Isocitrate Lyase (AceA) Is Required for Salmonella Persistence but Not for Acute Lethal Infection in Mice (original) (raw)
Abstract
Isocitrate lyase is required for fatty acid utilization via the glyoxylate shunt. Although isocitrate lyase is essential for Salmonella persistence during chronic infection, it is dispensable for acute lethal infection in mice. Substrate availability in the phagosome appears to evolve over time, with increasing fatty acid dependence during chronic infection.
Isocitrate lyase catalyzes the first step in the glyoxylate shunt, an anaplerotic carbon assimilatory pathway that allows the net synthesis of C4 dicarboxylic acids from C2 compounds such as acetate and fatty acids. In the presence of acetate and the absence of more readily utilized substrates, the tricarboxylic acid enzyme isocitrate dehydrogenase is down-regulated by phosphorylation (1, 11), allowing isocitrate to be converted to glyoxylate and succinate by the action of isocitrate lyase.
Isocitrate lyase encoded by the icl gene has been found to be essential for tuberculosis persistence in a murine model (7) but not for the acute phase of infection, suggesting that Mycobacterium tuberculosis utilizes fatty acids as a carbon source in the phagosomes of macrophages located within established granulomatous lesions. Fungal isocitrate lyase has also been found to be required for virulence in a mouse model of systemic candidiasis (5), leading to the suggestion that microbial isocitrate lyase might represent a novel target for broad-spectrum antimicrobial therapy (6). We therefore investigated whether isocitrate lyase and the glyoxylate shunt play an important role in the pathogenesis of the facultative intracellular bacterium Salmonella enterica serovar Typhimurium.
Relatively little is known regarding the carbon sources available to Salmonella within phagosomes. Measurements of gene expression during short-term (4- to 12-h) infection of nonactivated macrophage-like cell lines (3) have shown increased expression of genes coding for gluconate, glucuronate/galacturonate, and galactonate permeases as well as genes involved in subsequent steps in carbohydrate metabolism, suggesting that gluconate and related sugars might be utilized by Salmonella under these conditions. Expression of aceA, the single gene encoding isocitrate lyase in S. enterica serovar Typhimurium (13), was not elevated during these short-term macrophage experiments (3). Moreover, the fadF gene, which is required for β-oxidation of fatty acids, was not induced, and other investigators have reported that fadE (fadF) is not required for virulence following intragastric infection of _Salmonella_-susceptible BALB/c mice (10).
For the present study, the S. enterica serovar Typhimurium aceA gene was replaced with a cat (chloramphenicol acetyltransferase) cassette by the method of Datsenko and Wanner (2) by using the primers 5′-TGGGCGGCAAGGTGCTGGTCCCCACGCAGGAGGCGATTCAGAAACTGGTTGCTGCGCGTCAAGCCACTGGAGCACCTCAA and 5′-TTCGCTTATCCGGCCTACGCTATCTGTAGGCCCGGTAAGCGCAGCGCCACCGGGCATCAAACGGGGAGAGCCTGAGCAAA and transduced by bacteriophage P22 into wild-type S. enterica serovar Typhimurium ATCC 14028. The aceA::cat mutation was confirmed by PCR by using the flanking primers 5′-CTTAATCGACTTCCTCACCCTGCC and 5′-CTTAT CCGGCCTACGCTATCTGTA and by demonstrating the ability of the mutant strain to utilize 0.2% citrate and 0.2% glycerol, but not 0.4% acetate, as a sole carbon source (data not shown). The isogenic parental wild-type strain was able to grow on each of these carbon sources in minimal medium.
After 18 h of incubation, the aceA::cat S. enterica serovar Typhimurium mutant strain was found to survive in RAW264.7 murine macrophages as well as isogenic wild-type S. enterica serovar Typhimurium (data not shown), under experimental conditions essentially as previously described (14). Overt macrophage cell death induced by Salmonella prevented extending the assay to later time points, even when a multiplicity of infection as low as 1:1 was used.
The aceA::cat mutant S. enterica serovar Typhimurium strain was not found to be attenuated for virulence following either intraperitoneal inoculation of 103 CFU (Fig. 1, top) or atraumatic oral administration of 107, 108, or 109 CFU (Fig. 1, bottom) to 6- to 8-week-old female Ity r C3H/HeN mice (Charles River Laboratories). This demonstrated that isocitrate lyase and the glyoxylate shunt do not play an essential role in the acute murine Salmonella infection model.
FIG. 1.
Isocitrate lyase (AceA) is not required for acute lethal infection in mice. (Top) Survival of C3H/HeN mice infected intraperitoneally with 103 CFU of wild-type S. enterica serovar Typhimurium 14028s or an isogenic aceA mutant strain. An attenuated aroA mutant CL1509 was included as a control. (Bottom) Survival of C3H/HeN mice infected orally with 107, 108, or 109 CFU of wild-type S. enterica serovar Typhimurium 14028s or an isogenic aceA mutant strain. Murine challenge experiments were each performed twice with essentially identical results (five per group).
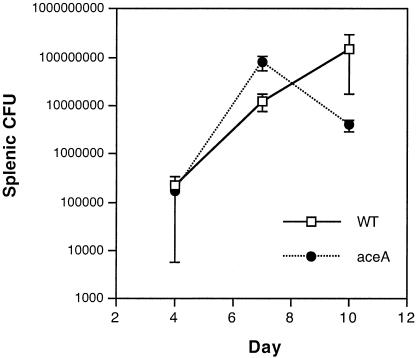
However, enumeration of splenic CFU in intraperitoneally infected animals suggested a possible mild growth defect at 10 days (Fig. 2), so competitive experiments in which a mixture of bacteria containing equivalent quantities of wild-type and aceA mutant S. enterica serovar Typhimurium were administered intraperitoneally were performed (4). These experiments were performed in an attenuated aroA mutant strain background (12) to allow infected animals to survive to later time points. At 14 days, a modest but significant defect in the competitive index of strains carrying an aceA mutation was evident (Table 1).
FIG. 2.
Salmonella splenic organ burden following intraperitoneal inoculation of mice. Splenic CFU were quantified in mice euthanized at selected intervals following intraperitoneal inoculation of C3H/HeN mice with 103 CFU of wild-type S. enterica serovar Typhimurium 14028s or an isogenic aceA mutant strain (six per group).
TABLE 1.
Fourteen-day competitive index (CI) of aroA aceA versus aroA mutant S. enterica serovar Typhimurium
| Inoculum (CFU) | Spleen | Liver | ||
|---|---|---|---|---|
| CI | P value | CI | P value | |
| 105 | 0.457 | 0.01 | 0.295 | 0.0003 |
| 107 | 0.173 | 0.0001 | 0.186 | <0.0001 |
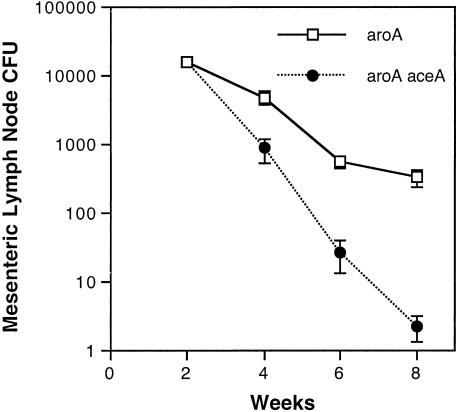
A recently published study demonstrated long-term persistence of S. enterica serovar Typhimurium within macrophages in mesenteric lymph nodes (MLN) following oral infection of Ity r 129Sv mice (8). We therefore repeated our competitive infection studies using oral inocula of 109 CFU in this _Salmonella_-resistant mouse strain. As shown in Fig. 3, the aroA mutant remained detectable in the MLN of 129Sv mice 2 months after infection, whereas the aceA aroA mutant strain was progressively cleared from the tissues.
FIG. 3.
Isocitrate lyase (AceA) is required for Salmonella persistence in MLN following oral inoculation of 129Sv mice. MLN CFU were quantified in 129 Sv mice euthanized at selected time intervals following oral inoculation with 109 CFU containing a mixture of equivalent quantities of aroA mutant S. enterica serovar Typhimurium CL1509 and an isogenic aceA aroA mutant derivative. This experiment was performed twice with virtually identical results (six per group).
Our results demonstrate that isocitrate lyase is required for Salmonella persistence during chronic infection but not for acute lethal infection in mice. These observations suggest that metabolic substrate availability in the phagosome may evolve over the course of infection, with an increasing dependence on fatty acid and acetate utilization occurring during chronic infection. When glucose or pyruvate is absent but fatty acids or acetate is present, isocitrate dehydrogenase is inactivated by phosphorylation, shunting carbon flow through isocitrate lyase (1, 11). Although the ability to utilize fatty acids is dispensable during the acute phase of salmonellosis, the glyoxylate shunt appears to play a critical role in the ability of Salmonella and other intracellular pathogens to persist in mammalian hosts (9).
Acknowledgments
We are grateful to the National Institutes of Health for their support of this work (AI39557, AI44486).
REFERENCES
- 1.Cozzone, A. J. 1998. Regulation of acetate metabolism by protein phosphorylation in enteric bacteria. Annu. Rev. Microbiol. 52**:**127-164. [DOI] [PubMed] [Google Scholar]
- 2.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97**:**6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eriksson, S., S. Lucchini, A. Thompson, M. Rhen, and J. C. Hinton. 2003. Unravelling the biology of macrophage infection by gene expression profiling of intracellular Salmonella enterica. Mol. Microbiol. 47**:**103-118. [DOI] [PubMed] [Google Scholar]
- 4.Ho, T. D., N. Figueroa-Bossi, M. Wang, S. Uzzau, L. Bossi, and J. M. Slauch. 2002. Identification of GtgE, a novel virulence factor encoded on the Gifsy-2 bacteriophage of Salmonella enterica serovar Typhimurium. J. Bacteriol. 184**:**5234-5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lorenz, M. C., and G. R. Fink. 2001. The glyoxylate cycle is required for fungal virulence. Nature 412**:**83-86. [DOI] [PubMed] [Google Scholar]
- 6.Lorenz, M. C., and G. R. Fink. 2002. Life and death in a macrophage: role of the glyoxylate cycle in virulence. Eukaryotic Cell 1**:**657-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McKinney, J. D., K. Honer zu Bentrup, E. J. Munoz-Elias, A. Miczak, B. Chen, W. T. Chan, D. Swenson, J. C. Sacchettini, W. R. Jacobs, Jr., and D. G. Russell. 2000. Persistence of Mycobacterium tuberculosis in macrophages and mice requires the glyoxylate shunt enzyme isocitrate lyase. Nature 406:735-738. [DOI] [PubMed] [Google Scholar]
- 8.Monack, D. M., D. M. Bouley, and S. Falkow. 2004. Salmonella typhimurium persists within macrophages in the mesenteric lymph nodes of chronically infected Nramp1+/+ mice and can be reactivated by IFNγ neutralization. J. Exp. Med. 199**:**231-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monack, D. M., A. Mueller, and S. Falkow. 2004. Persistent bacterial infections: The interface of the pathogen and the host immune system. Nat. Rev. Microbiol. 2**:**747-765. [DOI] [PubMed] [Google Scholar]
- 10.Spector, M. P., C. C. DiRusso, M. J. Pallen, F. Garcia del Portillo, G. Dougan, and B. B. Finlay. 1999. The medium-/long-chain fatty acyl-CoA dehydrogenase (fadF) gene of Salmonella typhimurium is a phase 1 starvation-stress response (SSR) locus. Microbiology 145(Pt. 1)**:**15-31. [DOI] [PubMed] [Google Scholar]
- 11.Stueland, C. S., K. Gorden, and D. C. LaPorte. 1988. The isocitrate dehydrogenase phosphorylation cycle. Identification of the primary rate-limiting step. J. Biol. Chem. 263**:**19475-19479. [PubMed] [Google Scholar]
- 12.Tsolis, R. M., A. J. Baumler, I. Stojiljkovic, and F. Heffron. 1995. Fur regulon of Salmonella typhimurium: identification of new iron-regulated genes. J. Bacteriol. 177**:**4628-4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson, R. B., and S. R. Maloy. 1987. Isolation and characterization of Salmonella typhimurium glyoxylate shunt mutants. J. Bacteriol. 169**:**3029-3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zaharik, M. L., V. L. Cullen, A. M. Fung, S. J. Libby, S. L. Kujat Choy, B. Coburn, D. G. Kehres, M. E. Maguire, F. C. Fang, and B. B. Finlay. 2004. The Salmonella enterica serovar Typhimurium divalent cation transport systems MntH and SitABCD are essential for virulence in an Nramp1 G169 murine typhoid model. Infect. Immun. 72**:**5522-5525. [DOI] [PMC free article] [PubMed] [Google Scholar]