Molecular Basis for the RIN4 Negative Regulation of RPS2 Disease Resistance (original) (raw)
Abstract
Recent studies have demonstrated that RPS2, a plasma membrane–localized nucleotide binding site/leucine-rich repeat protein from Arabidopsis thaliana, associates with RPM1 Interacting Protein 4 (RIN4) and that this association functions to modulate the RPS2-mediated defense pathway in response to the bacterial effector protein AvrRpt2. In addition to negatively regulating RPS2 activity, RIN4 is also a target of AvrRpt2, a Cys protease and cognate bacterial effector protein of RPS2. Nicotiana benthamiana has been employed as a heterologous expression system to characterize the RPS2–RIN4 association, defining the domains in RIN4 required for the negative regulation of RPS2 activity. Upon inoculation of N. benthamiana leaves with Agrobacterium tumefaciens expressing RPS2, a rapid hypersensitive response (HR) is detected with 22 h of infiltration. The HR can be blocked by infiltrating the leaf with A. tumefaciens expressing RPS2 in the presence of RIN4, recapitulating the ability of RIN4 to interfere with RPS2-mediated resistance in Arabidopsis. Moreover, in the presence of RIN4, the RPS2-mediated HR can be restored by the delivery of AvrRpt2 via A. tumefaciens. This assay has been developed as a phenotypic marker for (1) the HR-inducing phenotype associated with RPS2, (2) negative regulation of RPS2 by RIN4, and (3) the AvrRpt2-mediated disappearance of RIN4. Here, we present a series of deletion and site-directed mutation analyses to identify amino acids in RIN4 required for the RPS2–RIN4 association and to distinguish these from residues in RIN4 that serve as a target sequence for AvrRpt2. In addition to characterizing the RPS2–RIN4 association in N. benthamiana, we have moved forward to show that the biological relevance of these amino acid changes is applicable in Arabidopsis as well. To this end, we have identified specific amino acids within the C-terminal half of RIN4 that are required for RPS2 regulation and association.
INTRODUCTION
The molecular basis for resistance (R) protein–mediated bacterial disease resistance in plants involves the direct, or indirect, recognition of pathogen-derived virulence factors that activate a series of disease resistance signaling pathways, resulting in the induction of plant disease resistance (Staskawicz et al., 2001). After the delivery of a bacterial effector protein into the plant cytosol via the type III secretion system (TTSS), one of two general defense reactions may ensue. The defense response, commonly referred to as gene-for-gene resistance, occurs when effector proteins are recognized by the host plant, thus initiating an incompatible reaction. This results in abrogation of bacterial growth mediated by specific R protein signaling pathways. Conversely, the absence or inactivation of a plant R protein by a TTSS-delivered effector protein(s) results in host susceptibility. This situation results in the pathogen successfully evading host defense responses, allowing for colonization of the host plant (reviewed in Dangl and Jones, 2001).
The evolution of bacterial-plant innate immunity in higher plants has culminated in a sophisticated molecular surveillance system that is capable of recognizing bacterial effector proteins that are delivered to the plant host cell via a prokaryotic TTSS (Dangl and Jones, 2001; Van der Hoorn et al., 2002). However, the question still remains as to how various protein components of signaling pathways are assembled, activated, and subsequently regulated in response to the recognition of invading pathogens. R protein complex assembly and activation is slowly emerging as a model for defining the underlying molecular mechanisms of plant disease resistance. In recent years, two models have emerged as possible explanations as to how pathogens are either recognized by host plants and/or how they evade detection by the host's surveillance machinery. The direct recognition mechanism implies that R and avirulence (Avr) proteins physically interact, and this direct association specifies disease resistance. This model is based on the classical ligand-receptor association whereby bacterial effector proteins (i.e., ligand) interact with plant R proteins (i.e., receptor) (reviewed in Dangl and Jones, 2001). However, strong evidence for this hypothesis is rare, and only a few examples of this mode of recognition have been described (Jia et al., 2000; Deslandes et al., 2003).
A second mechanism for R and Avr interaction that has gained strong support in recent years is the guard hypothesis (Van der Biezen and Jones, 1998). This hypothesis predicts that R proteins function as part of larger surveillance machinery that has evolved to detect and respond to the actions of bacterial effector proteins. It is postulated that effector proteins function to suppress or inactivate basal resistance mechanisms in the host via the modification or elimination of essential components of basal resistance (Shirasu and Schulze-Lefert, 2003; Belkhadir et al., 2004b). In this manner, the effector protein indirectly disrupts R protein complexes, resulting in the activation of signal transduction pathways that lead to the induction of plant disease resistance.
The first experimental evidence in support of an indirect recognition event in the Avr–R protein associations came from Mackey et al. (2002) (2003), who identified a component of the RPM1-mediated disease resistance pathway that specifies resistance to pathogens expressing the effector proteins AvrB and/or AvrRpm1. This protein component, RIN4 (for RPM1 Interacting Protein 4), was shown to be phosphorylated in the presence of AvrB or AvrRpm1. This phosphorylation event in turn activates the RPM1 signaling cascade, leading to disease resistance. In the absence of either AvrB or AvrRpm1, RIN4 appears to function as a negative regulator of RPM1 function, keeping it in an inactive state (Mackey et al., 2003). Interestingly, recent work by Belkhadir et al. (2004a) also suggests that the activation of RPS2 is NDR1 independent, in contrast with the established requirement for NDR1 during AvrRpt2-dependent RPS2 activation. This work seems to indicate that RIN4 may function either cooperatively or independently of NDR1 to negatively regulate RPS2 in the absence of pathogen.
It has recently been shown that RIN4 functions in a second R–Avr association, suggesting that RIN4 has a dual specificity as it relates to regulating R protein–mediated resistance. Axtell and Staskawicz (2003) demonstrated that RIN4 also interacts with RPS2, the R protein conferring resistance to Pseudomonas syringae expressing AvrRpt2. As in the case of RPM1, RIN4 also functions as a negative regulator of RPS2 activation. However, converse to the mechanism of activation observed for RPM1, the RPS2–RIN4 association appears to function quite differently. Rather than the phosphorylation of RIN4 leading to activation, as is the case with RPM1, RPS2 activity appears to require the AvrRpt2-mediated disappearance of RIN4. This result seems to suggest that a physical association between RPS2 and RIN4, whether direct or indirect, serves to hold RPS2 in an inactive state. In turn, only the elimination of RIN4 by AvrRpt2 results in the activation of RPS2-mediated resistance responses. Using various mutant isoforms of AvrRpt2 that are rendered inactive through a series of catalytic triad mutations, it has since been determined that the AvrRpt2-mediated elimination of RIN4 is specific and requires a catalytically active AvrRpt2 enzyme (Axtell et al., 2003). Taken together with the results of Mackey et al. (2002) (2003), RIN4 appears to fulfill a role as a molecular switch regulating at least two independent R protein–mediated defense pathways in Arabidopsis thaliana.
The transient expression of gene products delivered by Agrobacterium tumefaciens is an efficient and robust tool to elucidate the genetic components required for disease resistance (Scofield et al., 1996). Moreover, transient expression systems can be further used to address the protein associations required for both the activation and inactivation of disease signaling pathways. To date, the use of Nicotiana benthamiana as a surrogate expression system for identifying and characterizing numerous components of disease resistance pathways and in determining the physical relationship(s) between various interactors is well documented (Mudgett and Staskawicz, 1999; Jin et al., 2002; Escobar et al., 2003; He et al., 2004; Zhang et al., 2004). The best characterized use of N. benthamiana as a system for monitoring RPS2 activity was demonstrated by Jin et al. (2002), who first described the heterologous recognition of RPS2 in N. benthamiana using a transient expression assay. These findings demonstrated that RPS2 is recognized when transiently expressed in N. benthamiana leaves via Agrobacterium delivery. This activation was shown to be specific and to require a functional RPS2 protein. These results further support the possibility that RPS2 is functionally capable of activating what might be an orthologous resistance pathway in tobacco.
In addition to the phenotype associated with the overexpression of RPS2 in N. benthamiana, Mudgett and Staskawicz (1999) reported on the recognition of the RPS2 cognate bacterial effector protein AvrRpt2 in the heterologous plant system N. tabacum. When overexpressed in N. tabacum leaves using Agrobacterium-mediated expression, AvrRpt2 alone induces a rapid, localized hypersensitive response (HR) within 30 h of infiltration, suggesting recognition of the effector protein within the plant cell. Although phenotypically and temporally distinct from the RPS2 HR (∼18 h), the AvrRpt2-induced HR (∼30 h) represents a somewhat complementary piece of the RPS2–RIN4 association that can be manipulated to further define the regulatory mechanisms associated with RPS2 activation. While manipulating various components of the RPS2 signaling pathway, such as AvrRpt2, either through silencing or overexpression, we can recapitulate various stages of the HR by expressing single or multiple protein components required for RPS2 activation. Using the N. benthamiana expression system, we sought to define the molecular basis for the RPS2–RIN4 association and the role of this association in the negative regulation of RPS2.
In this study, we report the identification of regions of RIN4 that are required for RPS2 association and characterize these domains in terms of identifying amino acids that appear to be critical for the negative regulation of RPS2 by RIN4, as well as those required for protein–protein interactions. Moreover, we have furthered our characterization in differentiating the domains of RIN4 required for RPS2 regulation from those that are targeted by AvrRpt2 proteolysis.
RESULTS
RIN4 Negatively Regulates RPS2 Activity
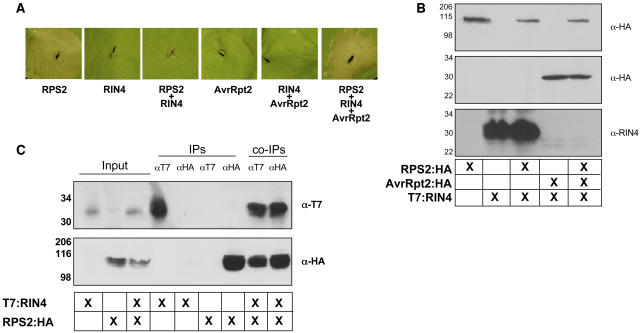
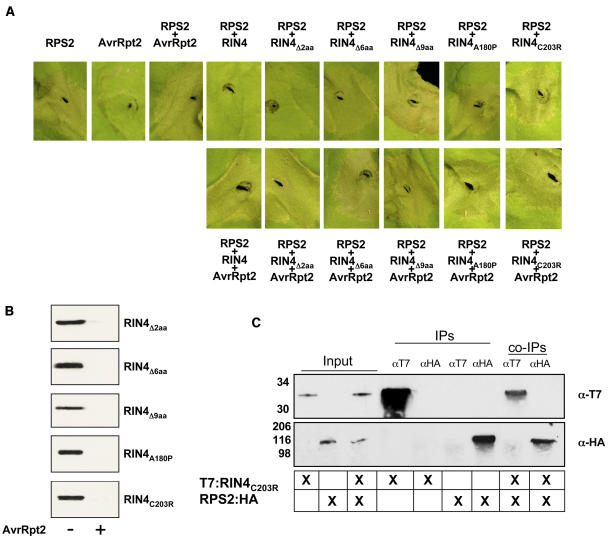
The first step in furthering our study of the RPS2–RIN4 association was to verify that we could recapitulate many of the phenotypes associated with RPS2-mediated disease resistance observed in Arabidopsis employing a heterologous system such as N. benthamiana. It has previously been reported that overexpression of RPS2 induces an HR when transiently expressed in N. benthamiana leaves (Jin et al., 2002). As shown in Figure 1A, when coexpressed with RPS2, RIN4 negatively regulates the HR-inducing activity of RPS2, suggesting that association of the two proteins may serve as a mechanism by which RIN4 keeps RPS2 in an inactive conformation. As described previously, the coexpression of RIN4 and AvrRpt2 by Agrobacterium-mediated expression results in the rapid elimination of RIN4, as observed by protein gel blot analysis (Figure 1B), as well as the restoration of the RPS2 HR (Figure 1A, last leaf panel).
Figure 1.
Coimmunoprecipitation of RPS2:HA and T7:RIN4 in N. benthamiana.
(A) RIN4 negatively regulates RPS2 activation. N. benthamiana leaves were hand-infiltrated with Agrobacterium strains expressing either 35S:RPS2:HA (OD600 = 0.1), 35S:T7:RIN4 (OD600 = 0.4), or 35S:AvrRpt2:HA (OD600 = 0.1). Immunoblot of HA- and T7-tagged proteins isolated 24 h postinoculation from wild-type N. benthamiana leaves hand-infiltrated with Agrobacterium strains expressing RPS2:HA, T7:RIN4, and AvrRpt2:HA confirmed expression of the various protein constructs (data not shown). Leaves were photographed 24 h postinoculation.
(B) Protein gel blot analysis of protein extracts isolated from N. benthamiana leaves as diagramed in (A). Leaf punches (7 mm) were taken at 20 h, and protein samples were prepared as described in Methods. Immunoblots were probed with either α-HA (RPS2/AvrRpt2) or α-T7 (RIN4). Sizes are indicated at the left of each blot.
(C) T7:RIN4 and RPS2:HA associate in planta. Immunoblots of immunoprecipitated proteins from the indicated tissue sources (center grid). “Input” lanes represent extracted samples incubated in the absence of antibody. “IP” indicates antibody used for immunoprecipitation and in all cases was both a positive and negative control for the fidelity of the antibody. “co-IP” refers to the coimmunoprecipitation experiment designed to detect the T7:RIN4 and RPS2:HA association in planta. Top panel, HA-HRP immunoblot; bottom panel, T7-HRP immunoblot. Protein sizes are indicated to the left of each blot.
To further establish that the RPS2–RIN4 association in a heterologous N. benthamiana expression system is a viable means for studying the protein–protein interactions, we also investigated whether these two proteins associate in planta, as first reported by Axtell and Staskawicz (2003) and Mackey et al. (2003) for Arabidopsis. Indeed, after transient coexpression of both proteins for 22 h in N. benthamiana leaves, we found that RPS2 and RIN4 can be reciprocally pulled down in a series of coimmunoprecipitation experiments (Figure 1C). This suggests that both proteins are expressed and can associate when transiently expressed from A. tumefaciens. These experiments demonstrate our success in establishing N. benthamiana as a viable system for furthering our study of the RPS2–RIN4 association.
The C-Terminal Domain of RIN4 Is Required for RPS2 Regulation and Association
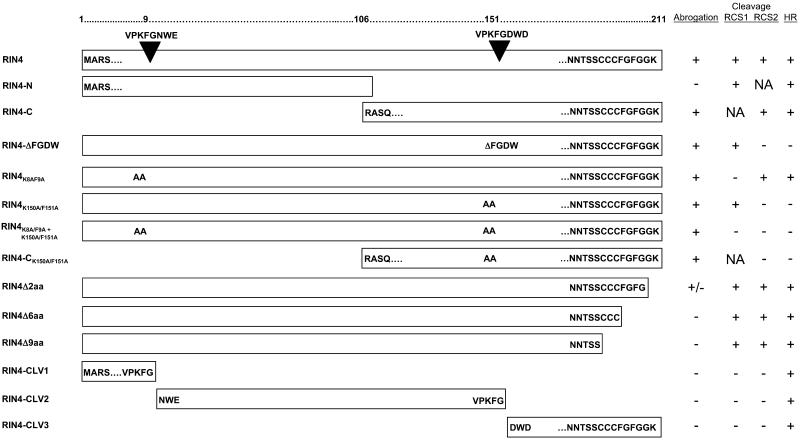
The RPS2–RIN4 association is correlated with two phenotypes: negative regulation and in planta association. We hypothesized that the negative regulation of RPS2 by RIN4 may be directly correlated with the association of these two proteins. To test this hypothesis, a series of stepwise deletions and site-directed mutations in RIN4 were made using PCR, and their ability to associate with, and negatively regulate, RPS2 was tested in N. benthamiana.
Constructs consisting of the N- and C-terminal halves of RIN4 were constructed (Figure 2) and examined for their ability to associate with RPS2. Using these basic deletion constructs, we asked if the resultant proteins were capable of interacting with RPS2. As shown in Figure 3A, only the C-terminal half of RIN4 (i.e., RIN4-C) retained the ability to negatively regulate RPS2 activity in a transient leaf assay, suggesting that the RPS2-interacting domain of RIN4 lies in the C-terminal half. Interestingly, both the N and C termini retain the ability to be eliminated by the Cys protease AvrRpt2 when coexpressed in N. benthamiana. These data reflect the fact that AvrRpt2 cleaves RIN4 at two sites, RIN4 cleavage site 1 (RCS1; amino acids 6 to 12) and RCS2 (amino acids 148 to 154) (Chisholm et al., 2005; Coaker et al., 2005). Thus, it appears that RIN4, although susceptible to AvrRpt2 cleavage in at least two distinct sites, possesses a more localized RPS2-interacting domain that is distinct from AvrRpt2 protease target sequences.
Figure 2.
Strategy for Identifying the Domain(s) of RIN4 Necessary for the Negative Regulation of RPS2 Function.
Using a PCR-based approach, deletion constructs were made in RIN4 corresponding to stepwise deletions, resulting in the elimination of amino acids at both the C and N termini of RIN4. Abrogation (+) refers to RIN4 ability to block RPS2 activation. Cleavage (+) refers to AvrRpt2 ability to cleave wild-type RIN4 at either the N- or C-terminal cleavage site (RCS1 or RCS2, respectively) or both and, where indicated, the various RIN4 deletion/mutant constructs. Where designated, “NA” refers to not applicable, indicating the respective cleavage site is absent in the construct. HR (+) refers to induction of the RPS2-mediated HR after delivery of AvrRpt2. Key amino acid residues are shown when necessary. Inverted triangles designate the AvrRpt2 cleavage sites present in both the N and C termini of RIN4.
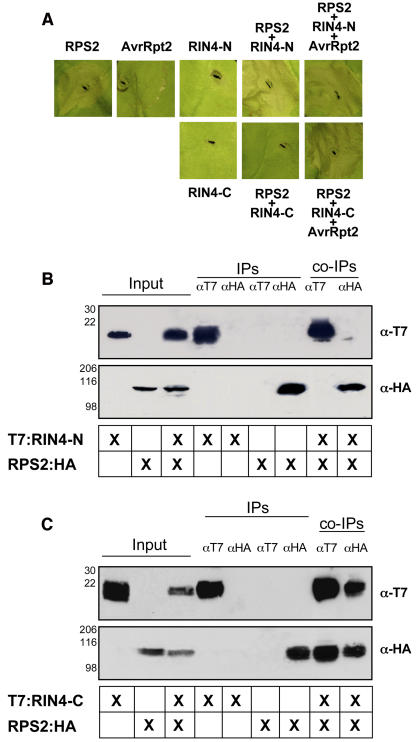
Figure 3.
Negative Regulation of RPS2 by RIN4: The Negative Regulatory Activity of RIN4 Is Localized in the C Terminus.
RPS2:HA and T7:RIN4 C and N termini constructs were transiently expressed in wild-type N. benthamiana plants via Agrobacterium-mediated transformation.
(A) Inoculation of N. benthamiana leaves with Agrobacterium expressing RPS2:HA resulted in the rapid (∼18 h) induction of the HR (first leaf panel, top). Leaf segments were photographed 24 h postinoculation. Coexpression of RPS2:HA and T7:RIN4-C resulted in a complete inhibition of the HR (second leaf panel, bottom). Conversely, coinoculation of N. benthamiana leaves with RPS2:HA and T7:RIN4-N failed to inhibit the induction of the HR (fourth leaf panel, top). Control inoculations with Agrobacterium expressing AvrRpt2 (second leaf panel, top) and the T7:RIN4 termini alone (i.e., RIN4-N and RIN4-C) showed only a slight HR phenotype encircling the periphery of the inoculation point or no reaction at all, respectively.
(B) The N terminus of RIN4 (i.e., T7:RIN4-N) does not coimmunoprecipitate RPS2:HA. Immunoblot of αHA and αT7 immunoprecipitated proteins isolated 24 h postinoculation from wild-type N. benthamiana leaves hand-infiltrated with Agrobacterium strains expressing RPS2:HA and the N-terminal half of RIN4 (i.e., T7:RIN4-N). Total protein extracts were immunoprecipitated with anti-HA (RPS2) and anti-T7 (RIN4) antibodies. Immunoprecipitated proteins were detected by immunoblotting with anti-T7 (top panel) and anti-HA (bottom panel) antibodies.
(C) The C terminus of RIN4 (i.e., T7:RIN4-C) coimmunoprecipitates RPS2:HA.
To examine whether RIN4 blocks RPS2 activation by physical association between the two proteins, coimmunoprecipitation experiments were performed using both RIN4-N and RIN4-C coinfiltrated with RPS2. As shown in Figure 3, a correlation exists between association (Figure 3C) and negative regulation (Figure 3A), as demonstrated by the ability of T7:RIN4-C to coimmunoprecipitate with RPS2:HA. Furthermore, the N terminus of RIN4 does not negatively regulate RPS2, and we were not able to detect an association between T7:RIN4-N and RPS2:HA (Figure 3B). Taken together, these results further support our hypothesis that the C-terminal half of RIN4 is required for both the regulation of, and association with, RPS2.
AvrRpt2 cleaves RIN4 at two sites in vitro (Coaker et al., 2005). To determine if any one of these cleavage products are alone capable of abrogating the RPS2-mediated HR, we made constructs that closely resemble these products (Figure 2; RIN4-CLV1/CLV2/CLV3) and expressed them in N. benthamiana using our transient expression system. As expected, when expressed in N. benthamiana leaves via Agrobacterium-mediated transient expression, none of the cleavage products were capable of abrogating the RPS2 HR (see Supplemental Figure 1B online). In agreement with these data is our finding that none of the RIN4 cleavage products coimmunoprecipitated with RPS2 (data not shown).
RIN4 Sequences Required for Negative Regulation of RPS2 Are Distinct from Those Required for Elimination by AvrRpt2
Once we established that the C terminus of RIN4 controls regulation of and physically associates with RPS2, we narrowed our focus to identify amino acids within the C terminus of RIN4 that are required for regulation and association (Figure 2). It has been suggested that conserved sequence motifs present in several potential effector proteins, and their cognate target substrates, may function as recognition sites for cleavage activity (Jones and Takemoto, 2004). In RIN4, for example, RCS1 and RCS2 are similar to the amino acid sequence surrounding the AvrRpt2 self-processing site (Jones and Takemoto, 2004; Chisholm et al., 2005).
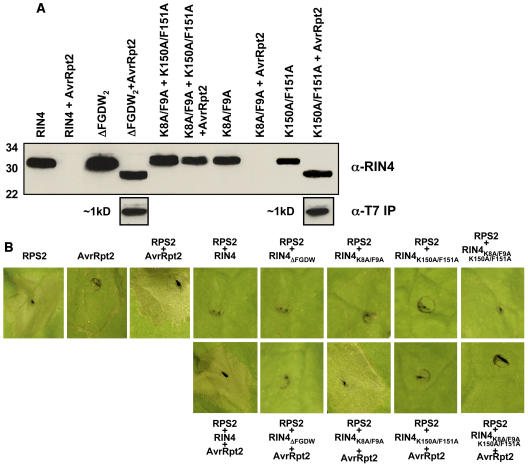
To determine if the residues required for AvrRpt2-mediated elimination of RIN4 are distinct from those required for RPS2 regulation and association, we first made a series of site-directed mutations in RCS2 of the RIN4-C construct to determine if these clones were still capable of regulating RPS2 function, as well as physically associating with RPS2 in vivo. Mutations in RCS2, specifically K150A/F151A and ΔFGDW, resulted in a loss of AvrRpt2-mediated elimination of RIN4 when transiently expressed in N. benthamiana. However, neither of these mutations affected the ability of RIN4 to negatively regulate RPS2 activity, as determined by the HR phenotype (Figure 4). These results suggest that RCS2 is not required for RPS2–RIN4 association.
Figure 4.
Amino Acid Residues Necessary for Cleavage of RIN4 by AvrRpt2 and Negative Regulation of RPS2 by RIN4 Are Distinct and Differentially Localized.
(A) Differential cleavage of transiently coexpressed cleavage site mutants of RIN4 in the presence of AvrRpt2. N. benthamiana leaves were coinfiltrated with Agrobacterium expressing AvrRpt2:HA (OD600 = 0.1) and various cleavage site mutants of T7:RIN4 (OD600 = 0.4). Twenty-four hours after coinfiltration, 7-mm leaf punches were harvested, ground in 3× Laemmli buffer, and analyzed by SDS-PAGE, followed by protein gel blotting with anti-RIN4 antibodies. Protein molecular mass sizes are shown at the left of the blot. The N-terminal T7-epitope tag on RIN4 was used to identify the N-terminal cleavage product released by coexpression with AvrRpt2. Parallel, duplicate samples were harvested at 24 h, homogenized in immunoprecipitation buffer (see Methods), and processed according to standard immunoprecipitation protocols as described in this study. Detection of the cleaved ∼1-kD T7-tagged N terminus was achieved by protein gel blot analysis using α-T7-HRP conjugated antibodies. The absence of a detectable RIN4 cleavage product in K150A/F151A + AvrRpt2 likely represents a lack of protein stability after cleavage at the C-terminal cleavage site.
(B) All cleavage site mutants retain their ability to negative regulate RPS2 activation. Top row: leaf segments in the absence of AvrRpt2 coinfiltration. Note that all cleavage site mutants of RIN4 retain the ability to block the RPS2-induced HR. Bottom row: leaf phenotypes in the presence of AvrRpt2 coinfiltration. RPS2 and AvrRpt2 control inoculations are shown as indicated. Leaf segments were photographed 24 h postinoculation.
To address the biological significance of these amino acid changes with regard to RIN4 function, mutations were also made within the context of the full-length RIN4 protein. As was observed with respect to the RIN4-C clone, the full-length RIN4 protein behaved similarly with regard to RPS2 negative regulation when both mutations were made in RCS2. However, changes in RCS2 inhibited elimination of RIN4 when coexpressed in the presence of AvrRpt2.
To test the hypothesis that cleavage of RIN4 results in a loss of association with RPS2, or repression of RPS2 activity, we made constructs corresponding to the three RIN4 cleavage products presumably generated by AvrRpt2 proteolysis (Figure 2, RIN4-CLV1/CLV2/CLV3). As expected, when coexpressed by Agrobacterium-mediated expression in N. benthamiana with RPS2, none of the cleavage products were capable of negatively regulating RPS2 activity (see Supplemental Figure 1B online; data not shown). This result strengthens the hypothesis that cleavage of RIN4 leads to a loss in the negative regulation imposed on RPS2 by RIN4.
RPS2–RIN4 Association Requires the Terminal Nine Amino Acids of RIN4
By employing a series of deletion analyses as well as an error-prone PCR mutagenesis approach, we successfully generated >250 RIN4 mutants (relevant ones shown; see supplemental data online) that could quickly be screened for their abilities to abrogate RPS2 activity and to coimmunoprecipitate RPS2. Using a stepwise deletion approach, we found that the removal of as few as two amino acids from the C terminus reduced regulation of RPS2 activity by RIN4 (Figure 5A). Deletion of the terminal two residues (i.e., G210 and K211) resulted in a slight, albeit noticeable, loss in RIN4's ability to regulate RPS2 activity. More striking, however, stepwise deletions of six (RIN4Δ6; 206-FGFGGK-211) and nine (RIN4Δ9; 203-CCCFGFGGK-211) amino acids from the terminus of RIN4 resulted in a complete loss of the regulatory activity of RIN4 on RPS2 (Figure 5A). Consistent with our hypothesis that RIN4 cleavage by AvrRpt2 is distinct from the regulatory activity of RIN4, all of the C-terminal deletion mutants were susceptible to AvrRpt2 cleavage (Figure 5).
Figure 5.
The C-Terminal Amino Acids in RIN4 Are Required for Regulating RPS2 Activation.
Stepwise deletions of two, six, and nine amino acids from the C terminus of RIN4, as well as a single amino acid change (A180P and C203R) results in a gradual (stepwise) or complete (A180P and C203R) loss in the regulatory function of RIN4 activity.
(A) N. benthamiana leaves were coinfiltrated with Agrobacterium expressing AvrRpt2:HA (OD600 = 0.1) and various C-terminal deletion mutants of T7:RIN4 (OD600 = 0.4). Top row: constructs infiltrated in the absence of Agrobacterium:AvrRpt2:HA. Bottom row: C-terminal deletion constructs coinfiltrated in the presence of AvrRpt2:HA. Control infiltrations (RPS2:HA and AvrRpt2:HA) are shown as indicated. All leaf segments were photographed 24 h postinoculation.
(B) Protein gel blot analysis of deletion and single site mutants alone and in the presence of AvrRpt2:HA.
(C) Coimmunoprecipitation: T7:RIN4C203R does not associate with RPS2:HA in planta.
Through our PCR-based approach, we identified complementary mutations that mimicked the effects of the deletion series at the C terminus of RIN4 as well. Single amino acid changes were generated in Cys203, Cys205, and ΔLys211 by error-prone PCR mutagenesis. When transiently coexpressed in N. benthamiana with RPS2, none of these proteins were capable of negatively regulating RPS2 activity. In addition to C-terminal amino acid changes and deletions that affected RIN4 activity on RPS2, one additional amino acid substitution, A180P, resulted in a protein that also was not able to regulate RPS2. Expression levels of this protein in N. benthamiana were comparable to wild-type RIN4, suggesting the protein was stable. However, we cannot assess whether the introduction of a Pro at this residue could simply be disruptive to the RIN4–RPS2 association via a gross alteration in RIN4 tertiary structure. In total, >10 mutant RIN4 proteins were analyzed that had mutations in the last nine amino acids (i.e., CCCFGFGGK), and all exhibited a loss in ability to negatively regulate RPS2 activity, further supporting the possibility that these residues are required for RPS2 regulation and association. Interestingly, localization of RIN4 and RIN4 Δ2, Δ6, and Δ9 expressed in N. benthamiana shows a gradual shift in membrane (i.e., insoluble) targeting (see Supplemental Figure 2 online) depending on the number of amino acids deleted from the C terminus. This result suggests that the extreme C terminus of RIN4 may be important for localization and that there may be a motif present in the terminal nine amino acids that serves as a substrate for possible posttranslational modifications and/or membrane targeting (see Discussion).
Mutations in RIN4 were also identified that had multiple amino acid changes within the C-terminal half of the protein. Of these, only proteins that had single, or multiple, changes within the last nine residues were impaired in their ability to regulate RPS2.
RIN4C203R and RIN4Δ9aa Are Unable to Associate with RPS2 in Planta
To further the hypothesis that the regulatory activity associated with RIN4 on RPS2 is due to a physical association between the two proteins, we performed coimmunoprecipitation experiments using RPS2:HA and two of the mutant T7:RIN4. As shown in Figure 5C, T7:RIN4(C203R) is unable to coimmunoprecipitate RPS2:HA. As a second confirmation in determining the significance of the terminal amino acids in RIN4, we also tested whether a deletion construct of the last nine amino acids in RIN4 also could interact with RPS2:HA. Again, this construct (i.e., RIN4Δ9aa) was unable to pull down RPS2:HA in reciprocal coimmunoprecipitation experiments (data not shown). Taken together with the results of the phenotypic assays (Figure 5A), we hypothesize that (1) RPS2 and RIN4 association requires residues within the nine terminal amino acid tail, and (2) this association is required for the regulation of RPS2 activity by RIN4.
RPS2 Regulation by RIN4 in Arabidopsis Is Analogous to the Heterologous Expression in N. benthamiana
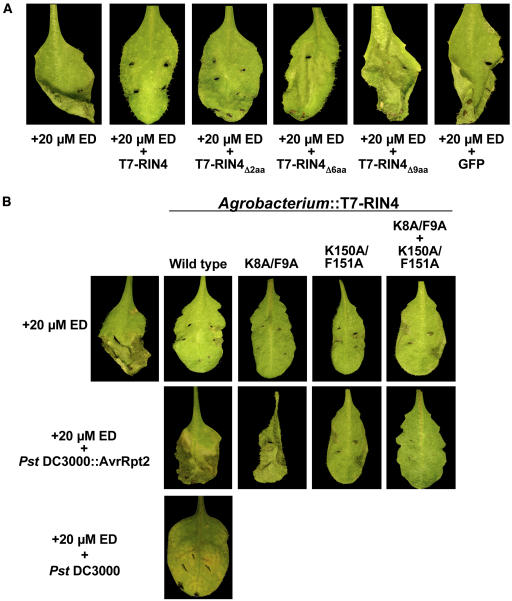
Next, as an initial step in translating the results obtained thus far back into Arabidopsis, we used an estradiol-inducible RPS2 Arabidopsis line (rps2/101C background) to study the phenotypic response of transient overexpression of RIN4 in parallel with the inducible expression of RPS2. Multiple independent homozygous pER8-RPS2:HA lines were identified that showed a clear induction of RPS2 after infiltration of 20 μM estradiol (ED), as assayed by HR and immunoblots (data not shown). When full-length RIN4 was coinfiltrated with ED, the induction of RPS2 was observed; however, RPS2 was held inactive due to its association with, and regulation by, RIN4 (Figure 6). A negative control coinoculation consisting of 20 μM ED plus A. tumefaciens expressing rGFP resulted in complete leaf collapse and cell death (Figure 6A, last panel). Coinfiltration of ED with Agrobacterium carrying T-DNAs for expression of T7:RIN4 Δ2, Δ6, or Δ9 failed to abrogate the effects of RPS2 activation and subsequent onset of the HR (Figure 6). The RCS mutants T7:RIN4K8A/F9A, K150A/F151A, and K8A/F9A/K150A/F151A were also able to abrogate the RPS2-induced HR when delivered via Agrobacterium in the presence of 20 μM ED (Figure 6B, top panels 3 to 5).
Figure 6.
Transient Overexpression of RIN4 in ED-Inducible RPS2:HA Arabidopsis Plants Blocks Induction of the RPS2-Mediated HR.
Recapitulation of abrogation/regulation experiments from N. benthamiana transient assays in Arabidopsis (rps2/pER8:RPS2:HA).
(A) Addition of 20 μM ED induces expression of RPS2:HA, followed by subsequent activation of the HR leading to cell death (first leaf panel). Coinoculation with various Agrobacterium-RIN4 clones results in the complete block of RPS2 activation (+20 μM ED + T7:RIN4) to a loss of negative regulation (second to fourth panels). Coinfiltration of ED in the presence of Agrobacterium expressing green fluorescent protein mimics RPS2 induction in the absence of RIN4 (control, fifth panel). Inoculation of rps2/pER8 vector only control plants with 20 μM ED showed no response (i.e., no HR; data not shown).
(B) Top row: induction (first panel) and abrogation (second panel) of the RPS2-mediated HR via transient expression in Arabidopsis by wild-type RIN4. Middle row: delivery of AvrRpt2 via the TTSS of Pseudomonas syringae pv tomato (Pst) results in the elimination of RIN4, release of the negative regulation of RPS2, and subsequent restoration of the HR (first panel). Bottom row: coinoculation of 20 μM ED and Pst DC3000 (–AvrRpt2) shows the onset of disease symptoms, evidenced by an absence of both pathogen recognition and the loss in induction of the RPS2-mediated HR. Results of cleavage site mutants are shown as indicated. Corresponding protein gel blot analysis is shown in Supplemental Figure 3 online.
To demonstrate the correlation between RIN4 disappearance and the release of negative regulation on RPS2, we further developed the use of the ED transient expression system in Arabidopsis to monitor both RIN4 elimination and RPS2 activation after delivery of AvrRpt2 via the TTSS of P. syringae pv tomato DC3000 (Pst). We first infiltrated plants with 20 μM ED and Agrobacterium delivering T7:RIN4. After a 24-h incubation, plants were hand inoculated with 104 colony-forming units/mL Pst expressing AvrRpt2. As shown in Figure 6B (middle row), restoration of the RPS2-mediated HR was observed (first panel), demonstrating both that the transiently expressed T7:RIN4 was eliminated by AvrRpt2 (protein gel blot data; see Supplemental Figure 3A online) and that this elimination of RIN4 relieves the negative regulatory effect RIN4 imposes on RPS2 activity.
Finally, we demonstrated that RIN4 cleavage site mutants could regulate RPS2 in the presence of AvrRpt2 (Figure 6B, middle row, panels 2 to 4). The RIN4 K8A/F9A mutant was eliminated in the presence of AvrRpt2 and therefore did not negatively regulate RPS2. RIN4 K150A/F151A and K8A/F9A/K150A/F151A were able to negatively regulate transiently induced RPS2:HA even after infection by Pst expressing AvrRpt2 as is evidenced by the absence of RPS2 activation and subsequent HR after delivery of AvrRpt2. Full disease symptoms were observed in both the K150A/F151A- and K8A/F9A/K150A/F151A-expressing Arabidopsis plants ∼4 d postinoculation with Pst DC3000 expressing AvrRpt2 (data not shown). Supporting protein gel blot data confirm the presence of the T7:RIN4 cleavage mutant constructs 24 h after delivery of AvrRpt2 by Pst DC3000 (see Supplemental Figure 3B online).
DISCUSSION
In this study, we demonstrate that the C terminus of RIN4 is required for association with, and regulation of, RPS2 and that this regulatory region is structurally distinct from that of AvrRpt2 cleavage sites localized in both the N and C termini of RIN4. Our initial focus was to identify critical amino acid residues in RIN4 that are required for regulating the activation of RPS2 and, as a likely prerequisite for this regulation, to identify those residues that are required for the physical association of RPS2 and RIN4. Using deletion analyses as well as error-prone PCR mutagenesis, we identified two distinct regions in RIN4 that appear to be required for RPS2 regulation. Using an N. benthamiana expression system, we have successfully demonstrated that a significant portion of the Arabidopsis RPS2-mediated resistance pathway can be recapitulated using transient expression in N. benthamiana. This cross-species reproducibility is significant given the rapidity with which these experiments can be performed using N. benthamiana as a host expression system. We screened in excess of 250 RIN4 mutants to determine alterations in their ability to both regulate RPS2 activation as well as to physically associate with RPS2 in planta. Furthermore, experiments in Arabidopsis confirmed the results observed in the heterologous system. This work further characterizes both the genetic and physical association of RPS2 and RIN4, defining not only the activation of RPS2, but also the negative regulation of RPS2 by RIN4.
The negative regulatory activity RIN4 imposes on RPS2 was first identified in a series of experiments that determined that the lethality of a rin4 mutant can be overcome in a rin4 rps2 double mutant (Mackey et al., 2003). Thus, we can infer that RIN4 holds RPS2 in an inactive state in the absence of the bacterial effector protein AvrRpt2. Upon delivery to the plant cell, AvrRpt2 specifically eliminates RIN4, resulting in the activation of RPS2, leading to the HR and disease resistance. These events provided the first genetic explanation for the requirement of RIN4 in preventing the activation of an R protein in the absence of its cognate bacterial effector protein (Belkhadir et al., 2004a).
Together with the fact that RPS2 and RIN4 associate in planta (Axtell et al., 2003) and that the overexpression of RPS2 in N. benthamiana leads to the initiation of a rapid HR phenotype (Jin et al., 2002), we hypothesized that a genetic screen designed to identify RIN4 mutants that were no longer capable of suppressing this HR could lead to the identification of important amino acid residues in RIN4 that are required for the association of RIN4 with RPS2. Indeed, as is shown in Figures 1 and 3, we have shown that the genetic and physical association between RIN4 and RPS2 observed in N. benthamiana is a valid system for studying the RPS2-mediated defense response.
The C Terminus of RIN4 Is Required for the Negative Regulation of RPS2
Our results further demonstrate that the C terminus of RIN4 is required for the negative regulation of RPS2 and, moreover, the association of these two proteins in planta. Based on our findings, however, we cannot conclude that amino acid residues within the C terminus of RIN4 serve as contact points between RPS2 and RIN4 or that they are merely required for this association. In fact, based on our mutation analyses of the amino acids within the C terminus of RIN4 that we identified as being required for RPS2 regulation and association with RPS2, we hypothesize that the Cys repeats (i.e., amino acids 203 to 205) may play a critical role in establishing the subcellular localization of RIN4 in the plant cell. Localization data (see Supplemental Figure 2 online) confirms that stepwise deletions of two, six, and nine amino acids from the C terminus of RIN4 alter its membrane localization. These data complement our results obtained from mutation analyses of the amino acids within the C terminus of RIN4 that we identified as being required for RPS2 regulation and association and suggests that membrane localization of RIN4 is required for association with RPS2. Taken together, we hypothesize that the Cys repeats (i.e., amino acids 203 to 205) may play a role in driving the subcellular localization of RIN4. In fact, a comparison of the Cys motifs present in the C terminus of RIN4 with analogous sequences in other membrane-targeted proteins suggests that these repeats in RIN4 could very likely serve as substrates for posttranslational modifications, such as _N_- or _S_-palmitoylation and/or prenylation (Resh, 2004; Smotrys and Linder, 2004). Indeed, Cys string motifs are well-documented substrates for the addition of both _N_- and _S_-palmitoyl fatty acids (Gundersen et al., 1994; Lane and Liu, 1997). Although there is no well-defined consensus sequence for these modifications other than the absolute requirement for Cys, our data demonstrating that a single amino acid change (i.e., C203R) inhibits the RPS2–RIN4 association are consistent with the possibility that the Cys string within the C terminus of RIN4 may in fact serve as a substrate for posttranslational modifications, such as palmitoylation and/or prenylation.
RIN4 Cleavage by AvrRpt2 Is Required for RPS2 Activation
The overall mechanism(s) by which AvrRpt2-mediated elimination of RIN4 leads to the activation of RPS2 is poorly understood. AvrRpt2 encodes a Cys protease (Axtell et al., 2003; Coaker et al., 2005) that eliminates RIN4, resulting in the destabilization of a RPS2-RIN4–containing complex, thereby leading to defense signaling. Jones and Takemoto (2004) noted that there are amino acid motifs in the N and C termini of RIN4 that are homologous to sequences adjacent to the cleavage site identified in AvrRpt2 (Mudgett and Staskawicz, 1999) and proposed that these RIN4 motifs are cleavage sites for AvrRpt2. Indeed, recent work has demonstrated that AvrRpt2 cleaves RIN4 at these sites in vitro (Coaker et al., 2005). In vitro, two RIN4 cleavage products are detected after incubation with active recombinant AvrRpt2 protein: the N terminus of RIN4 and an internal fragment of RIN4 between RCS1 and RCS2 (Coaker et al., 2005). The C-terminal fragment of RIN4 cannot be detected in vitro upon cleavage by AvrRpt2 and is likely unstable (Coaker et al., 2005). To assess RIN4 cleavage by AvrRpt2 in planta, we constructed a series of RIN4 mutant proteins. RIN4 RCS1 mutants were eliminated in the presence of AvrRpt2 in planta. We were able to detect two cleavage products generated from the RIN4 RCS2 mutant protein in the presence of AvrRpt2. These fragments corresponded to the approximate size of those that would be generated from a single cleavage event at RCS1. An identical result was obtained when RIN4 RCS2 mutant protein was incubated with AvrRpt2 in vitro (Coaker et al., 2005). Thus, it appears that upon cleavage by AvrRpt2 at RCS2, RIN4 is eliminated in planta. It is likely that cleavage at this site serves as a signal for RIN4 degradation in planta.
Here, we provide a detailed analysis of the RPS2–RIN4 association, identifying amino acid residues in RIN4 required for this association, and further determine that cleavage sites present in both the N and C termini are distinct from regions of RIN4 required for the regulation of RPS2. Although our data suggest that the cleavage and subsequent elimination of RIN4 by AvrRpt2 is functionally independent of the physical/structural requirement imposed by those amino acid residues that are necessary to regulate RPS2, we cannot exclude the possibility that there exists a complementary structural requirement. The primary focus of this study was to define the regulatory mechanism (i.e., RPS2-interacting sites) that exists in RIN4 and to distinguish these sites from AvrRpt2 cleavage sites. Our data suggest that RIN4 elimination (by AvrRpt2) and regulation (of RPS2) are two events that can occur independently of the other. However, the possibility exists that although cleavage and regulation are two distinct events, they may in fact be more intricately associated than we have investigated in this study. At present, our data seem to indicate that the RIN4 cleavage products are themselves not capable of negatively regulating RPS2, further supporting the hypothesis that cleavage of RIN4 by AvrRpt2 is required for activation of RPS2. However, it is possible that, with regard to the second cleavage site, there exists some overlap in the residues that bind RPS2 and those that are targeted by AvrRpt2. One of many possible scenarios is that cleavage of RIN4 by AvrRpt2 may induce a conformational change in the RPS2–RIN4 association that may ultimately lead to the release and elimination of RIN4 from RPS2, subsequently leading to the activation of RPS2. However, more work is needed to fully define the stoichiometry and regulatory mechanisms associated with the RPS2–RIN4 association.
Several plant disease R genes encode proteins predicted to have an N-terminal coiled-coil domain, a central putative nucleotide binding site domain, and a C-terminal Leu-rich repeat domain (reviewed in Dangl and Jones, 2001). These coiled-coil–nucleotide binding site–leucine-rich repeat (NB/LRR) proteins, including RPS2, recognize specific pathogen-derived effector proteins delivered by the type III secretion machinery and initiate a resistance response that often includes a type of cell death known as the HR. Recent studies have demonstrated that activation of the R protein–containing complex is due to the disruption of intramolecular interactions (Moffett et al., 2002; Leister et al., 2005). Moreover, these studies have also revealed the requirement for auxiliary proteins, including SGT1 (Takahashi et al., 2003), RAR1 (Tornero et al., 2002), and HSP90 (Hubert et al., 2003; Liu et al., 2004), further building support for the presence of multimeric R protein complexes. Indeed, silencing of SGT1 and HSP90 in N. benthamiana blocks both the HR-inducing activity of RPS2 as well as the physical association of RPS2 and RIN4 as determined by coimmunoprecipitation experiments (B. Day and B.J. Staskawicz, unpublished data).
In the second part of this study, we focused our efforts on the characterization of how cleavage sites present in RIN4 affect its ability to regulate RPS2 activity. Mutational analyses of these sites proved successful in allowing us to differentiate amino acid residues required for the elimination of RIN4 by AvrRpt2 (Chisholm et al., 2005) from those that are essential for the regulatory activity RIN4 imposes on RPS2. By mutating residues within the N-terminal cleavage site (site 1) of RIN4, we noted that elimination of the RIN4 protein in the presence of AvrRpt2 was similar to that as observed when wild-type RIN4 was used (Figure 4A, compare lanes 1 and 2 with lanes 7 and 8). However, when alterations were made to the residues within the C-terminal cleavage site (site 2), the RIN4 protein was not eliminated, but rather resulted in the generation of two cleavage products whose sizes corresponded to the expected fragment sizes generated by a single cleavage event at amino acids 8 and 9 (Figure 4A, compare lanes 1 and 2 with lanes 9 and 10). These results suggest that although two sites are present in RIN4, only cleavage at the second site is absolutely required for RIN4 elimination. As such, it is therefore a reasonable hypothesis that cleavage at this position could very likely disrupt the RPS2–RIN4 association, specifically within this C-terminal domain of RIN4.
RPS2 Activation Is Inhibited by the Transient Overexpression of RIN4 in Arabidopsis
The reconstruction of the transient expression experiments performed in N. benthamiana in Arabidopsis will ultimately provide us with a more absolute understanding of both the genetic and physical requirements for RIN4 regulation of RPS2 activation. To this end, we constructed a homologous transient expression system in Arabidopsis. Using an ED-inducible system (Zuo et al., 2000) to drive the expression of RPS2, we were able to recapitulate the experiments from N. benthamiana in Arabidopsis. In this regard, we have demonstrated that the simultaneous transient expression of T7:RIN4 by A. tumefaciens infection together with ED induction of RPS2 provided us with an excellent tool for testing the effect of our RIN4 deletion constructs on their ability to regulate RPS2 expression in Arabidopsis. As observed in the transient N. benthamiana expression system, C-terminal RIN4 deletion constructs (i.e., Δ9, Δ6, and Δ2 amino acids) were impaired in their ability to regulate RPS2 activation in Arabidopsis, as is evidenced by the initiation of the HR (Figure 6A). In addition to demonstrating the biological association, whether direct or indirect, between RIN4 and RPS2, we also demonstrate that RIN4's association with AvrRpt2 was functional in the transient Arabidopsis model as well. As shown in Figure 8B, when RIN4 is delivered by Agrobacterium in the presence of ED-induced RPS2, the transient induction of the HR is blocked. Interestingly, when Pst expressing AvrRpt2 is infiltrated into Arabidopsis leaves (i.e., 2 d after Agrobacterium delivery of RIN4), the HR is restored (Figure 6B, middle row, first panel), coinciding with the elimination of RIN4 (protein gel blot analysis; see Supplemental Figure 3 online) and, thus, the release of negative regulation of RPS2.
Ultimately, the goal of this research is to elucidate the mechanism(s) of R protein activation in response to pathogen infection. In this study, we have chosen to approach this problem from the standpoint of elucidating the mechanism(s) of regulation of R protein activation by investigating the physical association of RIN4 and RPS2. To this end, we have been successful in elucidating two critical aspects of RPS2 activation: RIN4 association with RPS2 and release of this negative regulation by AvrRpt2. It will be important to define the region(s) of RPS2 to which RIN4 binds. A recent article by Zhang et al. (2004) describing the transient activation of RPS4, a TIR-NB-LRR protein, has shown that RPS4 activity, when transiently expressed in N. benthamiana, requires the TIR-NB portion of the protein, whereas the LRR domain appears to be largely dispensable with regard to the HR-inducing activity. Similarly, the LRR domain of RPS2 has been shown to be dispensable for the HR induced by RPS2 overexpression (Tao et al., 2000), supporting the hypothesis that the LRR region may function in determining R gene specificity (Jia et al., 2000; Dodds et al., 2001). We are now in the process of identifying which region of RPS2 interacts with RIN4. By furthering these studies with regard to the RPS2–RIN4 association, we can now begin to specifically address the activation of RPS2 in terms of the modular structure/function relationship of R protein activity. Clearly, this will be an important part in further understanding the activity of not only RPS2, but the function and activation of other R proteins as well.
METHODS
Strains and Growth
Escherichia coli strains DH5α and Top10′ were grown on Luria-Bertani agar medium, as were binary constructs mobilized in Agrobacterium tumefaciens strain C58-C1, at 37°C and 28°C, respectively. Binary vector constructs were mobilized into A. tumefaciens by triparental matings according to standard protocols.
Nicotiana benthamiana plants were grown at 24°C in a growth cabinet under a 16-h-light/8-h-dark cycle.
Plasmid Construction
PCR was used to construct all epitope-tagged deletions and site-directed mutant constructs. For transient expression, RPS2, RIN4, and RIN4 deletion constructs were expressed from a modified pE1776 with a chimeric octopine and manopine synthase promoter engineered into a pBIN derivative (Ni et al., 1995). T7- and HA-epitope tags were added to all RPS2 and RIN4 constructs by PCR with gene-specific primers designed to incorporate a _Sal_I at the 5′ of each construct and the HA or FLAG epitope tag immediately after the final C-terminal amino acid of each construct, followed by a _Sac_I restriction site. The PCR products were subcloned into pCR-Blunt II TOPO (Invitrogen, Carlsbad, CA). RIN4-B and RIN4-C deletion constructs contain amino acids 1 to 106 and 107 to 211, respectively. All RIN4 clones were constructed with N-terminal T7-epitope tags. Primers used to construct Rps2:HA in p1776 were as follows: RPS2 forward, 5′-ctcgaggtcgactcgaaATGGATTTCATCTCATCTCTTATCGTTGGC-3′, RPS2 reverse, 5′-ctatgcgtacgcatagtcaggaacatcgtatgggtaGGGATAACTAGTATTTCCAACAAAGCGCGG-3′. (Note that nucleotides designated in lowercase are restriction sites added to facilitate cloning and are not part of native RPS2 or RIN4. In the case of RPS2, the HA-epitope tag sequence is underlined.) Primers for construction of RIN4, RIN4-N, RIN4-C, various deletion constructs, and site-directed mutants are as follows: RIN4 forward, 5′-gtcgacATGGCACGTTCGAATGTACCAAATTTGG-3′, and reverse, 5′-gagctcTCATTTTCCTCCAAAGCCAAAGCAGCAAC-3′; RIN4-N forward, 5′-gtcgacATGGCACGTTCGAATGTACCAAATTTGG-3′, and reverse, 5′-gagctcCTACTGAGAAGCTCTCCCTTGTCTTTTG-3′; RIN4-C forward, 5′-gtcgacATGAGAGCTTCTCAGAACAATAGT-3′, and reverse, 5′-gagctcTCATTTTCCTCCAAAGCCAAAGCAGCAA-3′; RIN4Δ2 reverse, 5′-gagctcTCATCCAAAGCCAAAGCAGCAACATGA-3′; RIN4Δ6 reverse, 5′-gagctcTCAGCAGCAACATGAGGAAGTGTTGTTC-3′; RIN4Δ9 reverse, 5′-gagctcTCATGAGGAAGTGTTGTTCGGGTTACGGG-3′; RIN4 K8A/F9A forward, 5′-gtcgacATGGCACGTTCGAATGTACCAGCAGCTGGAAACTGGGAAGCT-3′; RIN4 K150A/F151A forward, 5′-gtcgacGTCACAGTGGTGCCTGCAGCCGGTGACTGGGACGAGAACAACCC-3′.
Generation of single nucleotide changes, resulting in amino acid changes in the N- and C-terminal cleavage sites of RIN4, were performed using the Quick Change PCR mutagenesis kit (Stratagene, La Jolla, CA).
Construction of a modified pMD-1 (35S) binary vector harboring a T7-epitope tag was as follows: Primers corresponding to the T7-epitope tag (forward, 5′-tctagaATGGCTTCAATGACAGGTGGTCAACAAATGGGT-3′, and reverse, 5′-tctagaACCCATTTGTTGACCACCTGTCATTGAAGCCAT-3′) were mixed in equimolar concentrations, heated to 95°C, and then allowed to cool to room temperature. After cooling, primers were incubated for an additional hour at room temperature. The annealed primer mixture was ligated to _Xba_I-digested pMD-1, transformed into E. coli DH5α cells, and used as a cloning destination for all RIN4 constructs.
Sequence of all DNA constructs was confirmed by automated DNA sequencing using an ABI-3100 capillary DNA sequencer (Applied Biosystems, Foster City, CA).
Agrobacterium-Mediated Transient Expression
A. tumefaciens strain C58-C1 (pCH32) carrying the gene of interest expressed from the binary vector pMD-1 was infiltrated into leaves of N. benthamiana essentially as described by Tai et al. (1999). A. tumefaciens was grown overnight at 28°C on LB agar containing 100 μg/mL of rifampicin, 25 μg/mL of kanamycin, and 5 μg/mL of tetracycline. Cells were resuspended in induction media (10 mM Mes, pH 5.6, 10 mM MgCl2, and 150 μM acetosyringone) and incubated at room temperature for 1.5 h before inoculation. For RPS2:HA, A. tumefaciens was infiltrated at a final OD600 of 0.075. T7:RIN4 constructs were infiltrated at a final OD600 of 0.4. AvrRpt2:HA was infiltrated at a final OD600 of 0.1. In all cases, the final A. tumefaciens concentration infiltrated into plant leaves was normalized using an A. tumefaciens filler strain expressing pMD-1:GUS-intron to ensure that observed phenotypes were not due to differences in A. tumefaciens concentrations. Initial experiments were performed using both A. tumefaciens strains C58-C1 (armed) as well as D58-C1 (disarmed) to ensure that expressed proteins are the result of a successful plant genome integration event and not a result of bacterial expression.
Localization and Ultracentrifugation
N. benthamiana tissue (1.0 g) corresponding to Agrobacterium-infiltrated RIN4, RIN4Δ2, RIN4Δ6, and RIN4Δ9 was harvested at 15 h after infiltration. Leaf tissue was frozen in liquid nitrogen and ground to a fine powder. Samples were then transferred to a mortar and further homogenized in isolation buffer (250 mM Tris, pH 8.0, + 290 mM sucrose + 25 mM EDTA + 5 mM β-mercaptoethanol + 1 mM phenylmethylsulphonyl fluoride + 1× Complete protease inhibitor). Homogenized samples were precleared by centrifugation at 10,000_g_ for 20 min at 4°C. After centrifugation, a sample (∼500 μL) was removed and stored at −80°C. This sample is further defined as “total.” The remaining cleared sample was centrifuged in an ultracentrifuge at 100,000_g_ for 1 h at 4°C. After centrifugation, the resultant supernatant (i.e., “soluble”) was removed and stored at −80°C. The pellet (i.e., “membrane”) was resuspended in a volume of 3× Laemmli buffer equal to the original volume of the “total.” Before SDS-PAGE and protein gel blotting, samples were boiled for 5 min in 3× Laemmli buffer. SDS-PAGE and protein gel blot analyses were performed as described below.
Construction of ED-Inducible RPS2 Arabidopsis thaliana Plants
The RPS2 coding region was excised from plasmid pE1776-RPS2:His by restriction enzyme digestion using _Sal_I and _Spe_I. The ∼2.7-kb fragment was then ligated into vector pER8 (Zuo et al., 2000) digested with _Xho_I and _Spe_I to generate plasmid pER8-Rps2. Addition of an HA-epitope tag was as follows: HASpe2, 5′-ctagcTATCCGTACGACGTACCAGACTACGCAACTAGTTAAt-3′; HASpe3, 5′-ctagaTTAACTAGTTGCGTAGTCTGGTACGTCGTACGGATAg-3′.
Primers were mixed in equimolar concentrations, heated to 68°C, and then cooled at room temperature for 1 h to facilitate annealing. The annealed primers were ligated into _Spe_I-digested pER8-Rps2.
Ligation of the HA tag generated a C-terminal HA-tagged RPS2 in vector pER8. This plasmid, designated pER8-RPS2:HA, was mobilized in E. coli DH5α, then conjugated into A. tumefaciens strain GV3101 and used for Agrobacterium-mediated Arabidopsis transformation following standard protocols (Clough and Bent, 1998). rps2 mutant plants (i.e., 101C) were used as the genetic background for Arabidopsis transformations. Successful transformants were genotyped using PCR as an assay for monitoring transgene insertion with primers specific to the pER8 T-DNA.
ED was purchased from Sigma-Aldrich (St. Louis, MO) and applied at a final concentration of 20 μM, dissolved in 100% ethanol.
Immunoprecipitation
After Agrobacterium-mediated transient expression for 22 h, N. benthamiana leaves (∼0.3 g) were harvested and ground to a powder in liquid nitrogen. Ground tissues were resuspended in 3.0 mL of IP buffer (50 mM Hepes, pH 7.5, 50 mM NaCl, 10 mM EDTA, 5 mM DTT, 0.1% Triton X-100, and 1× Complete Protease Inhibitor [Roche, Mannheim, Germany]). The crude lysates were then spun at 20,000_g_ for 15 min at 4°C. After centrifugation, 1 mL of supernatant was used for each immunoprecipitation. Five microliters of either anti-HA (Covance, Princeton, NJ) or anti-T7 (Novagen, Madison, WI) antibody was used to capture the epitope-tagged proteins. After a 1-h incubation at 4°C, immunocomplexes were collected by the addition of 50 μL of protein G Sepharose-4 fast flow beads (Amersham, Piscataway, NJ) and incubated end-over-end for 4 h at 4°C. Immunocomplexes were then washed four times with 1 mL of wash buffer (IP buffer + 0.2% Triton X-100). After washing, the beads were resuspended in 3× SDS-PAGE loading buffer, boiled for 5 min, briefly centrifuged, and the supernatant removed for SDS-PAGE and protein gel blot analysis.
SDS-PAGE and Immunoblotting
Protein samples analyzed for experiments other than coimmunoprecipitation were isolated by homogenizing corresponding leaf disks in 3× Laemmli buffer (Laemmli, 1970) in a microfuge tube using a Kontes pestle (Fisher Scientific, Pittsburg, PA). Protein samples were separated by SDS-PAGE on 12% polyacrylamide gels and transferred for immunoblot analysis by electroblotting to nitrocellulose membranes according to standard protocols. Membranes were probed with anti-HA-HRP (Roche) or anti-T7-peroxidase (Novagen) to detect HA (RPS2 and AvrRpt2) and T7-epitope–tagged proteins (RIN4), respectively. All antibodies were used as recommended by their respective manufacturer. RIN4 polyclonal antiserum, where noted, was used at a 1:5000 dilution, as previously described (Axtell and Staskawicz, 2003; Mackey et al., 2003).
PCR Mutagenesis
Error-prone PCR mutagenesis was performed according to standard protocols (Vartanian et al., 1996), with slight modifications. Buffer conditions were as follows: 10 mM Tris-HCl, pH 8.3, + 50 mM KCl + 7 mM MgCl2 + 1 mM dCTP + 1 mM dTTP + 0.2 mM dATP + 0.2 mM dGTP. DNA template, pTOPO-RIN4C, was used at a final concentration of 40 pg/μL. M13 forward (−21) and M13 reverse primers were used to amplify mutagenized PCR products. Annealing and amplification conditions for PCR reactions were as follows: 94°C (1 min), 60°C (1 min), and 72°C (3 min), with a 20 cycle repetition. Amplified PCR products were digested with _Sal_I and _Sac_I restriction endonucleases and directly cloned into the pMD-1:T7 binary vector, transformed into E. coli DH5α, and subsequently conjugated into Agrobacterium strain C58-C1 as described above.
All DNA primers used in this study were purchased from Invitrogen.
Sequence data for all cDNAs from this article have been deposited with the EMBL/GenBank data libraries under the following accession numbers: RIN4, NM_113411; RPS2, NM_118742; AvrRpt2, Z21715.
Supplementary Material
[Supplemental Data]
Acknowledgments
This work was funded by Department of Energy Grant DE-FG03-88ER13917 and National Institutes of Health Grant R01 GM069680-01 to B.J.S. B.D. and S.T.C. were supported by National Institutes of Health-National Research Service Award Postdoctoral Fellowships. We thank all members of the Staskawicz lab for comments and critical reading of the manuscript.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Brian J. Staskawicz (stask@nature.berkeley.edu).
W⃞
Online version contains Web-only data.
References
- Axtell, M.J., Chisholm, S.T., Dahlbeck, D., and Staskawicz, B.J. (2003). Genetic and molecular evidence that the Pseudomonas syringae type III effector protein AvrRpt2 is a cysteine protease. Mol. Microbiol. 49**,** 1537–1546. [DOI] [PubMed] [Google Scholar]
- Axtell, M.J., and Staskawicz, B.J. (2003). Initiation of RPS2-specified disease resistance in Arabidopsis is coupled to the AvrRpt2-directed elimination of RIN4. Cell 112**,** 369–377. [DOI] [PubMed] [Google Scholar]
- Belkhadir, Y., Nimchuk, Z., Hubert, D.A., Mackey, D., and Dangl, J.L. (2004. a). Arabidopsis RIN4 negatively regulates disease resistance mediated by RPS2 and RPM1 downstream or independent of the NDR1 signal modulator and is not required for the virulence functions of bacterial type III effectors AvrRpt2 or AvrRpm1. Plant Cell 16**,** 2822–2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkhadir, Y., Subramaniam, R., and Dangl, J.L. (2004. b). Plant disease resistance protein signaling: NBS-LRR proteins and their partners. Curr. Opin. Plant Biol. 7**,** 391–399. [DOI] [PubMed] [Google Scholar]
- Chisholm, S.T., Dahlbeck, D., Krishnamurthy, N., Day, B., Sjolander, K., and Staskawicz, B.J. (2005). Molecular characterization of proteolytic cleavage sites in the Pseudomonas syringae effector AvrRpt2. Proc. Natl. Acad. Sci. USA 102**,** 2087–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough, S.J., and Bent, A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16**,** 735–743. [DOI] [PubMed] [Google Scholar]
- Coaker, G., Falick, A., and Staskawicz, B.J. (2005). A eukaryotic cyclophilin is required for the activation of a phytopathogenic bacterial effector protein. Science, in press. [DOI] [PubMed]
- Dangl, J.L., and Jones, J.D. (2001). Plant pathogens and integrated defence responses to infection. Nature 411**,** 826–833. [DOI] [PubMed] [Google Scholar]
- Deslandes, L., Olivier, J., Peeters, N., Feng, D.X., Khounlotham, M., Boucher, C., Somssich, I., Genin, S., and Marco, Y. (2003). Physical interaction between RRS1-R, a protein conferring resistance to bacterial wilt, and PopP2, a type III effector targeted to the plant nucleus. Proc. Natl. Acad. Sci. USA 100**,** 8024–8029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds, P.N., Lawrence, G.J., and Ellis, J.G. (2001). Six amino acid changes confined to the leucine-rich repeat beta-strand/beta-turn motif determine the difference between the P and P2 rust resistance specificities in flax. Plant Cell 13**,** 163–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar, C., Hernandez, L.E., Jimenez, A., Creissen, G., Ruiz, M.T., and Mullineaux, P.M. (2003). Transient expression of Arabidopsis thaliana ascorbate peroxidase 3 in Nicotiana benthamiana plants infected with recombinant potato virus X. Plant Cell Rep. 21**,** 699–704. [DOI] [PubMed] [Google Scholar]
- Gundersen, C.B., Mastrogiacomo, A., Faull, K., and Umbach, J.A. (1994). Extensive lipidation of a Torpedo cysteine string protein. J. Biol. Chem. 269**,** 19197–19199. [PubMed] [Google Scholar]
- He, X., Anderson, J.C., del Pozo, O., Gu, Y.Q., Tang, X., and Martin, G.B. (2004). Silencing of subfamily I of protein phosphatase 2A catalytic subunits results in activation of plant defense responses and localized cell death. Plant J. 38**,** 563–577. [DOI] [PubMed] [Google Scholar]
- Hubert, D.A., Tornero, P., Belkhadir, Y., Krishna, P., Takahashi, A., Shirasu, K., and Dangl, J.L. (2003). Cytosolic HSP90 associates with and modulates the Arabidopsis RPM1 disease resistance protein. EMBO J. 22**,** 5679–5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia, Y., McAdams, S.A., Bryan, G.T., Hershey, H.P., and Valent, B. (2000). Direct interaction of resistance gene and avirulence gene products confers rice blast resistance. EMBO J. 19**,** 4004–4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, H., Axtell, M.J., Dahlbeck, D., Ekwenna, O., Zhang, S., Staskawicz, B., and Baker, B. (2002). NPK1, an MEKK1-like mitogen-activated protein kinase kinase kinase, regulates innate immunity and development in plants. Dev. Cell 3**,** 291–297. [DOI] [PubMed] [Google Scholar]
- Jones, D.A., and Takemoto, D. (2004). Plant innate immunity—Direct and indirect recognition of general and specific pathogen-associated molecules. Curr. Opin. Immunol. 16**,** 48–62. [DOI] [PubMed] [Google Scholar]
- Laemmli, U.K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227**,** 680–685. [DOI] [PubMed] [Google Scholar]
- Lane, S.R., and Liu, Y. (1997). Characterization of the palmitoylation domain of SNAP-25. J. Neurochem. 69**,** 1864–1869. [DOI] [PubMed] [Google Scholar]
- Leister, R.T., Dahlbeck, D., Day, B., Li, Y., Chesnokova, O., and Staskawicz, B.J. (2005). Molecular genetic evidence for the role of SGT1 in the intramolecular complementation of Bs2 protein activity in Nicotiana benthamiana. Plant Cell 17**,** 1268–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y., Burch-Smith, T., Schiff, M., Feng, S., and Dinesh-Kumar, S.P. (2004). Molecular chaperone Hsp90 associates with resistance protein N and its signaling proteins SGT1 and Rar1 to modulate an innate immune response in plants. J. Biol. Chem. 279**,** 2101–2108. [DOI] [PubMed] [Google Scholar]
- Mackey, D., Belkhadir, Y., Alonso, J.M., Ecker, J.R., and Dangl, J.L. (2003). Arabidopsis RIN4 is a target of the type III virulence effector AvrRpt2 and modulates RPS2-mediated resistance. Cell 112**,** 379–389. [DOI] [PubMed] [Google Scholar]
- Mackey, D., Holt, B.F., Wiig, A., and Dangl, J.L. (2002). RIN4 interacts with Pseudomonas syringae type III effector molecules and is required for RPM1-mediated resistance in Arabidopsis. Cell 108**,** 743–754. [DOI] [PubMed] [Google Scholar]
- Moffett, P., Farnham, G., Peart, J., and Baulcombe, D.C. (2002). Interaction between domains of a plant NBS-LRR protein in disease resistance-related cell death. EMBO J. 21**,** 4511–4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudgett, M.B., and Staskawicz, B.J. (1999). Characterization of the Pseudomonas syringae pv. tomato AvrRpt2 protein: Demonstration of secretion and processing during bacterial pathogenesis. Mol. Microbiol. 32**,** 927–941. [DOI] [PubMed] [Google Scholar]
- Ni, M., Cui, D., Einstein, J., Narasimhulu, S., Vergara, C.E., and Gelvin, S.B. (1995). Strength and tissue specificity of chimeric promoters derived from the octopine and mannopine synthase genes. Plant J. 7**,** 661–676. [Google Scholar]
- Resh, M.D. (2004). Membrane targeting of lipid modified signal transduction proteins. Subcell. Biochem. 37**,** 217–232. [DOI] [PubMed] [Google Scholar]
- Scofield, S.R., Tobias, C.M., Rathjen, J.P., Chang, J.H., Lavelle, D.T., Michelmore, R.W., and Staskawicz, B.J. (1996). Molecular basis of gene-for-gene specificity in bacterial speck disease of tomato. Science 274**,** 2063–2065. [DOI] [PubMed] [Google Scholar]
- Shirasu, K., and Schulze-Lefert, P. (2003). Complex formation, promiscuity and multi-functionality: Protein interactions in disease-resistance pathways. Trends Plant Sci. 8**,** 252–258. [DOI] [PubMed] [Google Scholar]
- Smotrys, J.E., and Linder, M.E. (2004). Palmitoylation of intracellular signaling proteins: Regulation and function. Annu. Rev. Biochem. 73**,** 559–587. [DOI] [PubMed] [Google Scholar]
- Staskawicz, B.J., Mudgett, M.B., Dangl, J.L., and Galan, J.E. (2001). Common and contrasting themes of plant and animal diseases. Science 292**,** 2285–2289. [DOI] [PubMed] [Google Scholar]
- Tai, T.H., Dahlbeck, D., Clark, E.T., Gajiwala, P., Pasion, R., Whalen, M.C., Stall, R.E., and Staskawicz, B.J. (1999). Expression of the Bs2 pepper gene confers resistance to bacterial spot disease in tomato. Proc. Natl. Acad. Sci. USA 96**,** 14153–14158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, A., Casais, C., Ichimura, K., and Shirasu, K. (2003). HSP90 interacts with RAR1 and SGT1 and is essential for RPS2-mediated disease resistance in Arabidopsis. Proc. Natl. Acad. Sci. USA 100**,** 11777–11782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao, Y., Yuan, F., Leister, R.T., Ausubel, F.M., and Katagiri, F. (2000). Mutational analysis of the Arabidopsis nucleotide binding site-leucine-rich repeat resistance gene RPS2. Plant Cell 12**,** 2541–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornero, P., Chao, R.A., Luthin, W.N., Goff, S.A., and Dangl, J.L. (2002). Large-scale structure-function analysis of the Arabidopsis RPM1 disease resistance protein. Plant Cell 14**,** 435–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Biezen, E.A., and Jones, J.D.G. (1998). Plant disease resistance proteins and the “gene-for-gene” concept. Trends Biochem. Sci. 23**,** 454–456. [DOI] [PubMed] [Google Scholar]
- Van der Hoorn, R.A., De Wit, P.J., and Joosten, M.H. (2002). Balancing selection favors guarding resistance proteins. Trends Plant Sci. 7**,** 67–71. [DOI] [PubMed] [Google Scholar]
- Vartanian, J.P., Henry, M., and Wain-Hobson, S. (1996). Hypermutagenic PCR involving all four transitions and a sizeable proportion of transversions. Nucleic Acids Res. 24**,** 2627–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y., Dorey, S., Swiderski, M., and Jones, J.D.G. (2004). Expression of RPS4 in tobacco induces an AvrRps4-independent HR that requires EDS1, SGT1 and HSP90. Plant J. 40**,** 213–224. [DOI] [PubMed] [Google Scholar]
- Zuo, J., Niu, Q.W., and Chua, N.H. (2000). Technical advance: An estrogen receptor-based transactivator XVE mediates highly inducible gene expression in transgenic plants. Plant J. 24**,** 265–273. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
[Supplemental Data]