Direct Interaction of Jak1 and v-Abl Is Required for v-Abl-Induced Activation of STATs and Proliferation (original) (raw)
Abstract
In Abelson murine leukemia virus (A-MuLV)-transformed cells, members of the Janus kinase (Jak) family of non-receptor tyrosine kinases and the signal transducers and activators of transcription (STAT) family of signaling proteins are constitutively activated. In these cells, the v-Abl oncoprotein and the Jak proteins physically associate. To define the molecular mechanism of constitutive Jak-STAT signaling in these cells, the functional significance of the v-Abl–Jak association was examined. Mapping the Jak1 interaction domain in v-Abl demonstrates that amino acids 858 to 1080 within the carboxyl-terminal region of v-Abl bind Jak1 through a direct interaction. A mutant of v-Abl lacking this region exhibits a significant defect in Jak1 binding in vivo, fails to activate Jak1 and STAT proteins, and does not support either the proliferation or the survival of BAF/3 cells in the absence of cytokine. Cells expressing this v-Abl mutant show extended latency and decreased frequency in generating tumors in nude mice. In addition, inducible expression of a kinase-inactive mutant of Jak1 protein inhibits the ability of v-Abl to activate STATs and to induce cytokine-independent proliferation, indicating that an active Jak1 is required for these v-Abl-induced signaling pathways in vivo. We propose that Jak1 is a mediator of v-Abl-induced STAT activation and v-Abl induced proliferation in BAF/3 cells, and may be important for efficient transformation of immature B cells by the v-abl oncogene.
Activation of tyrosine kinases by translocation or retroviral transduction has been linked to the development of many lymphoid malignancies. The gag-Abl fusion protein encoded by the v-abl oncogene of Abelson murine leukemia virus (A-MuLV) is a non-receptor tyrosine kinase, which, unlike its cellular counterpart, c-Abl, is constitutively active. Although the A-MuLV primarily induces pre-B cell leukemias in vivo, the v-abl oncogene can transform other cell types such as NIH 3T3 fibroblasts in vitro (46). The mechanisms responsible for the cell type specificity of in vivo transformation by A-MuLV are not known.
Characterization of different domains of the v-Abl oncoprotein and the molecular interactions in which they participate has been a useful approach to elucidate the mechanism of v-Abl function. The majority of v-Abl substrates identified to date are proteins involved in the transduction of signals leading to gene transcription and cellular proliferation, including phosphoinositol 3-kinase (54), Ras/mitogen-activated protein (MAP) kinase (40, 49), Fes (14), Fos/Jun (8, 44), and c-Myc (48). It also appears that the regions of v-Abl required for transformation differ for bone marrow and fibroblast targets. The SH2 and protein tyrosine kinase domains, as well as the myristoylation signals at the N terminus of the protein, are required for the transformation of all v-Abl targets (24, 31, 42, 47). However, the C terminus of the protein is required for immortalization of bone marrow cells but is dispensable for fibroblast transformation (41, 47). The molecular basis for this differential requirement of the C-terminal region of v-Abl in immortalization of various targets has not been elucidated.
The primary target cells of A-MuLV in bone marrow are B-cell precursors that are normally dependent on cytokines produced by bone marrow stromal cells for proliferation and survival. Upon infection with A-MuLV, these cells arrest at the pre-B stage of differentiation (46) and become independent of cytokines for growth, suggesting that signaling pathways activated by v-abl can substitute for those activated by cytokines. Several proteins required for cytokine signaling have recently been identified and characterized (35). In an attempt to understand the molecular mechanism of the cytokine-independent growth of A-MuLV-transformed pre-B cells, we have previously shown that the Janus kinase (Jak)-STAT proteins involved in signaling by interleukin-4 (IL-4) and IL-7 are constitutively activated in these cells (9).
Constitutive activation of STATs has been implicated in several oncogenic processes, including human T-cell lymphotropic virus type 1 (HTLV-1) and v-_src_-generated tumors, in cells infected with herpesvirus saimiri, in some but not all Philadelphia chromosome-positive patients with Bcr-Abl-mediated leukemias, in anaplasic large-cell lymphoma, in lymphoid or myeloid leukemia and lymphoma cells, and in Sézary syndrome (Szs) (reviewed in reference 18). The molecular mechanism leading to constitutive STAT activation in these tumors, however, remains to be elucidated. Recent reports indicate that the SH2 and the SH3 domains of v-Src are required for STAT activation (6) and that Src can be immunoprecipitated with Stat3 and Jak1 (2, 3). Whether these interactions are sufficient to activate Stat3 remains to be shown.
Constitutive activation of the Jak-STAT components of IL-4 and IL-7 signaling in A-MuLV-transformed pre-B cells is not the product of an autocrine loop (9a). These observations are consistent with a model in which signals activated by v-Abl bypass the requirement for these cytokines to bind their receptors. A bypass mechanism is further supported by the finding that in A-MuLV-transformed pre-B cells, the Jak1 and Jak3 proteins are both activated in a v-Abl-dependent manner and are also found in physical association with v-Abl (9). To define the mechanism of v-Abl-dependent STAT activation in A-MuLV-transformed cells, we have examined the significance of v-Abl–Jak association in these cells. Association of v-Abl and Jak1 occurs through a direct interaction and requires a region in the carboxyl terminus of the v-Abl oncoprotein. Deletion of this region in v-Abl abrogates its ability to activate Jak1 and STAT proteins independent of cytokines and to support the proliferation of BAF/3 pro-B cells in the absence of IL-3 and reduces the efficiency of this oncoprotein to induce tumors in nude mice. Consistent with these structure-function studies, a kinase-inactive Jak1 protein inhibits the ability of v-Abl to activate STATs and to induce cytokine-independent growth. Taken together, these results imply that Jak1 is a downstream target of v-Abl and show that v-Abl–Jak1 interaction and Jak1 activity are necessary for v-Abl-induced STAT activation and cellular proliferation.
MATERIALS AND METHODS
Cell culture and cytokine treatment.
IL-7-dependent murine pre-B-cell line clone K (9) was cultured in RPMI 1640 medium supplemented with 10% fetal calf serum, 5 μM β-mercaptoethanol, 1 mM sodium pyruvate, and 1 mM l-glutamine. BAF/3 cells and BAF/3 transfectants stably expressing the thrombopoietin (TPO) receptor (see Fig. 2C) (11) were cultured in the same medium supplemented with 5% WEHI-3 supernatant. Recombinant murine gamma interferon (Genzyme, Cambridge, Mass.) was added at 33 U/ml for 15 min at 37°C. TPO stimulation was carried out at 200 ng/ml with recombinant human TPO (gift of Amgen, Thousand Oaks, Calif.).
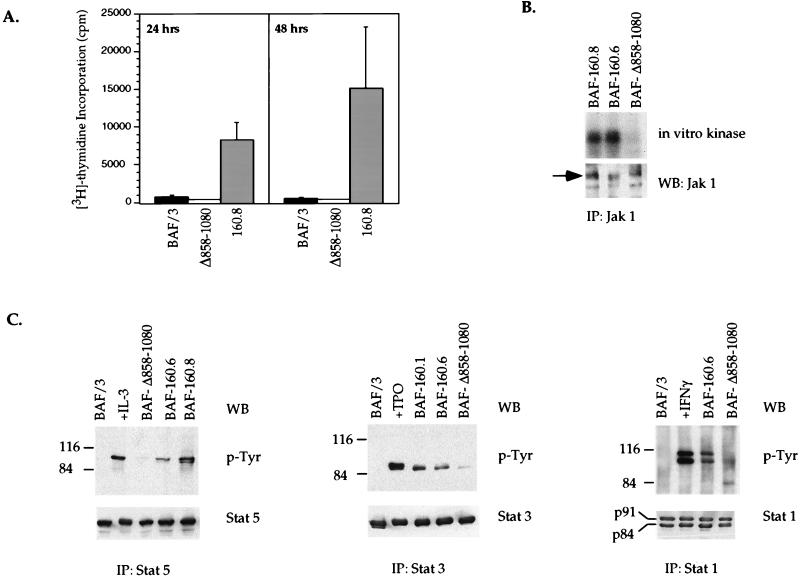
FIG. 2.
Requirement for the Jak1 binding domain of v-Abl in cytokine-independent proliferation, and the activation of Jak1 and STAT proteins in BAF/3 pro-B cells. (A) The Jak1 binding mutant (Δ858–1080) of v-Abl cannot support the proliferation of BAF/3 pro-B cells in the absence of IL-3. IL-3-dependent parental BAF/3 cells or BAF/3 transfectants expressing p160 v-Abl (160.8) or the Jak1 binding mutant (Δ858–1080) were washed and used in [3H]thymidine incorporation assays in the absence of IL-3 for 24 or 48 h. (B) The Δ858–1080 mutant of v-Abl cannot activate Jak1 in the absence of cytokines. Jak1 was immunoprecipitated (IP) from precleared lysates prepared from BAF/3 transfectants stably expressing wild-type (160.8 and 160.6) or Jak1 binding mutant (Δ858–1080) forms of v-Abl. Immune complexes were subjected to an in vitro kinase assay. Half of each sample was analyzed by SDS-PAGE (7% gel) and autoradiography, and the other half was blotted (WB) with an antibody to Jak1 to control for equal amounts of protein immunoprecipitated. (C) The Δ858–1080 mutant of v-Abl cannot activate STATs in the absence of cytokines. Stat1, Stat3, and Stat5 were immunoprecipitated (IP) from precleared lysates prepared from BAF/3 cells stably expressing the wild-type or Δ858–1080 mutant forms of v-Abl. Immune complexes were fractionated by SDS-PAGE and immunoblotted (WB) with an antiphosphotyrosine antibody. The blots were then stripped and reprobed with antibodies to specific STATs as indicated. As a control for tyrosine phosphorylation of specific STATs, parental BAF/3 cells were washed extensively and starved of IL-3 for 2 h. They were then either left untreated or treated for 15 min with WEHI (IL-3) conditioning medium, TPO (for BAF/3 cells stably expressing the TPO receptor), or gamma interferon (IFNγ).
Plasmids.
The glutathione _S_-transferase (GST) fusion constructs spanning various domains of v-Abl have been described previously (25, 55). The GST fusion construct expressing amino acids (aa) 706 to 857 was constructed by deleting the _Xho_I-_Sal_I fragment of GST-_Nar_I-_Sal_I (encoding aa 706 to 1080). The expression constructs for p160 v-Abl and c-Abl have been described previously (44, 55). The v-Abl expression vector lacking the entire Jak1 interaction domain (Δ858–1080) was constructed by deleting the _Xho_I-_Sal_I fragment from the p160 expression construct. The kinase-inactive Jak1 (Jak1 K896R) has been described previously (27). For inducible expression, the cDNA for this kinase-inactive Jak1 was subcloned in the MTCB6+ plasmid (45).
Stable transfections.
Transfections of BAF/3 cells were carried out by electroporation as described previously (11). The Abl expression constructs were cotransfected with a puromycin selectable marker, and stable transfectants were selected in 1 μg of puromycin per ml. Stable p160 BAF/3 cells expressing the inducible dominant negative Jak1 were selected in G418 (2 mg/ml).
Antibodies.
The anti-Jak1 antibody used in immunoblots and the Stat1, Stat3, Stat5, p62 Dok, and Ras antibodies were purchased from Santa Cruz Biotechnology, Santa Cruz, Calif. The Jak1 antibody used in immunoprecipitation and in vitro kinase assays and the Shc and the antiphosphotyrosine antibodies were purchased from Upstate Biotechnology, Lake Placid, N.Y. The Abl antibody was purchased from Calbiochem, Cambridge, Mass. The β-actin antibody was purchased from Sigma Immunochemicals, St. Louis, Mo. The anti-murine c-Myc antibody was a generous gift of Kathryn Calame, Columbia University.
Expression of GST fusion proteins and in vitro binding studies.
GST fusion proteins were expressed and purified as described by Frangioni and Neel (17). The amount of proteins bound to beads was estimated for each fusion protein following staining with Coomassie brilliant blue. Whole-cell extracts (1 mg of total protein) or 1 to 3 μg of purified Jak1 protein (a generous gift of Robert Schreiber, Washington University) was incubated with 50 μg of GST fusion protein in 300 to 500 μl of lysis buffer (0.5% Nonidet P-40, 50 mM Tris [pH 8.0], 10% glycerol, 0.1 mM EDTA, 1 mM dithiothreitol, 100 μM sodium orthovanadate, 200 mM NaCl, 1.2 mM phenylmethylsulfonyl fluoride). Incubation was done at 4°C overnight with constant agitation. Bound materials were washed extensively (four times with lysis buffer and three times with phosphate-buffered saline), eluted by boiling in Lammeli sample buffer, fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (7% polyacrylamide), transferred onto a nitrocellulose membrane, and blotted with an antibody to Jak1.
Whole-cell extracts, immunoprecipitation, and in vitro kinase assays.
Whole-cell extracts, immunoprecipitation, and in vitro kinase assays were done as described previously (9).
Proliferation assays.
Cells were washed extensively in medium without IL-3 and resuspended at a density of 2.5 × 104 cells in 100 μl of complete RPMI or complete RPMI containing 10−4 M ZnSO4 (final concentration) in 96-well plates. The cells were pulsed with 1 μCi of 3H (specific activity, 6.7 Ci/mmol; NEN, Boston, Mass.) during the last 4 h in culture, and 3H incorporation was quantified.
Nude-mouse injections.
Cells (5 × 106) were washed extensively and resuspended in 200 μl of PBS. Female nude mice (4 to 8 weeks old) were injected subcutaneously.
Ras assays.
Cells were starved of cytokines, and the assays were performed as described previously (10).
RESULTS
aa 858 to 1080 in the carboxyl-terminal portion of v-Abl are required for Jak1 association.
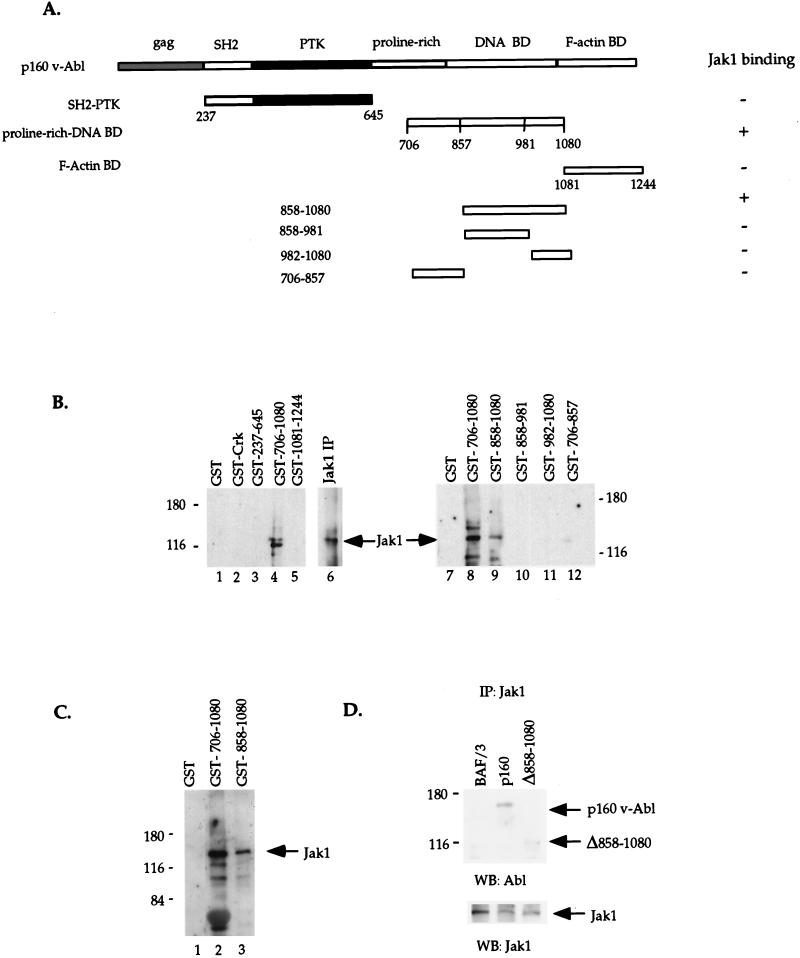
We have previously shown that in A-MuLV-transformed pre-B cells, 10 to 20% of the total cellular v-Abl associates with the Jak1 and Jak3 proteins (9). To identify the domain(s) of v-Abl essential for this interaction, different regions of Abl were expressed as GST fusion proteins for in vitro binding studies (Fig. 1A). The region spanning aa 237 to 645 (based on the p160 genome of A-MuLV, aa 1 is the starting methionine in gag [52]) corresponds to the N-terminal portion of v-Abl and includes the SH2 and kinase domains. The region containing aa 706 to 1080 includes proline-rich (SH3 binding) regions (16, 43) and a domain that, in c-Abl, exhibits DNA binding activity (25). aa 1081 to 1244 span a region that has been shown to bind F/G-actin in c-Abl and Bcr-Abl (32, 53). GST fusion proteins were generated, bound to glutathione-agarose beads, and incubated with whole-cell extracts prepared from a nontransformed pre-B-cell line (clone K). Materials bound to beads were washed and fractionated, and the interaction of the GST fusion proteins with Jak1 was examined by Western blotting with an antibody to Jak1.
FIG. 1.
Mapping the Jak1 interaction domain of v-Abl in vitro and in vivo. (A) GST fusion proteins spanning various domains of v-Abl, including the src homology 2 and the protein tyrosine kinase domains (SH2-PTK, aa 237 to 645), the proline-rich and the DNA binding domains (aa 706 to 1080), and the F-actin binding domain (aa 1081 to 1244), or smaller GST fusion proteins spanning aa 706 to 857, 858 to 981, and 982 to 1080 were expressed in bacteria and bound to glutathione beads. (B and C) Equal amounts of fusion proteins were incubated with 1 mg of whole-cell extracts prepared from a murine pre-B-cell line (clone K) (B) or 1 to 3 μg of purified Jak1 protein (C). Bound materials were washed extensively, eluted off the beads, fractionated by SDS-PAGE (7% polyacrylamide), and blotted with an antibody to Jak1. The GST moiety alone and the SH2 domain of Crk were used as controls for the specificity of the GST fusion protein-Jak1 interaction. Jak1 immunoprecipitates from the same whole-cell extracts (panel B, lane 6) were included as control for the Jak1 protein. (D) IL-3-dependent BAF/3 cells were stably transfected with v-Abl expression constructs encoding the wild-type p160 v-Abl or the Jak1 binding mutant Δ858–1080. A 1-mg portion of total protein extracts from the transfectants was immunoprecipitated (IP) with an antibody to Jak1. Immune complexes were fractionated by SDS-PAGE (7% polyacrylamide) and subjected to Western blotting (WB) with an Abl antibody. The blot was stripped and probed with a Jak1-specific antibody to control for the amount of Jak1 immunoprecipitated.
The GST-Abl fusion protein expressing the proline-rich and DNA binding domains (aa 706 to 1080) consistently associates with the Jak1 protein present in whole-cell extracts (Fig. 1B, lane 4). Preliminary experiments suggest that this region of Abl can also mediate interaction with Jak3 in cell extracts (data not shown). Interestingly, cytokine treatment before preparation of cell extracts does not affect the outcome of the in vitro binding experiments (data not shown), suggesting that prior activation of Jak1 is not necessary for the Jak1–Abl interaction. This is consistent with our previous observation that in _ts_-A-MuLV–pre-B cells, Jak1 and v-Abl coimmunoprecipitate at nonpermissive temperature when Jak1 is hypophosphorylated (9). The in vitro interaction of Abl and Jak1 is specific since unrelated proteins, including TFE3, Oct1, YY1, the two different SH2 domains of the regulatory subunit of PI3-kinase (p85), and the SH2 domain of Crk, expressed as fusion proteins do not bind Jak1 (Fig. 1B, lane 2, and data not shown). The GST-Abl fusion protein containing the SH2 and the kinase domains does not bind Jak1 consistently in similar in vitro binding assays. These data indicate that a domain in the carboxyl terminus of v-Abl can interact with Jak1 and that a second region of v-Abl may also participate in this interaction.
To further delineate the Jak1-interacting domain of Abl, various deletion mutants mutated in the region spanning aa 706 to 1080 were generated and analyzed in similar in vitro binding studies. The first 152-aa sequence in this region, aa 706 to 857, which contains one of the three proline-rich (SH3 binding) sequences previously defined as being in the C termini of Abl proteins (16, 43), does not interact with Jak1 in vitro (Fig. 1B, lane 12). In contrast, the 223-aa region which corresponds to the domain in c-Abl that binds DNA (25, 33), aa 858 to 1080, binds Jak1 when incubated with whole-cell extracts (lane 9). Two smaller deletion mutants mutated within this domain were generated to yield a fragment spanning aa 858 to 981 and the previously defined minimal c-Abl DNA binding domain, aa 982 to 1080. These latter GST fusion proteins do not bind Jak1 when incubated with whole-cell extracts (Fig. 1B, lanes 10 and 11). Thus, a 223-aa sequence in the carboxyl-terminal region of v-Abl is required for association with Jak1.
Association of the Abl carboxyl terminus with Jak1 in whole-cell extracts could occur through a direct interaction of these proteins or may require a third molecule. To distinguish between these two possibilities, in vitro binding studies with 1 to 3 μg of a purified Jak1 protein were performed. The Jak1 protein used in these experiments was histidine tagged and purified on appropriate columns. Immunoblotting of the materials bound to GST fusion proteins with an antibody to Jak1 shows that the GST fusion containing the proline-rich and DNA binding domains (aa 706 to 1080) or just the DNA binding domain (aa 858 to 1080) of Abl interacts with purified Jak1 (Fig. 1C). The lack of association between the GST moiety alone and purified Jak1 suggests that the above interaction is specific rather than an artifact of the amount of GST fusion protein or the purified Jak1 used. Analysis of total purified Jak1 protein left in the supernatant after incubation with GST fusion proteins compared to the amount brought down with GST fusion proteins shows that approximately 15% of purified Jak1 was associated with the GST fusion protein in the above in vitro binding experiment (data not shown).
To examine the requirement of the region spanning aa 858 to 1080 of v-Abl in Jak1 binding in vivo, stable BAF/3 pro-B-cell lines expressing either the full-length v-Abl (p160) or a C-terminal deletion mutant lacking the Jak1 binding domain (Δ858–1080) were generated. Clones expressing similar levels of v-Abl proteins were selected for further analysis. Immunoblotting of Jak1 immunoprecipitates from these cells with an Abl-specific antibody indicates that in contrast to wild-type p160 v-Abl, the Δ858–1080 mutant exhibits significant defects in its ability to associate with Jak1 in vivo (Fig. 1D). In addition, a peptide which contains only aa 858 to 1080 of v-Abl can bind Jak1 when expressed in 293T cells (data not shown), suggesting that this domain in the carboxyl-terminal portion of v-Abl is sufficient to bind Jak1 in vivo.
Taken together, these data indicate that the region spanning aa 858 to 1080 in the C terminus of v-Abl is required for Jak1 interaction both in vitro and in vivo. This is the first demonstration of a direct interaction between a Jak and another class of tyrosine kinases. Because the v-Abl oncoprotein exhibits constitutive kinase activity, these results led us to propose that the interaction of this protein with Jak proteins may activate Jak proteins independent of cytokines (see below).
The Jak1 binding domain of v-Abl is required for cytokine-independent proliferation and Jak-STAT signaling in BAF/3 pro-B cells.
Hematopoietic cells infected with A-MuLV or cells transfected with the v-Abl oncoprotein can grow in the absence of cytokines, including IL-3 (30). To address the requirement for the Jak1 binding domain of v-Abl in mitogenic pathways activated by this oncogene, the rate of DNA synthesis in BAF/3 cells expressing wild-type v-Abl or the Jak1 binding mutant (Δ858–1080) of v-Abl was assessed. 3H uptake assays 24 or 48 h after washing and seeding cells in the absence of IL-3 reveal a 20- to 30-fold-higher proliferation in cells expressing wild-type v-Abl than that in cells expressing the mutant protein (Fig. 2A). This defect in proliferation is reversible in the presence of IL-3, since IL-3-induced proliferation of cells expressing this mutant of v-Abl is comparable to or even slightly higher than that of parental BAF/3 cells (data not shown). BAF/3 p160 v-Abl transfectants remain viable and can proliferate continuously in the absence of IL-3. The differential proliferation and survival of the wild-type and mutant v-Abl transfectants cannot be explained simply by the differential enzymatic activities of the two proteins, since in vitro kinase assays demonstrate that the kinase activity of the mutant v-Abl is comparable to that of the wild-type protein (data not shown). This is consistent with recent studies indicating that the carboxyl-terminal portion of v-Abl does not play a regulatory role in the kinase activity of the protein (37). These data indicate that the Jak1 binding domain of v-Abl is required to render BAF/3 cells IL-3 independent for proliferation.
The growth properties of the v-Abl BAF/3 clones correlate with the activation status of Jak-STAT proteins in these cells. In vitro kinase assays performed on Jak1 immunoprecipitates from these transfectants suggest that Jak1 is a substrate of v-Abl. Jak1 activation in the absence of cytokine addition can be detected when wild-type v-Abl is expressed in these cells, while this activity is lost upon expression of the Δ858–1080 mutant (Fig. 2B). In p160 v-Abl transfectants, Stat1, Stat3, and Stat5 are constitutively activated as indicated by their state of tyrosine phosphorylation (Fig. 2C). Consistent with these observations, BAF/3 cells stably expressing p160 v-Abl also exhibit cytokine-independent STAT DNA binding activities as assessed by electrophoretic mobility shift assays, while such activities are absent in cells expressing the Δ858–1080 mutant (data not shown). In addition, supernatants taken from p160 v-Abl transfectants cannot induce STAT activation when added to parental BAF/3 cells, suggesting that constitutive phosphorylation of STATs observed in these transfectants is probably not the outcome of an autocrine loop (data not shown). The above observations indicate that the region spanning aa 858 to 1080 in the carboxyl terminus of v-Abl is required for cytokine-independent Jak1 and STAT activation by this oncoprotein.
An active Jak1 protein is required for v-Abl-induced activation of STATs and cytokine independent proliferation of BAF/3 cells expressing p160 v-Abl.
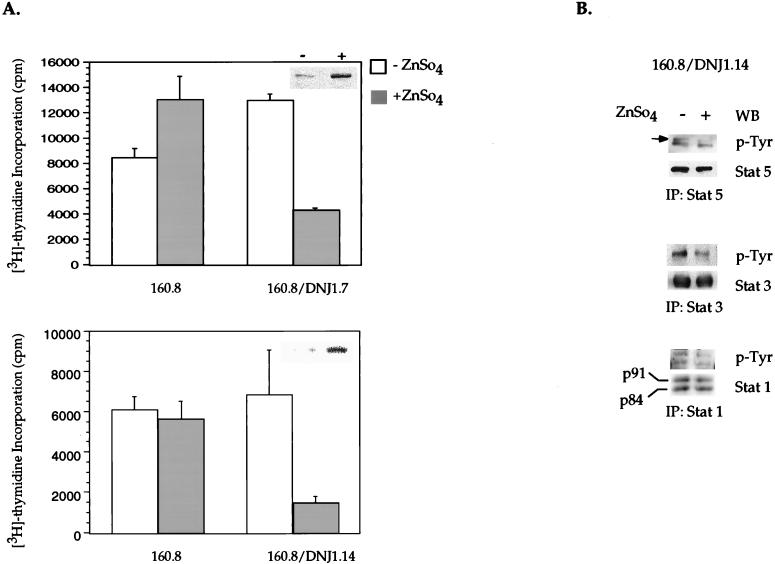
The role of Jak proteins in proliferation has not been extensively studied. The inability of the Jak1-binding mutant to activate STATs and to support the IL-3-independent growth of BAF/3 cells (Fig. 2A) is consistent with a model in which Jak1 may function as either a substrate or a mediator of v-Abl signaling to these pathways. To establish a link between Jak1 function and the differential ability of the wild-type and Jak1 binding mutant of v-Abl to confer cytokine-independent proliferation and STAT activation, a kinase-inactive mutant of Jak1 was generated by mutating a single lysine residue to arginine at position 896 in the ATP binding loop of the enzyme (27). The metallothionine promoter regulating the expression of kinase-inactive Jak1 allows two- to threefold induction of this protein when BAF/3 p160 v-Abl cells stably expressing the inducible kinase-inactive Jak1 expression construct are cultured overnight in the presence of ZnSO4. To examine the requirement for Jak1 function in v-Abl-induced cytokine-independent proliferation, 3H uptake assays were conducted with p160 v-Abl BAF/3 cells in the absence of IL-3, before and after induction of the kinase-inactive Jak1 protein. Concomitant with the induction of this Jak1 mutant (anti-Jak1 immunoblots [Fig. 3A, inserts]), an approximately three- to fourfold reduction in DNA synthesis was observed after various independent clones were cultured in the presence of ZnSO4. In contrast, proliferation of the parental p160-expressing BAF/3 clone was not affected in any significant manner after addition of ZnSO4 to the medium (Fig. 3A). STAT immunoprecipitates from cells induced to express the kinase-deficient Jak1 reveal that the reduction in the rate of DNA synthesis was accompanied by a decreased level of STAT tyrosine phosphorylation in these cells (Fig. 3B). The kinase-inactive Jak1 mutant can associate with v-Abl (data not shown). It is possible that this mutant exerts its effect as a dominant negative protein by competing with wild-type Jak1 for binding to v-Abl and activating downstream Jak1-dependent pathways. The above observations suggest that a functional Jak1 is required to relay cytokine-independent STAT activation and proliferation in the presence of v-Abl.
FIG. 3.
Functional requirement of Jak1 in v-Abl-induced mitogenesis and STAT activation independent of cytokines. (A) Induction of a kinase-inactive Jak1 leads to decreased v-Abl-induced, cytokine-independent proliferation. BAF/3 cells stably expressing p160 wild-type v-Abl (160.8) or two independently derived clones expressing p160 v-Abl and a construct encoding a kinase-inactive/dominant negative Jak1 (160.8/DNJ1.7 and 160.8/DNJ1.14) under the control of the metallothionine promoter were incubated overnight in 10−4 M (final concentration) ZnSO4. [3H]thymidine incorporation assays were performed after 24 h, as in Fig. 2A. Extracts were made from the same sample of cells to examine the induction of DNJ1 protein by Western blotting (insets). (B) Induction of dominant negative Jak1 leads to decreased STAT activation. Lysates from 160.8/DNJ1.14 cells were precleared, and Stat1, Stat3, and Stat5 were immunoprecipitated (IP); their phosphorylation was analyzed as described in the legend to Fig. 2C. WB, Western blotting.
Cells expressing the Jak1 binding mutant of v-Abl exhibit lower frequency and extended latency of tumor formation in nude mice.
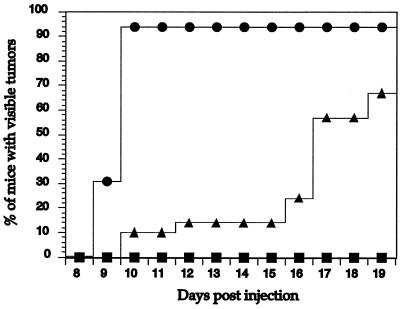
The above growth characteristics of BAF/3 cells expressing p160 v-Abl or the Jak1 binding mutant form of the protein (Δ858–1080) led us to examine the role of this domain in transformation. Because we have been unable to generate retroviruses that express the Jak1 binding mutant of v-Abl, we used the BAF/3 cells expressing wild-type or Δ858–1080 mutant v-Abl in nude-mouse injection assays. Mice were examined for 2 weeks for signs of visible tumor growth. Tumors could be detected within 10 days in 94% of the nude mice injected with wild-type v-Abl BAF/3 transfectants. In contrast, only 10% of the mice injected with cells expressing the Jak1 binding mutant of v-Abl showed visible tumor growth during this period (Fig. 4). Tumors were eventually visible 17 days postinjection in 57% of the mice that received cells expressing the mutant v-Abl. By day 19 postinjection, 67% of these mice showed visible sign of tumor growth. In addition to this delayed appearance, the tumors formed in mice injected with cells expressing the Δ858–1080 mutant of v-Abl were three- to fourfold smaller in mass compared to those generated by p160 transfectants (data not shown). Parental IL-3-dependent BAF/3 cells did not give rise to any tumors during the course of these experiments. Because p160 and the Jak1 binding mutant form of v-Abl show similar in vitro kinase activities (data not shown), the difference in tumorigenic potential of BAF/3 v-Abl transfectants cannot be explained based on differential enzymatic activities of the two proteins. Our observations suggest that the Jak1 binding domain of v-Abl is important in rendering BAF/3 cells highly tumorigenic in nude mice.
FIG. 4.
Reduced efficiency and extended latency of the Jak1 binding mutant of v-Abl in tumorigenesis. Nude mice were injected with either parental IL-3-dependent BAF/3 cells (■) or BAF/3 transfectants expressing wild-type (•) or Δ858–1080 mutant (▴) v-Abl and monitored for visible signs of growth during the 19-day period postinjection. Data from three experiments are pooled. The total numbers of mice injected per cell line were as follows: 5 for parental IL-3-dependent BAF/3 cells, 16 for BAF/3 transfectants expressing p160 v-Abl, and 22 for BAF/3 transfectants expressing Δ858–1080.
Jak1 may influence other signaling pathways downstream of v-Abl.
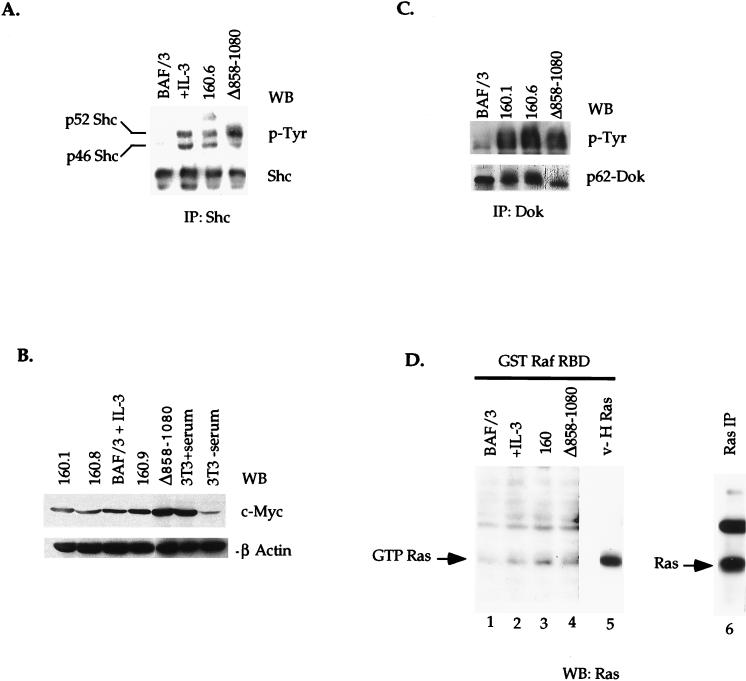
Although BAF/3 cells stably expressing p160 v-Abl or the Jak1 binding mutant (Δ858–1080) of the protein provide a useful in vivo system to analyze the requirement of the Jak1 binding domain in activation of downstream signaling pathways by v-Abl, it cannot be excluded that the difference in the abilities of these cells to proliferate in the absence of cytokines or to generate tumors in nude mice is due to a large deletion, which might impair other signaling pathways in addition to Jak-STAT. The region spanning aa 858 to 1080 of v-Abl has not been shown to bind any other signaling molecule thus far. The two identified PKC recognition sites (39), the majority of the cdc2 consensus sites (26), the SH3 binding regions (shown to bind Grb-2, Nck, and Crk) (16, 43), and the F/G-actin binding domain (32, 53) are still conserved when aa 858 to 1080 are deleted from v-Abl. To examine the impact of this deletion on protein folding and function, several other signaling pathways shown to be activated by v-Abl or those implicated in proliferation and cell survival were analyzed. Although the Jak1 binding mutant of v-Abl cannot confer cytokine-independent proliferation and survival to BAF/3 cells, deletion of the Jak1 binding domain of v-Abl does not lead to a reduction in the level of tyrosine-phosphorylated Shc, p62 Dok (13, 57), Abi-1 (50), or c-Myc (Fig. 5A to C and data not shown). These data suggest that the Jak1-Abl interaction is not required for the activation of these signaling pathways by v-Abl. It is interesting that the levels of c-Myc and phosphorylated p52 Shc are slightly increased in cells expressing the mutant v-Abl. The molecular mechanism underlying these increases is not clear.
FIG. 5.
Activation of other signaling pathways by the Δ858–1080 Jak1 binding mutant. (A and C) Lysates prepared from BAF/3 transfectants expressing p160 v-Abl or the Δ858–1080 mutant form of the protein were immunoprecipitated with anti-Shc (A) or anti-p62 Dok (C) antibodies. Immune complexes were fractionated by SDS-PAGE. The activation state of the proteins was examined by blotting the membranes with an antiphosphotyrosine antibody. The membranes were then stripped and reprobed with anti-Shc or anti-Dok antibodies. (B) A 30-μg portion of total protein cell lysate was fractionated on SDS-PAGE and immunoblotted with an antibody to c-Myc. Membranes were stripped and reprobed with an antibody to β-actin to control for protein loading. (D) Extracts from cytokine-starved cells (lanes 1 to 4) or pre-B cells transformed with the constitutively active form of Ras (v-H Ras [lane 5]) were incubated with glutathione beads coupled to GST Raf RBD fusion protein. Bound materials were washed, fractionated on SDS-PAGE (12.5% gel), and immunoblotted with an anti-Ras antibody. As a positive control for Ras protein, Ras was immunoprecipitated from v-H Ras-transformed pre-B cells (lane 6). The GST moiety alone does not bind Ras (data not shown).
It has become increasingly clear that in addition to STATs, Jak proteins may regulate other signaling proteins, including the Ras/MAPK pathway (56). To assess the level of the active (GTP-bound) form of Ras downstream of the wild-type v-Abl or the Jak1 binding mutant of v-Abl, GST pulldown assays were performed with the Ras-binding domain of Raf (GST Raf RBD). It has previously been established that this domain of Raf exhibits high-affinity interaction with GTP- and not GDP-bound Ras. This property of Raf RBD has been used to assess Ras activation (10). In BAF/3 transfectants expressing p160 v-Abl, a significant amount of GTP Ras can be captured with the GST Raf RBD fusion protein (Fig. 5D, lane 3). This level of GTP Ras is significantly higher than that detected in parental BAF/3 (lanes 1 and 2) but lower than that found in cells transformed with a constitutively active form of Ras (v-H Ras, lane 5). Interestingly, the level of GTP Ras appears to be slightly reduced downstream of the Jak1 binding mutant of v-Abl (lane 4), even though the kinase activity of this mutant is comparable to that of wild-type v-Abl (data not shown). The level of active Ras in cells expressing this mutant, however, is still higher than that detected in IL-3-treated parental BAF/3 cells (lane 2). These data suggest that the Jak1 binding domain of v-Abl may be required for maximum activation of Ras downstream of v-Abl.
DISCUSSION
In this study, we have taken two complementary approaches to examine the role of Jak-STAT activation in v-Abl function. First, we have defined a region in the carboxyl terminus of v-Abl that is required for Jak1 association both in vitro and in vivo. Deletion of this domain abrogates the ability of v-Abl to activate Jak1 and STATs in different cell lines. In addition, BAF/3 transfectants expressing the Jak1 binding mutant of v-Abl are unable to proliferate in the absence of IL-3 and are less tumorigenic in nude mice than are BAF/3 cells expressing the wild-type protein. Second, we have used a kinase-inactive mutant of Jak1 to show that Jak1 function is required for v-Abl to activate STATs and to stimulate proliferation of BAF/3 cells independent of cytokines. Together, these results indicate that Jak1 is important in several aspects of v-Abl function.
The Jak1 interaction domain of v-Abl maps to a novel region (aa 858 to 1080) in the carboxyl terminus of the protein, which was previously of unknown function. To our knowledge, no other signaling protein has been shown to interact with this domain. Although this region is required for Jak1 association, it is possible that other v-Abl domains or other Abl-interacting proteins contribute to Jak1 activation by v-Abl. Using in vitro binding assays, we can occasionally detect interaction of Jak1 with a GST fusion protein expressing the SH2 and the kinase domains of v-Abl. Our previous studies have demonstrated that the Abl kinase activity is required for v-Abl-dependent activation of the Jak-STAT pathway. Interestingly, association of Jak1 and v-Abl in vivo is diminished but not lost at nonpermissive temperature (9). It is therefore possible that the binding of Jak1 to the C terminus of v-Abl serves primarily to bring Jak1 close to the v-Abl catalytic (kinase) domain and that the interaction between v-Abl SH2 domain and tyrosine-phosphorylated Jak1 further stabilizes this complex.
Cells that are naturally dependent on cytokines for growth can, in the presence of v-Abl, proliferate independently of cytokines. Our observations suggest that Jak1 is required for v-Abl-mediated proliferation of BAF/3 cells. This is especially interesting because several signaling molecules previously suggested to be involved in proliferative signals by v-Abl, including Shc, p62 Dok, and myc, do not show a reduced level of activation in cells expressing the Jak1 binding mutant form of v-Abl. Therefore, it appears that Jak1 may play an important role in mitogenic signals downstream of v-Abl. Although recent studies suggest that STATs may be required for maximal proliferation induced by cytokines (29), it is not clear whether proliferation of v-Abl-expressing transfectants is dependent on STAT activation. In addition to STATs, Jaks may target other signaling pathways. For instance, a link between Jaks and Ras has been suggested (56). Consistent with a possible role for Jak1 in Ras regulation, we found that the level of the GTP-bound form of Ras is slightly diminished downstream of the Jak1 binding mutant of v-Abl. The level of GTP Ras in BAF/3 cells expressing this mutant, however, is still higher than that seen in parental cells. This may be due to the recruitment of signaling proteins such as Grb-2, which have been shown to bind the proline-rich sequences 3′ of the Abl kinase domain (43). Despite Ras activation, we and others did not detect a significant level of activated ERK1/ERK2 MAP kinases downstream of v-Abl (reference 36 and data not shown). It is possible that downstream of v-Abl, and in the context of BAF/3 cells, Ras is involved in regulation of other signaling proteins, such as PI3-kinase. Ras and PI3-kinase have been reported to regulate each other (12). Consistent with this, and in accord with a recent report suggesting the requirement for Jak1 in activation of PI3-kinase (1), we found a diminished level of p85 phosphorylation downstream of the Jak1 binding mutant of v-Abl (data not shown). Although the exact molecular mechanism underlying the proliferation defect in cells expressing the v-Abl mutant awaits further studies, our observations suggest that Jak1 activation downstream of v-Abl may serve to regulate multiple signaling pathways.
The ability of v-Abl to bind and activate Jak proteins led us to question whether this interaction is important in cellular transformation. Indeed, Jak proteins have been implicated in oncogenic processes (28, 38). A gain-of-function mutant allele of hopscotch, a homologue of Drosophila Jak, can also lead to transformation (19, 20). Our data clearly show that in BAF/3 cells, v-Abl–Jak1 association and Jak1 activation are important in cytokine-independent proliferation. However, factor-independent growth per se does not lead to transformation (7, 58). Signaling pathways activated by the abl oncogene therefore might not solely mimic events triggered by receptor-cytokine interaction. Because we have been unable to obtain A-MuLV that contains the Jak1 binding mutant of v-Abl, we addressed the importance of Jak activation by v-Abl in nude-mouse assays with BAF/3 cells. We found that BAF/3 cells expressing the Jak1 binding mutant of v-Abl are somewhat impaired in generating tumors in nude mice. Since these assays were done with a long-term tissue culture cell line, they do not necessarily completely mimic the transformation process in vivo. Cellular transformation appears to require the activation of several different complementary pathways. Since secondary genetic alterations tend to accumulate in long-term tissue culture cell lines, some of these pathways may already be active in the BAF/3 cell line, making these cells more permissive for transformation by the Jak1 binding mutant of v-Abl. Alternatively, since some tumors do eventually appear in these mice, the Jak1 binding domain of v-Abl may be important for high-efficiency tumor formation but not for absolute transformation. Analysis of primary cells expressing the mutant v-Abl and examination of Jak1-deficient mice for efficiency of v-Abl-induced transformation are needed to further address the importance of Jak1 in transformation by v-Abl.
Although the human oncogenic form of Abl, Bcr-Abl, and the murine oncogenic form v-Abl have many common characteristics, several structural and functional differences between the two have been reported. It appears that the activation of Jak-STAT signaling by these two forms of Abl may be another example of biological differences between these oncoproteins. Unlike v-Abl, Bcr-Abl does not associate with Jak proteins (5, 23). It is possible that sequences within the Bcr region or the Abl SH3 domain which are present in Bcr-Abl, but not v-Abl, contribute to protein folding or protein-protein interactions rendering C-terminal sequences inaccessible for Jak1 binding. Alternatively, the C terminus of Bcr-Abl might not be able to bind Jak proteins. Comparison of Abl protein sequences reveals that although there is a high degree of homology (99%) between murine and human c-Abl in the SH2 and kinase domains (15), the C-terminal region (corresponding to aa 858 to 1080 of v-Abl) are 68% identical. Therefore, it is possible that critical amino acids required for Jak1 binding within this region are not present in the human Bcr-Abl oncoprotein. Interestingly, constitutive activation of Jak proteins has not been consistently detected in all Bcr-Abl-expressing cells (4, 5, 23, 51). These data are consistent with the observation that kinase-inactive mutants of Jak2 do not block constitutive activation of Stat5 in Bcr-Abl-expressing BAF/3 cells (23). Thus, activation of STATs by Bcr-Abl may be Jak independent. It is possible that Bcr-Abl directly activates STATs or that it targets its kinase activity to STATs through a different interacting protein.
One of the paradoxes of v-Abl biology is that although A-MuLV can bind to most cell types, the tumors that develop in mice infected with this virus are almost exclusively pre-B-cell leukemias. The etiology of this specificity remains unknown. Accumulating evidence suggests that the carboxyl terminus of v-Abl may play an important role in regulating this function of the oncoprotein. Experiments in which various regions of src and abl oncogenes were used to generate hybrid retroviral genomes have shown that the C-terminal domain of v-Abl, in addition to the 3′ end of the Abl kinase domain, is sufficient to confer a pre-B-cell transforming property to src retroviruses (21). Although all C-terminal truncation mutants of A-MuLV examined thus far can transform NIH 3T3 cells, they exhibit reduced efficiency of bone marrow transformation. The majority of these mutants are also somewhat impaired in their ability to generate tumors in mice (22, 34). The Abl oncoprotein has been shown to activate many signaling pathways, several of which may be required for transformation. It is possible that in some cells (e.g., NIH 3T3 cells), signaling pathways that require the intact carboxyl terminus of v-Abl, such as the cytokine signaling pathway, are not essential for transformation, perhaps due to the activation of other compensatory pathways. Given our data and the transformation phenotype of carboxyl-terminal truncation mutants of A-MuLV, it would be interesting to examine whether activation of the Jak-STAT pathway by the carboxyl terminus of v-Abl may play a unique role in the selectivity with which A-MuLV functions in its in vivo targets.
ACKNOWLEDGMENTS
We thank Robert Schreiber for purified his-Jak1, Kathryn Calame for v-Abl expression vector and anti-murine c-Myc antibody, Frank McCormick for the GST Raf RBD construct, and Jacalyn Pierce for v-HRas-transformed pre-B cells. We are grateful to Binfeng Lu for assistance with nude-mouse injections and Pang-Dian Fan for Abi-1 activation assays. We thank Konstantina Alexandropoulos, Marion Dorsch, and members of the Rothman laboratory for critical reading of the manuscript.
This work was supported by Cancer Research Institute/Partridge Foundation Clinical Investigator Award (to P.B.R.) and NIH grants 2T32DK07328-17 (to N.N.D.) and CA43054 (to J.Y.J.W.).
REFERENCES
- 1.Burfoot M S, Rogers N C, Watling D, Smith J M, Pons S, Paonessaw G, Pellegrini S, White M F, Kerr I M. Janus kinase-dependent activation of insulin receptor substrate 1 in response to interleukin-4, oncostatin M, and the interferons. J Biol Chem. 1997;272:24183–24190. doi: 10.1074/jbc.272.39.24183. [DOI] [PubMed] [Google Scholar]
- 2.Campbell G, Yu C L, Jove R, Carter-Su C. Constitutive activation of JAK1 in Src-transformed cells. J Biol Chem. 1997;272:2591–2594. doi: 10.1074/jbc.272.5.2591. [DOI] [PubMed] [Google Scholar]
- 3.Cao X, Tay A, Guy G R, Tan Y H. Activation and association of Stat3 with Src in v-Src-transformed cell lines. Mol Cell Biol. 1996;16:1595–1603. doi: 10.1128/mcb.16.4.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlesso N, Frank D A, Griffin J D. Tyrosyl phosphorylation and DNA binding activity of signal transducers and activators of transcription (STAT) proteins in hematopoietic cell lines transformed by Bcr/Abl. J Exp Med. 1996;183:811–820. doi: 10.1084/jem.183.3.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chai S K, Nichols G L, Rothman P. Constitutive activation of JAKs and STATs in BCR-Abl-expressing cell lines and peripheral blood cells derived form leukemic patients. J Immunol. 1997;159:4720–4728. [PubMed] [Google Scholar]
- 6.Chaturvedi P, Sharma S, Reddy E P. Abrogation of interleukin-3 dependence of myeloid cells by the v-src oncogene requires SH2 and SH3 domains which specify activation of STATs. Mol Cell Biol. 1997;17:3295–3304. doi: 10.1128/mcb.17.6.3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen S-C, Redenius D, Young J C, Schwartz R C. Synergy of IL-7 and v-Ha-ras in the in vitro neoplastic progression of murine pre-B cells. Oncogene. 1993;8:2119–2125. [PubMed] [Google Scholar]
- 8.Cleveland J L, Dean M, Rosenberg N, Wang J Y J, Rapp U R. Tyrosine kinase oncogenes abrogate interleukin-3 dependence of murine myeloid cells through signaling pathways involving c-myc: conditional regulation of c-myc transcription by temperature-sensitive v-abl. Mol Cell Biol. 1989;9:5685–5695. doi: 10.1128/mcb.9.12.5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Danial N N, Pernis A, Rothman P B. Jak-STAT signaling induced by the v-Abl oncogene. Science. 1995;269:1875–1877. doi: 10.1126/science.7569929. [DOI] [PubMed] [Google Scholar]
- 9a.Danial, N. N., and P. B. Rothman. Unpublished results.
- 10.de Rooij J, Bos J L. Minimal Ras-binding domain of Raf1 can be used as an activation-specific probe for Ras. Oncogene. 1997;14:623–625. doi: 10.1038/sj.onc.1201005. [DOI] [PubMed] [Google Scholar]
- 11.Dorsch M, Fan P-D, Danial N N, Rothman P B, Goff S P. The thrombopoietin receptor can mediate proliferation without activation of the Jak-STAT pathway. J Exp Med. 1997;186:1947–1955. doi: 10.1084/jem.186.12.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Downward J. Role of phosphoinositide-3-OH kinase in Ras signaling. Adv Second Messenger Phosphoprotein Res. 1997;31:1–10. doi: 10.1016/s1040-7952(97)80004-3. [DOI] [PubMed] [Google Scholar]
- 13.Ellis C, Moran M, McCormick F, Pawson T. Phosphorylation of GAP and GAP-associated proteins by transforming and mitogenic tyrosine kinases. Nature. 1990;343:377–381. doi: 10.1038/343377a0. [DOI] [PubMed] [Google Scholar]
- 14.Ernst T J, Slattery K E, Griffin J D. p210Bcr/Abl and p160v-Abl induce an increase in the tyrosine phosphorylation of p93c-Fes. J Biol Chem. 1994;269:5764–5769. [PubMed] [Google Scholar]
- 15.Fainstein E, Einat M, Gokkel E, Marcelle C, Croce C M, Gale R P, Canaani E. Nucleotide sequence analysis of human ABL and BCR-ABL cDNAs. Oncogene. 1989;4:1477–1481. [PubMed] [Google Scholar]
- 16.Feller S M, Knudsen B, Hanafusa H. c-Abl kinase regulates the protein binding activity of c-Crk. EMBO J. 1994;13:2341–2351. doi: 10.1002/j.1460-2075.1994.tb06518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frangioni J V, Neel B G. Solubilization and purification of enzymatically active glutathione S-transferase (pGEX) fusion proteins. Anal Biochem. 1993;210:179–187. doi: 10.1006/abio.1993.1170. [DOI] [PubMed] [Google Scholar]
- 18.Garcia R, Jove R. Activation of STAT transcription factors in oncogenic tyrosine kinase signaling. J Biomed Sci. 1998;5:79–85. doi: 10.1007/BF02258360. [DOI] [PubMed] [Google Scholar]
- 19.Hanratty W P, Dearolf C R. The Drosophila tumorous-lethal hematopoietic oncogene is a dominant mutation in the hopscotch locus. J Gen Genet. 1993;238:33–37. doi: 10.1007/BF00279527. [DOI] [PubMed] [Google Scholar]
- 20.Harrison D A, Binari R, Nahreini T S, Gilman M, Perrimon N. Activation of a Drosophila Janus kinase (JAK) causes hematopoietic neoplasia and developmental defects. EMBO J. 1995;14:2857–2865. doi: 10.1002/j.1460-2075.1995.tb07285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hevezi P, Alin K, Goff S P. Transforming activity and tissue tropism of hybrid retroviral genomes containing portions of the v-Abl and v-src oncogenes. Oncogene. 1993;8:2413–2423. [PubMed] [Google Scholar]
- 22.Huebner R C, Engelman A, Schiff L, Rosenberg N. Abelson virus sequences important in lymphoid transformation. In: Klineman N, Witte O N, Howard M, editors. B cell development. New York, N.Y: Raven Press; 1988. pp. 257–270. [Google Scholar]
- 23.Ilaria R L, Jr, Van Etten R A. P120 and P190BCR/ABL induce the tyrosine phosphorylation and DNA binding activity of multiple specific STAT family members. J Biol Chem. 1996;271:31704–31710. doi: 10.1074/jbc.271.49.31704. [DOI] [PubMed] [Google Scholar]
- 24.Jackson P, Baltimore D. N-terminal mutations activate the leukemogenic potential of the myristoylated form of c-abl. EMBO J. 1989;8:449–456. doi: 10.1002/j.1460-2075.1989.tb03397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kipreos E T, Wang J Y J. Cell cycle-regulated binding of c-Abl tyrosine kinase to DNA. Science. 1992;256:382–385. doi: 10.1126/science.256.5055.382. [DOI] [PubMed] [Google Scholar]
- 26.Kipreos E T, Wang J Y J. Differential phosphorylation of c-Abl tyrosine kinase in cell cycle determined by cdc2 kinase and phosphatase activity. Science. 1990;248:217–220. doi: 10.1126/science.2183353. [DOI] [PubMed] [Google Scholar]
- 27.Krishnan K, Pine R, Krolewski J J. Kinase-deficient forms of Jak 1 and Tyk 2 inhibit interferon α signaling in a dominant manner. Eur J Biochem. 1997;247:298–305. doi: 10.1111/j.1432-1033.1997.00298.x. [DOI] [PubMed] [Google Scholar]
- 28.Lacronique V, Boureux A, Della Valle V, Poirel H, Quang C T, Mauchauffe M, Berthou C, Lessard M, Berger R, Ghysdael J, Bernard O A. A TEL-JAK2 fusion protein with constitutive kinase activity in human leukemia. Science. 1997;278:1309–1312. doi: 10.1126/science.278.5341.1309. [DOI] [PubMed] [Google Scholar]
- 29.Leonard W J, O’Shea J J. JAKS and STATS: biological implications. Annu Rev Immunol. 1998;16:293–322. doi: 10.1146/annurev.immunol.16.1.293. [DOI] [PubMed] [Google Scholar]
- 30.Mathey-Prevot B, Nabel G, Palacios R, Baltimore D. Abelson virus abrogation of interleukin-3 dependence in a lymphoid cell line. Mol Cell Biol. 1986;6:4133–4135. doi: 10.1128/mcb.6.11.4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mayer B J, Jackson P K, Van Etten R A, Baltimore D. Point mutations in the abl SH2 domain coordinately impair phosphotyrosine binding in vitro and transforming activity in vivo. Mol Cell Biol. 1992;12:609–618. doi: 10.1128/mcb.12.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McWhirther J R, Wang J Y J. Activation of tyrosine kinase and microfilament-binding functions of c-Abl by bcr sequences in bcr/abl fusion proteins. Mol Cell Biol. 1991;11:1553–1565. doi: 10.1128/mcb.11.3.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miao Y-J, Wang J Y J. Binding of A/T-rich DNA by three high mobility group-like domains in c-Abl tyrosine kinase. J Biol Chem. 1996;271:22823–22830. doi: 10.1074/jbc.271.37.22823. [DOI] [PubMed] [Google Scholar]
- 34.Murtach K, Skladany G, Hoag J, Rosenberg N. Abelson murine leukemia virus variants with increased oncogenic potential. J Virol. 1986;60:599–606. doi: 10.1128/jvi.60.2.599-606.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Shea J J. Jaks, STATs, cytokine signal transduction, and immunoregulation: are we there yet? Immunity. 1997;7:1–11. doi: 10.1016/s1074-7613(00)80505-1. [DOI] [PubMed] [Google Scholar]
- 36.Owen-Lynch P J, Wong A K Y, Whetton A D. v-Abl-mediated apoptosis suppression is associated with SHC phosphorylation without concomitant mitogen-activated protein kinase activation. J Biol Chem. 1995;270:5956–5962. doi: 10.1074/jbc.270.11.5956. [DOI] [PubMed] [Google Scholar]
- 37.Parmar K, Rosenberg N. Ras complements the carboxyl terminus of v-Abl protein in lymphoid transformation. J Virol. 1996;70:1009–1015. doi: 10.1128/jvi.70.2.1009-1015.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peeters P, Raynaud S D, Cools J, Wlodarska I, Grosgeorge J, Philip P, Monpoux F, Rompaey L V, Baens M, Van den Berghe H, Marynen P. Fusion of TEL, the ETS-variant gene 6 (ETV6), to the receptor-associated kinase Jak2 as a result of t(9;12) in a lymphoid and t(9;15;12) in a myeloid leukemia. Blood. 1997;90:2535–2540. [PubMed] [Google Scholar]
- 39.Pendergast A M, Traugh J A, Witte O N. Normal cellular and transformation-associated abl proteins share common sites for protein kinase C phosphorylation. Mol Cell Biol. 1987;7:4280–4289. doi: 10.1128/mcb.7.12.4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pendergast A M, Quilliam L A, Cripe L D, Bassing G H, Dai Z, Li N, Batzer A, Rabun K M, Der C J, Schlessinger J, Gishizky M. BCR-ABL-induced oncogenesis is mediated by direct interaction with the SH2 domain of the Grb-2 adaptor protein. Cell. 1993;75:175–185. [PubMed] [Google Scholar]
- 41.Prywes R, Foulkes J G, Rosenberg N, Baltimore D. Sequences of the A-MuLV protein needed for fibroblast and lymphoid cell transformation. Cell. 1983;34:569–579. doi: 10.1016/0092-8674(83)90389-6. [DOI] [PubMed] [Google Scholar]
- 42.Prywes R, Hoag J, Rosenberg N, Baltimore D. Protein stabilization explains the gag requirement for transformation of lymphoid cells by Abelson murine leukemia virus. J Virol. 1985;54:123–132. doi: 10.1128/jvi.54.1.123-132.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ren R, Ye Z-S, Baltimore D. Abl protein-tyrosine kinase selects the Crk adapter as a substrate using SH3-binding sites. Genes Dev. 1994;8:783–795. doi: 10.1101/gad.8.7.783. [DOI] [PubMed] [Google Scholar]
- 44.Renshaw M W, Kipreos E T, Albrecht M R, Wang J Y J. Oncogenic v-Abl tyrosine kinase can inhibit or stimulate growth depending on the cell context. EMBO J. 1992;11:3941–3951. doi: 10.1002/j.1460-2075.1992.tb05488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Renshaw M W, Lea-Chou E, Wang J Y J. Rac is required for v-Abl tyrosine kinase to activate mitogenesis. Curr Biol. 1995;6:76–83. doi: 10.1016/s0960-9822(02)00424-4. [DOI] [PubMed] [Google Scholar]
- 46.Rosenberg N. abl-mediated transformation, immunoglobulin gene rearrangements and arrest of B lymphocyte differentiation. Semin Cancer Biol. 1994;5:95–102. [PubMed] [Google Scholar]
- 47.Rosenberg N, Witte O N. Abelson murine leukemia virus mutants with alteration in the virus-specific p120 molecule. J Virol. 1980;33:340–348. doi: 10.1128/jvi.33.1.340-348.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sawyers C L, Callahan W, Witte O N. Dominant negative MYC blocks transformation by ABL oncogenes. Cell. 1992;70:901–910. doi: 10.1016/0092-8674(92)90241-4. [DOI] [PubMed] [Google Scholar]
- 49.Sawyers C L, McLaughlin J, Witte O N. Genetic requirement for Ras in the transformation of fibroblasts and hematopoietic cells by the Bcr-Abl oncogene. J Exp Med. 1995;181:307–313. doi: 10.1084/jem.181.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shi Y, Alin K, Goff S P. Abl-interactor-1, a novel SH3 protein binding to the carboxy-terminal portion of the Abl protein, suppresses v-Abl transforming activity. Genes Dev. 1995;9:2583–2597. doi: 10.1101/gad.9.21.2583. [DOI] [PubMed] [Google Scholar]
- 51.Shuai K, Halpern J, Hoeve J T, Rao X, Sawyers C L. Constitutive activation of STAT5 by the BCR-ABL oncogene in chronic myelogenous leukemia. Oncogene. 1996;13:247–254. [PubMed] [Google Scholar]
- 52.Van Beveren C, Coffin J, Hughes S. Pages 852–866. In: Weiss R, Teich N, Varmus H, Coffin J, editors. RNA tumor viruses. Vol. 2. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1985. [Google Scholar]
- 53.Van Etten R A, Jackson P K, Baltimore D, Sanders M C, Matsudaira P T, Janmey P A. The COOH terminus of the c-Abl tyrosine kinase contains distinct F- and G-actin binding domains with bundling activity. J Cell Biol. 1989;124:235–240. doi: 10.1083/jcb.124.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Varticovski L, Daley G Q, Jackson P, Baltimore D, Cantley L C. Activation of phosphatidylinositol 3-kinase in cells expressing abl oncogene variants. Mol Cell Biol. 1991;11:1107–1113. doi: 10.1128/mcb.11.2.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Welch P J, Wang J Y J. A C-terminal protein-binding domain in the retinoblastoma protein regulates nuclear c-Abl tyrosine kinase in the cell cycle. Cell. 1993;75:779–790. doi: 10.1016/0092-8674(93)90497-e. [DOI] [PubMed] [Google Scholar]
- 56.Winston L A, Hunter T. Intracellular signalling: putting JAKs on the kinase MAP. Curr Biol. 1996;6:668–671. doi: 10.1016/s0960-9822(09)00445-x. [DOI] [PubMed] [Google Scholar]
- 57.Yamanashi Y, Baltimore D. Identification of the Abl and rasGAP-associated 62 kDa protein as a docking protein, Dok. Cell. 1997;88:205–211. doi: 10.1016/s0092-8674(00)81841-3. [DOI] [PubMed] [Google Scholar]
- 58.Young J C, Gishizky M L, Witte O N. Hyperexpression of interleukin-7 is not necessary or sufficient for transformation of a pre-B-lymphoid cell line. Mol Cell Biol. 1991;11:854–863. doi: 10.1128/mcb.11.2.854. [DOI] [PMC free article] [PubMed] [Google Scholar]