Staphylococcus aureus Serotype 5 Capsular Polysaccharide Is Antiphagocytic and Enhances Bacterial Virulence in a Murine Bacteremia Model (original) (raw)
Abstract
Controversy persists over the role that the capsular polysaccharide plays in the pathogenesis of Staphylococcus aureus infections. To address this issue, we compared the mouse virulence of S. aureus Reynolds and capsule-defective mutant strains cultivated under conditions of high or low capsule expression. Strain Reynolds cells cultivated on Columbia salt agar plates expressed ∼100-fold more type 5 capsular polysaccharide than did cells cultivated in Columbia salt broth. The relative virulence of strain Reynolds and its capsule-defective mutants after growth on either solid or liquid medium was examined in mice challenged intraperitoneally or intravenously. The results indicated that agar-grown Reynolds cells were cleared from the bloodstream of mice less readily than broth-grown Reynolds cells. When the parental and mutant strains were cultivated on solid medium, strain Reynolds sustained a higher level of bacteremia than did the capsular mutants. We performed in vitro opsonophagocytic killing assays to determine whether staphylococcal virulence for mice correlated with resistance to phagocytosis. S. aureus Reynolds cultivated on solid medium was susceptible to phagocytic killing only in the presence of specific capsular antibodies and complement. Strain Reynolds grown in broth showed opsonic requirements for phagocytic killing that were similar to those of the capsular mutants (grown in broth or on agar); i.e., the bacteria were opsonized for phagocytosis by nonimmune serum with complement activity. These studies indicate that optimal expression of capsule enhances bacterial virulence in the mouse model of bacteremia, probably by rendering the organisms resistant to opsonophagocytic killing by leukocytes.
Capsular polysaccharides are produced by ∼90% of Staphylococcus aureus strains. Although 11 capsular serotypes have been described, most isolates of S. aureus belong to capsule types 5 or 8 (4, 13, 15, 22). Previous studies from our laboratory utilized Tn_918_ mutagenesis of strain Reynolds to isolate mutants that were altered in type 5 capsular polysaccharide (CP5) expression (2). Initial results comparing the virulence of wild-type strains and that of mutant strains revealed that the capsule did not enhance staphylococcal virulence in several animal models of infection (2, 5). In addition, we reported that broth-grown S. aureus strains expressing serotype 5 or 8 capsules did not resist opsonophagocytic killing by human polymorphonuclear leukocytes (29). In contrast, Karakawa et al. (14) reported that microcapsules elaborated by type 5 and 8 S. aureus strains were antiphagocytic. Nilsson et al. (19) recently showed that mice inoculated with S. aureus expressing CP5 had a higher frequency of arthritis and a more severe form of the disease than animals inoculated with nonencapsulated mutant strains. Furthermore, macrophages were able to ingest and kill nonencapsulated S. aureus mutants to a greater degree than the parental strain could. Thus, the role of the capsule in staphylococcal virulence seems to be dependent on the particular assay or animal model of infection tested.
S. aureus serotype CP5 and CP8 expression is greatly influenced by environmental and bacterial growth conditions, such as the culture medium and the growth phase of the organism (8, 23). Growth of S. aureus under iron limitation or on solid medium augmented production of CP8 (18). We demonstrated that S. aureus harvested from endocardial vegetations of infected rabbits expressed a high level of CP8, similar to that of organisms cultivated on agar (18). CP5 production was found to be inhibited by high levels of yeast extract, alkaline growth conditions, CO2, and anaerobiosis (8, 12, 23) but enhanced by growth of the bacterium in milk (24).
The objective of this study was to reexamine the virulence of the serotype 5 strain Reynolds when its capsule expression was optimized by cultivation on solid medium. The results indicate that the parental strain is more virulent than the capsule-defective mutants in a mouse bacteremia model of staphylococcal infection. We attribute the enhanced virulence to the antiphagocytic nature of the bacterial CP, since in vitro assays indicate that the parental strain resists phagocytic killing by human polymorphonuclear leukocytes in nonimmune serum. Capsule-deficient mutant strains (or the parental strain grown in broth) were opsonized for phagocytosis by complement alone.
MATERIALS AND METHODS
Bacteria.
The S. aureus isolates used for this study include the parental strain Reynolds, Tn_918_-insertional mutant JL236, and mutant JL243 (derived by mutagenesis with ethyl methanesulfonate) (2). The strains were maintained in skim milk at −70°C and cultivated for 24 h at 37°C in Columbia medium (Difco Laboratories, Detroit, Mich.) supplemented with 2% NaCl. Solid medium was prepared by the addition of 15 g of Bacto Agar (Difco Laboratories) per liter. Broth-grown staphylococcal cultures were incubated end over end in screw-cap glass tubes (16 by 150 mm) containing 6 ml of medium; the tubes were rotated at 65 rpm on a multipurpose rotator (model 151; Scientific Industries, Inc.). The bacteria were harvested, washed once in PBS (10 mM sodium phosphate–0.15 M NaCl [pH 7.3]), and suspended to an optical density at 650 nm of 0.4. Bacteria were diluted to the appropriate concentration for testing, and CFU were verified by plate counts performed in duplicate on tryptic soy agar (Becton Dickinson Microbiology Systems, Cockeysville, Md.).
CP5 quantitation.
An enzyme-linked immunosorbent assay (ELISA) inhibition method (18) was used to quantitate CP5 expression by S. aureus. The assay is based on the ability of encapsulated bacteria (trypsinized to remove protein A) or purified CP5 to absorb capsular antibodies from immune serum. The method is sensitive to CP5 (<1 ng/ml) and is useful for quantitating capsule production by different S. aureus strains or by the same strain grown under different conditions.
Briefly, wells of a microtiter plate were coated with purified CP5 (4 μg/ml) coupled to poly-l-lysine by the cyanuric chloride method (11). After 18 h at 4°C, the microtiter plate was washed and blocked at 4°C overnight with 0.05% skim milk. Suspensions of S. aureus cells were trypsinized (1 mg of trypsin/ml of 0.1 M phosphate buffer, pH 8) for 60 min at 37°C to remove protein A, washed, and serially diluted threefold from ∼5 × 107 to ∼5 × 104 CFU/ml. Polyclonal CP5-specific antiserum was diluted 1:20,000 and incubated overnight at 4°C with serial dilutions of the bacteria or purified CP (1 μg/ml to 0.1 ng/ml). Samples were centrifuged, and the absorbed serum samples (supernatants) were added to the coated and blocked microtiter plate. Following a 2-h incubation with absorbed or nonabsorbed serum samples, the plates were washed with PBS-Tween and alkaline phosphatase-conjugated goat anti-rabbit immunoglobulin G (Organon Teknika-Cappel, Durham, N.C.; 1:3,000) was added to each well. After 2 h at ambient temperature, the plate was again washed and _p_-nitrophenol phosphate substrate was added. Following a 30-min incubation at room temperature, the reaction was stopped with 3 M NaOH, and absorbance at 405 nm was measured by an ELISA reader (Biotek, Inc., Burlington, Vt.). The concentration of sample (bacterial cells) that resulted in 50% inhibition of antibody binding was determined, and the CP content of the sample was calculated from the CP5 standard curve. Specificity was shown by experiments demonstrating that only CP5, but not CP8, inhibited the reaction.
Analysis of cell wall extracts.
Cell wall extracts made from S. aureus protoplasts were prepared by the method of Cheung and Fischetti (6). Bacteria harvested from either Columbia salt agar (CSA) plates or Columbia salt broth (CSB) were suspended in 0.6 ml of digestion buffer (30% raffinose in 0.05 M Tris [pH 7.5] with 0.145 M NaCl) containing 100 μg of lysostaphin (Aplin & Barrett, Trowbridge, United Kingdom) and 10 μg of DNase (Worthington Diagnostics, Freehold, N.J.). The samples were incubated with gentle rotation for 2 h at 37°C. The protoplasts were collected by centrifugation at 8,000 × g for 10 min, and the supernatant was stored at −70°C. The samples were electrophoresed in a sodium dodecyl sulfate–9% polyacrylamide gel and stained with Coomassie brilliant blue.
Animal experiments.
Female CD-1 mice, 8 to 10 weeks old, were obtained from Charles River Laboratories, Kingston, Mass. For lethality studies, five groups of 9 to 11 CD-1 mice were challenged intraperitoneally (i.p.) with serial dilutions of S. aureus grown on CSA plates. The inocular sizes ranged from ∼1010 to ∼108 CFU/mouse. Mortality was assessed on a daily basis for 3 days. The 50% lethal doses (LD50s) were estimated by using a probit model of the dose-response relationship. The null hypothesis of common LD50s was tested by the likelihood ratio test. Sublethal bacteremia was initiated by challenging groups of 8 to 20 mice by the intravenous (i.v.) route with ∼2 × 106 CFU/mouse or by the i.p. route with ∼2 × 107 CFU/mouse. After inoculation separate groups of animals were bled from the tail at specified times, and the bacteremia levels were estimated by quantitative plate counts performed in duplicate on tryptic soy agar plates with 5% sheep blood (Becton Dickinson Microbiology Systems). Statistical significance was determined with the Welch modification of the unpaired Student’s t test.
Opsonophagocytic killing assay.
The in vitro opsonophagocytic killing of S. aureus by human polymorphonuclear leukocytes (PMNs) was determined as described previously (29). Antibodies to CP5 were elicited by immunization of rabbits with strain Reynolds, and antibodies to noncapsular cell wall determinants were removed by absorption of the serum with trypsinized (to remove protein A) cells of acapsular mutant JL243. Rabbit serum containing antibodies to noncapsular staphylococcal cell wall antigens was obtained by immunizing rabbits with killed cells of either mutant JL240, strain Lafferty, or strain NT857 (29). These sera were referred to as teichoic acid antisera because each immunoprecipitated with purified teichoic acid. All rabbit sera were heat inactivated at 56°C for 30 min prior to use. Pooled normal human serum was obtained by venipuncture from a group of eight healthy adult volunteers. Human serum from volunteers immunized with a conjugate vaccine composed of CP5 linked covalently to recombinant Pseudomonas aeruginosa exotoxoid A (9) was kindly provided by Ali Fattom, Nabi, Rockville, Md. Guinea pig serum (Pel-Freez Biologicals) was used as a source of nonimmune serum complement. Percent killing was defined as the reduction in CFU per milliliter after 60 or 120 min of incubation compared with that at time zero. The data presented are the means of at least two independent experiments.
RESULTS
Quantitation of CP5 expression by S. aureus cultivated on solid or in liquid medium.
Culture conditions have a profound effect on the level of CP5 production by S. aureus Reynolds (Table 1). Broth-grown cells expressed only ∼1% of the amount of cell-associated CP5 generated by cells grown on solid medium, a result consistent with our previous report on the relative expression of CP8 by S. aureus Becker when cultivated on solid or in liquid medium (18). The CP5-deficient mutant strain JL236 expressed ∼9% of wild-type levels of CP5, and the acapsular mutant JL243 produced negligible amounts of CP5.
TABLE 1.
CP5 expression measured by ELISA inhibition for S. aureus serotype 5 strains
| Strain | Growth medium | No. of trials | CP5 (μg/1010 CFU) |
|---|---|---|---|
| Reynolds | Agar | 2 | 263 ± 40 |
| Reynolds | Broth | 5 | 2.6 ± 1.0 |
| JL236 | Agar | 1 | 22.6 |
| JL243 | Agar | 1 | 1.34 × 10−4 |
Analysis of cell wall extracts made from S. aureus cultivated on agar or in broth.
Figure 1 shows a comparison of the cell wall-associated proteins expressed by S. aureus Reynolds, JL236, and JL243 cultivated either on CSA or in CSB. The proteins associated with the cell wall of staphylococci cultivated on solid medium differed from those expressed by broth-grown cells. As expected, the banding patterns for the broth-grown parental and mutant strains were identical, as were the banding patterns for the parental and mutant strains grown on solid medium. The conclusion from this experiment is that there are many differences, in addition to capsule expression, between cells cultivated in liquid or on solid medium. Thus, we have compared the virulence of strain Reynolds cells cultivated in broth or on agar with the virulence of the CP5 mutant strains (JL236 and JL243) cultivated under the same conditions.
FIG. 1.
Cell wall extracts were made from S. aureus strains cultivated on CSA (A) or CSB (B). Samples were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and the gel was stained with Coomassie brilliant blue. Positions of molecular mass markers run on the gel are indicated on the right.
Effect of CP5 production on staphylococcal lethality.
S. aureus Reynolds and acapsular mutant JL243 were cultivated on CSA plates at 37°C for 24 h. Suspensions of each bacterial strain were prepared, and groups of 9 to 11 mice were challenged i.p. with either strain at inoculum sizes ranging from 1010 to 108 CFU/mouse. After 24 h the LD50 of agar-grown strain Reynolds was 6.2 × 108 CFU (95% confidence interval = 3.8 × 108 to 9.9 × 108 CFU). At the same time point, only three of nine mice given the highest challenge dose (9.3 × 109 CFU) of mutant JL243 had died, yielding an extrapolated LD50 of 9.9 × 109 CFU. The difference (P < 10−5) in lethality induced by these two bacterial strains at 24 h likely reflects their relative abilities to be cleared effectively by host phagocytic cells. By day 3 after challenge, the LD50 of strain Reynolds was 3.9 × 108 CFU (95% confidence interval = 2.6 × 108 to 5.9 × 108 CFU), whereas that for mutant JL243 was 4.1 × 109 CFU (95% confidence interval = 2.6 × 109 to 6.3 × 109 CFU; P = 1.5 × 10−5). These results confirm an observation made earlier by others that rodents are very resilient to high doses of S. aureus administered i.p. (1).
Effect of CP5 production on bacteremia.
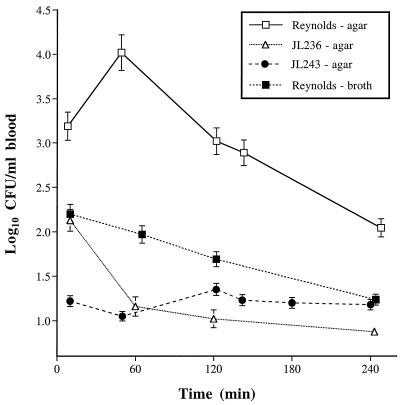
To provide a more sensitive measure of staphylococcal virulence, groups of 9 to 20 mice were inoculated i.p. with sublethal doses (∼2 × 107 CFU) of S. aureus, and their bacteremia levels were monitored over time. As shown in Fig. 2, bacterial concentrations in the blood of animals challenged with agar-grown strain Reynolds were significantly higher (P ≤ 0.0019) at all time points than those of mice challenged with broth-grown strain Reynolds. Similarly, the bacteremia levels in mice infected with agar-grown strain Reynolds were significantly greater (P < 0.0002) at each time point than those of mice challenged with the mutant strain JL236 or JL243 grown on similar medium. At 24 h after challenge, cultures of blood from the infected animals were sterile (<10 CFU/ml; not shown). These findings suggest that maximal CP5 expression by S. aureus allowed the inoculum to disseminate more effectively from the peritoneal cavity to the bloodstream.
FIG. 2.
Results of quantitative blood cultures from groups of 9 to 20 mice challenged i.p. with 2 × 107 CFU of S. aureus. The values shown are means ± SEM (error bars).
In a separate experiment groups of 8 to 19 mice were inoculated i.v. with ∼2 × 106 CFU of S. aureus. Blood was collected from the animals at time points ranging from 10 min to 24 h after inoculation, and quantitative blood cultures were performed on the samples. As shown in Fig. 3, there was a very rapid initial clearance of both wild-type and mutant strains of S. aureus from the blood following i.v. injection. However, the bacteremia levels were significantly higher for mice challenged with the parental strain Reynolds at 10 min (P < 0.0001), 60 min (P ≤ 0.024), and 180 min (P < 0.031) than for those challenged with each of the mutant strains. At 120 min after challenge, strain Reynolds was recovered from the blood in significantly greater numbers than was strain JL236 (P = 0.0302), but the difference in bacteremia levels between the parental strain and mutant JL243 did not reach significance (P = 0.0986). By 4 h after challenge, the results of the quantitative blood cultures from all of the mice were similar, achieving a steady-state concentration of ∼102 CFU/ml of blood. By 24 h the blood cultures showed <10 CFU/ml (not shown).
FIG. 3.
Results of quantitative blood cultures from groups of 8 to 19 mice challenged i.v. with 2 × 106 CFU of S. aureus. The values shown are means ± SEM (error bars).
Effect of CP5 production on opsonophagocytic killing by human PMNs in vitro.
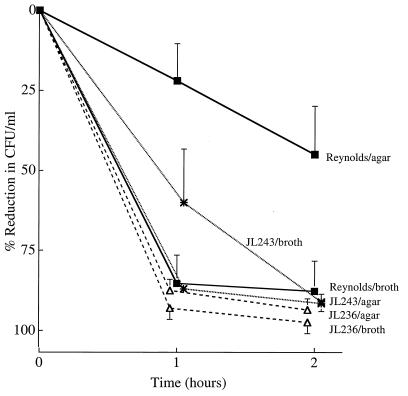
To determine whether the relative virulence of the parental and mutant strains correlated with their resistance to phagocytosis, we examined the opsonic requirements for phagocytic killing of each S. aureus strain by human PMNs. When the staphylococcal strains were cultivated in broth culture, each isolate was opsonized for phagocytic killing by either capsular antibodies and complement or complement alone (Fig. 4). Capsular antibodies alone (no complement activity) showed less opsonic activity, although the inoculum was reduced by >60% after 1 or 2 h of incubation with PMNs. These opsonic requirements for phagocytic killing are similar to those previously reported for S. aureus serotype 5 and 8 strains grown in broth culture (29).
FIG. 4.
In vitro opsonophagocytic killing of S. aureus strains (cultivated in CSB) by human PMNs in the presence of rabbit CP5 antiserum (ab). Nonimmune guinea pig serum was added as a complement (C′) source. The values shown are means ± SEM (error bars).
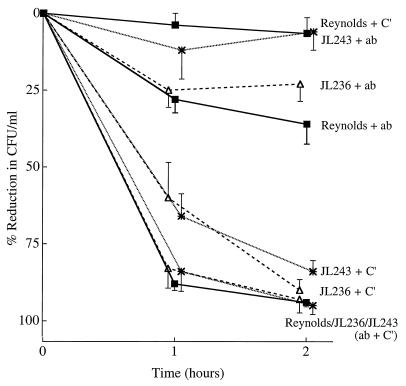
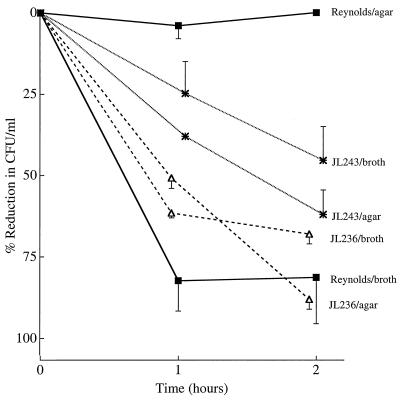
When strain Reynolds was cultivated on solid medium to enhance CP5 expression, it was susceptible to phagocytic killing only in the presence of both capsular antibodies and complement (Fig. 5). Little or no killing (5.5% reduction in CFU per milliliter) occurred when strain Reynolds was incubated with complement alone. In contrast, mutants JL243 and JL236 cultivated on agar were still efficiently opsonized for phagocytosis by either capsular antibodies and complement or complement alone (Fig. 5). When the PMNs were incubated with S. aureus and heat-inactivated CP5-specific serum (no added complement), the reduction in CFU per milliliter was related to the amount of CP5 produced by each strain; i.e., 36% of strain Reynolds cells were killed, 23% of mutant JL236 cells were killed, and only 6% of mutant JL243 cells were killed by the PMNs after 120 min of incubation. Clearly the target antigen recognized by this antiserum on the capsule-deficient mutant strains was diminished.
FIG. 5.
In vitro opsonophagocytic killing of S. aureus strains (cultivated on CSA plates) by human PMNs in the presence of rabbit capsular type 5 antiserum (ab). Nonimmune guinea pig serum was added as a complement (C′) source. The values shown are means ± SEM (error bars).
To determine whether antibodies to noncapsular cell wall determinants like peptidoglycan and teichoic acid could substitute for capsular antibodies in opsonizing serotype 5 S. aureus for phagocytosis, additional experiments comparing S. aureus strains cultivated in broth or on agar plates were performed. As shown in Fig. 6, broth-grown staphylococci were killed by phagocytes in the presence of antibodies to noncapsular cell wall antigens (teichoic acid antibodies) and complement (>92% killing) or complement alone (≥90% killing [Fig. 5]). Teichoic acid antibodies alone (no complement) were much less efficient in opsonizing the broth-grown staphylococcal strains for phagocytosis (44, 29, and 12% killing for strains Reynolds, JL236, and JL243, respectively).
FIG. 6.
In vitro opsonophagocytic killing of S. aureus strains (cultivated in CSB) by human PMNs in the presence of sera raised against nontypeable S. aureus strains (NT857, JL240, and Lafferty). The antisera contain antibodies (ab) to cell wall antigens such as teichoic acid, but the sera are negative for CP5 antibodies. The values shown are means ± SEM (error bars). C′, complement.
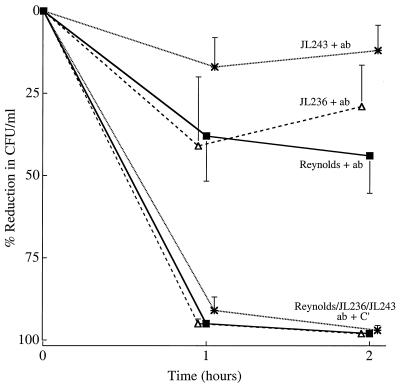
Opsonophagocytic killing was poor (47% reduction in CFU per milliliter) when agar-grown strain Reynolds was opsonized with teichoic acid antibodies and complement (Fig. 7). In contrast, >92% of mutant JL243 and JL236 cells (cultivated on CSA plates) were killed by the PMNs in the presence of teichoic acid antibodies and complement. Teichoic acid antibodies alone were poorly opsonic for each of the S. aureus strains cultivated on agar (Fig. 7).
FIG. 7.
In vitro opsonophagocytic killing of S. aureus strains (cultivated on CSA plates) by human PMNs in the presence of sera raised against nontypeable S. aureus strains (NT857, JL240, and Lafferty). The antisera contain antibodies (ab) to cell wall antigens such as teichoic acid, but the sera are negative for CP5 antibodies. The values shown are means ± SEM (error bars). C′, complement.
Normal humans have serum antibodies to S. aureus cell wall components, including peptidoglycan, teichoic acid, CP5, and CP8 (3, 7, 9, 26, 28). Because phagocytic clearance is the host’s primary defense against the staphylococcus, we wanted to determine whether pooled fresh, normal human serum was opsonic for strain Reynolds and its mutants. As shown in Fig. 8, fresh normal human serum with complement activity (unheated) was opsonic for the capsule-negative mutant strains whether they were cultivated in broth or on agar plates (>91% killing). Similarly, 88% of the broth-grown strain Reynolds inoculum was killed by the PMNs after a 2-h incubation. However, if strain Reynolds was harvested from agar plates, only 22 and 45% of the inoculum was opsonized for killing in fresh normal human serum after 1 and 2 h of incubation, respectively. The differences in opsonophagocytic killing between broth- and agar-grown S. aureus Reynolds were significant at 1 h (P = 0.01) but not at 2 h (P = 0.10). Likewise, when agar-grown strain Reynolds was contrasted with the mutant strains grown on agar, the differences in killing were significant at 1 h (P = 0.02) but not at 2 h (P = 0.13 and P = 0.14, comparing strain Reynolds with mutants JL236 and JL243, respectively). When we tested the opsonic activity of a pool of sera from human volunteers immunized with a conjugate vaccine composed of CP5 linked covalently to recombinant P. aeruginosa exotoxoid A, 88% ± 3.6% and 95% ± 1.2% (means ± standard errors of the means [SEM]) of the agar-grown Reynolds inoculum was killed in the opsonophagocytic assay after 1 and 2 h, respectively (data not shown in Fig. 8). When the same immune serum was heat inactivated, it lost all opsonic activity against agar-grown Reynolds cells (0% killing).
FIG. 8.
In vitro opsonophagocytic killing of S. aureus strains by human PMNs in the presence of pooled, fresh normal human serum. The values shown are means ± SEM (error bars).
The pool of serum from nonimmunized adults was heated to inactivate complement and retested in the opsonophagocytic killing assay. As shown Fig. 9, 81% of broth-grown Reynolds cells were killed in the presence of heated normal serum, whereas there was no killing of agar-grown Reynolds cells under the same conditions (P = 0.0178 for the 2-h values). The mutant strains JL236 and JL243 cultivated in broth or on agar showed an intermediate level of opsonophagocytic killing in heated human serum, ranging from 47 to 88% killing (Fig. 9). The differences in killing between agar-grown strain Reynolds (0%) and agar-grown mutants JL236 (88%) and JL243 (61.5%) at 2 h were significant (P < 0.02).
FIG. 9.
In vitro opsonophagocytic killing of S. aureus strains by human PMNs in the presence of pooled, heat-inactivated, normal human serum. The values shown are means ± SEM (error bars).
DISCUSSION
Expression of S. aureus cell-associated and secreted antigens is highly influenced by environmental culture conditions. Many staphylococcal exoproteins are produced in the postexponential growth phase, whereas cell-associated proteins are preferentially produced during exponential growth (16, 21). Alpha-toxin expression has been shown to be dependent on growth rate (25), temperature, osmolarity, and concentrations of oxygen and CO2 (20). Cheung and Fischetti (6) reported that S. aureus cells cultivated on agar showed a greater abundance of cell-wall-associated, high-molecular-weight proteins than broth-grown cells. We confirmed the findings of the latter study and observed that S. aureus cells grown on agar plates produced ∼100-fold more CP5 than did broth-grown cells. This fact has undoubtedly influenced the results from different laboratories that have focused on the role of the S. aureus capsule in virulence. Broth-grown S. aureus cultures were used for challenge in the animal virulence studies reported by Baddour et al. (5) and Albus et al. (2). Both of these studies failed to show that capsule expression enhanced staphylococcal virulence. In contrast, the recent study reported by Nilsson et al. (19) utilized the same S. aureus isolates as the previous two studies, but the bacterial strains were cultivated on agar. These investigators showed that the parental strain was more virulent than the capsule-defective mutants in a mouse model of arthritis. It is likely that both culture conditions and the type of infection model tested influenced the conclusions of these studies.
Different laboratories have also reported conflicting data regarding the antiphagocytic properties of the S. aureus capsule as measured in an in vitro opsonophagocytic killing assay (14, 29). Similarly, these differences may be due to variability in bacterial growth conditions, which are now recognized to greatly influence in vitro capsule expression (8, 12, 18, 23, 24).
In the present study, we report that capsule type 5 S. aureus Reynolds cultivated under conditions of maximum CP5 expression was more virulent for mice than mutant strains defective in CP5 expression. Only modest differences attributable to encapsulation were evident when virulence was assessed in the lethal peritonitis model or when bacteremia was quantified following i.v. challenge with S. aureus. The most striking, biologically relevant difference in staphylococcal virulence was observed when bacteremia levels were measured after i.p. challenge of the mice (Fig. 2). Our data suggest that the abundant capsule expressed by S. aureus cultivated on solid medium allowed the organism within the peritoneal cavity to escape local defenses and transit more efficiently through the lymphatics to the bloodstream of the animals.
The mouse virulence of the S. aureus strains under study correlated with their resistance to opsonophagocytic killing by human PMNs; i.e., when cultivated under conditions of high CP5 expression, strain Reynolds was killed only when it was opsonized by complement and capsular antibodies. In contrast, organisms with little capsule (broth-grown strain Reynolds and mutants JL236 and JL243 cultivated in broth or on agar) were effectively opsonized for phagocytosis by complement alone. Copious amounts of CP5 may mask other cell wall antigens like teichoic acid and peptidoglycan that are targets for complement deposition. Verbrugh et al. (27) demonstrated by transmission electron microscopy that C3b bound to the cell wall of the highly encapsulated strain M beneath the capsular layer and was physically separated from complement receptors on the PMN membrane. The large capsule produced by this serotype 1 S. aureus strain physically masked complement deposited on the cell wall. When strain M was mixed with type-specific antibodies and complement, the deposition of complement not only on the cell wall but throughout the capsular layer led to efficient phagocytic uptake and killing. Whether CP5 is produced by strain Reynolds in quantities sufficient to mask complement deposited on its cell wall remains to be determined.
Overall, the results of this study indicate that the role of the S. aureus capsule in bacterial virulence is markedly influenced by the in vitro bacterial culture conditions. When capsule expression was maximized, the parental strain Reynolds was more virulent for mice than capsule-defective mutant strains. Furthermore, there was a correlation between mouse virulence of the wild-type and mutant strains and their resistance to opsonophagocytic killing by human PMNs. These findings are consistent with recent studies that show that antibodies to the S. aureus capsule are protective in disseminated staphylococcal infections of mice (10) or rats (17).
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant AI-29040 from the National Institute of Allergy and Infectious Diseases and by the William F. Milton Fund of Harvard University.
We thank Derek Frederickson and Thuyanh Dang Le for their technical assistance. We acknowledge Ali Fattom for providing us with human serum from volunteers immunized with the S. aureus capsule-conjugate vaccine.
REFERENCES
- 1.Adlam C, Anderson J C, Arbuthnott J P, Easmon C S F, Noble W C. Animal and human models of staphylococcal infection. In: Easmon C S F, Adlam C, editors. Staphylococci and staphylococcal infections. Vol. 1. New York, N.Y: Academic Press; 1983. pp. 357–384. [Google Scholar]
- 2.Albus A, Arbeit R D, Lee J C. Virulence of Staphylococcus aureus mutants altered in type 5 capsule production. Infect Immun. 1991;59:1008–1014. doi: 10.1128/iai.59.3.1008-1014.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albus A, Fournier J M, Wolz C, Boutonnier A, Ranke M, Hoiby N, Hochkeppel H, Doring G. Staphylococcus aureus capsular types and antibody response to lung infection in patients with cystic fibrosis. J Clin Microbiol. 1988;26:2505–2509. doi: 10.1128/jcm.26.12.2505-2509.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arbeit R D, Karakawa W W, Vann W F, Robbins J B. Predominance of two newly described capsular polysaccharide types among clinical isolates of Staphylococcus aureus. Diagn Microbiol Infect Dis. 1984;2:85–91. doi: 10.1016/0732-8893(84)90002-6. [DOI] [PubMed] [Google Scholar]
- 5.Baddour L M, Lowrance C, Albus A, Lowrance J H, Anderson S K, Lee J C. Staphylococcus aureus microcapsule expression attenuates bacterial virulence in a rat model of experimental endocarditis. J Infect Dis. 1992;165:749–753. doi: 10.1093/infdis/165.4.749. [DOI] [PubMed] [Google Scholar]
- 6.Cheung A L, Fischetti V A. Variation in the expression of cell wall proteins of Staphylococcus aureus grown on solid and liquid media. Infect Immun. 1988;56:1061–1065. doi: 10.1128/iai.56.5.1061-1065.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christensson B, Boutonnier A, Rydiing U, Fournier J M. Diagnosing Staphylococcus aureus endocarditis by detecting antibodies against S. aureus capsular polysaccharide type 5 and 8. J Infect Dis. 1991;163:530–533. doi: 10.1093/infdis/163.3.530. [DOI] [PubMed] [Google Scholar]
- 8.Dassy B, Stringfellow W T, Lieb M, Fournier J M. Production of type 5 capsular polysaccharide by Staphylococcus aureus grown in a semi-synthetic medium. J Gen Microbiol. 1991;137:1155–1162. doi: 10.1099/00221287-137-5-1155. [DOI] [PubMed] [Google Scholar]
- 9.Fattom A, Schneerson R, Watson D C, Karakawa W W, Fitzgerald D, Pastan I, Li X, Shiloach J, Bryla D A, Robbins J B. Laboratory and clinical evaluation of conjugate vaccines composed of Staphylococcus aureus type 5 and type 8 capsular polysaccharides bound to Pseudomonas aeruginosa recombinant exoprotein A. Infect Immun. 1993;61:1023–1032. doi: 10.1128/iai.61.3.1023-1032.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fattom A I, Sarwar J, Ortiz A, Naso R. A Staphylococcus aureus capsular polysaccharide (CP) vaccine and CP-specific antibodies protect mice against bacterial challenge. Infect Immun. 1996;64:1659–1665. doi: 10.1128/iai.64.5.1659-1665.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gray B M. ELISA methodology for polysaccharide antigens: protein coupling of polysaccharides for adsorption to plastic tubes. J Immunol. 1979;28:187–192. doi: 10.1016/0022-1759(79)90340-5. [DOI] [PubMed] [Google Scholar]
- 12.Herbert S, Worlitzsch D, Dassy B, Boutonnier A, Fournier J-M, Bellon G, Dalhoff A, Doring G. Regulation of Staphylococcus aureus capsular polysaccharide type 5: CO2 inhibition in vitro and in vivo. J Infect Dis. 1997;176:431–438. doi: 10.1086/514061. [DOI] [PubMed] [Google Scholar]
- 13.Hochkeppel H K, Braun D G, Vischer W, Imm A, Sutter S, Staeubli U, Guggenheim R, Kaplan E L, Boutonnier A, Fournier J M. Serotyping and electron microscopy studies of Staphylococcus aureus clinical isolates with monoclonal antibodies to capsular polysaccharide types 5 and 8. J Clin Microbiol. 1987;25:526–530. doi: 10.1128/jcm.25.3.526-530.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karakawa W W, Sutton A, Schneerson R, Karpas A, Vann W F. Capsular antibodies induce type-specific phagocytosis of capsulated Staphylococcus aureus by human polymorphonuclear leukocytes. Infect Immun. 1988;56:1090–1095. doi: 10.1128/iai.56.5.1090-1095.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karakawa W W, Vann W F. Capsular polysaccharides of Staphylococcus aureus. Semin Infect Dis. 1982;4:285–293. [Google Scholar]
- 16.Kornblum J, Kreiswirth B N, Projan S J, Ross H, Novick R P. Agr: a polycistronic locus regulating exoprotein synthesis in Staphylococcus aureus. In: Novick R P, editor. Molecular biology of the staphylococci. New York, N.Y: VCH Publishers, Inc.; 1990. pp. 373–402. [Google Scholar]
- 17.Lee J C, Park J-S, Shepherd S E, Carey V, Fattom A. Protective efficacy of antibodies to the Staphylococcus aureus type 5 capsular polysaccharide in a modified model of endocarditis in rats. Infect Immun. 1997;65:4146–4151. doi: 10.1128/iai.65.10.4146-4151.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee J C, Takeda S, Livolsi P J, Paoletti L C. Effects of in vitro and in vivo growth conditions on expression of type-8 capsular polysaccharide by Staphylococcus aureus. Infect Immun. 1993;61:1853–1858. doi: 10.1128/iai.61.5.1853-1858.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nilsson I-M, Lee J C, Bremell T, Ryden C, Tarkowski A. The role of staphylococcal polysaccharide microcapsule expression in septicemia and septic arthritis. Infect Immun. 1997;65:4216–4221. doi: 10.1128/iai.65.10.4216-4221.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohlsen K, Koller K-P, Hacker J. Analysis of expression of the alpha-toxin gene (hla) of Staphylococcus aureus by using a chromosomally encoded hla::lacZ gene fusion. Infect Immun. 1997;65:3606–3614. doi: 10.1128/iai.65.9.3606-3614.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Recsei P, Kreiswirth B, O’Reilly M, Schlievert P, Gruss A, Novick R P. Regulation of exoprotein gene expression in Staphylococcus aureus by agr. Mol Gen Genet. 1986;202:58–61. doi: 10.1007/BF00330517. [DOI] [PubMed] [Google Scholar]
- 22.Sompolinsky D, Samra Z, Karakawa W W, Vann W F, Schneerson R, Malik Z. Encapsulation and capsular types in isolates of Staphylococcus aureus from different sources and relationship to phage types. J Clin Microbiol. 1985;22:828–834. doi: 10.1128/jcm.22.5.828-834.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stringfellow W T, Dassy B, Lieb M, Fournier J M. Staphylococcus aureus growth and type 5 capsular polysaccharide production in synthetic media. Appl Environ Microbiol. 1991;57:618–621. doi: 10.1128/aem.57.2.618-621.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sutra L, Rainard P, Poutrel B. Phagocytosis of mastitis isolates of Staphylococcus aureus and expression of type-5 capsular polysaccharide are influenced by growth in the presence of milk. J Clin Microbiol. 1990;28:2253–2258. doi: 10.1128/jcm.28.10.2253-2258.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Totake K, Ichikawa Y. Correlation between the production of α-toxin and growth rate in Staphylococcus aureus. Microbiol Immunol. 1983;27:389–394. doi: 10.1111/j.1348-0421.1983.tb00597.x. [DOI] [PubMed] [Google Scholar]
- 26.Verbrugh H, Peters R, Rozenberg-Arska M, Peterson P, Verhoef J. Antibodies to cell wall peptidoglycan of Staphylococcus aureus in patients with serious staphylococcal infections. J Infect Dis. 1981;144:1–9. doi: 10.1093/infdis/144.1.1. [DOI] [PubMed] [Google Scholar]
- 27.Verbrugh H A, Peterson P K, Nguyen B Y, Sisson S P, Kim Y. Opsonization of encapsulated Staphylococcus aureus: the role of specific antibody and complement. J Immunol. 1982;129:1681–1687. [PubMed] [Google Scholar]
- 28.Wergeland H I, Haaheim L R, Natas O B, Wesenberg F, Oeding P. Antibodies to staphylococcal peptidoglycan and its peptide epitopes, teichoic acid, and lipoteichoic acid in sera from blood donors and patients with staphylococcal infections. J Clin Microbiol. 1989;27:1286–1291. doi: 10.1128/jcm.27.6.1286-1291.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu S, Arbeit R D, Lee J C. Phagocytic killing of encapsulated and microencapsulated Staphylococcus aureus by human polymorphonuclear leukocytes. Infect Immun. 1992;60:1358–1362. doi: 10.1128/iai.60.4.1358-1362.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]