Interaction of Glycogen Synthase Kinase 3β with the DF3/MUC1 Carcinoma-Associated Antigen and β-Catenin (original) (raw)
Abstract
The DF3/MUC1 mucin-like glycoprotein is highly overexpressed in human carcinomas. Recent studies have demonstrated that the cytoplasmic domain of MUC1 interacts with β-catenin. Here we show that MUC1 associates with glycogen synthase kinase 3β (GSK3β). GSK3β binds directly to an STDRSPYE site in MUC1 and phosphorylates the serine adjacent to proline. Phosphorylation of MUC1 by GSK3β decreases binding of MUC1 to β-catenin in vitro and in vivo. GSK3β-mediated phosphorylation of MUC1 had no apparent effect on β-catenin levels or the transcriptional coactivation function of β-catenin. The results, however, demonstrate that MUC1 expression decreases binding of β-catenin to the E-cadherin cell adhesion molecule. Negative regulation of the β-catenin–MUC1 interaction by GSK3β is associated with restoration of the complex between β-catenin and E-cadherin. These findings indicate that GSK3β decreases the interaction of MUC1 with β-catenin and that overexpression of MUC1 in the absence of GSK3β activity inhibits formation of the E-cadherin–β-catenin complex.
The Drosophila segment polarity gene product Armadillo (22) is regulated by the serine/threonine kinase Zeste-White 3 (ZW3)/shaggy (4, 49, 50). Activation of the Wnt/Wingless pathway is associated with downregulation of ZW3/shaggy and a decrease in phosphorylation of Armadillo (33). In the absence of a Wnt/Wingless signal, ZW3/shaggy mediates a decrease in the stability of Armadillo (34, 58). Studies in Xenopus laevis have demonstrated that Wnt signaling involves the ZW3/shaggy homolog Xgsk-3 and regulates formation of the early dorsal-ventral axis (6, 10, 37). Xgsk-3 phosphorylates β-catenin, the vertebrate Armadillo homolog, and thereby decreases β-catenin levels (62). In mammalian cells, expression of Wnt-1 is also associated with stabilization and accumulation of β-catenin (12) by a mechanism involving inhibition of glycogen synthase kinase 3β (GSK3β). These findings have supported a conserved role for the regulation of β-catenin/Armadillo by the GSK3β/Xgsk-3/ZW3-related kinases.
β-Catenin is a component of the adherens junction of mammalian epithelial cells and through α-catenin links the cadherin cell adhesion molecules to the actin cytoskeleton. Other studies have demonstrated that β-catenin binds directly to the adenomatous polyposis coli (APC) gene product (43, 44, 53). The cadherins and APC form independent complexes with β-catenin (14, 44). The interaction between APC and β-catenin alters cell adhesion (2) and regulates β-catenin turnover (27). Importantly, phosphorylation of APC by GSK3β enhances the interaction of APC and β-catenin (45). Moreover, cells that express certain APC mutants or are APC deficient exhibit increased levels of cytosolic β-catenin (27). Free β-catenin forms complexes with members of the T-cell factor/leukocyte enhancing factor 1 (Tcf/LEF-1) family of transcription factors (3, 13, 24) and thereby activates gene expression (5, 39, 56). Thus, loss of APC-mediated regulation of β-catenin in transformed cells is associated with constitutive activation of β-catenin–Tcf/LEF-1 transcriptional complexes (18, 25, 42). These findings have supported a role for β-catenin as a transcriptional coactivator.
Other studies have demonstrated that β-catenin interacts with the DF3/MUC1 mucin-like glycoprotein (61). MUC1 is expressed on the apical borders of secretory epithelial cells and at high levels throughout the entire membrane and cytoplasm of carcinoma cells (7, 19, 35). The N-terminal ectodomain of MUC1 consists of variable numbers of 20-amino-acid tandem repeats that are subject to O glycosylation (8, 48). The C-terminal region includes a transmembrane domain and a 72-amino-acid cytoplasmic tail that contains sites for tyrosine phosphorylation (32, 63). The finding that increased expression of MUC1 on carcinoma cells reduces cell-cell and cell-extracellular matrix interactions (20, 57, 60) has supported a role for MUC1 in cell adhesion. Also, like E-cadherin and APC, MUC1 binds directly to β-catenin (61). SXXXXXSSL sites in E-cadherin (amino acids 840 to 848) and APC (seven motifs) are responsible for interactions with β-catenin (43, 44, 53). A similar motif (SAGNGGSSL) in the cytoplasmic domain of MUC1 has been identified as a β-catenin binding site (61). The formation of a complex between MUC1 and β-catenin may differ from β-catenin complexes with E-cadherin and APC, which are linked to the cytoskeleton by α-catenin (14). In this context, there is little if any α-catenin in the MUC1–β-catenin complex (61). These findings have supported a potentially distinct role for binding of β-catenin to MUC1.
The present studies demonstrate that MUC1 interacts directly with GSK3β. A TDRSP motif in the MUC1 cytoplasmic domain (CD) has been identified as a site for GSK3β phosphorylation. The results also demonstrate that GSK3β regulates the interaction between MUC1 and β-catenin.
MATERIALS AND METHODS
Cell culture.
Human ZR-75-1 breast carcinoma cells were grown in RPMI 1640 medium containing 10% heat-inactivated fetal bovine serum, 100 μg of streptomycin per ml, 100 U of penicillin per ml, and 2 mM l-glutamine. 293, HeLa, and SW480 cells were cultured in Dulbecco’s modified Eagle’s medium (high glucose; Sigma) with 10% heat-inactivated fetal bovine serum, 100 μg of streptomycin per ml, and 100 U of penicillin per ml.
Cell lysate.
Subconfluent cells were lysed in ice-cold lysis buffer (50 mM Tris-HCl [pH 7.6], 150 mM NaCl, 0.1% Nonidet P-40, 10 μg of leupeptin per ml, 10 μg of aprotinin per ml, 1 mM phenylmethylsulfonyl fluoride, and 1 mM dithiothreitol) for 30 min on ice. Lysates were cleared by centrifugation at 14,000 × g for 20 min.
Immunoprecipitation and immunoblotting.
Lysates were incubated with anti-MUC1 (monoclonal antibody [MAb] DF3 [19]), anti-GSK3β (Transduction Laboratories, Lexington, Ky.), anti-E-cadherin (Santa Cruz Biotechnology, Santa Cruz, Calif.), or mouse immunoglobulin G (IgG; Zymed Laboratories Inc., San Francisco, Calif.) for 2 h at 4°C. The immune complexes were precipitated with protein G-agarose (Pharmacia Biotech, Piscataway, N.J.) for 1 h at 4°C. After being washed with lysis buffer, the immunoprecipitates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were transferred to nitrocellulose membranes. The membranes were blocked with 5% nonfat dry milk in phosphate-buffered saline (PBS) containing 0.05% Tween 20 and then incubated with anti-MUC1, anti-GSK3β, or anti-β-catenin (Zymed). Immunoblotting was also performed with a rabbit antibody prepared against the MUC1 cytoplasmic domain (anti-MUC1/CD). Reactivity was detected by the use of horseradish peroxidase-conjugated second antibodies and chemiluminescence (ECL; Amersham Life Science).
Generation of histidine-tagged MUC1/CD fusion proteins.
Constructs expressing the full-length (72-amino-acid) MUC1 CD or N- or C-terminal regions of the CD (MUC1/CD, N-MUC1/CD, and C-MUC1/CD, respectively) were amplified from the DF3/MUC1 cDNA (23, 48) and cloned into the pET28(+) vector. The MUC1/CD construct was also subjected to site-directed mutagenesis, with a QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, Calif.) being used to generate proteins with S→A mutations at the STDRSP domain. The mutations were confirmed by DNA sequencing. The His-tagged MUC1/CD, N-MUC1/CD, and C-MUC1/CD proteins were purified by Ni2+ affinity column chromatography (Novagen Inc., Madison, Wis.). The purified fusion proteins were dialyzed against 10 mM Tris-HCl, pH 7.4, to remove imidazole.
Binding studies.
Glutathione _S_-transferase (GST) or GST-MUC1/CD (61) bound to glutathione beads was incubated with 0.1 μg of GSK3β (Transduction Laboratories) in PBS–0.2% Triton X-100 for 1 h at 4°C. After being washed, proteins were separated by SDS-PAGE and transferred to nitrocellulose membranes. The proteins were analyzed by immunoblotting with anti-GSK3β. For binding competition studies, purified His-MUC1/CD was incubated with GSK3β in the absence or presence of the MUC1/CD STDRSPYE peptide (10 μg) or in the presence of the control MNRRGSIK peptide (10 μg) for 1 h at 4°C. Protein G-conjugated anti-GSK3β antibody was added to precipitate the protein complex. The precipitates were subjected to immunoblot analysis with the anti-MUC1/CD polyclonal antibody.
In vitro phosphorylation.
Purified MUC1/CD (wild-type or mutant) proteins were incubated with 0.1 μg of GSK3β in 20 μl of kinase reaction buffer (20 mM Tris-HCl [pH 7.6], 10 mM MgCl2, 5 mM dithiothreitol). The reaction was initiated by addition of 10 μCi of [γ-32P]ATP at 30°C. After 15 min, the reaction was stopped by adding 2× SDS-PAGE sample buffer and boiling for 5 min. Phosphorylated proteins were separated by SDS-PAGE and visualized by autoradiography.
Transient-transfection studies.
Kinase-inactive GSK3β [GSK3β(KI)] was constructed by site-directed mutagenesis of a GSK3β cDNA (52) to replace lysine 85 and lysine 86 with methionine and isoleucine, respectively. 293 cells were transfected with pcDNA3, pcDNA3/CMV-MUC1, and either pcDNA3/CMV-His-GSK3β or pcDNA3/CMV-His-GSK3β(KI) constructs in the presence of Lipofectamine (Life Technologies, Inc.). HeLa cells were transfected with pcDNA3 vector, pcDNA3/CMV-His-GSK3β, or pcDNA3/CMV-His-GSK3β(KI). 293 and SW480 cells were transfected with pTOPFLASH or pFOPFLASH reporter constructs (18). Cell lysates were prepared at 48 h after transfection.
Cell fractionation.
Cell fractionation was performed as described elsewhere (30). Cells were harvested by scraping them into ice-cold PBS. After being washed with PBS at 4°C, cells were lysed in cold PBS containing 0.1% Triton X-100, 0.1% Nonidet P-40, and protease inhibitor cocktail (Boehringer-Mannheim, Indianapolis, Ind.). Nuclei were pelleted by centrifugation at 1,000 × g for 10 min at 4°C. The supernatant was then centrifuged at 100,000 × g for 60 min at 4°C. The resulting supernatant was collected as the cytoplasmic fraction.
RESULTS
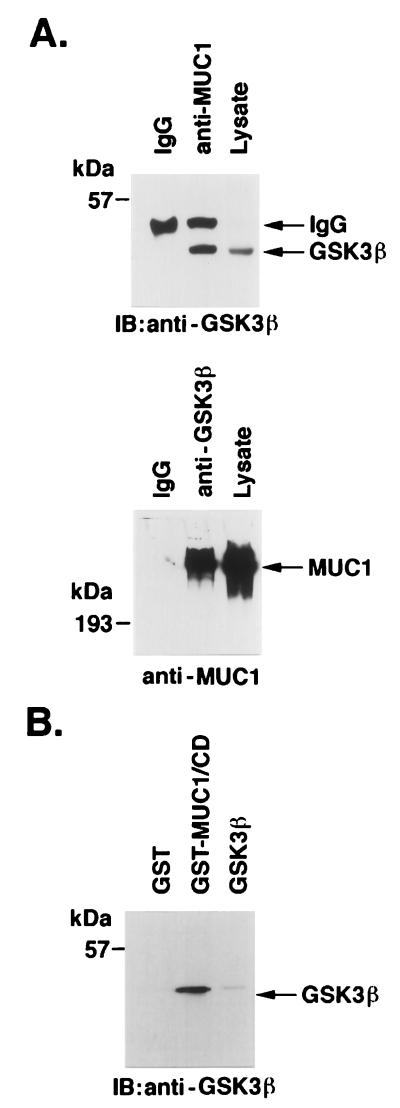
To determine whether DF3/MUC1 associates with GSK3β, we subjected MAb DF3 (anti-MUC1) immunoprecipitates to immunoblotting with anti-GSK3β. The results demonstrated coprecipitation of GSK3β and MUC1 (Fig. 1A). In the reciprocal experiment, analysis of anti-GSK3β immunoprecipitates by immunoblotting with the anti-MUC1 antibody confirmed association of MUC1 and GSK3β (Fig. 1A). To assess whether binding is direct, purified GSK3β was incubated with a GST fusion protein that contains the MUC1 CD (GST-MUC1/CD) (61). The adsorbate was subjected to immunoblot analysis with anti-GSK3β. The finding that GSK3β binds to GST-MUC1/CD but not to GST alone supported a direct interaction (Fig. 1B).
FIG. 1.
Interaction of MUC1 and GSK3β. (A) Lysates from ZR-75-1 cells were subjected to immunoprecipitation with anti-MUC1 (MAb DF3; upper panel) or anti-GSK3β (lower panel). Mouse IgG was used as a control. The immunoprecipitates were analyzed by immunoblotting with anti-GSK3β (upper panel) or anti-MUC1 (lower panel). (B) GST and GST-MUC1/CD were incubated with purified GSK3β. Proteins precipitated with glutathione-Sepharose 4B beads were separated by SDS-PAGE, transferred to nitrocellulose membranes, and immunoblotted with anti-GSK3β. Purified GSK3β was directly subjected to immunoblot analysis with anti-GSK3β as a control. The positions of molecular size markers are shown on the left of the gels.
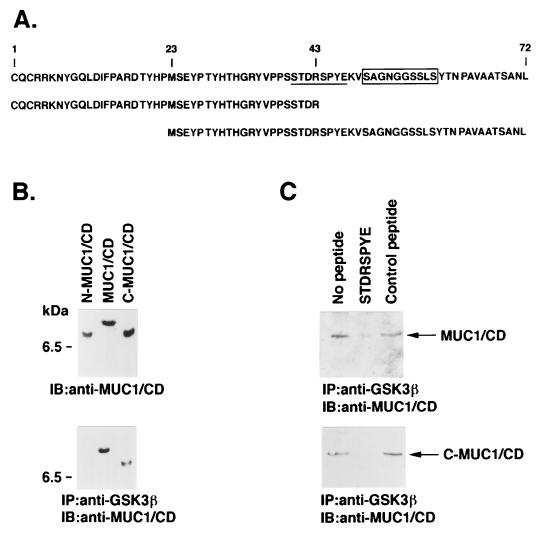
To identify the site in MUC1 that binds to GSK3β, His-tagged proteins were prepared from the N-terminal (N-MUC1/CD) and C-terminal (C-MUC1/CD) regions of the CD (Fig. 2A and B, upper panel). Purified GSK3β was incubated with full-length His-MUC1/CD and the two fragments. Immunoprecipitation with anti-GSK3β and analysis of the precipitates with anti-MUC1/CD demonstrated binding of GSK3β with MUC1/CD and C-MUC1/CD but not with the N-MUC1/CD fragment (Fig. 2B, lower panel). Previous studies have demonstrated that GSK3β phosphorylates an SXXXS site in the APC protein (45). Since a similar (STDRS) site is present in MUC1/CD, we synthesized an STDRSPYE peptide as a potential competitor for interactions between GSK3β and MUC1. The results demonstrate that preincubation of GSK3β with STDRSPYE inhibits the binding of GSK3β and MUC1/CD (Fig. 2C). By contrast, there was little effect on this interaction when a control MNRRGSIK peptide was used (Fig. 2C, upper panel). The STDRSPYE peptide, but not the control peptide, also blocked binding of GSK3β to C-MUC1/CD (Fig. 2C, lower panel). These findings indicated that GSK3β interacts with the STDRSPYE site in MUC1.
FIG. 2.
GSK3β interacts with the STDRSPYE site in MUC1/CD. (A) Amino acid sequences of the MUC1/CD, N-MUC1/CD, and C-MUC1/CD proteins. The 72-amino-acid CD is reflected by numbering from the N terminus of the expressed MUC1/CD protein. The β-catenin binding sequence is boxed, and the GSK3β binding and phosphorylation site is underlined. (B) The purified N-MUC1/CD, full-length MUC1/CD, and C-MUC1/CD proteins were subjected to immunoblotting (IB) with an anti-MUC1/CD antibody (upper panel). Purified N-MUC1/CD, MUC1/CD, and c-MUC1/CD were incubated with purified GSK3β. Complexes immunoprecipitated (IP) with anti-GSK3β were subjected to immunoblotting with anti-MUC1/CD (lower panel). The position of a molecular size standard is shown on the left of both panels. (C) Purified MUC1/CD (upper panel) and C-MUC1/CD (lower panel) were incubated with purified GSK3β in the absence of competing peptide and in the presence of the STDRSPYE peptide or an irrelevant control peptide. Anti-GSK3β immunoprecipitates were analyzed by immunoblotting with anti-MUC1/CD.
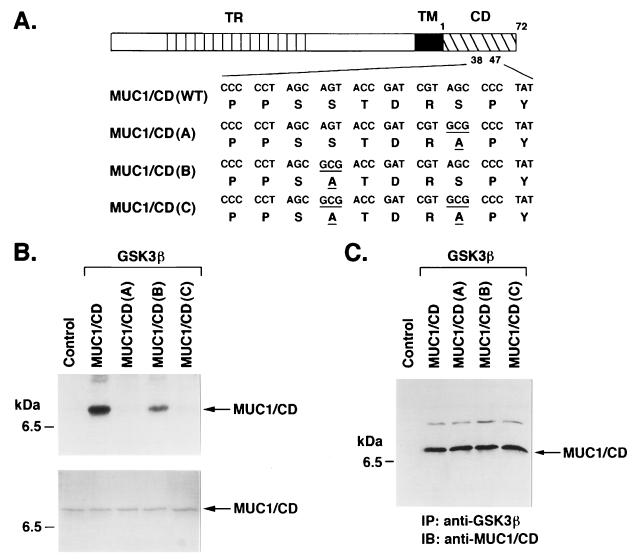
To determine whether MUC1/CD is a substrate for GSK3β, we incubated the N-MUC1/CD and C-MUC1/CD fragments with purified GSK3β and [γ-32P]ATP. Analysis of the reaction products by SDS-PAGE and autoradiography demonstrated phosphorylation of only C-MUC1/CD (data not shown). Previous work has shown that SP sites are substrates for GSK3β phosphorylation (21, 40). A single SP site in C-MUC1/CD is located in the STDRSPYE domain. Mutation of this domain in MUC1/CD to STDRAPYE [designated MUC1/CD(A)] (Fig. 3A) abrogated GSK3β-mediated phosphorylation of MUC1/CD (Fig. 3B). By contrast, mutation to an ATDRSPYE sequence [MUC1/CD(B)] (Fig. 3A) had little effect on phosphorylation (Fig. 3B). As expected, the ATDRAPYE double mutant [MUC1/CD(C)] (Fig. 3A) also failed to serve as a substrate for GSK3β (Fig. 3B). To assess whether binding of GSK3β to MUC1/CD is affected by the S→A mutations, we incubated GSK3β with wild-type MUC1/CD and the three mutants. Analysis of anti-GSK3β immunoprecipitates by immunoblotting with anti-MUC1/CD demonstrated that the S→A mutations have little if any effect on GSK3β binding (Fig. 3C). These findings indicate that GSK3β phosphorylates serine in the TDRSPYE domain of MUC1/CD.
FIG. 3.
GSK3β phosphorylates MUC1/CD at the TDRSPYE domain. (A) Wild-type and mutant forms of MUC1/CD. TR, tandem repeat; TM, transmembrane. Numbers (1 to 72) reflect amino acids in the CD. Underlined codons and amino acids are those that differ from the wild type. (B) Purified MUC1/CD proteins were incubated with purified GSK3β and [γ32P]ATP. As a control, MUC1/CD was incubated with [γ32P]ATP and no GSK3β. The reaction products were analyzed by SDS-PAGE and autoradiography (upper panel). Equal loading of the MUC1/CD proteins was assessed by Coomassie blue staining (lower panel). The position of a molecular size standard is shown on the left. (C) Purified MUC1/CD proteins were incubated with purified GSK3β. The proteins were subjected to immunoprecipitation (IP) with anti-GSK3β, and the precipitates were analyzed by immunoblotting (IB) with anti-MUC1/CD. The control lane represents incubation of MUC1/CD and GSK3β, immunoprecipitation with mouse IgG, and immunoblot analysis of the precipitates with anti-MUC1/CD.
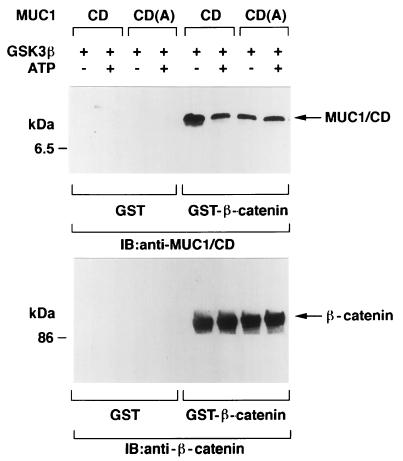
Previous studies have demonstrated that phosphorylation of APC by GSK3β enhances binding of β-catenin to APC (45). To assess the effects of GSK3β-mediated phosphorylation of MUC1/CD, we incubated MUC1/CD with GSK3β in the presence and absence of ATP. After phosphorylation of MUC1/CD, GST or GST–β-catenin was added for a 1-h incubation at 4°C. Proteins precipitated with glutathione-Sepharose 4B beads were subjected to immunoblot analysis with anti-MUC1/CD. There was no apparent binding of unphosphorylated or phosphorylated MUC1/CD to GST (Fig. 4). By contrast, incubation of MUC1/CD with GST–β-catenin demonstrated that binding of β-catenin is inhibited by GSK3β-mediated phosphorylation of MUC1/CD (Fig. 4, upper panel). Similar studies with the MUC1/CD(A) mutant also demonstrated decreased binding of β-catenin compared to that of wild-type MUC1/CD. The extent of β-catenin binding to MUC1/CD(A) was unaffected by prior incubation of MUC1/CD(A) with GSK3β and ATP (Fig. 4, upper panel), consistent with the finding that MUC1/CD(A) is not a substrate for GSK3β. As a control, analysis of adsorbates to the glutathione beads with anti-β-catenin demonstrated that equal amounts of GST–β-catenin were precipitated from the various reaction mixtures (Fig. 4, lower panel). These findings indicate that modification of the serine in TDRSPYE by phosphorylation or mutation to alanine decreases in vitro binding of MUC1 to β-catenin.
FIG. 4.
GSK3β-mediated phosphorylation of the TDRSPYE site in vitro reduces binding of MUC1 to β-catenin. MUC1/CD and MUC1/CD(A) were incubated with (+) or without (−) purified GSK3β and ATP for 15 min at 30°C. The MUC1/CD and MUC1/CD(A) proteins were then incubated with GST or GST–β-catenin for 1 h at 4°C. Proteins precipitated with glutathione-Sepharose 4B beads were subjected to immunoblot (IB) analysis with anti-MUC1 (upper panel) and anti-β-catenin (lower panel). The positions of molecular size standards are shown on the left of the gels.
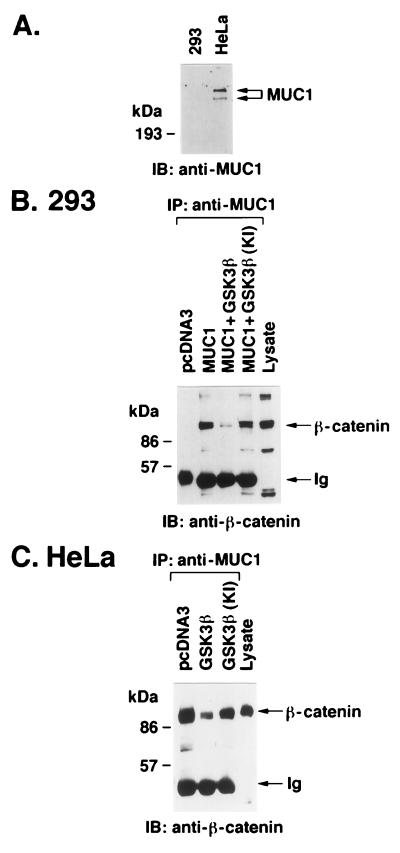
To determine whether GSK3β regulates the interaction between MUC1 and β-catenin in vivo, transfection studies were performed in 293 and HeLa cells. 293 cells, in contrast to HeLa cells, have undetectable levels of MUC1 (Fig. 5A). After transfection of vectors expressing MUC1 and GSK3β, 293 cells were subjected to immunoprecipitation with anti-MUC1 and the precipitates were analyzed for binding of MUC1 to β-catenin. The results demonstrate that GSK3β decreased the interaction between MUC1 and β-catenin compared to control cells transfected with MUC1 alone (Fig. 5B). By contrast, expression of MUC1 with the kinase-inactive GSK3β(KI) had little effect on the interaction with β-catenin (Fig. 5B). In studies with the MUC1-positive HeLa cells, transfections were performed with vectors expressing the kinase-active and -inactive GSK3βs. Analysis of anti-MUC1 immunoprecipitates from HeLa cells transfected with the kinase-active GSK3β demonstrated a decrease in the interaction of MUC1 and β-catenin compared to that seen in cells transfected with the empty vector (Fig. 5C). Moreover, expression of the kinase-inactive GSK3β(KI) had little effect on the interaction of MUC1 and β-catenin (Fig. 5C). These findings support a model in which GSK3β downregulates binding of MUC1 and β-catenin.
FIG. 5.
GSK3β downregulates the interaction between MUC1 and β-catenin. (A) Lysates from 293 and HeLa cells were subjected to immunoblot (IB) analysis with anti-MUC1. (B) 293 cells were transiently transfected with pcDNA3 (10 μg), pcDNA3 plus MUC1 (5 μg each), MUC1 plus GSK3β (5 μg each), or MUC1 plus GSK3β(KI) (5 μg each). After 48 h, the cells were harvested and lysates were subjected to immunoprecipitation (IP) with anti-MUC1. The immunoprecipitates were analyzed by immunoblotting with anti-β-catenin. As a control, 293 cell lysate was directly analyzed by immunoblotting with anti-β-catenin (last lane). (C) HeLa cells were transiently transfected with pcDNA3 (10 μg), GSK3β (10 μg), or GSK3β(KI) (10 μg). After 48 h, lysates were prepared from the transfected cells and proteins were immunoprecipitated with anti-MUC1. The immunoprecipitates were analyzed by immunoblotting with anti-β-catenin. HeLa cell lysate was directly analyzed by immunoblotting with anti-β-catenin. The positions of molecular size standards are shown to the left of all panels.
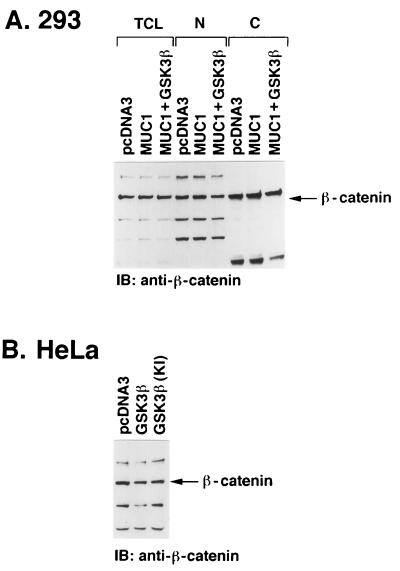
To assess whether the interaction of MUC1 and GSK3β affects β-catenin levels, 293 cells were transfected with MUC1 in the absence and presence of GSK3β. Analysis of whole-cell lysates demonstrated little if any change in the total pool of β-catenin in cells that overexpress MUC1 (Fig. 6A). Similar results were obtained with cells that overexpress both MUC1 and GSK3β (Fig. 6A). Because these findings do not exclude the possibility that β-catenin is redistributed intracellularly, cytoplasmic and nuclear fractions of the transfectants were analyzed for β-catenin levels. The results demonstrate that overexpression of MUC1 with or without GSK3β has little if any effect on the distribution of β-catenin in the cytoplasm and nucleus (Fig. 6A). Similar findings were obtained with MUC1-positive HeLa cells transfected to express GSK3β or GSK3β(KI) (Fig. 6B and data not shown). These findings suggest that overexpression of MUC1 and GSK3β is not associated with redistribution or degradation of β-catenin.
FIG. 6.
Effect of MUC1 and GSK3β on β-catenin levels. (A) 293 cells were transiently transfected with pcDNA3, pcDNA3/MUC1, or MUC1/GSK3β. After 48 h, the cells were harvested and total cell lysates (TCL) were subjected to immunoblot (IB) analysis with anti-β-catenin. The cell lysates were also separated into nuclear (N) and cytoplasmic (C) fractions that were analyzed by immunoblotting with anti-β-catenin. (B) HeLa cells were transfected with pcDNA3, pcDNA3/GSK3β, or pcDNA3/GSK3β(KI). Total cell lysates were analyzed by immunoblotting with anti-β-catenin.
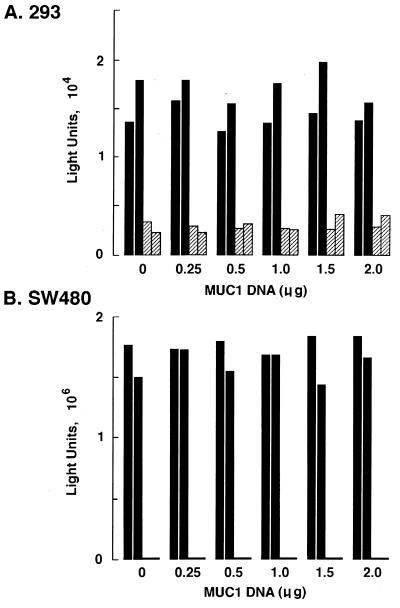
β-Catenin binds to the Tcf/LEF family of transcription factors (3, 13, 24). The functional interaction between β-catenin and Tcf has been assessed by activation of reporter constructs containing the Tcf motif (18, 25). To investigate whether MUC1 expression affects the transactivation function of β-catenin, 293 cells were transfected with pcDNA3/MUC1 and the β-catenin–Tcf luciferase reporter construct (pTOPFLASH) (18, 25). As a control, transfections were performed with a similar reporter containing a mutant or nonfunctional Tcf motif (pFOPFLASH) (18, 25). Transcriptional activity of the pTOPFLASH reporter in 293 cells was approximately 5 to 10 times that of pFOPFLASH (data not shown). Cotransfection with pcDNA3/MUC1 had no apparent effect on pTOPFLASH transcription (Fig. 7A). The constitutive transcriptional activity of the Tcf reporter gene in 293 cells contrasted with the inactivity of this construct in HeLa cells (data not shown). Therefore, to confirm the findings in 293 cells, we studied the effects of MUC1 expression in SW480 cells, which exhibit a high basal level of transcription of pTOPFLASH (18, 25). The results demonstrate that transfection of pcDNA3/MUC1 alone or with pcDNA3/GSK3β has no apparent effect on pTOPFLASH activity (Fig. 7B). These findings indicate that the binding of β-catenin to MUC1 and the regulation of this interaction by GSK3β are involved in functions other than transcriptional activation or repression of the β-catenin–Tcf complex.
FIG. 7.
Effect of MUC1 on the cotransfection function of β-catenin. 293 (A) and SW480 (B) cells were transiently transfected with 0.3 μg of pTOPFLASH (solid bars) or pFOPFLASH (hatched bars), the indicated amounts of MUC1 vector, 0.3 μg of pCATCONTROL, and pcDNA3 to a total of 2.5 μg of plasmid DNA. After 48 h, the cells were harvested and cell lysates were assayed for luciferase activity. The results of two independent experiments are shown.
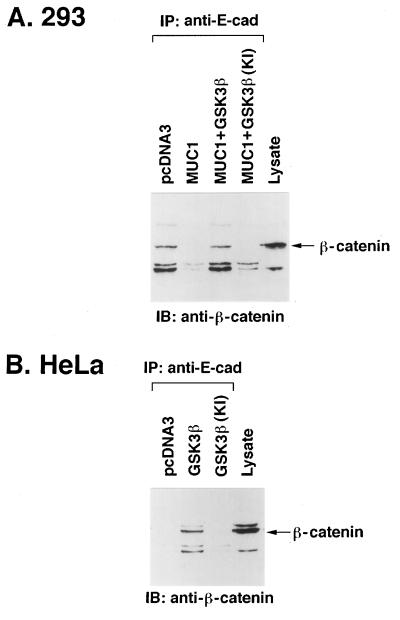
β-Catenin also interacts with the cell adhesion molecule E-cadherin (14). The N-terminal domain of β-catenin interacts with α-catenin and thereby links the E-cadherin–β-catenin complex to the cytoskeleton (14). To determine whether MUC1 influences the interaction between E-cadherin and β-catenin, 293 cells were transfected with pcDNA3/MUC1 and anti-E-cadherin immunoprecipitates were assayed for β-catenin. The results show that MUC1 expression decreases binding of β-catenin to E-cadherin (Fig. 8A). By contrast, coexpression of MUC1 and GSK3β resulted in restoration of the E-cadherin–β-catenin interaction (Fig. 8A). The effects of GSK3β were dependent on its kinase function, since coexpression of MUC1 and GSK3β(KI) was associated with a decrease in binding of β-catenin to E-cadherin (Fig. 8A). Other studies were performed in MUC1-positive HeLa cells by transfecting pcDNA3/GSK3β or pcDNA3/GSK3β(KI). Transfection of GSK3β, which decreases binding of β-catenin to MUC1 (Fig. 5C), increased the association of β-catenin with E-cadherin (Fig. 8B). As a control, transfection of GSK3β(KI) had no apparent effect on binding of β-catenin and E-cadherin (Fig. 8B). These results collectively demonstrate that the interaction of GSK3β and MUC1 decreases binding of β-catenin to MUC1 and stimulates the association of β-catenin and E-cadherin.
FIG. 8.
Regulation of β-catenin–E-cadherin complexes by MUC1 and GSK3β. (A) 293 cells were transfected with pcDNA3 (10 μg), pcDNA3 plus MUC1 (5 μg each), MUC1 plus GSK3β (5 μg each), or MUC1 plus GSK3β(KI) (5 μg each). (B) HeLa cells were transfected with pcDNA3 (10 μg), GSK3β (10 μg), or GSK3β(KI) (10 μg). After 48 h, the transfected cells were harvested and cell lysates subjected to immunoprecipitation (IP) with anti-E-cadherin. The precipitates were analyzed by immunoblotting (IB) with anti-β-catenin. As controls, cell lysates were directly subjected to immunoblot analysis with anti-β-catenin (last lanes).
DISCUSSION
Regulation of β-catenin by APC and GSK3β.
β-Catenin forms a complex with the APC tumor suppressor by binding to 15-amino-acid and 20-amino-acid tandem repeats in the central region of APC (43, 53). Interaction with the 20-amino-acid repeat is associated with degradation of β-catenin (27). Accordingly, the central 20-amino-acid repeat region of APC contains sites for GSK3β phosphorylation that regulate the binding and subsequent degradation of β-catenin (45). The N terminus of β-catenin also contains a consensus motif for GSK3β phosphorylation that when mutated results in stabilization of the protein (62). The finding that N-terminal deletion mutants of β-catenin bind to APC but are resistant to degradation has indicated that GSK3β-mediated phosphorylation of N-terminal sites may be essential for β-catenin degradation (14). Indeed, elevated levels of β-catenin in tumor cell lines have been associated with mutations of the GSK3β phosphorylation motif in the N terminus (15, 25, 41, 42). These observations have suggested that GSK3β induces the degradation of β-catenin by phosphorylating sites in both APC and β-catenin. The GSK3β phosphorylation site in β-catenin is also required for ubiquitination of β-catenin and, thereby, targeting of the protein to the proteosome (1). Thus, downregulation of GSK3β by Wnt signaling increases β-catenin levels by reducing the formation of ubiquitinated β-catenin intermediates (1).
Interactions of GSK3β and β-catenin with MUC1.
The present findings demonstrate that GSK3β interacts with the MUC1 CD. GSK3β associates with an STDRSPYE site in MUC1 that is similar to the GSK3β phosphorylation motif SXXXS in the APC protein (45). The present results also demonstrate that GSK3β phosphorylates MUC1 on the serine in the TDRSPYE domain. In contrast to the observation that GSK3β enhances binding of β-catenin to APC (45), GSK3β-mediated phosphorylation of MUC1 decreases binding of β-catenin. Modification of the serine in TDRSPYE by phosphorylation or by mutation reduced, but did not completely eliminate, the interaction between MUC1 and β-catenin. Thus, signals other than GSK3β-mediated phosphorylation may contribute to regulation of the MUC1–β-catenin complex. The site in MUC1 for GSK3β binding and phosphorylation is adjacent to the β-catenin binding site (Fig. 2A). The finding that overexpression of the kinase-inactive GSK3β(KI) somewhat diminishes binding of MUC1 and β-catenin in cells suggests that the association of GSK3β with MUC1 may displace β-catenin. Nonetheless, overexpression of the kinase-active GSK3β was more effective in abrogating the interaction between MUC1 and β-catenin, consistent with the involvement of GSK3β-mediated phosphorylation. The finding that GSK3β inhibits the association of MUC1 and β-catenin in vitro and in vivo is in contrast to the effects of GSK3β that enhance the interaction between APC and β-catenin. Also, whereas GSK3β-mediated phosphorylation of APC promotes β-catenin degradation, we observed no apparent difference in β-catenin levels following overexpression of MUC1 and GSK3β. These findings indicate that GSK3β-mediated regulation of β-catenin is dependent at least in part on whether β-catenin is associated with APC or MUC1. In this context, APC and MUC1 may interact with different pools of β-catenin. Alternatively, the interaction between GSK3β and APC or MUC1 could determine whether the associated β-catenin is subject to ubiquitination and targeting to the proteosome.
Functional significance of the interactions among MUC1, β-catenin, and GSK3β.
The GSK3β-mediated downregulation of the interaction between MUC1 and β-catenin could, in the absence of β-catenin degradation, contribute to the available pools of free β-catenin. APC regulates the formation of β-catenin–Tcf complexes and the downstream transcriptional activation mediated by their binding to Tcf elements (18, 25). By contrast, the present studies demonstrate that overexpression of MUC1 has no detectable effect on activation of the Tcf-reporter construct. Similar results were obtained in the setting of both MUC1 and GSK3β overexpression (data not shown). These findings indicate that the regulation of the transcriptional costimulator function of β-catenin is not influenced by the interaction of MUC1 and β-catenin. This conclusion is supported by studies in 293 (full-length APC) (43) and SW480 (truncated mutant APC) (51) cells. However, the observation that wild-type APC regulates β-catenin levels in SW480 cells, but not HT-29 cells (26), raises the possibility that MUC1-mediated regulation of the β-catenin transcriptional coactivator functions differ in other cell types.
Interaction of β-catenin with MUC1 and E-cadherin.
β-Catenin also plays a role in the formation of adherens junctions of mammalian epithelial cells by connecting E-cadherin to α-catenin and, thereby, the cytoskeleton (28). E-cadherin functions in homotypic recognition and the control of cell mobility (55). The formation of complexes between E-cadherin and β-catenin, or the closely related γ-catenin, is essential for cell adhesive function (16, 29, 31). The present findings support a functional relationship between E-cadherin and MUC1 through competition for binding to β-catenin. Thus, overexpression of MUC1 in the MUC1-negative 293 cells downregulates the interaction between E-cadherin and β-catenin. Importantly, however, overexpression of MUC1 and GSK3β, which decreases binding of β-catenin to MUC1, restores the interaction between β-catenin and E-cadherin. These findings are supported by studies in the MUC1-positive HeLa cells, which exhibit β-catenin binding to MUC1 but not E-cadherin. In these cells, overexpression of GSK3β decreases binding of β-catenin to MUC1 and stimulates the interaction of β-catenin and E-cadherin. Collectively, the results support a model in which GSK3β controls the distribution of at least certain pools of β-catenin for binding to MUC1 or E-cadherin. It is of interest that previous studies have demonstrated that E-cadherin and APC compete for binding to β-catenin (14). However, whereas overexpression of MUC1 in 293 cells decreased binding of β-catenin to E-cadherin, there was no apparent effect of MUC1 on the interaction between β-catenin and APC (data not shown). These findings suggest that MUC1 and E-cadherin may exhibit cross talk through GSK3β-regulated binding to β-catenin.
Aberrant regulation of MUC1 expression in human carcinomas.
Downregulation of E-cadherin expression in human tumors (47) is associated with the loss of an invasion suppressor function (59), progression from adenoma to carcinoma (36), and development of familial gastric cancers (9). Decreased expression of β-catenin has also been observed in certain tumors (38, 46, 54). Disruption of the adherens junction and cell adhesion has thus been proposed as a mechanism that may be important in tumor development (16). MUC1 is highly overexpressed in diverse human carcinomas (19) and has been implicated in the suppression of cell-cell interactions (20). Other studies have suggested that MUC1 expression affects E-cadherin-mediated cell adhesion (17). The present findings support a potential role for MUC1 in abrogating the availability of β-catenin for interactions with E-cadherin. Accordingly, downregulation of GSK3β by Wnt signaling would in this model subvert E-cadherin function by titrating binding of β-catenin to MUC1. MUC1 is normally expressed at the apical borders of glandular epithelial cells (19). By contrast, the polarization of MUC1 expression is lost in carcinoma cells that express the protein at high levels over the entire cell surface (11, 19). The apical border of normal glandular epithelium is devoid of cell-cell interactions as a consequence of the positioning of this surface along secretory ducts. However, aberrant overexpression of MUC1 in carcinoma cells may confer perturbation of an antiadhesive function of MUC1 to the entire cell surface. The present findings support a competitive interaction between MUC1 and E-cadherin, through β-catenin binding, that could disrupt E-cadherin-mediated cell-cell interactions at sites of MUC1 expression. In epithelial cells, disruption of the interaction between E-cadherin and β-catenin by overexpression of MUC1 may be important in the progression to carcinoma.
ACKNOWLEDGMENTS
We thank Rolf Kemler for GST–β-catenin, James Woodgett for GSK3β, and Kenneth Kinzler for the pTOPFLASH, pFOPFLASH, and pCATCONTROL constructs.
REFERENCES
- 1.Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. β-Catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 1997;16:3797–3804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barth A I M, Pollack A L, Altschuler Y, Mostov K E, Nelson W J. NH2-terminal deletion of β-catenin results in stable colocalization of mutant β-catenin with adenomatous polyposis coli protein and altered MDCK cell adhesion. J Cell Biol. 1997;136:693–706. doi: 10.1083/jcb.136.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Behrens J, von Kries J P, Kühl M, Bruhn L, Wedlich D, Grosschedi R, Birchmeier W. Functional interaction of β-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- 4.Bourouis M, Moore P, Ruel L Y G, Heitzler P, Simpson P. An early embryonic product of the gene shaggy encodes a serine/threonine protein kinase related to the CDC28/cdc2+ subfamily. EMBO J. 1990;9:2877–2884. doi: 10.1002/j.1460-2075.1990.tb07477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunner E, Peter O, Schweizer L, Basler K. Pangolin encodes a Lef-1 homologue that acts downstream of Armadillo to transduce the Wingless signal in Drosophila. Nature. 1997;385:829–833. doi: 10.1038/385829a0. [DOI] [PubMed] [Google Scholar]
- 6.Dominguez I, Itoh K, Sokol S Y. Role of glycogen synthase kinase 3β as a negative regulator of dorsoventral axis formation in Xenopus embryos. Proc Natl Acad Sci USA. 1995;92:8498–8502. doi: 10.1073/pnas.92.18.8498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedman E L, Hayes D G, Kufe D W. Reactivity of monoclonal antibody DF3 with a high molecular weight antigen expressed in human ovarian carcinomas. Cancer Res. 1986;46:5189–5194. [PubMed] [Google Scholar]
- 8.Gendler S, Taylor-Papadimitriou J, Duhig T, Rothbard J, Burchell J A. A highly immunogenic region of a human polymorphic epithelial mucin expressed by carcinomas is made up of tandem repeats. J Biol Chem. 1988;263:12820–12823. [PubMed] [Google Scholar]
- 9.Guilford P, Hopkins J, Harraway J, McLeod M, McLeod N, Harawira P, Taite H, Scoular R, Miller A, Reeve A E. E-cadherin germline mutations in familial gastric cancer. Nature. 1998;392:402–405. doi: 10.1038/32918. [DOI] [PubMed] [Google Scholar]
- 10.He X, Saint-Jeannet J-P, Woodgett J R, Varmus H E, Dawid I. Glycogen synthase kinase-3 and dorsoventral patterning in Xenopus embryos. Nature. 1995;374:617–622. doi: 10.1038/374617a0. [DOI] [PubMed] [Google Scholar]
- 11.Hilkens J, Buijs F, Hilgers J, Hageman P, Calafat J, Sonnenberg A, van der Valk M. Monoclonal antibodies against human milk-fat globule membranes detecting differentiation antigens of the mammary gland and its tumors. Int J Cancer. 1984;34:197–206. doi: 10.1002/ijc.2910340210. [DOI] [PubMed] [Google Scholar]
- 12.Hinck L, Nelson W J, Papkoff J. Wnt-1 modulates cell-cell adhesion in mammalian cells by stabilizing β-catenin binding to the cell adhesion protein cadherin. J Cell Biol. 1994;124:729–741. doi: 10.1083/jcb.124.5.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huber O, Korn R, McLaughlin J, Ohsugi M, Hermann B G, Kemler R. Nuclear localization of β-catenin by interaction with transcription factor LEF-1. Mech Dev. 1996;59:3–10. doi: 10.1016/0925-4773(96)00597-7. [DOI] [PubMed] [Google Scholar]
- 14.Hulsken J, Birchmeier W, Behrens J. E-cadherin and APC compete for interaction with β-catenin and the cytoskeleton. J Cell Biol. 1994;127:2061–2069. doi: 10.1083/jcb.127.6.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawanishi J, Kato J, Sasaki K, Fujii S, Watanabe N, Niitsu Y. Loss of E-cadherin-dependent cell-cell adhesion due to mutation of the β-catenin gene in a human cancer cell line, HSC-39. Mol Cell Biol. 1995;15:1175–1181. doi: 10.1128/mcb.15.3.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kintner C. Regulation of embryonic cell adhesion by the cadherin cytoplasmic domain. Cell. 1992;69:225–236. doi: 10.1016/0092-8674(92)90404-z. [DOI] [PubMed] [Google Scholar]
- 17.Kondo K, Kohno N, Yokoyama A, Hiwada K. Decreased MUC1 expression induces E-cadherin-mediated cell adhesion of breast cancer cell lines. Cancer Res. 1998;58:2014–2019. [PubMed] [Google Scholar]
- 18.Korinek V, Barker N, Morin P J, van Wichen D, de Weger R, Kinzler K W, Vogelstein B, Clevers H. Constitutive transcriptional activation by a β-catenin–Tcf complex in APC−/− colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- 19.Kufe D, Inghirami G, Abe M, Hayes D, Justi-Wheeler H, Schlom J. Differential reactivity of a novel monoclonal antibody (DF3) with human malignant versus benign breast tumors. Hybridoma. 1984;3:223–232. doi: 10.1089/hyb.1984.3.223. [DOI] [PubMed] [Google Scholar]
- 20.Ligtenberg M J L, Buijs F, Vos H L, Hilkens J. Suppression of cellular aggregation by high levels of episialin. Cancer Res. 1992;52:2318–2324. [PubMed] [Google Scholar]
- 21.Mandelkow E-M, Drewes G, Biernat J, Gustke N, Van Lint J, Vandenheede J R, Mandelkow E. Glycogen synthase kinase-3 and the Alzheimer-like state of microtubule-associated protein tau. FEBS Lett. 1992;314:315–321. doi: 10.1016/0014-5793(92)81496-9. [DOI] [PubMed] [Google Scholar]
- 22.McCrea P D, Turck C W, Gumbiner B. A homolog of the armadillo protein in Drosophila (plakoglobin) associated with E-cadherin. Science. 1991;254:1359–1361. doi: 10.1126/science.1962194. [DOI] [PubMed] [Google Scholar]
- 23.Merlo G R, Siddiqui J, Cropp C, Liscia D S, Lidereau R, Callahan R, Kufe D. Frequent alteration of the DF3 tumor-associated antigen gene in primary human breast carcinomas. Cancer Res. 1989;49:6966–6971. [PubMed] [Google Scholar]
- 24.Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Destrée O, Clevers H. XTcf-3 transcription factor mediates β-catenin-induced axis formation in Xenopus embryos. Cell. 1996;86:391–399. doi: 10.1016/s0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- 25.Morin P J, Sparks A B, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler K W. Activation of β-catenin–Tcf signaling in colon cancer by mutations in β-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 26.Morin P J, Vogelstein B, Kinzler K W. Apoptosis and APC in colorectal tumorigenesis. Proc Natl Acad Sci USA. 1996;93:7950–7954. doi: 10.1073/pnas.93.15.7950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munemitsu S, Albert I, Souza B, Rubinfeld B, Polakis P. Regulation of intracellular beta-catenin levels by the adenomatous polyposis coli (APC) tumor-suppressor protein. Proc Natl Acad Sci USA. 1995;92:3046–3050. doi: 10.1073/pnas.92.7.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagafuchi A, Ishihara S, Tsukita S. The roles of catenins in cadherin-mediated cell adhesion: functional analysis of E-cadherin–alpha catenin fusion molecules. J Cell Biol. 1994;127:235–245. doi: 10.1083/jcb.127.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagafuchi A, Takeichi M. Transmembrane control of cadherin-mediated cell adhesion: a 94 kDa protein functionally associated with a specific region of the cytoplasmic domain of E-cadherin. Cell Regul. 1989;1989:37–44. doi: 10.1091/mbc.1.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neufeld K L, White R. Nuclear and cytoplasmic localizations of the adenomatous polyposis coli protein. Proc Natl Acad Sci USA. 1997;94:3034–3039. doi: 10.1073/pnas.94.7.3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ozawa M, Ringwald M, Kemler R. Uvomorulin-catenin complex formation is regulated by a specific domain in the cytoplasmic region of the cell adhesion molecule. Proc Natl Acad Sci USA. 1990;87:4246–4250. doi: 10.1073/pnas.87.11.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pandey P, Kharbanda S, Kufe D. Association of the DF3/MUC1 breast cancer antigen with Grb2 and the Sos/Ras exchange protein. Cancer Res. 1995;55:4000–4003. [PubMed] [Google Scholar]
- 33.Peifer M, Pai L-M, Casey M. Phosphorylation of Drosophila adherens junction protein armadillo: roles for wingless signal and zeste-white 3 kinase. Dev Biol. 1994;166:543–556. doi: 10.1006/dbio.1994.1336. [DOI] [PubMed] [Google Scholar]
- 34.Peifer M, Sweeton D, Casey M, Wieschaus E. Wingless signal and zeste-white 3 kinase trigger opposing changes in the intracellular distribution of armadillo. Development. 1994;120:369–380. doi: 10.1242/dev.120.2.369. [DOI] [PubMed] [Google Scholar]
- 35.Perey L, Hayes D F, Maimonis P, Abe M, O’Hara C, Kufe D W. Tumor selective reactivity of a monoclonal antibody prepared against a recombinant peptide derived from the DF3 human breast carcinoma-associated antigen. Cancer Res. 1992;52:2563–2568. [PubMed] [Google Scholar]
- 36.Perl A-K, Wilgenbus P, Dahl U, Semb H, Christofori G. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature. 1998;392:190–193. doi: 10.1038/32433. [DOI] [PubMed] [Google Scholar]
- 37.Pierce S B, Kimelman D. Regulation of Spemann organizer formation by the intracellular kinase Xgsk-3. Development. 1995;121:755–765. doi: 10.1242/dev.121.3.755. [DOI] [PubMed] [Google Scholar]
- 38.Pierceall W E, Woodard A S, Morrow J S, Rimm D, Fearon E R. Frequent alteration in E-cadherin and α- and β-catenin expression in human breast cancer cell lines. Oncogene. 1995;11:1319–1325. [PubMed] [Google Scholar]
- 39.Riese J, Yu X, Munnerlyn A, Eresh S, Hsu S-C, Grosschedl R, Bienz M. LEF-1, a nuclear factor coordinating signaling inputs from wingless and decapentaplegic. Cell. 1997;88:777–787. doi: 10.1016/s0092-8674(00)81924-8. [DOI] [PubMed] [Google Scholar]
- 40.Roach P J. Multisite and hierarchal protein phosphorylation. J Biol Chem. 1991;266:14139–14142. [PubMed] [Google Scholar]
- 41.Robbins P F, El-Gamil M, Li Y F, Kawakami Y, Loftus D, Appella E, Rosenberg S A. A mutated β-catenin gene encodes a melanoma-specific antigen recognized by tumor infiltrating lymphocytes. J Exp Med. 1996;183:1185–1192. doi: 10.1084/jem.183.3.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rubinfeld B, Robbins P, El-Gamil M, Albert I, Porfiri E, Polakis P. Stabilization of β-catenin by genetic defects in melanoma cell lines. Science. 1997;275:1790–1792. doi: 10.1126/science.275.5307.1790. [DOI] [PubMed] [Google Scholar]
- 43.Rubinfeld B, Souza B, Albert I, Muller O, Chamberlain S H, Masiarz F R, Munemitsu S, Polakis P. Association of the APC gene product with β-catenin. Science. 1993;262:1731–1734. doi: 10.1126/science.8259518. [DOI] [PubMed] [Google Scholar]
- 44.Rubinfeld B, Souza B, Albert I, Munemitsu S, Polakis P. The APC protein and E-cadherin form similar but independent complexes with α-catenin, β-catenin, and plakoglobin. J Biol Chem. 1995;270:5549–5555. doi: 10.1074/jbc.270.10.5549. [DOI] [PubMed] [Google Scholar]
- 45.Rubinfeld B, Albert I, Porfiri E, Fiol C, Munemitsu S, Polakis P. Binding of GSK3β to the APC–β-catenin complex and regulation of complex assembly. Science. 1996;272:1023–1026. doi: 10.1126/science.272.5264.1023. [DOI] [PubMed] [Google Scholar]
- 46.Shimazui T, Schalken J A, Giroldi L A, Jansen C F J, Akaza H, Koiso K, Debruyne F M J, Bringuier P P. Prognostic value of cadherin-associated molecules (α-, β-, and γ-catenins and p120cas) Cancer Res. 1996;56:4154–4158. [PubMed] [Google Scholar]
- 47.Shiozaki, H., H. Oka, M. Inoue, S. Tamura, and M. Monden. 1996. E-cadherin mediated adhesion system in cancer cells. Cancer 77(Suppl.):1605–1613. [DOI] [PubMed]
- 48.Siddiqui J, Abe M, Hayes D, Shani E, Yunis E, Kufe D. Isolation and sequencing of a cDNA coding for the human DF3 breast carcinoma-associated antigen. Proc Natl Acad Sci USA. 1988;85:2320–2323. doi: 10.1073/pnas.85.7.2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Siegfried E, Chou T, Perrimon N. Wingless signaling acts through zeste-white 3, the Drosophila homolog of glycogen synthase kinase-3, to regulate engrailed and establish cell fate. Cell. 1992;71:1167–1179. doi: 10.1016/s0092-8674(05)80065-0. [DOI] [PubMed] [Google Scholar]
- 50.Siegfried E, Wilder E L, Perrimon N. Components of wingless signaling in Drosophila. Nature. 1994;367:76–80. doi: 10.1038/367076a0. [DOI] [PubMed] [Google Scholar]
- 51.Smith K J, Johnson K A, Bryan T M, Hill D E, Markowitz S, Willson J K, Paraskeva C, Peterson G M, Hamilton S R, Vogelstein B, Kinzler K W. The APC gene product in normal and tumor cells. Proc Natl Acad Sci USA. 1993;90:2846–2850. doi: 10.1073/pnas.90.7.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stambolic V, Woodgett J. Mitogen inactivation of glycogen synthase kinase-3β in intact cells via serine 9 phosphorylation. Biochem J. 1994;303:701–704. doi: 10.1042/bj3030701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Su L-K, Vogelstein B, Kinzler K W. Association of the APC tumor suppressor protein with catenins. Science. 1993;262:1734–1737. doi: 10.1126/science.8259519. [DOI] [PubMed] [Google Scholar]
- 54.Takayama T, Shiozaki H, Shibamoto S, Oka H, Kimura Y, Tamura S, Inoue M, Monden T, Ito F, Monden M. β-Catenin expression in human cancers. Am J Pathol. 1996;148:39–46. [PMC free article] [PubMed] [Google Scholar]
- 55.Takeichi M. Cadherins: a molecular family important in selective cell-cell adhesion. Annu Rev Biochem. 1990;59:237–252. doi: 10.1146/annurev.bi.59.070190.001321. [DOI] [PubMed] [Google Scholar]
- 56.van de Wetering M, Cavallo R, Booijes D, van Beest M, van Es J, Loureiro J, Ypma A, Hursh D, Jones T, Bejsovec A, Peifer M, Mortin M, Clevers H. Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell. 1997;88:789–799. doi: 10.1016/s0092-8674(00)81925-x. [DOI] [PubMed] [Google Scholar]
- 57.van de Wiel-van Kemenade E, Ligtenberg M J L, de Boer A J, Buijs F, Vos H L, Melief C J M, Hilkens J, Figdor C G. Episialin (MUC1) inhibits cytotoxic lymphocyte-target cell interaction. J Immunol. 1993;151:767–776. [PubMed] [Google Scholar]
- 58.van Leeuwen F, Nusse R. Biological activity of soluble wingless protein in cultured Drosophila cells. Nature. 1994;368:342–344. doi: 10.1038/368342a0. [DOI] [PubMed] [Google Scholar]
- 59.Vleminckx K, Vakaet L J, Mareel M, Fiers W, van Roy F. Genetic manipulation of E-cadherin expression by epithelial tumor cells reveals an invasion suppressor role. Cell. 1991;66:107–119. doi: 10.1016/0092-8674(91)90143-m. [DOI] [PubMed] [Google Scholar]
- 60.Wesseling J, van der Valk S W, Hilkens J. A mechanism for inhibition of E-cadherin-mediated cell-cell adhesion by the membrane-associated mucin episialin/MUC1. Mol Biol Cell. 1996;7:565–577. doi: 10.1091/mbc.7.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yamamoto M, Bharti A, Li Y, Kufe D. Interaction of the DF3/MUC1 breast carcinoma-associated antigen and β-catenin in cell adhesion. J Biol Chem. 1997;272:12492–12494. doi: 10.1074/jbc.272.19.12492. [DOI] [PubMed] [Google Scholar]
- 62.Yost C, Torres M, Miller J R, Huang E, Kimelman D, Moon R T. The axis-inducing activity, stability, and subcellular distribution of β-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev. 1996;10:1443–1454. doi: 10.1101/gad.10.12.1443. [DOI] [PubMed] [Google Scholar]
- 63.Zrihan-Licht S, Baruch A, Keydar I, Wreschner D H. Phosphorylation of the MUC1 breast cancer membrane proteins: cytokine receptor-like molecules. FEBS Lett. 1994;356:130–137. doi: 10.1016/0014-5793(94)01251-2. [DOI] [PubMed] [Google Scholar]