Human T-Cell Leukemia Virus Type 1 Tax and Cell Cycle Progression: Role of Cyclin D-cdk and p110Rb (original) (raw)
Abstract
Human T-cell leukemia virus type 1 is etiologically linked to the development of adult T-cell leukemia and various human neuropathies. The Tax protein of human T-cell leukemia virus type I has been implicated in cellular transformation. Like other oncoproteins, such as Myc, Jun, and Fos, Tax is a transcriptional activator. How it mechanistically dysregulates the cell cycle is unclear. Previously, it was suggested that Tax affects cell-phase transition by forming a direct protein-protein complex with p16INK4a, thereby inactivating an inhibitor of G1-to-S-phase progression. Here we show that, in T cells deleted for p16INK4a, Tax can compel an egress of cells from G0/G1 into S despite the absence of serum. We also show that in undifferentiated myocytes, expression of Tax represses cellular differentiation. In both settings, Tax expression was found to increase cyclin D-cdk activity and to enhance pRb phosphorylation. In T cells, a Tax-associated increase in steady-state E2F2 protein was also documented. In searching for a molecular explanation for these observations, we found that Tax forms a protein-protein complex with cyclin D3, whereas a point-mutated and transcriptionally inert Tax mutant failed to form such a complex. Interestingly, expression of wild-type Tax protein in cells was also correlated with the induction of a novel hyperphosphorylated cyclin D3 protein. Taken together, these findings suggest that Tax might directly influence cyclin D-cdk activity and function, perhaps by a route independent of cdk inhibitors such as p16INK4a.
Human T-cell leukemia virus type 1 (HTLV-1) is the etiological agent for adult T-cell leukemia and various neurological disorders termed HTLV-1-associated myelopathy (HAM) and tropical spastic paraparesis (TSP) (reviewed in reference 36). HTLV-1 encodes a 40-kDa _trans_-activator protein, Tax, which activates transcription through three 21-bp cyclic AMP responsive elements found in the viral long terminal repeat (LTR; 6, 10, 22, 39, 78). Tax has been implicated as the critical viral protein for transformation of T cells (90). Tax induces tumors and leukemia in transgenic mice in vivo and immortalizes cultured T cells (1, 4, 24, 25, 28, 29, 38, 54, 59, 69, 75).
The exact mechanism through which Tax exerts its oncogenic potential is not known. It is known, however, that Tax can modulate the expression of several cellular genes that are involved in cellular proliferation. For example, Tax upregulates the expression of interleukin-2, interleukin-2 receptor, c-fos, c-Jun, erg-1, and granulocyte-macrophage colony-stimulating factor (1, 4, 24, 25, 38, 54, 69, 75). Tax can also repress the expression of β-polymerase, the p53 promoter, and some functions of c-myc and Bax (7, 40, 71, 85). Additionally, Tax can cooperate with oncoproteins, such as Ras, in cellular transformation (65) and can induce morphological changes in cells via its association with intermediate filaments (83). Tax also associates with other cellular proteins (42, 60; reviewed in reference 23). How these findings fit together into the transformation process remains to be investigated further.
Cell cycle dysregulation is a hallmark of transformed cells (reviewed in references 35, 37, and 64). In this regard, many viral oncoproteins target components of the cell cycle, thus impairing orderly phase progression. Normal transition from one phase of the cell cycle to the next is regulated at “checkpoints” which are, in part, governed by cyclin-dependent kinases (CDKs) assembled with partner cyclins. Active CDK-cyclin complexes are further regulated by phosphorylation-dephosphorylation events mediated through CDK-activating kinases and phosphatases. CDKs can also be inactivated through physical binding with CDK inhibitory proteins (CKIs) (reviewed in references 27, 37, 55, 58, 63, 72, and 74).
D- and E-type cyclins are important in cell cycle transition from G1 to S (reviewed in references 27, 72, and 73). In this regard, the cyclin D-cdk complex is believed to function by specific phosphorylation of the retinoblastoma tumor suppressor gene product (pRb) (51, 68). Hypophosphorylated pRb binds members of the E2F transcription factor family, leading to inactivation of E2F function and reduced expression of genes (such as dihydrofolate reductase, DNA polymerase α, and cyclins) that are critical for S-phase events (reviewed in references 61 and 73). Hyperphosphorylation of pRb disables its E2F-binding, thereby influencing the passage of cells from G1 into S and cellular proliferation (14, 20). Thus, regulation of pRb phosphorylation by cyclin D-cdk and CDK inhibitors such as p16INK4a, p21CIP1, and p27Kip1 is critical in controlling overall cellular metabolism (reviewed in reference 74).
In considering how viruses evolve mechanisms to overcome pRb-mediated cell cycle control, three possibilities come to mind. First, viruses could affect events upstream of pRb by targeting factors such as CDKs or CKIs. Indeed, recent reports have shown that HTLV-1 Tax might affect transformation in some cells by a physical binding to p16INK4a (49, 80). Second, viral oncoproteins such as adenovirus E1a, human papillomavirus E7, or simian virus 40 large-T antigen can inactivate pRb either through physical binding (11, 15, 89) or through protein destabilization (5, 44). Third, viruses could act downstream of pRb by directly increasing the activity of E2F. Hence, ectopic expression of E2F was sufficient to drive cells from G1 into S (43, 66). In principle, then, viruses can subvert cell cycle regulation in one or more ways.
In HTLV-1 leukemias, normal cell cycling is dysregulated. One suggested explanation for this is the binding of Tax protein to p16INK4a and the consequential inactivation of the inhibitory effect of p16INK4a on cdk4 (49, 80). In many cells, however, the gene for p16INK4a is deleted, and in others the expression of p16INK4a is very low (80). Therefore, we wondered whether a dominant effect of Tax on cell cycle progression might occur through alternate paths. Here, using myoblasts and two different T-cell lines, we describe an effect of Tax on G1-to-S-phase transition (80). We show that Tax mediates an increase in cyclin D3-dependent cdk4 and cdk6 activity in cells null for p16INK4a. This increased kinase function was accompanied by p110Rb hyperphosphorylation and elevated expression of E2F2. At the same time, we also observed a physical association between Tax and cyclin D3, which was correlated with increased phosphorylation of the latter. Taken together, these findings suggest that Tax might activate cyclin D-cdk in a way that is separate from its ability to complex with p16INK4a. We propose that Tax has two effects: inactivation of a CDK inhibitor (i.e., p16INK4a) and activation of cyclin D-cdk through a CKI-independent route(s).
MATERIALS AND METHODS
Cells and viruses.
JPX9, a derivative of Jurkat cells, contains an inducible Tax cDNA under the control of the metallothionein promoter (62). Tax expression can be induced with zinc (120 μM ZnCl2) or cadmium (20 μM CdCl2). JPX9m cells are counterparts of JPX9 cells that contain a functionally inactive Tax point mutant (i.e., a single arginine insertion at residue 62 [79a]) that is similarly regulated by either zinc or cadmium. JPX9, JPX9m, and Jurkat cells were cultured in RPMI 1640 with 10% fetal calf serum (FCS). SS8tet Tax cells are human CD4+ T cells expressing the tax gene under the control of a tetracycline-repressible promoter. This cell line was obtained by transduction of the SS8BTP cell line (87) with a rhadinovirus vector expressing Tax (69a). Tax is repressed when these cells are cultured in the presence of 1 μg of tetracycline per ml (doxycycline). SS8tet Tax cells were propagated in RPMI 1640-GC medium (Vitromex) with 10% FCS and 20 U of interleukin-2 (IL-2) per ml. JEG-3 human choriocarcinoma cells and vaccinia viral strains were from the American Type Culture Collection (Rockville, Md.). JEG-3 cells were cultured in minimal essential medium (MEM) containing 10% FCS. HeLa S3 human cervical carcinoma epithelial cells were cultured in S-MEM containing 5% FCS. vTF7-3 vaccinia strain WR stocks were amplified in HeLa S3 cells, and titers of the virus were determined on BSC-1 cells by plaquing (16). For protein expression, cells were infected at a multiplicity of infection of 10 as previously described (18). HeLa, C2C12, U2OS, and 293T cells (from G. P. Nolan, Stanford University, Palo Alto, Calif.) were cultured in Dulbecco modified Eagle medium (DMEM) containing 10% FCS. MT4 is a human T-cell line transformed with HTLV-1.
Plasmids.
The Tax expression plasmid HpX contains the Tax cDNA under the control of the HTLV-1 LTR. DNAs for the expression of recombinant proteins in mammalian cell lines were generous gifts from P. Hinds, Harvard Medical School, Boston, Mass. (pCMV-cyclin D1 and pCMV-cyclin D3), and C. Z. Giam, U.S. Uniformed Health Services, Bethesda, Md. (pET-11d-TaxH6). pTM3-TaxH6 was constructed by in-frame insertion into pTM3 of an _Nco_I-_Bam_HI fragment containing the full-length Tax coding sequence with a carboxyl-terminal six-His tag from pET-11d-TaxH6 (17, 56). pGEX-KG-p16 was a gift from Jack Dixon and pGEX-p19 was a gift from Charles J. Sherr (St. Jude Children’s Research Hospital). Maltose-binding protein (MBP)–Tax fusion protein was constructed by an in-frame insertion of the tax cDNA into the pMAL vector (Invitrogen).
Purification of fusion proteins.
Escherichia coli was grown overnight in 50 ml of Luria-Bertani (LB) medium with 100 μg of ampicillin per ml. This overnight culture was inoculated into 500 ml of LB-ampicillin medium and cultured for an additional 1 h at 37°C. Fusion proteins were induced by treatment with 0.1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) for an additional 4 h. Cells were collected by centrifugation at 5,800 × g for 10 min. Bacterial pellets were resuspended into 30 ml of column buffer (10 mM Tris-HCl [pH 8.0], 200 mM NaCl, 2 mM EDTA, 1 mM dithiothreitol [DTT], and protease inhibitor cocktail [aprotinin, leupeptin, Pefabloc SC, and EDTA; Boehringer Mannheim]). The cells were lysed by sonication (Branson) with 10 pulses of 30 s. Sonicated cells were clarified by centrifuging at 9,000 × g for 30 min at 4°C.
MBP and MBP-Tax fusion proteins were purified with amylose resin according to the manufacturer’s protocol. Resins were washed extensively first with column buffer and then with phosphate-buffered saline (PBS) to remove nonspecifically associated proteins and were subsequently equilibrated with buffer B (20 mM HEPES [pH 7.9], 20 mM KCl, 1 mM MgCl2, 17% glycerol, 2 mM DTT).
In vitro binding assays.
Equal amounts of amylose-immobilized MBP alone or MBP-Tax fusion proteins were incubated with 5 μl of glutathione _S_-transferase (GST)-p16 or GST-p19 for 3 h at 4°C. The resins were washed five times with buffer B containing 0.1 M KCl. The bound proteins were eluted with buffer B containing 0.25 M KCl. The eluates were precipitated with trichloroacetic acid, resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and analyzed by Western blotting.
Transfections.
C2C12 cells were transfected with a Tax expression plasmid and a neomycin resistance plasmid by using Lipofectamine reagent (Gibco-BRL); they were then either selected with G418 to establish stable cell lines or harvested in order to prepare cellular extracts. JEG-3 and 293T cells were transfected with calcium phosphate (26).
Flow cytometry.
JPX9 cells were synchronized by serum starvation for 20 h and then maintained with or without zinc for 16 h. The cells were washed in PBS, resuspended in 0.5 ml of solution (0.1% sodium citrate, 0.1% Triton, propidium iodide [50 μg/ml]), and incubated at 4°C for 30 min. The DNA content was analyzed by fluorescence-activated cell sorting (FACS). SS8tet Tax cells (5 × 105) were cultured in either the presence (1 μg) or absence of doxycycline and serum starved for 20 h. The cells were then analyzed as described above.
[3H]thymidine incorporation.
JPX9, JPX9m, and Jurkat cells were synchronized by serum starvation for 20 h and treated with or without zinc for 16 h. Cells were then plated into a 96-well plate and maintained in RPMI containing 1 μCi of [3H]thymidine per 200 μl for 10 h. Cells were harvested with a cell harvester, and [3H]thymidine incorporation was determined by scintillation counting.
Antibodies.
Monoclonal antibody against Tax was from the AIDS Research and Reference Program Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health. Polyclonal rabbit antiserum against Tax was prepared in our laboratory. Antisera against cyclin D1, cyclin D3, cdk4, cdk6, p16, p19, and E2F2 were from Santa Cruz Biotechnology. Monoclonal antibodies against pRb and myogenin were from PharMingen. The competitor E2F2 peptide was from Santa Cruz Biotechnology.
Rb kinase assay.
Kinase assays with GST-Rb as substrate were performed as described previously (12). Cells (5 × 105) were washed in PBS and resuspended in 500 μl of IP buffer (50 mM HEPES [pH 8.0], 150 mM NaCl, 1 mM EDTA, 1 mM DTT, 0.1% Tween 20) containing 10% glycerol and protease inhibitor cocktail (Boehringer Mannheim). Cell lysates were quick frozen on dry ice. Lysates were thawed and incubated on ice for 2 h and then microcentrifuged at 14,000 rpm for 10 min. Lysates were precleared by incubation with protein A-protein G-agarose for 2 h at 4°C and then centrifuged, followed by equilibration with specific antiserum at 4°C overnight. After incubation with antibody, protein A-G-protein agarose was added at 4°C for an additional 2 h. Immune complexes on beads were then washed three times with IP buffer and twice with Rb kinase buffer (50 mM HEPES [pH 8.0], 10 mM MgCl2, 1 mM DTT). The beads were resuspended into 30 μl of Rb kinase buffer containing 2 μg of GST-Rb (Santa Cruz Biotechnology), 2.5 mM EGTA, 10 mM β-glycerophosphate, 0.1 mM sodium orthovanadate, 1 mM NaF, 20 μM ATP, and 5 μCi of [γ-32P]ATP and then incubated at 30°C for 30 min. The reaction was stopped by the addition of SDS sample buffer. The samples were boiled for 5 min, resolved by SDS–10% PAGE, and visualized and quantified with a phosphorimager (Fuji).
Protein analysis.
For Western blot analysis, 2 × 106 cells were washed with PBS and resuspended in radioimmunoprecipitation assay buffer (150 mM NaCl, 1% Nonidet P-40, 0.5% desoxycholate, 0.1% SDS, 50 mM Tris-HCl [pH 8.0]). Identical amounts of proteins were resolved by SDS-PAGE and transferred to Immobilon-P (Millipore) membrane, which was blocked with buffer (0.2% I-block [Tropix] in 1× PBS and 0.1% Tween 20). Membranes were incubated overnight with primary antisera. Reactive proteins were developed with secondary antibodies conjugated to alkaline phosphatase and visualized by using chemiluminescence according to the manufacturer’s protocol (Tropix).
For immunoprecipitation, cells were lysed in buffer (0.15 M NaCl, 50 mM Tris-HCl [pH 7.4], 0.5% [vol/vol] Nonidet P-40) containing 0.1 mM phenylmethylsulfonyl fluoride, and 2.5 μg each of aprotinin, leupeptin, and pepstatin A per ml for 30 min at 4°C. Cellular debris was pelleted at 14,000 × g for 10 min and discarded. Clarified extracts were incubated with either polyclonal anti-Tax, anti-cyclin D3, or anti-cyclin D1 for 16 h and then with 50 μl of 50% (vol/vol) protein A-protein G-Sepharose for an additional 4 h. The bound complexes were washed three times with 1 ml of lysis buffer, and the proteins were resolved by SDS-PAGE.
PCR analysis.
Cells (5 × 105) were resuspended in 100 μl of lysis buffer (50 mM KCl, 15 mM Tris-HCl [pH 8.0], 2.5 mM MgCl2, 0.5% Tween 20, 120 μg proteinase K per ml), incubated at 56°C for 1 h, and then boiled for 10 min. Then 5-μl portions of the extract were used in 100 μl of PCR mixture. Analysis of p15INK4b genomic DNA was done with two different set of primers: one set for the first exon, 5′-CGCGAGGAGAACAAGGGCAT-3′ and 5′-ATCGCGCGCCTCCCGAAAC-3′, and one set for the second exon, 5′-TGGGCAGCGCCCGCGTG-3′ and 5′-AGTCCCCCGTGGCTGTGC-3′. Primers for the actin gene were used as a control: sense, 5′-ATCAAGATCCTGACCGAGCG-3′, and antisense, 5′-TACTTGCGCTCAGGAGGAGC-3′.
RESULTS
Tax induces progression from G0/G1 to S.
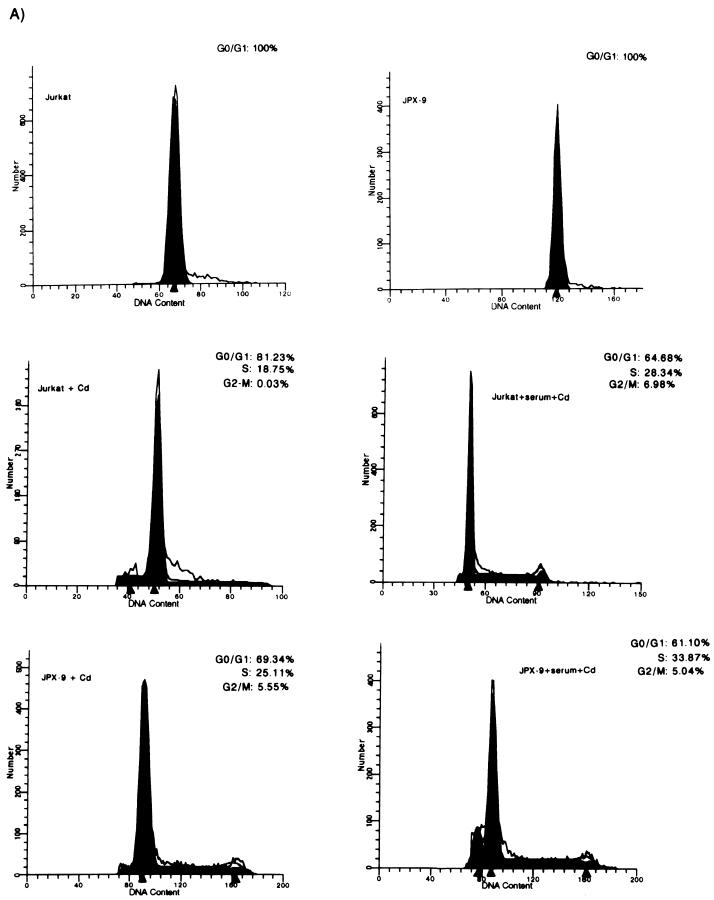
The effect of Tax expression on cell cycle progression was studied by FACS. We analyzed Jurkat and JPX9 (a cell line derived from Jurkat that has been engineered to express Tax upon treatment with either cadmium or zinc [62]) (Fig. 1A). JPX9 and Jurkat cultures were synchronized by cultivation in serum-free medium for 20 h, after which virtually 100% of cells were found in G0/G1 (Fig. 2A, top panels). Under this serum-free condition, when JPX-9 cells were treated with cadmium, Tax expression was induced (Fig. 1A). Induction of Tax was correlated with 30% of cells (S and G2/M, 25.11 and 5.55%, respectively) egressing from G0/G1 (Fig. 2A, bottom left panel). By contrast, parallel treatment of Jurkat cells produced a much-reduced progression of cells (19% of cells exited from G0/G1) (Fig. 2A, middle left panel). Although treatment with divalent cation (Cd2+) has some general effects on cellular metabolism, induction of Tax synthesis accounts for the additional 11% of cells that exited G0/G1. When the cells were treated with serum plus cadmium (Fig. 2A, middle right and bottom right panels), similar fractions of Jurkat (35%) (Fig. 2A, middle right panel) or JPX9 (39%) (Fig. 2A, bottom right panel) cells exited from G0/G1 into later phases. These results are consistent with Tax providing a cell cycle growth function which is redundant with that of serum. In the absence of serum, Tax expression is seen to be sufficient to drive a significant number of cells from G0/G1 into S.
FIG. 1.
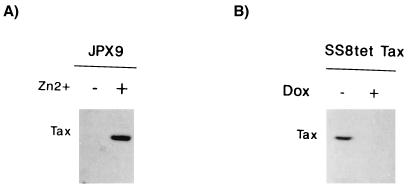
Inducible expression of Tax in JPX9 and SS8tet Tax T cells. Expression of Tax was assessed by Western blotting with a monoclonal antibody. (A) Tax was induced in JPX9 with zinc (+). −, Mock-treated cells. (B) Expression of Tax was repressed in SS8tet Tax cells with 1 μg of doxycycline (Dox) added to the culture medium (+). −, Mock-treated cells.
FIG. 2.
Tax induces the progression of cells from G0/G1 into S phase. The effect of Tax on the cell cycle was examined in either JPX9 or SS8tet Tax cells by FACS. (A) The DNA contents of synchronized cadmium-treated JPX9 cells expressing Tax in the presence (+serum+Cd) or absence (+Cd) of serum were analyzed by propidium iodide staining and compared to the identically treated parental Jurkat cell line with (+serum+Cd) or without (+Cd) serum. The DNA contents of JPX9 and Jurkat cells without cadmium and without serum are shown. Open line reflects actual values. Filled areas are the values interpreted from the MODFIT program. The short peaks to the left in the bottom panels are indicative of apoptotic cells. (B) The DNA contents of SS8tet Tax cells expressing (−Dox) or not expressing (+Dox) Tax were analyzed by propidium iodide staining. The cells were serum starved for 20 h and then stained with propidium iodide and analyzed by flow cytometry. In these profiles, the gating was such that apoptotic cells were not graphed. However, a significantly higher number of apoptotic cells was seen with cells expressing Tax. (C) Control DNA profiles of the parental cell line for SS8tet Tax (SS8 BPT) were examined after serum starvation for 20 h and treatment with (+Dox) or without (−Dox) doxycycline.
To verify that Tax has a general (as opposed to cell-specific) effect on cell cycling, we repeated this study in a second Tax-inducible cell line. Here, the cell cycling profile of a second human CD4+ T-lymphocyte (SS8tet Tax) line that has tax under the control of a tetracycline-regulated promoter was analyzed. In this system, Tax expression is repressed when SS8tet Tax cells are propagated in the presence of tetracycline, and removal of tetracycline induces Tax synthesis (Fig. 1B). We cultured SS8tet Tax with or without doxycycline (a tetracycline analog) in serum-free medium for 20 h. The relative DNA contents of cells after propidium iodide staining were quantified by FACS (Fig. 2B). We found that in cells with the SS8 background, Tax expression increased by more than twofold the relative fraction of cells that progressed from G0/G1 into S (16.03% of the cells in S phase for Tax-expressing cells versus 7.51% of the cells in S phase for non-Tax-expressing cells) (Fig. 2B). Figure 2C shows control doxycycline treatment of the parental SS8PBT cells from which the SS8tet Tax cells were derived. These analyses show that doxycycline treatment alone has little effect on cell cycle progression (Fig. 2C, compare right and left panels).
Tax increases cyclin D-cdk4 and cyclin D-cdk6 activities.
The above results suggest that Tax can accelerate phase progression from G0/G1 into S. This transition is, in part, controlled by the activity of G1 cyclin-cdk’s which include D cyclins associated with either cdk4 or cdk6 and E cyclins associated with cdk2 (reviewed in references 27, 37, and 64). Previously, it was shown that Tax physically complexes with p16INK4a, resulting in abrogation of the inhibitory effects of this CKI (49, 80). However, most human T cells have very low ambient levels of p16INK4a, leading us to wonder whether, in addition to the removal of this negative effect, Tax might not also exert a positive effect in increasing directly G1 cyclin-cdk function. To address this hypothesis, we analyzed cells (Jurkat and JPX9) (Fig. 3) which are null for p16INK4a (80) for a Tax effect on cdk4 and cdk6.
FIG. 3.
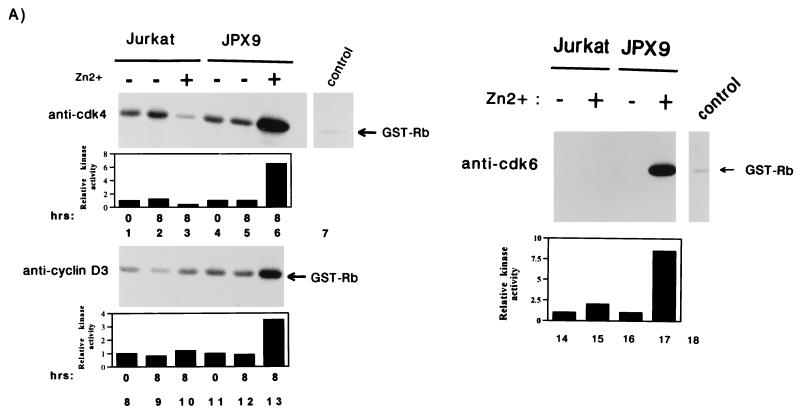
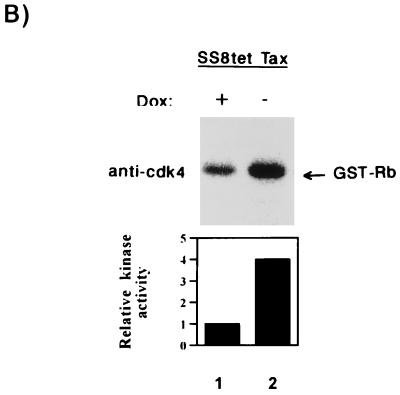
Activation of cyclin D-cdk4 and cyclin D-cdk6 kinases by Tax. (A) Jurkat or JPX9 cells were synchronized by serum starvation for 20 h. Cells propagated in the presence (+; lanes 3, 6, 10, 13, 15, and 17) or absence (−; lanes 1, 2, 4, 5, 8, 9, 11, 12, 14, and 16) of zinc were harvested at the indicated time (in hours). Cell extracts were immunoprecipitated with anti-cdk4 (lanes 1 to 6), anti-cyclin D3 (lanes 8 to 13), and anti-cdk6 (lanes 14 to 17), or a control irrelevant (lanes 7 and 18) antibody. The immunoprecipitates were tested for kinase activity with GST-Rb as substrate. In the top left panel, the cdk4 activities are shown; in the bottom left panel, the cyclin D3-associated kinase activities are shown. In the right panel (lanes 14 to 17) extracts were harvested after 8 h of induction with zinc, and the cdk6 kinase activities were assayed. (B) In vitro kinase assays with GST-Rb as substrate with anti-cdk4 immunoprecipitates from exponentially growing SS8tet Tax cells expressing (−Dox; lane 2) or not expressing (+Dox; lane 1) Tax. Quantitations of each series of activities are shown in bar graphs.
We conducted in vitro assays with pRb, a physiological substrate for cdk4 and cdk6, as the phosphate accepter. Extracts were prepared from synchronized Jurkat and JPX9 cells treated for 8 h with or without zinc. JPX9 cells cultured with zinc synthesize Tax (see Fig. 1A) and, when correlated with Tax induction, we observed that cdk4-associated and cyclin D3-associated kinase activities increased by 6.5- and 3.5-fold, respectively (Fig. 3A, lanes 6 and 13). In comparison, Jurkat cells treated in parallel either with or without zinc showed no increase in kinase activity (Fig. 3A, lanes 3 and 10). A similar observation was made for cdk6. The cdk6-specific pRb-phosphorylating activity increased by 8.5-fold when Tax expression was induced with zinc (Fig. 3A, lane 17) but not in the control cells (Fig. 3, lane 15).
To verify these findings in a different cellular background, we analyzed SS8tet Tax cells. Extracts prepared from cells propagated either with or without doxycycline were immunoprecipitated, and kinase assays were performed with GST-Rb as the substrate. In the absence of doxycycline (Tax synthesis is induced), we observed a fourfold increase in cdk4 activity (Fig. 3B, lane 2) compared to control cells propagated in the presence of doxycycline (Fig. 3B, lane 1). In SS8tet Tax cells, no significant change in cdk6 activity was correlated with Tax induction (59a). Thus, the relative contributions of cdk4 and cdk6 appear to vary in different cells. Taken together, these results suggest that even in p16INK4a-deleted cells, Tax can modulate cell cycling through cdk4 and/or cdk6.
Tax expression correlates with pRb hyperphosphorylation.
D-type cyclin-cdks phosphorylate pRb (14, 20, 45). pRb is hypophosphorylated in G1 and becomes increasingly phosphorylated just before S (reviewed in reference 33). Hypophosphorylated pRb binds and negatively regulates members of the E2F transcription factor family. Phosphorylation of pRb dissociates it from E2F, resulting in the release of the latter, which in turn activates transcription of S-phase-specific genes. Because pRb is one downstream substrate for cyclin D-cdk4 and cyclin D-cdk6, we investigated whether the activation of these kinases by Tax expression results in enhanced pRb phosphorylation.
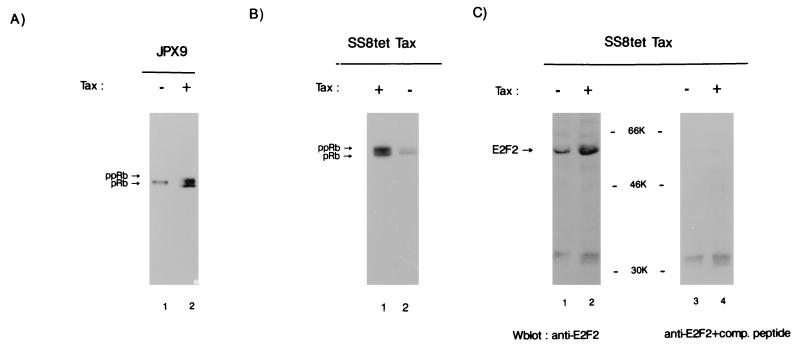
Tax was induced by treatment with zinc of synchronized JPX9 cells. Total cellular extract was analyzed by SDS-PAGE followed by immunoblotting with anti-Rb (Fig. 4A). In the absence of induction a single hypophosphorylated pRb band was observed in JPX9 cells (Fig. 4A, lane 1). In contrast, when Tax was induced with zinc nearly 50% of the Rb species became hyperphosphorylated (ppRb; Fig. 4A, lane 2). This observation is consistent with a Tax effect on cdk4 and cdk6 resulting in downstream phosphorylation of pRb.
FIG. 4.
Tax induces hyperphosphorylation of Rb. (A) The status of Rb phosphorylation was determined by Western blotting with anti-Rb in JPX9 cells synchronized first by serum starvation and then cultured with (+; lane 2) or without (−; lane 1) zinc. Cells were harvested 8 h after addition of zinc. pRb, Hypophosphorylated Rb; ppRb, hyperphosphorylated Rb. (B) Rb was detected by Western blotting in SS8tet Tax cells expressing Tax (+) and in SS8tet Tax cells not expressing Tax (−) (lanes 1 and 2) with anti-Rb. ppRb is the hyperphosphorylated form of Rb; pRb is the hypophosphorylated form. (C) Increased expression of E2F2 upon Tax induction. E2F2 was examined in extracts from SS8tet Tax cultured in the absence (+; lanes 2 and 4) or presence (−; lanes 1 and 3) of doxycycline by Western blotting by using an E2F2-specific antibody (left panel) or E2F2-specific antibody competed by mixing with a 2-μg/ml solution of the immunizing E2F2 peptide (right panel). The immunizing peptide competed specifically with a protein of an apparent size of 53 kDa but not with nonspecific lower-molecular-mass bands.
Effects of Tax on Rb phosphorylation were also verified in SS8tet Tax cells. In SS8tet Tax, when Tax was not expressed, both pRb and ppRb were found (Fig. 4B, lane 2). However, upon Tax induction, the ratio of hyperphosphorylated versus hypophosphorylated Rb changed from 0.3:1 (Fig. 4B, lane 2) to 1.2:1 (Fig. 4B, lane 1), a finding consistent with a fourfold increase in the former species. Thus, while ppRb is basally more abundant in SS8tet Tax (Fig. 4B) than in JPX9 (Fig. 4A), Tax synthesis in both cells results consistently in increased phosphorylation of pRb.
pRb hyperphosphorylation correlates with increased E2F activities. This has been characterized best for the E2F1 protein (reviewed in reference 77). Tax induction in JPX9 and SS8tet Tax cells was found to result in the upregulation of several E2F-responsive genes (59a). This is consistent with Tax-associated changes in cyclin D-cdk4 and cyclin D-cdk6 and with phosphorylated pRb as described above (Fig. 3 and 4). To define better which member of the E2F family might be correlated with Tax expression, we immunoblotted SS8tet Tax cells to determine the amounts of E2F1 and E2F2. While Tax expression did not alter the ambient amounts of E2F1 (data not shown), it resulted in more than a threefold increase in the amount of E2F2 (Fig. 4C, compare lanes 1 and 2). Peptide competition experiments confirmed that the induced protein is indeed E2F2 (Fig. 4C, lanes 3 and 4).
Tax affects differentiation of myocytes into myotubes.
The activities of D cyclins and Rb play important roles in cellular differentiation. For example, ectopic expression of cyclins D2 and D3 in 32D myeloid cells inhibits differentiation (46). Overexpression of cyclin D1 also prevents MyoD-activated expression of muscle-specific genes (67, 76). Similarly, Rb provides an essential function in maintaining the postmitotic state of differentiated myotubes (30, 70). Thus, myogenic differentiation is linked to increased amounts of Rb mRNA and the hypophosphorylated form of Rb protein (30, 50, 86). Accordingly, the myocyte-myotube system affords another measure of the generality of Tax, cyclin D-cdk, and Rb interaction in a cellular background distinct from that of T cells.
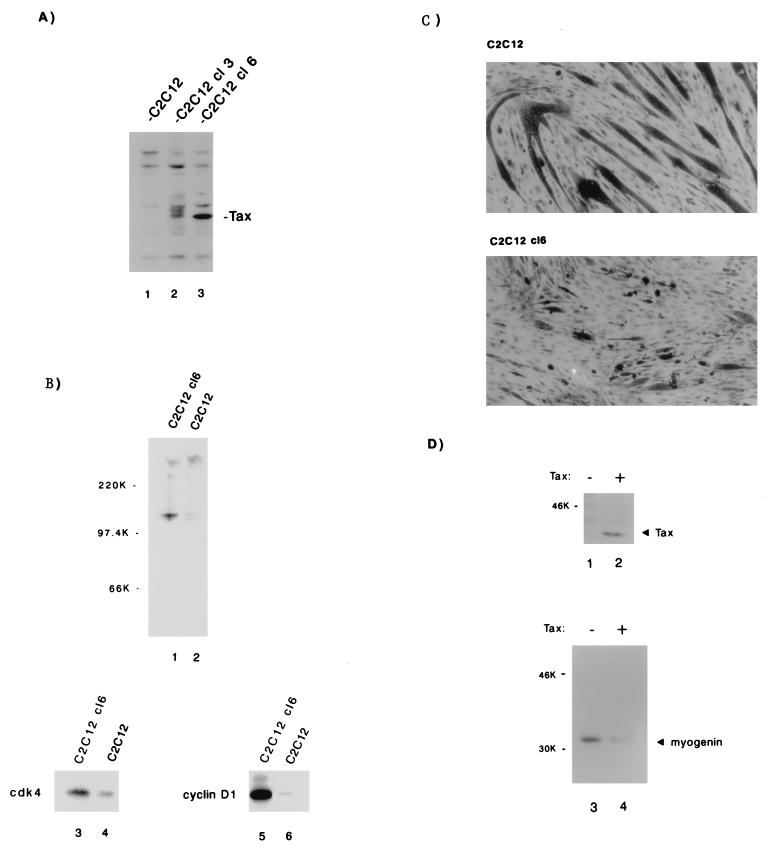
We selected for C2C12 myocytes that express Tax stably. Three Tax-expressing cell lines were independently isolated and in follow-up analyses showed similar results. For illustrative purposes, two cell lines (C2C12 cl3 and C2C12 cl6) are shown in Fig. 5A. Of the two lines, the C2C12 cl6 expresses more Tax protein. C2C12 cl6 (Fig. 5A, lane 3) was compared to a C2C12 line that was selected only for G418 resistance (Fig. 5A, lane 1). Both cell lines were cultured until 95% confluence; Rb status and cdk4- and cyclin D1-associated activities were then assayed. When we compared C2C12 cl6 to C2C12, we found that the latter expressed pRb and ppRb at a 1:1 ratio, whereas the former expressed the two forms at a 1:10 ratio (Fig. 5B, compare lanes 1 and 2). In vitro kinase assays of cdk4 (Fig. 5B, lanes 3 and 4)- or cyclin D1 (Fig. 5B, lanes 5 and 6)-associated activities showed relative increases of 3- and 10-fold in C2C12 cl6 compared to C2C12. Thus, consistent with the results found for T cells, Tax expression in muscle cells is also correlated with increased cyclin D-cdk activity and pRb hyperphosphorylation.
FIG. 5.
Tax induces cyclin D1-cdk4 kinase activity in C2C12 myoblasts and inhibits the differentiation of myoblasts into myotubes. (A) C2C12 cells stably expressing Tax were engineered by cotransfection of a vector encoding Tax under the control of the HTLV-1 LTR and a vector expressing neomycin resistance. The control cells were transfected with neomycin resistance vector alone. Stable expression of Tax (lanes 2 and 3) in two illustrative cell lines (C2C12 cl3 and C2C12 cl6) is shown by Western blotting with Tax-specific serum. (B) C2C12 cl6 and the C2C12 vector-selected control were cultured to 95% confluence, harvested, and probed for Rb expression (lanes 1 and 2) by Western blotting. Compared to C2C12, the hyperphosphorylated form predominates in C2C12 cl6 (upper panel). The lower panels show Rb-kinase assays performed with immunoprecipitates of normalized cell extracts with anti-cdk4 (lanes 3 and 4) or anti-cyclin D1 (lanes 5 and 6) antibody. (C) C2C12 vector-selected (top; non-Tax-expressing) and C2C12 cl6 (bottom; Tax-expressing) cells were grown in DMEM 2% horse serum (differentiating medium). After 6 days cells were fixed in staining solution. Long dark tubular morphology indicates differentiation of cells into myotubes. (D) Expression of Tax in C2C12 myoblasts decreases the differentiation specific marker, myogenin. C2C12 cells were transiently lipofected either with 2 μg of Tax expression vector (+) or with 2 μg of a control vector (−). At 15 h after lipofection, cells were cultured in differentiating medium (DMEM–2% horse serum) for an additional 48 h. Cell extracts were normalized and prepared as described in Materials and Methods, resolved by SDS-PAGE, and analyzed by immunoblotting with a monoclonal Tax antibody (upper panel) or a monoclonal myogenin antibody (lower panel).
Next, we asked whether the biochemical consequences of Tax expression in muscle cells correlate with morphological differentiation. Are the effects of Tax on cyclin D-cdk and pRb reflected in the ability of myocytes to become myotubes? To address this question, we propagated C2C12 cl6 and C2C12 control lines in differentiation-inducing medium and then compared the morphological features. When the cells were stained, it was evident that C2C12 abundantly differentiated into myotubes, whereas C2C12 cl6 showed only rare changes (Fig. 5C).
Muscle cell differentiation can also be defined with biochemical markers. To this end, we asked whether Tax expression correlates with changes in muscle-specific proteins. To avoid biases that might emerge from clonal selection, we introduced transiently into C2C12 cells either a Tax or a control vector; immunoblotting verified that cells that received Tax DNA, but not cells that received vector DNA, expressed Tax protein (Fig. 5D, compare lanes 2 and 1). At 16 h after transfection, cells were shifted into differentiation-inducing medium for an additional 48 h. Cells were then assessed by Western blotting for myogenin, a basic helix-loop-helix protein which is synthesized early in myotube morphogenesis. In agreement with the morphological changes presented in Fig. 5C, the immunoblot results in Fig. 5D show that Tax-expressing myocytes (Fig. 5C, lane 4) had fivefold less myogenin than control cells (Fig. 5C, lane 3).
Tax but not a Tax point-mutant binds cyclin D3.
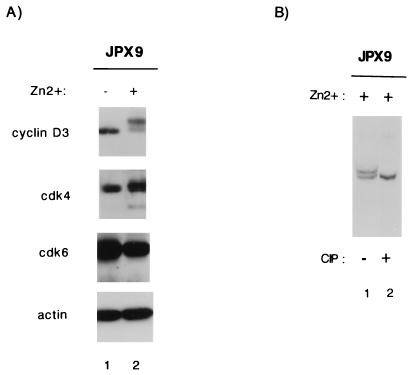
The T-cell and myocyte results point to a common effect of Tax on cyclin D-cdk’s. To understand better how Tax might influence cyclin D-cdk’s, we checked JPX9 cells to see whether the ambient expression of these proteins is affected by Tax. Synchronized JPX9 cells treated with or without zinc were analyzed by Western blotting with anti-cyclin D3, anti-cdk4, anti-cdk6, or anti-actin antibody. Fig. 6A shows that none of the expression profiles for cyclin D3, cdk4, or cdk6 changed significantly in JPX9 cells, whether treated or not with zinc. Unexpectedly, we noted that in Tax-expressing cells, anti-cyclin D3 antibody revealed two closely migrating bands, with the slower-migrating band being predominant. This mobility change in cyclin D3 suggested a modification in phosphorylation. Indeed, when the lower-mobility cyclin D3 band was treated with calf intestinal phosphatase (CIP), it was converted to the faster-migrating moiety (Fig. 6B). While this finding is intriguing, how Tax affects this phosphorylation event and how this alteration might influence G1 cyclin-cdk activity remain to be investigated further.
FIG. 6.
Effect of Tax on cyclin D-cdk4 and cyclin D-cdk6. (A) JPX9 cells were synchronized by serum starvation and then cultured with (+; lane 2) or without (−; lane 1) zinc for 8 h. Cell extracts were prepared and assayed by Western blotting for the expression of cyclin D3, cdk4, cdk6, or actin. Note that upon zinc induction, cyclin D3 appears as a doublet with a mass predominance in the slower-migrating form. (B) Sensitivity of the slower migrating form of cyclin D3 in JPX9 cells to phosphatase. Cell extracts from JPX9 cells were treated for 8 h with zinc (lanes 1 and 2); one extract was further incubated for 1 h at 0°C with 40 U of CIP (lane 2). Extracts were analyzed by Western blotting with anti-cyclin D3 antibody. The sample in lane 2 showed a distinct change in relative migration upon CIP treatment.
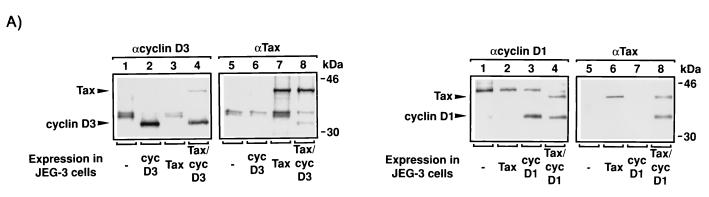
The phosphorylation of cyclin D3 prompted us to explore whether Tax might directly contact this protein. To address this possibility, we coexpressed cyclin D3 and/or cyclin D1 with Tax in JEG-3 choriocarcinoma cells with the vaccinia virus-T7 RNA polymerase. The cells were subsequently labeled with [35S]methionine (Fig. 7A, left and right panels), and protein complexes were immunoprecipitated with anti-cyclin D3, anti-cyclin D1, or anti-Tax antibody. In reciprocal assays, we found that coexpression of Tax with either cyclin D3 or cyclin D1 resulted in the recovery of protein-protein complexes (Fig. 7A). Thus, cyclins D1 and D3 were found in anti-Tax immunoprecipitates, and Tax was present in immunoprecipitates that contain either cyclin D1 or cyclin D3.
FIG. 7.
Tax coimmunoprecipitates with cyclin D1 and/or cyclin D3. (A) Intracellular complex of Tax with cyclin D3 or D1. In the left panel, each lane represents JEG-3 cells transiently transfected with 5 μg of pTM3-TaxH6 (lanes 3, 4, 7, and 8) and/or 5 μg of pCMV-cyclin D3 (cyc D3; lanes 2, 4, 6, and 8), infected with 2 × 106 PFU of vTF7-3 vaccinia strain WR for 18 h, and subsequently labeled with [35S]methionine. Protein complexes were identified by immunoprecipitation under native conditions with polyclonal antisera raised against either cyclin D3 (αcyclin D3) or Tax (αTax). Results are representative of two different experiments. In the right panel, JEG-3 cells were transiently transfected with 5 μg of pTM3-TaxH6 (lanes 2, 4, 6, and 8) and/or 5 μg of pCMV-cyclin D1 (cyc D1) (lanes 3, 4, 7, and 8), infected with 2 × 106 PFU of vTF7-3 vaccinia strain WR for 18 h and subsequently labeled with [35S]methionine. Protein complexes were identified by immunoprecipitation under native conditions with a polyclonal antisera raised against either cyclin D1 (αD1) or Tax (αTax). Results are representative of three different experiments. (B) In the top panel, detection of Tax coimmunoprecipitated with cyclin D1 or cyclin D3 by Western blotting is shown. Each lane represents 5 × 106 293T cells transiently transfected for 48 h with 20 μg of pCMV-Tax (lanes 1 to 6) and 20 μg of either pCMV-cyclin D1 (lanes 1 to 4) or pCMV-cyclin D3 (lanes 5 and 6). Protein complexes were immunoprecipitated under native conditions with polyclonal antisera raised against either Tax (αTax; lanes 1 and 2), cyclin D1 (αcyc D1; lanes 3 and 4) or cyclin D3 (αcyc D3; lanes 5 and 6), and the presence of Tax was detected by Western blotting with polyclonal antisera against Tax (αTax). Results are representative of two different experiments. In the middle and bottom panels, each lane represents 5 × 106 293T cells transiently transfected with 20 μg of pCMV-Tax (lanes 1 to 4) and 20 μg of either pCMV-cyclin D1 (middle panel) or pCMV-cyclin D3 (bottom panel) for 48 h. Protein complexes were immunoprecipitated under native conditions with polyclonal antisera raised against either cyclin D1 (αcyc D1; middle panel, lanes 1 and 2), Tax (αTax; middle panel, lanes 3 and 4; bottom panel, lanes 3 and 4), or cyclin D3 (αcyc D3; bottom panel, lanes 1 and 2). The presence of either cyclin D1 (middle panel) or cyclin D3 (bottom panel) was detected by Western blotting with polyclonal antisera raised against cyclin D1 (middle panel) or against cyclin D3 (bottom panel). Results are representative of two different experiments.
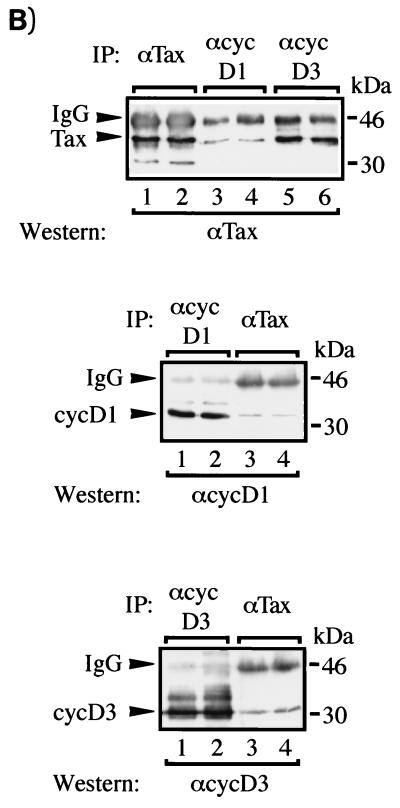
To confirm that the proteins coimmunoprecipitating with Tax are cyclin D3 and cyclin D1, the experiments were repeated in 293T cells. Transiently transfected cells were lysed; proteins were immunoprecipitated, and the identities of coimmunoprecipitated proteins were verified by Western blotting. Antiserum to Tax was used to demonstrate that both cyclin D1 and cyclin D3 coimmunoprecipitated with Tax (Fig. 7B, lanes 3 to 6). More Tax protein was recovered with anti-cyclin D3 than with anti-cyclin D1 antibody. We do not have a clear explanation for this difference, which was confirmed in reciprocal immunoprecipitations with anti-Tax antibody (see Fig. 7B, middle and bottom panels). Thus, the results obtained with JEG-3 and 293T cells are in good agreement.
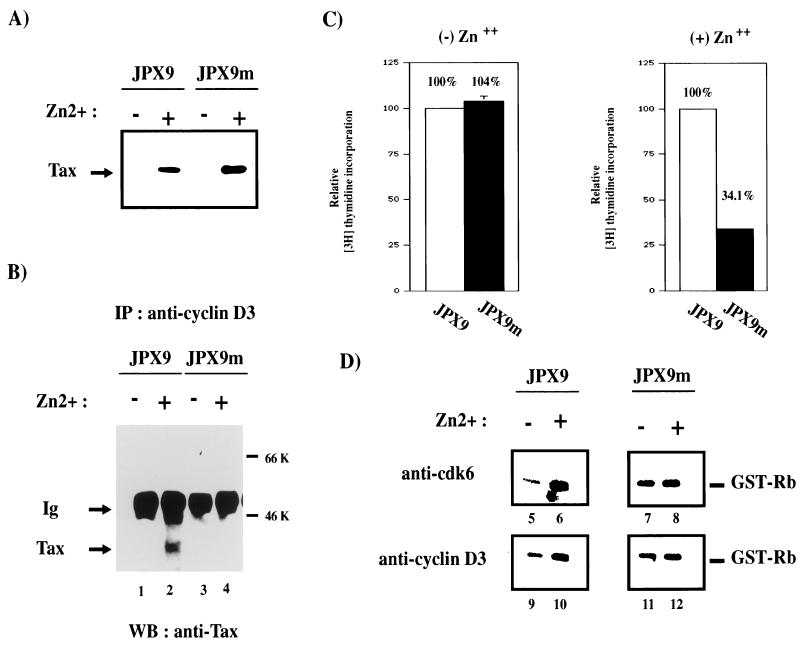
The generality and specificity of Tax and cyclin D3 interactions were examined further in T cells. We asked whether Tax and an inactive point-mutated Tax protein would associate differently with cyclin D3. We also sought to determine whether active and inactive Tax proteins would affect cell cycle progression from G1 to S differently. To address these issues, we compared JPX9 with JPX9m cells. JPX9m is identical to JPX9 except that it expresses a Tax mutant protein that is inactivated by the insertion of a single arginine at position 62 (79a). Extracts from induced JPX9 and JPX9m cells were immunoprecipitated with anti-cyclin D3 antibody. The results showed that wild-type Tax but not point-mutated Tax coimmunoprecipitated with cyclin D3 (Fig. 8B, lane 4). Thus, a single insertion at position 62 of Tax prevents its interaction with cyclin D3. We next asked how the loss of association between Tax mutant and cyclin D3 might be reflected in cell cycle progression and in kinase activities. JPX9 and JPX9m cells were therefore serum starved to halt cells in G1 phase. Cells were then treated or not treated with zinc, and cell cycle progression into S phase was monitored by measuring the incorporation of [3H]thymidine. As shown in Fig. 8C, induction of functional Tax protein by zinc in JPX9 cells efficiently resulted in the incorporation of [3H]thymidine. In contrast, induction of nonfunctional Tax in JPX9m cells resulted in 66% less incorporation of [3H]thymidine (Fig. 8C, right panel). This finding supports a role for functional Tax protein in compelling cell cycle progression from G1 to S. To assess further the effect of Tax on cyclin D-kinase activities, extracts were prepared from JPX9 and JPX9m cells with or without zinc induction; cyclin D-kinase activities were assayed after immunoprecipitation. Increased cdk6- and cyclin D3-associated kinase activities (Fig. 8D, lanes 5 and 6 and lanes 9 and 10) were seen in JPX9 samples upon Tax induction. In contrast, JPX9m samples (with or without zinc) exhibited no difference in kinase activities (Fig. 8D, lanes 7 and 8 and lanes 11 and 12). Anti-cdk4 results (59a) were similar to the anti-cdk6 profiles.
FIG. 8.
A Tax point mutant neither coimmunoprecipitates with cyclin D3 nor induces increased cyclin D3-cdk6 kinase activity. (A) Inducible expression of Tax in JPX9 and JPX9m cell lines. Expression of Tax wild-type and Tax mutant was assessed by Western blotting with a monoclonal antibody to Tax. +, Tax wild type and mutant induced in JPX9 and JPX9m strains with zinc; −, control mock-treated cells. JPX9m expresses a transcriptionally inactive Tax mutant that contains a single amino acid (arginine) inserted at position 62. (B) JPX9 or JPX9m cells were treated (lanes 2 and 4) or mock treated with zinc (lanes 1 and 3) for 16 h. After solubilization in lysis buffer, complexes were immunoprecipitated under native conditions with polyclonal antisera against cyclin D3. The presence of Tax in complexes was assessed by Western blotting with a monoclonal antibody to Tax. (C) Tax mutant protein in JPX9m does not compel G1-to-S progression as measured by [3H]thymidine incorporation. JPX9 and JPX9m cells were synchronized by serum starvation and then treated with or without zinc for 16 h. Cells were then plated into a 96-well plate and labeled with [3H]thymidine. Cell cycle progression from G1 to S phase was measured by [3H]thymidine incorporation. The relative values for JPX9 and JPX9m cells were normalized against parallel values obtained in similarly treated Jurkat cells, with thymidine incorporation for JPX9 set at 100%. (D) Comparison of kinase activity of cyclin D3-cdk6 in cells expressing Tax mutant or Tax wild type. In vitro kinase assays with GST-Rb as substrate were performed. JPX9 or JPX9m cells were synchronized by serum starvation for 20 h. Cells grown in the presence (+; lanes 6, 8, 10, and 12) or absence (−; lanes 5, 7, 9, and 11) of zinc for 8 h were harvested, and cell extracts were immunoprecipitated with either anti-cdk6 (lanes 5 to 8) or anti-cyclin D3 (lanes 9 to 10) antibody. The immunoprecipitates were tested for kinase activity with GST-Rb as the substrate.
p19INK4d does not bind Tax.
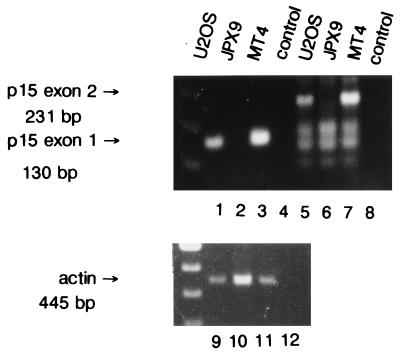
p16INK4a is one member of a family of INK4 inhibitors. This family has three additional members, each capable of inhibiting the kinase activities of cdk4 and cdk6 (9, 31, 32, 34). Previously, Tax was shown to interact with p16INK4a (49, 80). Although the p16INK4a gene is deleted in JPX9 cells, this does not rule out the possibility that some of the findings described above could occur as a consequence of Tax interaction with another members of the INK4 family. We therefore examined this question. Because the gene for p15INK4b is located on chromosome 9 adjacent to the p16INK4a gene and because both are frequently deleted together in human tumors (32, 74), we asked whether JPX9 cells might also be deleted for p15INK4b. Hence, DNAs were prepared from several cell lines and probed by PCR with primers specific for either the first exon or the second exon of p15INK4b. Figure 9 shows that neither the first exon nor the second exon of p15INK4b could be detected in JPX9 DNA (Fig. 9, lanes 2 and 6). Control reactions showed that signals for p15INK4b were easily seen in U2OS and MT4 DNAs (Fig. 9, lanes 1, 5, 3, and 7). An actin control product was also clearly amplified from all DNAs (Fig. 9, lanes 9 to 11).
FIG. 9.
PCR amplification of the p15INK4b gene from JPX9 cells. Cellular DNAs were prepared from various cell lines: U2OS (lanes 1, 5, and 9), JPX9 (lanes 2, 6, and 10), and MT4 (lanes 3, 7, and 11). p15INK4b-specific signal was assayed by amplification with two pairs of primers expected to produce a fragment of 130 bp in the first exon (lanes 1 to 4) and a fragment of 231 bp in the second exon (lanes 5 to 8) of p15INK4b, respectively. A set of primers amplifying a fragment of 445 bp (lanes 9 to 12) in the actin gene was used as a positive control.
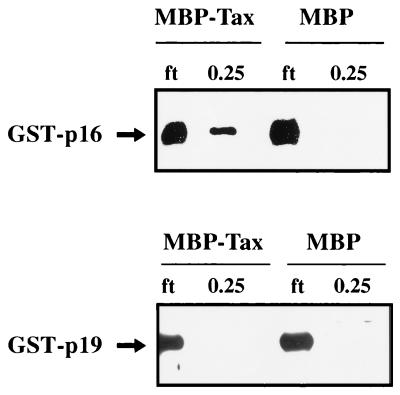
We next addressed the binding properties of p19INK4d and p18INK4c for Tax. These two proteins share 48 and 38% protein homologies, respectively, with p16INK4a (9, 31, 34). We compared, in parallel, the affinity of Tax for p16INK4a or p19INK4d in affinity assays (Fig. 10). The results showed that GST-p16, but not GST-p19, associated with Tax (Fig. 10). When the same experiment was repeated with GST-p18, no detectable signal for p18INK4c was seen in elutions from MBP-Tax resin (59a).
FIG. 10.
Binding of p16INK4a but not p19INK4d to Tax. Amylose-bound MBP-Tax fusion protein (lanes 1, 2, 5, and 6) and MBP protein (lane 3, 4, 5, and 6) were equilibrated for 3 h with 5 μl of extracts containing either GST-p16 (lanes 1 to 4) or GST-p19 (lanes 5 to 8). The resins were packed into columns, and flow-through fractions (ft; lanes 1, 3, 5, and 7) were collected. The resins were then extensively washed with buffer containing 0.1 M KCl. The final wash fraction did not contain any detectable GST-p16 or GST-p19 signal (data not shown). Beads were then eluted with buffer containing 0.25 M KCl (lanes 2, 4, 6, and 8). The presence of p16INK4a or p19INK4d in the elutions was assessed by Western blotting with anti-p16 or anti-p19 antibody.
DISCUSSION
In this study we demonstrate that Tax accelerates phase progression from G1 to S in T cells and influences the differentiation status of muscle cells. We propose that the two processes are linked through a common effect of Tax on G1 cyclin-cdk’s, pRb, and E2F2.
Expression of Tax compels cells to exit from G1 despite an absence of serum. This is correlated with an increase in cyclin D-cdk4 and cyclin D-cdk6 activities. We show in several settings that Tax affects events downstream of G1 cyclin-cdk’s, including hyperphosphorylation of pRb and elevation in E2F2 activity. Thus, a compatible scenario is one in which upstream effects of Tax on cyclin D-cdk’s lead stepwise to E2F2-dependent activation of S-phase genes, thus influencing phase progression. Without excluding a role for CKIs, the demonstration of a physical association (Fig. 7 and 8) between Tax and cyclins D3 and D1 provides another possible molecular mechanism though which Tax can influence G1 cdk’s. Indeed, the association of oncoprotein with cyclins has been previously described, and this association has been invoked as one mechanism through which oncoproteins induce cell cycle dysregulation. Thus, it has been shown that adenovirus E1A and human papillomaviruses E7 oncoproteins associate with cyclin E-p33cdk2 and/or cyclin A-p33cdk2 (3, 21, 53, 82).
Relevant to how Tax might transform cells, there is ample evidence for a role of G1 cyclin-cdk’s in tumorigenesis. For example, there are chromosomal inversions at the cyclin D1 gene (CCDN1) in cases of parathyroid adenoma (2, 57). In B-cell neoplasms, a translocation of the CCDN1 gene to the immunoglobulin heavy-chain locus has also been described (19, 84). Additionally, amplification of CCDN1 in breast, gastric, and esophageal carcinomas resulting in cyclin D1 overexpression has been reported (reviewed in reference 8, 41, 48). Similarly, the gene for cyclin D3 (CCND3) has also been found to be rearranged in several lymphoproliferative disorders (reviewed in reference 37). Furthermore, although there are no described mutations in cdk4 or cdk6 in human tumors, both kinases are frequently overexpressed in several types of tumors (81). Amplification of the gene for cdk4 is also found in various cancers (47). Thus, if Tax indeed affects the G1 cyclin-cdk functions, then it is reasonable that this effect would contribute to cellular transformation.
Previously Tax was shown to bind p16INK4a (49, 80). In such a setting, Tax affects the G1-to-S transition as a consequence of the removal of a negative influence. However, this observation does not exclude that Tax might have a separate and directly positive effect on the G1 cyclin D-cdk’s. Our finding that in p16INK4a-null Jurkat and JPX9 cells (80), Tax increases cyclin D-cdk4 and -cdk6 activities is compatible with an activation route separate from the sequestration of this particular CKI. While another INK4 family member might intercede with Tax in JPX9 cells, the facts that p15INK4b is also deleted and that Tax does not bind either p19INK4d or p18INK4c make this explanation unlikely.
How then might Tax influence D cyclin-cdk’s? We find that it is not from a direct effect of Tax on the transcription of cyclin D promoters. We have consistently failed to observe any increase in such promoter activity as a consequence of Tax expression (59a). Indeed, we do not find any steady-state changes in cyclin D3, cdk4, or cdk6 proteins in cells that express Tax (Fig. 6). Instead, we consider that the effect of Tax on cyclin D-cdk activity likely occurs at a posttranslational step since an interaction between Tax and cyclin D3 (and more weakly with cyclin D1) and a Tax-associated phosphorylation of D3 were observed. Phosphorylation of cyclins has been described previously for cyclins D1 and E. In both instances, the phosphorylated form appears when the cyclins are actively complexed to cognate cdk’s (52, 79). Diehl et al. have recently suggested that phosphorylation of cyclin D1 on threonine 286 might be an autoregulatory mechanism that modulates degradation via the ubiquitin-proteasome pathway (13). It is unclear to us whether the Tax-associated cyclin D3 phosphorylation might also reflect a function or mechanism similar to that described for cyclin D1, in addition to others. However, in our experiments we did not observe a reduction in ambient D3 levels upon Tax expression.
Because we saw an increase in cyclin D-cdk activities in cells expressing Tax and because we found an interaction of Tax with cyclin D3, it is tempting to speculate on the mechanistic significance of these observations. Direct binding by Tax could, through unknown processes, affect cyclin D3 phosphorylation, and thus changing the phosphorylation of cyclin D3 might alter its recognition by CKIs, which might partially explain the alterations in kinase activities. Alternatively, phosphorylated cyclin D3 might qualitatively contribute to making the cyclin-cdk complexes more stable and more potent. That the interaction of Tax with cyclin D3 is physiologically relevant is supported by the fact that a single amino acid point mutation in Tax abolishes binding to cyclin D3 and the enhancement of cyclin D-associated kinase activities (Fig. 8). That these Tax effects are general is suggested by consistent findings in both T cells and myocytes.
It is very difficult to drive serum-starved quiescent cells from G1 into S phase. Currently, only the ectopic expression of E2F and myc (reviewed in reference 88) has shown this capacity. Tax appears to be another oncoprotein that provides a G1-to-S serum-independent transiting function. We suggest that this property is a consequence of the ability of Tax to dually inhibit p16INK4a and enhance cyclin D-cdk activities.
ACKNOWLEDGMENTS
We thank M. Benkirane, R. Chun, V. Giordano, D. Jin, I. Quinto, E. Rich, and H. Xiao for critical readings of manuscript. We thank C. J. Sherr for the pGEX-p19 and pGEX-p18 plasmids.
R.G. and I.S. were supported by DGF (SFB-466).
ADDENDUM IN PROOF
Recently we have demonstrated that Tax can subvert a cellular M-phase checkpoint (D. Jin et al., Cell 93:81–91, 1998).
REFERENCES
- 1.Alexandre C, Charnay P, Verrier B. Transactivation of Krox-20 and Krox-24 promoters by the HTLV-1 Tax protein through common regulatory elements. Oncogene. 1991;6:1851–1857. [PubMed] [Google Scholar]
- 2.Arnold A, Kim H G, Gaz R D, Eddy R L, Fukushima Y, Byers M G, Shows T B, Kronenberg H M. Molecular cloning and chromosomal mapping of DNA rearranged with the parathyroid hormone gene in a parathyroid adenoma. J Clin Invest. 1989;83:2034–2040. doi: 10.1172/JCI114114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arroyo M, Bagchi S, Raychaudhuri P. Association of the human papillomavirus type 16 E7 protein with the S-phase-specific E2F-cyclin A complex. Mol Cell Biol. 1993;13:6537–6546. doi: 10.1128/mcb.13.10.6537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ballard D W, Bohnlein E, Lowenthal J W, Wano Y, Franza B R, Greene W C. HTLV-I tax induces cellular proteins that activate the kappa B element in the IL-2 receptor alpha gene. Science. 1988;241:1652–1655. doi: 10.1126/science.241.4873.1652. [DOI] [PubMed] [Google Scholar]
- 5.Boyer S N, Wazer D E, Band V. E7 protein of human papilloma virus-16 induces degradation of retinoblastoma protein through the ubiquitin-proteasome pathway. Cancer Res. 1996;56:4620–4624. [PubMed] [Google Scholar]
- 6.Brady J, Jeang K T, Duvall J, Khoury G. Identification of p40x-responsive regulatory sequences within the human T-cell leukemia virus type I long terminal repeat. J Virol. 1987;61:2175–2181. doi: 10.1128/jvi.61.7.2175-2181.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brauweiler A, Garrus J E, Reed J C, Nyborg J K. Repression of bax gene expression by the HTLV-1 Tax protein: implications for suppression of apoptosis in virally infected cells. Virology. 1997;231:135–140. doi: 10.1006/viro.1997.8509. [DOI] [PubMed] [Google Scholar]
- 8.Buckley M F, Sweeney K J, Hamilton J A, Sini R L, Manning D L, Nicholson R I, deFazio A, Watts C K, Musgrove E A, Sutherland R L. Expression and amplification of cyclin genes in human breast cancer. Oncogene. 1993;8:2127–2133. [PubMed] [Google Scholar]
- 9.Chan F K, Zhang J, Cheng L, Shapiro D N, Winoto A. Identification of human and mouse p19, a novel CDK4 and CDK6 inhibitor with homology to p16ink4. Mol Cell Biol. 1995;15:2682–2688. doi: 10.1128/mcb.15.5.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen I S, Slamon D J, Rosenblatt J D, Shah N P, Quan S G, Wachsman W. The x gene is essential for HTLV replication. Science. 1985;229:54–58. doi: 10.1126/science.2990037. [DOI] [PubMed] [Google Scholar]
- 11.DeCaprio J A, Ludlow J W, Figge J, Shew J Y, Huang C M, Lee W H, Marsilio E, Paucha E, Livingston D M. SV40 large tumor antigen forms a specific complex with the product of the retinoblastoma susceptibility gene. Cell. 1988;54:275–283. doi: 10.1016/0092-8674(88)90559-4. [DOI] [PubMed] [Google Scholar]
- 12.DeGregori J, Leone G, Ohtani K, Miron A, Nevins J R. E2F-1 accumulation bypasses a G1 arrest resulting from the inhibition of G1 cyclin-dependent kinase activity. Genes Dev. 1995;9:2873–2887. doi: 10.1101/gad.9.23.2873. [DOI] [PubMed] [Google Scholar]
- 13.Diehl J A, Zindy F, Sherr C J. Inhibition of cyclin D1 phosphorylation on threonine-286 prevents its rapid degradation via the ubiquitin-proteasome pathway. Genes Dev. 1997;11:957–972. doi: 10.1101/gad.11.8.957. [DOI] [PubMed] [Google Scholar]
- 14.Dowdy S F, Hinds P W, Louie K, Reed S I, Arnold A, Weinberg R A. Physical interaction of the retinoblastoma protein with human D cyclins. Cell. 1993;73:499–511. doi: 10.1016/0092-8674(93)90137-f. [DOI] [PubMed] [Google Scholar]
- 15.Dyson N, Howley P M, Munger K, Harlow E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science. 1989;243:934–937. doi: 10.1126/science.2537532. [DOI] [PubMed] [Google Scholar]
- 16.Earl P L, Cooper N, Moss B. Preparation of cell culture and vaccinia virus stocks. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: Greene Publishing Associates and Wiley-Interscience; 1991. pp. 16.16.1–16.16.7. [Google Scholar]
- 17.Elroy-Stein O, Fuerst T R, Moss B. Cap-independent translation of mRNA conferred by encephalomyocarditis virus 5′ sequence improves the performance of the vaccinia virus/bacteriophage T7 hybrid expression system. Proc Natl Acad Sci USA. 1989;86:6126–6130. doi: 10.1073/pnas.86.16.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elroy-Stein O, Moss B. Gene expression using vaccinia/T7 RNA polymerase hybrid system. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: Greene Publishing Associates and Wiley-Interscience; 1991. pp. 16.19.1–16.19.9. [Google Scholar]
- 19.Erikson J, Finan J, Tsujimoto Y, Nowell P C, Croce C M. The chromosome 14 breakpoint in neoplastic B cells with the t(11;14) translocation involves the immunoglobulin heavy chain locus. Proc Natl Acad Sci USA. 1984;81:4144–4148. doi: 10.1073/pnas.81.13.4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ewen M E, Sluss H K, Sherr C J, Matsushime H, Kato J, Livingston D M. Functional interactions of the retinoblastoma protein with mammalian D-type cyclins. Cell. 1993;73:487–497. doi: 10.1016/0092-8674(93)90136-e. [DOI] [PubMed] [Google Scholar]
- 21.Faha B, Harlow E, Lees E. The adenovirus E1A-associated kinase consists of cyclin E-p33cdk2 and cyclin A-p33cdk2. J Virol. 1993;67:2456–2465. doi: 10.1128/jvi.67.5.2456-2465.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Felber B K, Paskalis H, Kleinman-Ewing C, Wong-Staal F, Pavlakis G N. The pX protein of HTLV-I is a transcriptional activator of its long terminal repeats. Science. 1985;229:675–679. doi: 10.1126/science.2992082. [DOI] [PubMed] [Google Scholar]
- 23.Franklin A A, Nyborg J K. Mechanisms of Tax regulation of human T cell leukemia virus type I gene expression. J Biomed Sci. 1995;2:17–29. doi: 10.1007/BF02257921. [DOI] [PubMed] [Google Scholar]
- 24.Fujii M, Niki T, Mori T, Matsuda T, Matsui M, Nomura N, Seiki M. HTLV-1 Tax induces expression of various immediate early serum responsive genes. Oncogene. 1991;6:1023–1029. [PubMed] [Google Scholar]
- 25.Fujii M, Sassone-Corsi P, Verma I M. c-fos promoter trans-activation by the tax1 protein of human T-cell leukemia virus type I. Proc Natl Acad Sci USA. 1988;85:8526–8530. doi: 10.1073/pnas.85.22.8526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gorman C M, Moffat L F, Howard B H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982;2:1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grana X, Reddy E P. Cell cycle control in mammalian cells: role of cyclins, cyclin dependent kinases (CDKs), growth suppressor genes and cyclin-dependent kinase inhibitors (CKIs) Oncogene. 1995;11:211–219. [PubMed] [Google Scholar]
- 28.Grassmann R, Berchtold S, Radant I, Alt M, Fleckenstein B, Sodroski J G, Haseltine W A, Ramstedt U. Role of human T-cell leukemia virus type 1 X region proteins in immortalization of primary human lymphocytes in culture. J Virol. 1992;66:4570–4575. doi: 10.1128/jvi.66.7.4570-4575.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grossman W J, Kimata J T, Wong F H, Zutter M, Ley T J, Ratner L. Development of leukemia in mice transgenic for the tax gene of human T-cell leukemia virus type I. Proc Natl Acad Sci USA. 1995;92:1057–1061. doi: 10.1073/pnas.92.4.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gu W, Schneider J W, Condorelli G, Kaushal S, Mahdavi V, Nadal-Ginard B. Interaction of myogenic factors and the retinoblastoma protein mediates muscle cell commitment and differentiation. Cell. 1993;72:309–324. doi: 10.1016/0092-8674(93)90110-c. [DOI] [PubMed] [Google Scholar]
- 31.Guan K L, Jenkins C W, Li Y, Nichols M A, Wu X, O’Keefe C L, Matera A G, Xiong Y. Growth suppression by p18, a p16INK4/MTS1- and p14INK4B/MTS2-related CDK6 inhibitor, correlates with wild-type pRb function. Genes Dev. 1994;8:2939–2952. doi: 10.1101/gad.8.24.2939. [DOI] [PubMed] [Google Scholar]
- 32.Hannon G J, Beach D. p15INK4B is a potential effector of TGF-beta-induced cell cycle arrest. Nature. 1994;371:257–261. doi: 10.1038/371257a0. [DOI] [PubMed] [Google Scholar]
- 33.Hinds P W, Weinberg R A. Tumor suppressor genes. Curr Opin Genet Dev. 1994;4:135–141. doi: 10.1016/0959-437x(94)90102-3. [DOI] [PubMed] [Google Scholar]
- 34.Hirai H, Roussel M F, Kato J Y, Ashmun R A, Sherr C J. Novel INK4 proteins, p19 and p18, are specific inhibitors of the cyclin D-dependent kinases CDK4 and CDK6. Mol Cell Biol. 1995;15:2672–2681. doi: 10.1128/mcb.15.5.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hirama T, Koeffler H P. Role of the cyclin-dependent kinase inhibitors in the development of cancer. Blood. 1995;86:841–854. [PubMed] [Google Scholar]
- 36.Hollsberg P, Hafler D A. Seminars in medicine of the Beth Israel Hospital, Boston. Pathogenesis of diseases induced by human lymphotropic virus type I infection. N Engl J Med. 1993;328:1173–1182. doi: 10.1056/NEJM199304223281608. [DOI] [PubMed] [Google Scholar]
- 37.Hunter T, Pines J. Cyclins and cancer. II: cyclin D and CDK inhibitors come of age. Cell. 1994;79:573–582. doi: 10.1016/0092-8674(94)90543-6. [DOI] [PubMed] [Google Scholar]
- 38.Inoue J, Seiki M, Taniguchi T, Tsuru S, Yoshida M. Induction of interleukin 2 receptor gene expression by p40x encoded by human T-cell leukemia virus type 1. EMBO J. 1986;5:2883–2888. doi: 10.1002/j.1460-2075.1986.tb04583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jeang K T, Shank P R, Kumar A. Transcriptional activation of homologous viral long terminal repeats by the human immunodeficiency virus type 1 or the human T-cell leukemia virus type I tat proteins occurs in the absence of de novo protein synthesis. Proc Natl Acad Sci USA. 1988;85:8291–8295. doi: 10.1073/pnas.85.21.8291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jeang K T, Widen S G, Semmes IV O J, Wilson S H. HTLV-I trans-activator protein, tax, is a trans-repressor of the human beta-polymerase gene. Science. 1990;247:1082–1084. doi: 10.1126/science.2309119. [DOI] [PubMed] [Google Scholar]
- 41.Jiang W, Kahn S M, Tomita N, Zhang Y J, Lu S H, Weinstein I B. Amplification and expression of the human cyclin D gene in esophageal cancer. Cancer Res. 1992;52:2980–2983. [PubMed] [Google Scholar]
- 42.Jin D Y, Teramoto H, Giam C Z, Chun R F, Gutkind J S, Jeang K T. A human suppressor of c-Jun N-terminal kinase 1 activation by tumor necrosis factor alpha. J Biol Chem. 1997;272:25816–25823. doi: 10.1074/jbc.272.41.25816. [DOI] [PubMed] [Google Scholar]
- 43.Johnson D G, Schwarz J K, Cress W D, Nevins J R. Expression of transcription factor E2F1 induces quiescent cells to enter S phase. Nature. 1993;365:349–352. doi: 10.1038/365349a0. [DOI] [PubMed] [Google Scholar]
- 44.Jones D L, Munger K. Analysis of the p53-mediated G1 growth arrest pathway in cells expressing the human papillomavirus type 16 E7 oncoprotein. J Virol. 1997;71:2905–2912. doi: 10.1128/jvi.71.4.2905-2912.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kato J, Matsushime H, Hiebert S W, Ewen M E, Sherr C J. Direct binding of cyclin D to the retinoblastoma gene product (pRb) and pRb phosphorylation by the cyclin D-dependent kinase CDK4. Genes Dev. 1993;7:331–342. doi: 10.1101/gad.7.3.331. [DOI] [PubMed] [Google Scholar]
- 46.Kato J Y, Sherr C J. Inhibition of granulocyte differentiation by G1 cyclins D2 and D3 but not D1. Proc Natl Acad Sci USA. 1993;90:11513–11517. doi: 10.1073/pnas.90.24.11513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khatib Z A, Matsushime H, Valentine M, Shapiro D N, Sherr C J, Look A T. Coamplification of the CDK4 gene with MDM2 and GLI in human sarcomas. Cancer Res. 1993;53:5535–5541. [PubMed] [Google Scholar]
- 48.Lammie G A, Peters G. Chromosome 11q13 abnormalities in human cancer. Cancer Cells. 1991;3:413–420. [PubMed] [Google Scholar]
- 49.Low K G, Dorner L F, Fernando D B, Grossman J, Jeang K T, Comb M J. Human T-cell leukemia virus type 1 Tax releases cell cycle arrest induced by p16INK4a. J Virol. 1997;71:1956–1962. doi: 10.1128/jvi.71.3.1956-1962.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martelli F, Cenciarelli C, Santarelli G, Polikar B, Felsani A, Caruso M. MyoD induces retinoblastoma gene expression during myogenic differentiation. Oncogene. 1994;9:3579–3590. [PubMed] [Google Scholar]
- 51.Matsushime H, Quelle D E, Shurtleff S A, Shibuya M, Sherr C J, Kato J Y. D-type cyclin-dependent kinase activity in mammalian cells. Mol Cell Biol. 1994;14:2066–2076. doi: 10.1128/mcb.14.3.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matsushime H, Roussel M F, Ashmun R A, Sherr C J. Colony-stimulating factor 1 regulates novel cyclins during the G1 phase of the cell cycle. Cell. 1991;65:701–713. doi: 10.1016/0092-8674(91)90101-4. [DOI] [PubMed] [Google Scholar]
- 53.McIntyre M C, Ruesch M N, Laimins L A. Human papillomavirus E7 oncoproteins bind a single form of cyclin E in a complex with cdk2 and p107. Virology. 1996;215:73–82. doi: 10.1006/viro.1996.0008. [DOI] [PubMed] [Google Scholar]
- 54.Miyatake S, Seiki M, Yoshida M, Arai K. T-cell activation signals and human T-cell leukemia virus type I-encoded p40x protein activate the mouse granulocyte-macrophage colony-stimulating factor gene through a common DNA element. Mol Cell Biol. 1988;8:5581–5587. doi: 10.1128/mcb.8.12.5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morgan D O. Principles of CDK regulation. Nature. 1995;374:131–134. doi: 10.1038/374131a0. [DOI] [PubMed] [Google Scholar]
- 56.Moss B, Elroy-Stein O, Mizukami T, Alexander W A, Fuerst T R. Product review. New mammalian expression vectors. Nature. 1990;348:91–92. doi: 10.1038/348091a0. [DOI] [PubMed] [Google Scholar]
- 57.Motokura T, Bloom T, Kim H G, Juppner H, Ruderman J V, Kronenberg H M, Arnold A. A novel cyclin encoded by a bcl1-linked candidate oncogene. Nature. 1991;350:512–515. doi: 10.1038/350512a0. [DOI] [PubMed] [Google Scholar]
- 58.Murray A. Cell cycle checkpoints. Curr Opin Cell Biol. 1994;6:872–876. doi: 10.1016/0955-0674(94)90059-0. [DOI] [PubMed] [Google Scholar]
- 59.Nerenberg M, Hinrichs S H, Reynolds R K, Khoury G, Jay G. The tat gene of human T-lymphotropic virus type 1 induces mesenchymal tumors in transgenic mice. Science. 1987;237:1324–1329. doi: 10.1126/science.2888190. [DOI] [PubMed] [Google Scholar]
- 59a.Neuveut, C. Unpublished data.
- 60.Neuveut C, Jin D Y, Semmes O J, Diella F, Callahan R, Jeang K T. Divergent subcellular locations of HTLV-1 Tax and INT-6: a contrast between in vitro protein-protein binding and intracellular protein colocalization. J Biomed Sci. 1997;4:229–234. doi: 10.1007/BF02253422. [DOI] [PubMed] [Google Scholar]
- 61.Nevins J R. E2F: a link between the Rb tumor suppressor protein and viral oncoproteins. Science. 1992;258:424–429. doi: 10.1126/science.1411535. [DOI] [PubMed] [Google Scholar]
- 62.Ohtani K, Nakamura M, Saito S, Nagata K, Sugamura K, Hinuma Y. Electroporation: application to human lymphoid cell lines for stable introduction of a transactivator gene of human T-cell leukemia virus type I. Nucleic Acids Res. 1989;17:1589–1604. doi: 10.1093/nar/17.4.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peter M, Herskowitz I. Joining the complex: cyclin-dependent kinase inhibitory proteins and the cell cycle. Cell. 1994;79:181–184. doi: 10.1016/0092-8674(94)90186-4. [DOI] [PubMed] [Google Scholar]
- 64.Peters G. The D-type cyclins and their role in tumorigenesis. J Cell Sci Suppl. 1994;18:89–96. doi: 10.1242/jcs.1994.supplement_18.13. [DOI] [PubMed] [Google Scholar]
- 65.Pozzatti R, Vogel J, Jay G. The human T-lymphotropic virus type I tax gene can cooperate with the ras oncogene to induce neoplastic transformation of cells. Mol Cell Biol. 1990;10:413–417. doi: 10.1128/mcb.10.1.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Qin X Q, Livingston D M, Kaelin W, Jr, Adams P D. Deregulated transcription factor E2F-1 expression leads to S-phase entry and p53-mediated apoptosis. Proc Natl Acad Sci USA. 1994;91:10918–10922. doi: 10.1073/pnas.91.23.10918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rao S S, Chu C, Kohtz D S. Ectopic expression of cyclin D1 prevents activation of gene transcription by myogenic basic helix-loop-helix regulators. Mol Cell Biol. 1994;14:5259–5267. doi: 10.1128/mcb.14.8.5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Resnitzky D, Gossen M, Bujard H, Reed S I. Acceleration of the G1/S phase transition by expression of cyclins D1 and E with an inducible system. Mol Cell Biol. 1994;14:1669–1679. doi: 10.1128/mcb.14.3.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ruben S, Poteat H, Tan T H, Kawakami K, Roeder R, Haseltine W, Rosen C A. Cellular transcription factors and regulation of IL-2 receptor gene expression by HTLV-I tax gene product. Science. 1988;241:89–92. doi: 10.1126/science.2838905. [DOI] [PubMed] [Google Scholar]
- 69a.Schmitt V I, Rosin O, Rohwer P, Gossen M, Grassmann R. Stimulation of cyclin-dependent kinase activity and G1-to-S-phase transition in human lymphocytes by the human T-cell leukemia/lymphotropic virus type 1 Tax protein. J Virol. 1998;72:633–640. doi: 10.1128/jvi.72.1.633-640.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schneider J W, Gu W, Zhu L, Mahdavi V, Nadal-Ginard B. Reversal of terminal differentiation mediated by p107 in Rb−/− muscle cells. Science. 1994;264:1467–1471. doi: 10.1126/science.8197461. [DOI] [PubMed] [Google Scholar]
- 71.Semmes O J, Barret J F, Dang C V, Jeang K T. Human T-cell leukemia virus type I tax masks c-Myc function through a cAMP-dependent pathway. J Biol Chem. 1996;271:9730–9738. doi: 10.1074/jbc.271.16.9730. [DOI] [PubMed] [Google Scholar]
- 72.Sherr C J. G1 phase progression: cycling on cue. Cell. 1994;79:551–555. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- 73.Sherr C J. D-type cyclins. Trends Biochem Sci. 1995;20:187–190. doi: 10.1016/s0968-0004(00)89005-2. [DOI] [PubMed] [Google Scholar]
- 74.Sherr C J, Roberts J M. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- 75.Siekevitz M, Feinberg M B, Holbrook N, Wong-Staal F, Greene W C. Activation of interleukin 2 and interleukin 2 receptor (Tac) promoter expression by the trans-activator (tat) gene product of human T-cell leukemia virus, type I. Proc Natl Acad Sci USA. 1987;84:5389–5393. doi: 10.1073/pnas.84.15.5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Skapek S X, Rhee J, Spicer D B, Lassar A B. Inhibition of myogenic differentiation in proliferating myoblasts by cyclin D1-dependent kinase. Science. 1995;267:1022–1024. doi: 10.1126/science.7863328. [DOI] [PubMed] [Google Scholar]
- 77.Slansky J E, Farnham P J. Introduction to the E2F family: protein structure and gene regulation. Curr Top Microbiol Immunol. 1996;208:1–30. doi: 10.1007/978-3-642-79910-5_1. [DOI] [PubMed] [Google Scholar]
- 78.Sodroski J, Rosen C, Goh W C, Haseltine W. A transcriptional activator protein encoded by the x-lor region of the human T-cell leukemia virus. Science. 1985;228:1430–1434. doi: 10.1126/science.2990028. [DOI] [PubMed] [Google Scholar]
- 79.Steiner P, Philipp A, Lukas J, Godden-Kent D, Pagano M, Mittnacht S, Bartek J, Eilers M. Identification of a Myc-dependent step during the formation of active G1 cyclin-cdk complexes. EMBO J. 1995;14:4814–4826. doi: 10.1002/j.1460-2075.1995.tb00163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79a.Sugamora, K. Personal communication.
- 80.Suzuki T, Kitao S, Matsushime H, Yoshida M. HTLV-1 Tax protein interacts with cyclin-dependent kinase inhibitor p16INK4A and counteracts its inhibitory activity towards CDK4. EMBO J. 1996;15:1607–1614. [PMC free article] [PubMed] [Google Scholar]
- 81.Tam S W, Theodoras A M, Shay J W, Draetta G F, Pagano M. Differential expression and regulation of cyclin D1 protein in normal and tumor human cells: association with Cdk4 is required for cyclin D1 function in G1 progression. Oncogene. 1994;9:2663–2674. [PubMed] [Google Scholar]
- 82.Tommasino M, Adamczewski J P, Carlotti F, Barth C F, Manetti R, Contorni M, Cavalieri F, Hunt T, Crawford L. HPV16 E7 protein associates with the protein kinase p33CDK2 and cyclin A. Oncogene. 1993;8:195–202. [PubMed] [Google Scholar]
- 83.Trihn D, Jeang K T, Semmes O J. HTLV-1 Tax and cytokeratin-Tax-expressing cells show morphological changes in keratin-containing cytoskeletal networks. J Biomed Sci. 1997;4:47–53. doi: 10.1007/BF02255593. [DOI] [PubMed] [Google Scholar]
- 84.Tsujimoto Y, Yunis J, Onorato-Showe L, Erikson J, Nowell P C, Croce C M. Molecular cloning of the chromosomal breakpoint of B-cell lymphomas and leukemias with the t(11;14) chromosome translocation. Science. 1984;224:1403–1406. doi: 10.1126/science.6610211. [DOI] [PubMed] [Google Scholar]
- 85.Uittenbogaard M N, Giebler H A, Reisman D, Nyborg J K. Transcriptional repression of p53 by human T-cell leukemia virus type I Tax protein. J Biol Chem. 1995;270:28503–28506. doi: 10.1074/jbc.270.48.28503. [DOI] [PubMed] [Google Scholar]
- 86.Wang J, Nadal-Ginard B. Regulation of cyclins and p34CDC2 expression during terminal differentiation of C2C12 myocytes. Biochem Biophys Res Commun. 1995;206:82–88. doi: 10.1006/bbrc.1995.1012. [DOI] [PubMed] [Google Scholar]
- 87.Weber F, Meinl E, Drexler K, Czlonkowska A, Huber S, Fickenscher H, Muller-Fleckenstein I, Fleckenstein B, Wekerle H, Hohlfeld R. Transformation of human T-cell clones by herpesvirus saimiri: intact antigen recognition by autonomously growing myelin basic protein-specific T cells. Proc Natl Acad Sci USA. 1993;90:11049–11053. doi: 10.1073/pnas.90.23.11049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Weinberg R A. E2F and cell proliferation: a world turned upside down. Cell. 1996;85:457–459. doi: 10.1016/s0092-8674(00)81244-1. [DOI] [PubMed] [Google Scholar]
- 89.Whyte P, Buchkovich K J, Horowitz J M, Friend S H, Raybuck M, Weinberg R A, Harlow E. Association between an oncogene and an anti-oncogene: the adenovirus E1A proteins bind to the retinoblastoma gene product. Nature. 1988;334:124–129. doi: 10.1038/334124a0. [DOI] [PubMed] [Google Scholar]
- 90.Yoshida M. HTLV-1 Tax: regulation of gene expression and disease. Trends Microbiol. 1993;1:131–135. doi: 10.1016/0966-842x(93)90127-d. [DOI] [PubMed] [Google Scholar]