 mice, and mice lacking both perforin and Fas (P0L0) to examine the potential role of Fas-mediated cytotoxicity in host defenses to listerial infections in vivo and in vitro. The results of in vivo experiments indicate that Fas-dependent CMC complements perforin-dependent CMC and plays a significant role in the control and clearance of L. monocytogenes from the spleens and livers of mice during primary and secondary infections. In vitro analyses demonstrated that lysis of _L. monocytogenes_-infected hepatocytes by immune CD8+ T cells is mediated primarily by Fas. Taken together, these results demonstrate a significant role for the Fas-dependent CMC pathway in immunity to L. monocytogenes.
MATERIALS AND METHODS
Mice.
Perforin−/− mice (P0, H-2b) and WT control mice were generated from altered strain 129 embryonic stem cells injected into C57BL/6 (B6, H-2b) blastocysts as previously described (55). B6, B6-MRL-Fas_lpr/lpr_ (L0, H-2b), MRL, and MRL-Fas.lpr/lpr (L0, H-2b) mice were purchased from Jackson Laboratory (Bar Harbor, Maine). B6-MRL-Fas_lpr/lpr_ mice were crossed with P0 mice to produce perforin−/−, Fas_lpr/lpr_ mice (P0L0). The progeny lpr and perforin genotypes were determined by PCR performed on genomic DNA extracted from tail tissue. Mice were maintained in an ultraclean isolated mouse room in filter-top cages and cared for in accordance with the guidelines set forth by the Institute of Laboratory Animals Resources, National Research Council. Sex-matched, 5- to 10-week-old mice were used for in vivo studies.
Perforin and Fas genotype analysis.
Tail tissue was collected from WT, P0, L0, and P0L0 progeny mice and incubated overnight at 56°C in digestion buffer composed of 20 mM Tris (pH8.0), 0.1 M EDTA, 0.1 M NaCl, 1% sodium dodecyl sulfate, and 0.03% proteinase K. The digestion products were then centrifuged, and the supernatants were sequentially subjected to phenol, phenol-chloroform, and chloroform extractions followed by isopropanol precipitation of the genomic DNA. Purified genomic DNA was then subjected to PCR with two sets of primers which identified perforin and Fas genotypes. The perforin primers used were 5′-CGTGAGAGGTCAGCATCCTTC-3′ (primer P1), 5′-TGGCCTAGGGTTCACATCCAG-3′ (primer P2), and 5′-ATATTGGCTGCAGGGTCGCTC-3′ (primer P3). The Fas primers used were 5′-ACAAACGCAGTCAAATCTGCT-3′ (primer L1), 5′-AGTTTACCATAAGAAAGGTTA-3′ (primer L2), and 5′-TTTAGTAAAAGGTTACAAAAG-3′ (primer L3). PCR analysis with the perforin primers yielded a 500-bp (primers P1 plus P2) product from the WT allele or 1.6-kbp (primers P1 plus P2) and 350-bp (primers P1 plus P3) products from the mutant allele. PCR analysis with the Fas primers yielded a 175-bp (primers L1 plus L3) product from the WT allele or a 400-bp (primers L1 plus L2) product from the Fas_lpr_ allele. P0 × L0 F1 mice which had perforin−/− and Fas_lpr/lpr_ genotypes were used as P0L0 mice.
Bacteria.
The EGD and 10403S strains of L. monocytogenes were cultured, maintained, and stored as previously described (57). Virulence was maintained by periodic passage in mice. For i.v. inoculation or in vitro infection, bacteria from mid-log-phase cultures were washed and diluted with phosphate-buffered saline or tissue culture medium, respectively. Comparable results were obtained in experiments with the EGD and 10403S strains of L. monocytogenes.
Preparation of primary hepatocytes.
Hepatocytes were prepared after perfusion of mouse livers by a two-step technique described previously (21). Briefly, livers perfused with collagenase were teased apart and the resultant cell suspension was centrifuged twice at 30 × g for 4 min at 4°C. The purified cell population obtained was composed of ≥96% hepatocytes as previously reported (21). Hepatocytes were cultured in HEPES-buffered RPMI 1640 medium (BioWhittaker, Walkersville, Md.) supplemented with 1 mM sodium pyruvate, 10−7 M recombinant human insulin (Humulin R; Eli Lilly and Co., Indianapolis, Ind.), and 10% heat-inactivated fetal bovine serum (Sterile Systems, Inc., Logan, Utah).
Preparation of CD8-enriched splenic T cells.
Splenocytes were prepared as described elsewhere (21). Briefly, spleens were dissected from mice infected i.v. with 104 CFU of L. monocytogenes 10 to 12 days previously and single-cell suspensions were prepared in RPMI 1640 (BioWhittaker). Erythrocytes were lysed by NH4Cl treatment, and the resultant erythrocyte-depleted splenocytes were diluted to 2 × 106 viable cells/ml in HEPES-buffered RPMI 1640 with 10% heat-inactivated fetal bovine serum, 1 mM l-glutamine, 5 × 10−5 M 2-mercaptoethanol, 5 μg of gentamicin per ml, and 20 U of recombinant human interleukin-2 (Hoffmann-La Roche, Nutley, N.J.) per ml. The splenocytes were cultured for 2 days at 37°C in humidified 5% CO2. After the incubation, nonadherent cells were harvested and passed over nylon wool columns to obtain T-cell-enriched populations. CD8-enriched T-lymphocyte subpopulations were prepared by two cycles of treatment with antibody and complement as described previously (37). The resultant CD8-enriched T-cell subpopulations were approximately 70% CD8+ (4% CD4+) by cytofluorometric analysis, and adoptive transfer of CD8-enriched splenocytes into naive mice before challenge with L. monocytogenes conferred a 2- to 3-log-unit reduction in bacterial titers in the spleen and liver (data not shown).
Cytolytic assay.
Cytotoxicity directed against hepatocyte target cells was determined as previously described (15, 21). Briefly, hepatocytes derived from B6 (Fas+) or P0L0 (Fas−) mice were seeded into 96-well tissue culture plates (104 cells/well). On the following day, the hepatocyte cultures were inoculated with L. monocytogenes, centrifuged to promote contact between the bacteria and cells, and incubated at 37°C. After 4 h, gentamicin (final concentration, 5 μg/ml) was added to kill extracellular bacteria. B6 (perforin+) or P0 (perforin−) _L. monocytogenes_-immune CD8-enriched T cells at various effector-to-target-cell (E/T) ratios were added 1 to 2 h thereafter, and the cells were cocultured for an additional 16 to 18 h at 37°C. Specific hepatocyte lysis was estimated from the aspartate aminotransferase (AST) activity in the culture supernatants as previously described (11, 21). AST activity was quantified by the Clinical Chemistry Laboratory at the University of Pittsburgh Medical Center. The percent specific cytotoxicity was calculated as [(experimental AST − spontaneous AST)/(total AST − spontaneous AST)] × 100. Total AST activity (150 to 200 IU/liter) was determined by lysing the cells with 0.05% Triton X-100. Supernatants derived from T cells cultured alone had AST activity of less than 3 IU/liter.
CFU reduction assay.
Effector cell lysis of _L. monocytogenes_-infected target cells exposes intracellular L. monocytogenes to extracellular gentamicin, resulting in bacterial-cell death. Viable L. monocytogenes cells remaining at the end of the cytolytic assays were quantified as described previously (21). Briefly, after a 16- to 18-h coculture, the culture supernatants were removed and the remaining hepatocyte monolayer was lysed with 0.05% Triton X-100 in Trypticase soy broth (BBL Microbiology Systems). Serial 10-fold dilutions of each lysate were plated on Trypticase soy agar for quantitation of the number of CFU per well.
Primary infection.
Mice were infected i.v. with 2 × 103 CFU of L. monocytogenes. P0 and P0L0 mice were matched with WT B6 mice; L0 mice were matched with WT MRL mice. Bacterial titers in the spleen and liver were quantified at various times postinfection. Entire organs were removed and homogenized in 1% Triton X-100 in distilled H2O, and serial 10-fold dilutions of organ homogenates were plated on brain heart infusion agar. The number of CFU per organ was determined after incubating the agar plates at 37°C for 24 h. The L. monocytogenes detection limits were 50 CFU per spleen and 100 CFU per liver.
Secondary infection.
Mice were inoculated i.v. with 2 × 103 CFU of L. monocytogenes. Four weeks later, immunized mice and age- and sex-matched nonimmune, control mice were challenged i.v. with 2 × 105 CFU of L. monocytogenes. All mice used in these experiments had B6 backgrounds. Bacterial titers in organs dissected at 60 h postchallenge were determined as described for primary infections. Log10 protection per organ dissected from immunized mice was calculated as (mean log10 CFU/organ from controls) − (log10 CFU/organ from immunized mice). The L. monocytogenes detection limits were 50 CFU per spleen and 100 CFU per liver.
Statistical analysis.
Significant differences between groups were determined with StatView 4.51 software (Abacus Concepts Inc., Berkeley, Calif.). The results of primary infection were compared by analysis of variance followed by post hoc testing with Fisher’s protected least significant difference to identify significant differences between mean CFU per day postinfection. The data from secondary-infection experiments was compared in an unpaired t test.
RESULTS
Absence of perforin and Fas diminishes host defenses against primary listeriosis.
The roles of perforin and Fas in host defenses against L. monocytogenes were examined in vivo with WT, P0, L0, and P0L0 mice. P0 × L0 F1 mice were generated and screened by PCR to determine their perforin and Fas genotypes. Offspring confirmed as having both perforin−/− and Fas_lpr/lpr_ genotypes were used as P0L0 mice. The consequences of cytolytic pathway deficiencies on the course of primary listeriosis were determined by measuring bacterial titers in the spleens and livers of mice inoculated i.v. with a sublethal dose of L. monocytogenes. Figure 1A and B demonstrate the effect of a lack of perforin on the course of listeriosis. Bacterial loads in the spleens of WT and P0 mice were similar during the first 3 days postinfection. However, L. monocytogenes titers in the spleens of P0 mice were 4- to 17-fold higher than in WT mice between days 5 and 14 postinfection and bacterial clearance was significantly delayed (P < 0.02). At 24 h postinfection, mean titers in livers of P0 mice had increased eightfold compared to those in livers of WT mice; by day 5, both WT and P0 mice had cleared the bacteria from their livers. These results are consistent with previous studies (23) and suggest that perforin-dependent CMC plays a significant role in host resistance to L. monocytogenes in the spleen. The ability of P0 mice to eliminate L. monocytogenes from their organs, however, indicates that perforin-independent mechanisms are also operative.
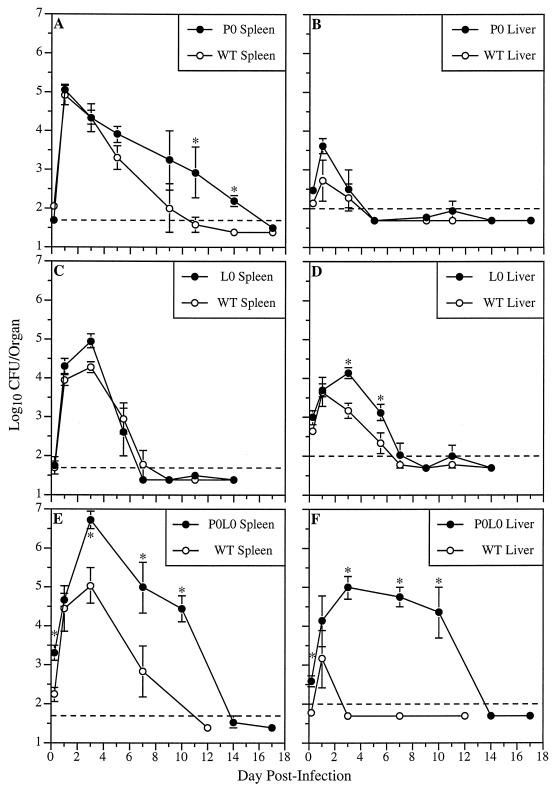
FIG. 1.
L. monocytogenes titers in the spleens and livers of WT, P0, L0, and P0L0 mice during primary infection. Mice were inoculated i.v. with 2 × 103 CFU of L. monocytogenes, and bacterial titers in their spleens (A, C, and E) and livers (B, D, and F) were determined at various times postinfection. Dashed lines indicate the limits of detection (50 CFU/spleen and 100 CFU/liver). Data represent the mean log10 CFU per organ ± standard error of the mean (SEM) derived from three mice per time point. Asterisks denote significant increases in mean L. monocytogenes titers compared with the relevant WT controls (P < 0.05).
Figure 1C and D demonstrate the effect of Fas deficiency on the course of primary L. monocytogenes infection. Between 3 and 5 days postinfection, bacterial titers in the livers of L0 mice were six- to ninefold greater than those in the livers of WT mice. L. monocytogenes titers were slightly higher in the spleens of L0 mice on day 3 postinfection. Lack of Fas did not affect the rate of bacterial clearance from either the spleen or the liver. These results suggest that a Fas-dependent mechanism may complement the activity of perforin-mediated protection and plays a significant role in host resistance to primary L. monocytogenes infections of the liver.
The complementary roles of Fas and perforin in host defenses against primary listeriosis were examined in P0L0 mice (Fig. 1E and F). Compared to WT mice at 4 h postinfection, P0L0 mice had significantly higher bacterial titers in their spleens and livers. Between days 3 and 10 postinfection, the spleens of P0L0 mice exhibited a 50- to 560-fold increase in L. monocytogenes titers over WT levels and also showed a significant delay in bacterial clearance (P < 0.02). Moreover, in contrast to the rapid clearance of L. monocytogenes from the livers of WT animals, bacterial titers in the livers of P0L0 mice remained between 104 and 105 total CFU through day 10 postinfection. Bacterial titers in the liver reached levels below the limit of detection by day 14 postinfection. Taken together, the results of primary-infection experiments indicate that (i) lack of perforin delays the clearance of L. monocytogenes from the spleen; (ii) a deficiency in Fas permits greater replication of L. monocytogenes in the liver; and (iii) absence of both perforin- and Fas-dependent CMC pathways drastically impairs control and elimination of L. monocytogenes from both the spleen and liver.
The absence of both perforin and Fas inhibits immunity to secondary listeriosis.
We then examined the resistance of immunized mice to secondary listerial challenge 4 weeks after primary infection. Nonimmune WT, L0, P0, and P0L0 mice inoculated with a lethal dose of L. monocytogenes had similar titers of bacteria in their spleens and livers at 60 h postinfection (Fig. 2). Relative to these controls, similarly challenged immune WT, L0, and P0 mice exhibited significant reductions in the titers of bacteria recovered from their spleens and livers. In contrast, P0L0 mice undergoing secondary infection maintained high bacterial titers in their spleens and livers. Table 1 lists the antilisterial resistance expressed by immunized WT, L0, P0, and P0L0 mice during secondary challenge. P0L0 mice exhibited significantly less protection against secondary infection than did mice deficient in either Fas or perforin alone. Immune L0 and P0 mice expressed similar levels of protection in the liver to those in WT mice, while L0 mice maintained equivalent protection in the spleen. These results indicate that both Fas- and perforin-dependent CMC pathways play significant and complementary roles in protective immunity to secondary listeriosis.
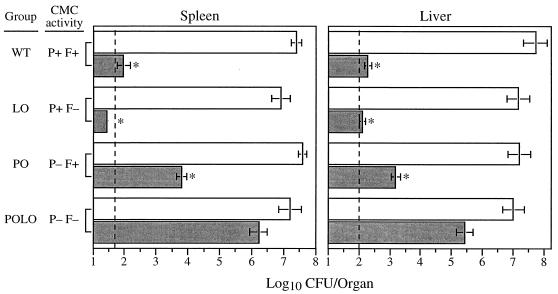
FIG. 2.
Resistance to secondary L. monocytogenes infection of WT, L0, P0, and P0L0 mice. Mice were inoculated i.v. with 2 × 103 CFU of L. monocytogenes. Four weeks later, nonimmune and immunized mice were challenged i.v. with 2 × 105 organisms. Values represent the mean log10 CFU ± SEM in spleens and livers determined at 60 h postchallenge. Data were combined from four independent experiments. CMC activity refers to that mediated by perforin (P) or Fas (F). Dashed lines indicate the limits of detection (50 CFU/spleen and 100 CFU/liver). Asterisks indicate a significant reduction in mean titers per organ compared to nonimmune controls (P < 0.01).
TABLE 1.
Anti-L. monocytogenes protection upon secondary challenge of WT, L0, P0, and P0L0 mice
| Immune group (no. of mice) | CMC activitye | Log10 protection/organa | |
|---|---|---|---|
| Spleen | Liver | ||
| WT (13) | P+ F+ | 5.32 ± 0.21 | 5.16 ± 0.26 |
| L0 (8) | P+ F− | 5.31 ± 0.23 | 4.94 ± 0.27 |
| P0 (13) | P− F+ | 3.80 ± 0.16b | 4.03 ± 0.29c |
| P0L0 (6) | P− F− | 0.99 ± 0.28d | 1.57 ± 0.54d |
Perforin-independent lysis of _L. monocytogenes_-infected hepatocytes in vitro.
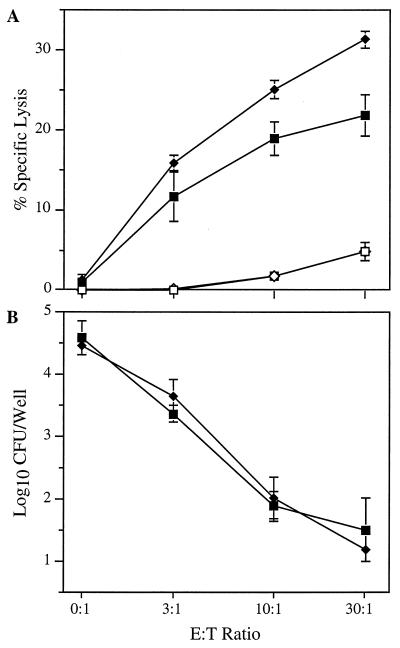
_L. monocytogenes_-infected hepatocytes are targets of cell-mediated immunity in vivo and in vitro (18, 21). The significant increase in the replication of L. monocytogenes in the livers of L0 and P0L0 mice during primary infection suggests that the absence of Fas expression by hepatocytes may impair the biological response of cytolytic effector cells. The potential roles of perforin expressed by immune CD8+ T cells and Fas exhibited on the surface of target cells were further investigated in vitro by using _Listeria_-infected primary hepatocyte cultures in anti-Listeria cytotoxic T-lymphocyte (CTL) assays. Immune CD8-enriched T cells derived from either WT or P0 mice were cocultured with hepatocytes derived from WT mice. Specific lysis of hepatocytes was estimated from the AST activity released into the culture supernatant. Immune CD8-enriched T cells derived from P0 and WT mice lysed _Listeria_-infected hepatocytes equally well (Fig. 3A). Significant lysis did not occur in cocultures that contained uninfected hepatocytes. Thus, lysis of _Listeria_-infected hepatocytes cocultured with immune CD8+ T cells occurred in a perforin-independent manner.
FIG. 3.
Perforin-independent lysis of _L. monocytogenes_-infected hepatocytes by immune CD8+ T lymphocytes in vitro. Immune, CD8-enriched T lymphocytes derived from B6 (squares) or P0 (diamonds) mice were cocultured with _L. monocytogenes_-infected (solid symbols) or uninfected (open symbols) hepatocytes from B6 mice for 18 h in the presence of gentamicin. (A) The percent specific lysis of _L. monocytogenes_-infected or uninfected target cells was estimated from the AST activity released into the culture supernatant. (B) The number of surviving intracellular L. monocytogenes organisms per well was calculated from the number of CFU recovered from hepatocyte monolayers at the end of the incubation period. Data are the means ± standard deviations of quadruplicate determinations and are representative of four experiments.
Gentamicin added to the culture medium kills intracellular L. monocytogenes exposed to the extracellular environment. Quantitation of CFU per well remaining at the end of the incubation period provides an alternate means of assessing the effector cell activity against _Listeria_-infected target cells. Specific lysis of infected hepatocytes inversely correlated with a reduction in the number of viable intracellular L. monocytogenes organisms recovered from the wells (Fig. 3). A >99% reduction of the intracellular L. monocytogenes at the highest E/T ratio suggests that essentially all of the hepatocytes infected with L. monocytogenes were lysed during the assay period regardless of whether the effector T cells were from WT or P0 mice.
Absence of Fas severely abrogates lysis of _L. monocytogenes_-infected hepatocytes in vitro.
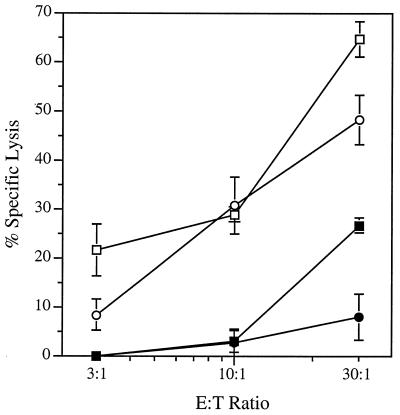
Cultured mouse hepatocytes undergo apoptosis rapidly after the addition of anti-Fas antibody (41). Intraperitoneal injection of anti-Fas antibody into WT mice but not into Fas-deficient mice induced rapid apoptosis of hepatocytes, massive liver destruction, and sudden death (43). To determine the role of Fas in the perforin-independent lysis of _Listeria_-infected hepatocytes by immune CD8+ T cells in vitro, additional cytolytic assays were performed with hepatocytes derived from WT (Fas+) and P0L0 (Fas−) mice (Fig. 4). In agreement with the data shown in Fig. 3A, lysis of _Listeria_-infected Fas+ hepatocytes by WT and by perforin-deficient CD8+ T cells was comparable over the range of E/T ratios tested. The absence of Fas expression by _Listeria_-infected hepatocytes derived from P0L0 mice, however, severely impaired the specific cytolytic activity exhibited by both effector T-cell populations. The activity exhibited by WT effector T cells cocultured with _Listeria_-infected Fas− hepatocytes at a 30:1 ratio was reduced 60% relative to the activity expressed in cocultures that contained _Listeria_-infected Fas+ hepatocytes. Lysis of _Listeria_-infected Fas− hepatocytes by immune, perforin-deficient CD8+ T cells was comparable to the background lysis of uninfected hepatocytes (data not shown). The results of these experiments indicate that Fas- and perforin-dependent pathways play major and minor roles, respectively, in the lysis of _Listeria_-infected hepatocytes by immune CD8+ T cells in vitro.
FIG. 4.
Fas-dependent lysis of _L. monocytogenes_-infected hepatocytes by _L. monocytogenes_-immune CD8+ T cells in vitro. Immune, CD8-enriched T cells derived from B6 (squares) or P0 (circles) mice were cocultured for 18 h with _L. monocytogenes_-infected hepatocytes from B6 (open symbols) or P0L0 (solid symbols) mice. Lytic activity was estimated from the AST activity detected in the medium. Data are the means ± SEM of quadruplicate determinations; a second experiment yielded similar results.
DISCUSSION
CTL lyse target cells via two independent effector pathways that rely upon either perforin or Fas (22, 27, 31, 34, 50). Because of the potential complementary nature of these pathways, the specific contribution of either pathway to cell-mediated immunity has been difficult to assess. Kägi et al. (23) demonstrated the importance of perforin in host resistance to L. monocytogenes. In experiments with P0 mice, they found that perforin plays a significant role in the protection expressed in the spleen during primary and secondary infections and in the protection conferred upon the spleen by adoptive transfer of immune CD8+ T cells. In each case, however, the absence of perforin had a relatively minor effect on the course of listerial infections in the liver or on the overall susceptibility of P0 mice to L. monocytogenes. The specific effector mechanisms that contributed to the continued resistance of these mice remained to be delineated.
Fas (CD95) is a single, 43-kDa transmembrane protein belonging to the tumor necrosis factor/nerve growth factor receptor superfamily (42, 58). Activated CTL expressing cell surface Fas ligand (CD95L) can induce apoptosis in target cells expressing Fas (51). The overwhelming evidence gathered to date demonstrates the role of Fas-dependent cytotoxicity in controlling T-cell development and the homeostatic regulation of peripheral immune responses (26, 35, 49). Indeed, a recent study suggests that Fas is involved in T-cell death and in termination of the antigen-specific response of T lymphocytes to L. monocytogenes after the resolution of infection (13). Although some experimental evidence supports a protective role for Fas-dependent cytotoxicity during viral infection (1, 33, 40, 53), most of the evidence has not demonstrated a role in immunity to intracellular pathogens, immunopathology, or transplant rejection (4, 12, 26, 32, 45, 54, 56). This may be due to the complementary nature of cytotoxic pathways which remain active in mice deficient in a single cytolytic mechanism.
To examine the potential role of Fas in host defenses against primary and secondary L. monocytogenes infections, we generated mice lacking both Fas and perforin and compared them to perforin knockout, Fas-deficient, and WT mice. The results of our study confirm those of Kägi et al. (23) demonstrating the function of perforin in primary and secondary host defenses against L. monocytogenes, especially in the spleen. In addition, our results demonstrate a significant role for Fas in host resistance to both primary and secondary listerial infections, complementing the perforin-mediated response to L. monocytogenes in WT mice and compensating for the lack of perforin in P0 mice. The critical role of Fas was particularly evident in comparison of the resolution of infections in P0L0 mice, deficient in both perforin and Fas, to that in P0 mice, which retained Fas-mediated cytolytic activity. P0L0 mice were much more susceptible to infection, exhibiting a marked elevation in the bacterial burden and an extended delay in clearance of L. monocytogenes from the spleen and liver. The failure of previous investigators (13) to detect the increased susceptibility of Fas-deficient mice to primary listerial infections undoubtedly reflects the complementary nature of the Fas- and perforin-mediated cytolytic pathways in host defenses against intracellular L. monocytogenes.
Recently, Harty and Bevan reported evidence suggesting that both hepatocytes and macrophages infected with L. monocytogenes were targets of CD8+-T-cell-mediated cytotoxicity in vivo (18). Their findings correlate with our in vitro experiments demonstrating the classical MHC class I-restricted lysis of _L. monocytogenes_-infected hepatocytes by immune CD8+ T cells in culture (21). Our results showing significant increases in the proliferation of L. monocytogenes in the livers of L0 and P0L0 mice suggest that Fas plays an important role in the lysis of infected hepatocytes by _L. monocytogenes_-specific T cells. This hypothesis is supported by in vitro experiments demonstrating the critical function of Fas in the lysis of _L. monocytogenes_-infected hepatocytes by immune CD8+ T cells (Fig. 4). _Listeria_-specific effector cells generated from WT and P0 mice were comparable in their ability to lyse _L. monocytogenes_-infected hepatocytes. Target cell death induced by either P0 or WT CD8+ T cells was greatly reduced, however, when the cells were cocultured with _L. monocytogenes_-infected hepatocytes derived from P0L0 (i.e., Fas−) mice. The relative importance of Fas in CTL-mediated lysis of infected hepatocytes may be due, in part, to the high level of Fas normally expressed on the cell surface (41, 43). The lytic activity retained by WT, immune CD8+ T cells cocultured with _Listeria_-infected, Fas− hepatocytes (i.e., at a 30:1 E/T ratio) suggests that perforin-dependent lysis of infected hepatocytes also has a function, albeit a subordinate one, in protective immunity against L. monocytogenes expressed within the liver.
The eventual clearance of L. monocytogenes primary infection and the remaining minimal protection against secondary challenge of P0L0 mice indicates that cytolytic mechanisms mediated by perforin and Fas are not absolute prerequisites for the resolution of infection; rather, additional effector mechanisms must also be operative. In this regard, several recent studies suggest that cytokines critical for host defenses against L. monocytogenes, e.g., IFN-γ and TNF-α, may contribute to the elimination of bacteria by acting directly on infected cell populations. Indeed, we previously reported that the replication of L. monocytogenes within hepatocytes was diminished in mice inoculated with recombinant murine IFN-γ (17). Infected animals given monoclonal anti-IFN-γ, on the other hand, exhibited a marked increase in hepatocyte-associated L. monocytogenes. These results are supported by in vitro experiments in which IFN-γ treatment restricted the replication of L. monocytogenes in cultures of freshly isolated mouse hepatocytes (17) and the purported mouse hepatocyte cell line TIB-75 (52). More recently, White and Harty reported adoptive transfer experiments in which TNF-α played a critical role in the protective immunity conferred by an _L. monocytogenes_-specific CD8+-T-cell line (56). Thus, in addition to lysing infected target cells, CD8+ T cells may influence the titers of listeriae within infected organs by elaborating cytokines such as IFN-γ and TNF-α.
Until now, evidence for the role of Fas-dependent cytotoxicity in host defenses against intracellular bacterial pathogens has been lacking. In fact, recent reports suggest that neither Fas nor perforin is a critical factor in protective immunity to Mycobacterium tuberculosis infections in animal models (8, 32). However, a comparison of the anti-listerial responses exhibited by mice deficient in perforin, Fas, or both perforin and Fas implicates Fas in the immune response to primary and secondary listerial infections of the spleen and liver. The results of this comparison suggest that Fas-mediated lysis of infected cells complements the perforin-dependent pathway of cytotoxicity in the control and clearance of L. monocytogenes. Most probably, the difference between our results and those reported by investigators studying M. tuberculosis (8, 32) reflects, in part, the cell type and intracellular location of the organisms, i.e., the phagocytic vacuoles of macrophages versus the cytoplasm of hepatocytes for M. tuberculosis and L. monocytogenes, respectively. In light of the experiments reported here, it now appears that the biological functions of Fas include providing a perforin-independent mechanism for lysing target cells infected with an intracellular bacterial pathogen. Based on the results of these experiments, we speculate that Fas may play an important role in host defenses against other intracellular pathogens, particularly those infecting the liver.
ACKNOWLEDGMENTS
We extend our grateful appreciation to Chau-Ching Liu for her critical review of the manuscript and acknowledge Athanasia Sagnimeni for her excellent technical assistance.
This research was supported by National Institutes of Health grants to S.H.G. (DK44367), W.R.C. (AI32512-03), and J.F.M. (AI38955); an American Cancer Society grant to J.F.M. (IM-791), and training grants to E.R.J. (T32-AI07323 and T32-AI07126).
REFERENCES
- 1.Ando K, Hiroishi K, Kaneko T, Moriyama T, Muto Y, Kayagaki N, Yagita H, Okumura K, Imawari M. Perforin, Fas/Fas ligand, and TNF-alpha pathways as specific and bystander killing mechanisms of hepatitis C virus-specific human CTL. J Immunol. 1997;158:5283–5291. [PubMed] [Google Scholar]
- 2.Baldridge J R, Barry R A, Hinrichs D J. Expression of systemic protection and delayed-type hypersensitivity to Listeria monocytogenes is mediated by different T-cell subsets. Infect Immun. 1990;58:654–658. doi: 10.1128/iai.58.3.654-658.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berke G. The binding and lysis of target cells by cytotoxic lymphocytes: molecular and cellular aspects. Annu Rev Immunol. 1994;12:735–773. doi: 10.1146/annurev.iy.12.040194.003511. [DOI] [PubMed] [Google Scholar]
- 4.Braun M Y, Lowin B, French L, Acha-Orbea H, Tschopp J. Cytotoxic T cells deficient in both functional fas ligand and perforin show residual cytolytic activity yet lose their capacity to induce lethal acute graft-versus-host disease. J Exp Med. 1996;183:657–661. doi: 10.1084/jem.183.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunt L M, Portnoy D A, Unanue E R. Presentation of Listeria monocytogenes to CD8+ T cells requires secretion of hemolysin and intracellular bacterial growth. J Immunol. 1990;145:3540–3546. [PubMed] [Google Scholar]
- 6.Clark W R, Walsh C M, Glass A A, Hayashi F, Matloubian M, Ahmed R. Molecular pathways of CTL-mediated cytotoxicity. Immunol Rev. 1995;146:33–44. doi: 10.1111/j.1600-065x.1995.tb00682.x. [DOI] [PubMed] [Google Scholar]
- 7.Cohen P L, Eisenberg R A. Lpr and gld: single gene models of systemic autoimmunity and lymphoproliferative disease. Annu Rev Immunol. 1991;9:243–269. doi: 10.1146/annurev.iy.09.040191.001331. [DOI] [PubMed] [Google Scholar]
- 8.Cooper A M, D’Souza C, Frank A A, Orme I M. The course of Mycobacterium tuberculosis infection in the lungs of mice lacking expression of either perforin- or granzyme-mediated cytolytic mechanisms. Infect Immun. 1997;65:1317–1320. doi: 10.1128/iai.65.4.1317-1320.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Czuprynski C J, Brown J F. Effects of purified anti-Lyt-2 mAb treatment on murine listeriosis: comparative roles of Lyt-2+ and L3T4+ cells in resistance to primary and secondary infection, delayed-type hypersensitivity and adoptive transfer of resistance. Immunology. 1990;71:107–112. [PMC free article] [PubMed] [Google Scholar]
- 10.De Libero G, Kaufmann S H. Antigen-specific Lyt-2+ cytolytic T lymphocytes from mice infected with the intracellular bacterium Listeria monocytogenes. J Immunol. 1986;137:2688–2694. [PubMed] [Google Scholar]
- 11.Feutren G, Lacour B, Bach J F. Immune lysis of hepatocytes in culture: accurate detection by aspartate aminotransferase release measurement. J Immunol Methods. 1984;75:85–94. doi: 10.1016/0022-1759(84)90227-8. [DOI] [PubMed] [Google Scholar]
- 12.Franco M A, Tin C, Rott L S, VanCott J L, McGhee J R, Greenberg H B. Evidence for CD8+ T-cell immunity to murine rotavirus in the absence of perforin, fas, and gamma interferon. J Virol. 1997;71:479–486. doi: 10.1128/jvi.71.1.479-486.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuse Y, Nishimura H, Maeda K, Yoshikai Y. CD95 (Fas) may control the expansion of activated T cells after elimination of bacteria in murine listeriosis. Infect Immun. 1997;65:1883–1891. doi: 10.1128/iai.65.5.1883-1891.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gregory S H, Barczynski L K, Wing E J. Effector function of hepatocytes and Kupffer cells in the resolution of systemic bacterial infections. J Leukocyte Biol. 1992;51:421–424. doi: 10.1002/jlb.51.4.421. [DOI] [PubMed] [Google Scholar]
- 15.Gregory S H, Jiang X, Wing E J. Lymphokine-activated killer cells lyse Listeria-infected hepatocytes and produce elevated quantities of interferon-gamma. J Infect Dis. 1996;174:1073–1079. doi: 10.1093/infdis/174.5.1073. [DOI] [PubMed] [Google Scholar]
- 16.Gregory S H, Sagnimeni A J, Wing E J. Bacteria in the bloodstream are trapped in the liver and killed by immigrating neutrophils. J Immunol. 1996;157:2514–2520. [PubMed] [Google Scholar]
- 17.Gregory S H, Wing E J. IFN-gamma inhibits the replication of Listeria monocytogenes in hepatocytes. J Immunol. 1993;151:1401–1409. [PubMed] [Google Scholar]
- 18.Harty J T, Bevan M J. CD8 T-cell recognition of macrophages and hepatocytes results in immunity to Listeria monocytogenes. Infect Immun. 1996;64:3632–3640. doi: 10.1128/iai.64.9.3632-3640.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harty J T, Bevan M J. Specific immunity to Listeria monocytogenes in the absence of IFN gamma. Immunity. 1995;3:109–117. doi: 10.1016/1074-7613(95)90163-9. [DOI] [PubMed] [Google Scholar]
- 20.Harty J T, Schreiber R D, Bevan M J. CD8 T cells can protect against an intracellular bacterium in an interferon gamma-independent fashion. Proc Natl Acad Sci USA. 1992;89:11612–11616. doi: 10.1073/pnas.89.23.11612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang X, Gregory S H, Wing E J. Immune CD8+ T lymphocytes lyse Listeria monocytogenes-infected hepatocytes by a classical MHC class I-restricted mechanism. J Immunol. 1997;158:287–293. [PubMed] [Google Scholar]
- 22.Ju S T, Cui H, Panka D J, Ettinger R, Marshak-Rothstein A. Participation of target Fas protein in apoptosis pathway induced by CD4+ Th1 and CD8+ cytotoxic T cells. Proc Natl Acad Sci USA. 1994;91:4185–4189. doi: 10.1073/pnas.91.10.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kägi D, Ledermann B, Burki K, Hengartner H, Zinkernagel R M. CD8+ T cell-mediated protection against an intracellular bacterium by perforin-dependent cytotoxicity. Eur J Immunol. 1994;24:3068–3072. doi: 10.1002/eji.1830241223. [DOI] [PubMed] [Google Scholar]
- 24.Kägi D, Ledermann B, Burki K, Seiler P, Odermatt B, Olsen K J, Podack E R, Zinkernagel R M, Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- 25.Kägi D, Ledermann B, Burki K, Zinkernagel R M, Hengartner H. Molecular mechanisms of lymphocyte-mediated cytotoxicity and their role in immunological protection and pathogenesis in vivo. Annu Rev Immunol. 1996;14:207–232. doi: 10.1146/annurev.immunol.14.1.207. [DOI] [PubMed] [Google Scholar]
- 26.Kägi D, Seiler P, Pavlovic J, Ledermann B, Burki K, Zinkernagel R M, Hengartner H. The roles of perforin- and Fas-dependent cytotoxicity in protection against cytopathic and noncytopathic viruses. Eur J Immunol. 1995;25:3256–3262. doi: 10.1002/eji.1830251209. [DOI] [PubMed] [Google Scholar]
- 27.Kägi D, Vignaux F, Ledermann B, Burki K, Depraetere V, Nagata S, Hengartner H, Golstein P. Fas and perforin pathways as major mechanisms of T cell-mediated cytotoxicity. Science. 1994;265:528–530. doi: 10.1126/science.7518614. [DOI] [PubMed] [Google Scholar]
- 28.Kaufmann S H. Immunity to intracellular bacteria. Annu Rev Immunol. 1993;11:129–163. doi: 10.1146/annurev.iy.11.040193.001021. [DOI] [PubMed] [Google Scholar]
- 29.Kaufmann S H, Ladel C H. Role of T cell subsets in immunity against intracellular bacteria: experimental infections of knock-out mice with Listeria monocytogenes and Mycobacterium bovis BCG. Immunobiology. 1994;191:509–519. doi: 10.1016/S0171-2985(11)80457-2. [DOI] [PubMed] [Google Scholar]
- 30.Ladel C H, Flesch I E, Arnoldi J, Kaufmann S H. Studies with MHC-deficient knock-out mice reveal impact of both MHC I- and MHC II-dependent T cell responses on Listeria monocytogenes infection. J Immunol. 1994;153:3116–3122. [PubMed] [Google Scholar]
- 31.Lancki D W, Hsieh C S, Fitch F W. Mechanisms of lysis by cytotoxic T lymphocyte clones. Lytic activity and gene expression in cloned antigen-specific CD4+ and CD8+ T lymphocytes. J Immunol. 1991;146:3242–3249. [PubMed] [Google Scholar]
- 32.Laochumroonvorapong P, Wang J, Liu C C, Ye W, Moreira A L, Elkon K B, Freedman V H, Kaplan G. Perforin, a cytotoxic molecule which mediates cell necrosis, is not required for the early control of mycobacterial infection in mice. Infect Immun. 1997;65:127–132. doi: 10.1128/iai.65.1.127-132.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin M T, Stohlman S A, Hinton D R. Mouse hepatitis virus is cleared from the central nervous systems of mice lacking perforin-mediated cytolysis. J Virol. 1997;71:383–391. doi: 10.1128/jvi.71.1.383-391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lowin B, Hahne M, Mattmann C, Tschopp J. Cytolytic T-cell cytotoxicity is mediated through perforin and Fas lytic pathways. Nature. 1994;370:650–652. doi: 10.1038/370650a0. [DOI] [PubMed] [Google Scholar]
- 35.Lynch D H, Ramsdell F, Alderson M R. Fas and FasL in the homeostatic regulation of immune responses. Immunol Today. 1995;16:569–574. doi: 10.1016/0167-5699(95)80079-4. [DOI] [PubMed] [Google Scholar]
- 36.Mackaness M B. Cellular resistance to infection. J Exp Med. 1962;116:381–406. [PubMed] [Google Scholar]
- 37.Magee D M, Wing E J. Antigen-specific production of colony-stimulating factors by Listeria monocytogenes-immune, L3T4-positive cells. J Infect Dis. 1988;157:941–949. doi: 10.1093/infdis/157.5.941. [DOI] [PubMed] [Google Scholar]
- 38.Magee D M, Wing E J. Cloned L3T4+ T lymphocytes protect mice against Listeria monocytogenes by secreting IFN-gamma. J Immunol. 1988;141:3203–3207. [PubMed] [Google Scholar]
- 39.Mielke M E, Ehlers S, Hahn H. T cell subsets in DTH, protection and granuloma formation in primary and secondary Listeria infection in mice: superior role of Lyt-2+ cells in acquired immunity. Immunol Lett. 1988;19:211–215. doi: 10.1016/0165-2478(88)90144-7. [DOI] [PubMed] [Google Scholar]
- 40.Nakamoto Y, Guidotti L G, Pasquetto V, Schreiber R D, Chisari F V. Differential target cell sensitivity to CTL-activated death pathways in hepatitis B virus transgenic mice. J Immunol. 1997;158:5692–5697. [PubMed] [Google Scholar]
- 41.Ni R, Tomita Y, Matsuda K, Ichihara A, Ishimura K, Ogasawara J, Nagata S. Fas-mediated apoptosis in primary cultured mouse hepatocytes. Exp Cell Res. 1994;215:332–337. doi: 10.1006/excr.1994.1349. [DOI] [PubMed] [Google Scholar]
- 42.Oehm A, Behrmann I, Falk W, Pawlita M, Maier G, Klas C, Li-Weber M, Richards S, Dhein J, Trauth B C, Ponsting H, Krammer P H. Purification and molecular cloning of the APO-1 cell surface antigen, a member of the tumor necrosis factor/nerve growth factor receptor superfamily. Sequence identity with the Fas antigen. J Biol Chem. 1992;267:10709–10715. [PubMed] [Google Scholar]
- 43.Ogasawara J, Watanabe-Fukunaga R, Adachi M, Matsuzawa A, Kasugai T, Kitamura Y, Itoh N, Suda T, Nagata S. Lethal effect of the anti-Fas antibody in mice. Nature. 1993;364:806–809. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- 44.Rakhmilevich A L. Evidence for a significant role of CD4+ T cells in adoptive immunity to Listeria monocytogenes in the liver. Immunology. 1994;82:249–254. [PMC free article] [PubMed] [Google Scholar]
- 45.Renggli J, Hahne M, Matile H, Betschart B, Tschopp J, Corradin G. Elimination of P. berghei liver stages is independent of Fas (CD95/Apo-I) or perforin-mediated cytotoxicity. Parasite Immunol. 1997;19:145–148. doi: 10.1046/j.1365-3024.1997.d01-190.x. [DOI] [PubMed] [Google Scholar]
- 46.Roberts A D, Ordway D J, Orme I M. Listeria monocytogenes infection in beta-2 microglobulin-deficient mice. Infect Immun. 1993;61:1113–1116. doi: 10.1128/iai.61.3.1113-1116.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosen H, Gordon S, North R J. Exacerbation of murine listeriosis by a monoclonal antibody specific for the type 3 complement receptor of myelomonocytic cells. Absence of monocytes at infective foci allows Listeria to multiply in nonphagocytic cells. J Exp Med. 1989;170:27–37. doi: 10.1084/jem.170.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sasaki T, Mieno M, Udono H, Yamaguchi K, Usui T, Hara K, Shiku H, Nakayama E. Roles of CD4+ and CD8+ cells, and the effect of administration of recombinant murine interferon gamma in listerial infection. J Exp Med. 1990;171:1141–1154. doi: 10.1084/jem.171.4.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Singer G G, Abbas A K. The fas antigen is involved in peripheral but not thymic deletion of T lymphocytes in T cell receptor transgenic mice. Immunity. 1994;1:365–371. doi: 10.1016/1074-7613(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 50.Stalder T, Hahn S, Erb P. Fas antigen is the major target molecule for CD4+ T cell-mediated cytotoxicity. J Immunol. 1994;152:1127–1133. [PubMed] [Google Scholar]
- 51.Suda T, Takahashi T, Golstein P, Nagata S. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell. 1993;75:1169–1178. doi: 10.1016/0092-8674(93)90326-l. [DOI] [PubMed] [Google Scholar]
- 52.Szalay G, Hess J, Kaufmann S H. Restricted replication of Listeria monocytogenes in a gamma interferon-activated murine hepatocyte line. Infect Immun. 1995;63:3187–3195. doi: 10.1128/iai.63.8.3187-3195.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Topham D J, Tripp R A, Doherty P C. CD8+ T cells clear influenza virus by perforin or Fas-dependent processes. J Immunol. 1997;159:5197–5200. [PubMed] [Google Scholar]
- 54.Walsh C M, Hayashi F, Saffran D C, Ju S T, Berke G, Clark W R. Cell-mediated cytotoxicity results from, but may not be critical for, primary allograft rejection. J Immunol. 1996;156:1436–1441. [PubMed] [Google Scholar]
- 55.Walsh C M, Matloubian M, Liu C C, Ueda R, Kurahara C G, Christensen J L, Huang M T, Young J D, Ahmed R, Clark W R. Immune function in mice lacking the perforin gene. Proc Natl Acad Sci USA. 1994;91:10854–10858. doi: 10.1073/pnas.91.23.10854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.White D W, Harty J T. Perforin-deficient CD8+ T cells provide immunity to Listeria monocytogenes by a mechanism that is independent of CD95 and IFN-gamma but requires TNF-alpha. J Immunol. 1998;160:898–905. [PubMed] [Google Scholar]
- 57.Wing E J, Waheed A, Shadduck R K. Changes in serum colony-stimulating factor and monocytic progenitor cells during Listeria monocytogenes infection in mice. Infect Immun. 1984;45:180–184. doi: 10.1128/iai.45.1.180-184.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yonehara S, Ishii A, Yonehara M. A cell-killing monoclonal antibody (anti-Fas) to a cell surface antigen co-downregulated with the receptor of tumor necrosis factor. J Exp Med. 1989;169:1747–1756. doi: 10.1084/jem.169.5.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]