Infectious Clones and Vectors Derived from Adeno-Associated Virus (AAV) Serotypes Other Than AAV Type 2 (original) (raw)
Abstract
Adeno-associated viruses (AAVs) are single-stranded dependent parvoviruses being developed as transducing vectors. Although at least five serotypes exist (AAV types 1 to 5 [AAV1 to -5]), only AAV2, AAV3, and AAV4 have been sequenced, and the vectors in use were almost all derived from AAV2. Here we report the cloning and sequencing of a second AAV3 genome and a new AAV serotype designated AAV6 that is related to AAV1. AAV2, AAV3, and AAV6 were 82% identical at the nucleotide sequence level, and AAV4 was 75 to 78% identical to these AAVs. Significant sequence variation was noted in portions of the capsid proteins that presumably are responsible for serotype-specific functions. Vectors produced from AAV3 and AAV6 differed from AAV2 vectors in host range and serologic reactivity. The AAV3 and AAV6 vector serotypes were able to transduce cells in the presence of serum from animals previously exposed to AAV2 vectors. Our results suggest that vectors based on alternative AAV serotypes will have advantages over existing AAV2 vectors, including the transduction of different cell types, and resistance to neutralizing antibodies against AAV2. This could be especially important for gene therapy, as significant immunity against AAV2 exists in human populations and many protocols will likely require multiple vector doses.
Adeno-associated viruses (AAVs) are 4.7-kb single-stranded DNA viruses that depend on helper viruses such as adenovirus for replication. Five primate AAV serotypes have been characterized in the literature and are designated AAV types 1 to 5 (AAV1 to -5) (3, 4, 27, 41). Serological studies suggest that AAV1 to -3 and AAV5 frequently infect human populations (6, 21, 40), while AAV4 infects monkeys (40). The AAV2 and AAV3 viral genomes have been sequenced in their entirety (11, 38, 46, 57) and found to have 82% overall sequence homology. The recently sequenced AAV4 genome was slightly more divergent, with 75 and 78% homology to AAV2 and AAV3, respectively (14). No DNA sequence data has been published for AAV1 or AAV5.
Transducing vectors have been constructed from cloned proviral genomes of the AAV2 serotype and used to transfer genes into a wide variety of mammalian cells (39). The advantages of AAV vectors include particle stability, the ability to integrate into host chromosomes, and the potential for transducing normal cells in vitro and in vivo. However, many problems remain with AAV vectors, including wide variability in transduction efficiencies among different cell types (2, 5, 23, 25, 39, 44), poor in vivo transduction rates after prior vector exposure (24, 59), the large numbers of vector particles required for transduction (20, 25, 34, 47), and the possibility that some transduction events may lead to transient gene expression, perhaps from episomal vector genomes (1, 5). As our understanding of the basic biology of AAV vectors improves, several of these problems may eventually be overcome with improved vector designs and transduction protocols. Another approach is to take advantage of distinct, naturally occurring AAV isolates that may have inherent advantages over existing AAV2 vectors.
Vectors based on other AAV serotypes could prove especially useful for transducing cells that are resistant to AAV2 infection. Although an AAV2 receptor gene has not been cloned, a 150-kDa glycoprotein has been identified as a candidate receptor that binds to the virus (37). This protein was not detected in cells resistant to AAV2 infection, and binding studies suggest that each AAV serotype uses a different receptor (37). Thus, vectors based on other AAV serotypes may have a host range distinct from AAV2 vectors. An AAV4-based vector was recently tested in a variety of cell lines and found to have a transduction pattern distinct from AAV2 vectors, suggesting that AAV4 may use a different receptor (14). Another advantage of new AAV vector serotypes could be the evasion of host immune responses directed against AAV2. Infection by wild-type AAV results in the production of neutralizing and complement-fixing antibodies (6, 21, 40), and 50 to 80% of adults have neutralizing antibodies to AAV, with antibodies against AAV2 being the predominant serotype (7, 40). Although the effects of these antibodies on in vivo gene transfer have not been studied in humans, the presence of high-titer neutralizing antibodies is likely to severely decrease transduction rates. The fact that additional transduction events were not observed after readministration of AAV2 vectors in animals suggests that the host immune response can completely prevent transduction (24, 59). As each AAV serotype elicits a distinct humoral response, this problem might be overcome by using different vector serotypes. It is also possible that other vector serotypes will have improved particle-to-infectivity ratios or different integration properties, allowing more efficient transduction.
Here we describe the isolation and sequencing of new infectious clones of AAV. One clone is from the AAV3 serotype, while the other is from a new AAV isolate found as a contaminant in a laboratory adenovirus stock. We have designated this new isolate AAV6, which by sequence analysis appears to be related to AAV1. During the completion of this project, Muramatsu et al. (38) reported the sequencing of their own AAV3 clone; for clarity, therefore, we refer to their published isolate as AAV3A and to ours as AAV3B. Vectors based on AAV3B and AAV6 were produced and found to have potential advantages over AAV2 vectors. Each vector serotype transduced a variety of cell lines at different rates, suggesting that they have unique host ranges and may use distinct cellular receptors. AAV3B and AAV6 vectors were resistant to the neutralizing effects of anti-AAV2 antibodies, demonstrating their usefulness in avoiding host immune responses against AAV2.
MATERIALS AND METHODS
Cell culture.
Human embryonic kidney 293 (22) and 293T (16) cells, IB3 human bronchial epithelial cells (61), HT-1080 human fibrosarcoma cells (45), MHF2 normal human fibroblasts (NIGMS GM05387), COS1 simian virus 40 (SV40)-transformed African green monkey kidney cells (ATCC CRL 1650), and BHK21 baby hamster kidney cells (33) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 10% heat-inactivated (56°C for 30 min) fetal bovine serum (FBS) (HyClone, Logan, Utah), 1.25 μg of amphotericin per ml, 100 U of penicillin per ml, and 100 μg of streptomycin per ml at 37°C in a 10% CO2 atmosphere. CHO-K1 AA8 Chinese hamster ovary cells (ATCC CRL 1859) were grown in minimal essential medium α medium (GIBCO BRL, Grand Island, N.Y.) with 10% FBS and antibiotics. K-562 cells (32) were cultured in RPMI medium with 10% FBS and antibiotics. MHF2 fibroblasts (GM05387) were obtained from the National Institute of General Medical Sciences Human Genetic Mutant Cell Repository (Camden, N.J.). CHO-K1 AA8 cells and COS1 cells were obtained from the American Type Culture Collection (ATCC) (Rockville, Md.).
Virus and vector stocks.
Wild-type virus stocks of AAV1, AAV3, and AAV6 used for cloning and sequencing were prepared by infecting 293 cells with the AAV isolate and adenovirus, freeze-thawing infected cell lysates 3 days later, and pelleting the cell debris by centrifugation at 5,800 × g for 30 min. The crude lysates were concentrated by pelleting through sucrose, and the AAV6 stock was further purified on a cesium chloride gradient as described previously (48). AAV1 (ATCC VR-645) and AAV3 (ATCC VR-681) were obtained from ATCC, and AAV6 was obtained from a laboratory adenovirus type 5 stock after wild-type AAV was identified in vector stocks prepared with this adenovirus helper.
Large virus stocks were prepared from cloned AAV2, AAV3B, and AAV6 proviral DNA in adenovirus-infected 293 cells. The AAV2 stock was purified on CsCl gradients 3 days after transfection of pAV2 (30). The AAV3B and AAV6 stocks were made by transfecting first with pAAV3B or pAAV6, respectively, preparing crude lysates 3 days later, and then infecting new adenovirus-infected 293 cells with these lysates and purifying virions on CsCl 3 days later. Crude lysates and CsCl gradients (see Fig. 1) were prepared as described (see above and reference 48). Fractions of 10 drops (approximately 400 μl) were collected from the CsCl gradients, and the amount of full-length, linear viral DNA was determined by alkaline gel electrophoresis and Southern analysis. The refractive index of each fraction was measured by refractometry.
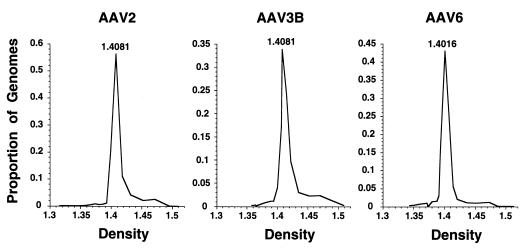
FIG. 1.
Cesium chloride gradient fractionation of AAV serotypes. AAV2, AAV3B, and AAV6 virus stocks produced from infectious clones were purified on CsCl gradients. The number of AAV genomes in each gradient fraction was determined by Southern analysis, and the density of each fraction was determined by refractometry. Each fraction genome number was calculated as a proportion of the total present in the gradient and plotted against the density of the fraction. The density of the fraction with the greatest AAV signal is indicated.
AAV vector stocks were prepared from 293 or 293T cells and purified on CsCl gradients as described previously (48). pA2LAPSN with pRepCap2, pA3LAPSN with pRepCap3, and pA6LAPSN with pRepCap6 were used to produce AAV2-LAPSN, AAV3-LAPSN, and AAV6-LAPSN, respectively. The vector particle number was calculated by measuring the number of full-length vector genomes per milliliter of stock by Southern analysis (47).
Purification and cloning of viral DNA.
Viral DNA was isolated from wild-type virus stocks prepared as crude lysates and concentrated through sucrose (AAV1 and AAV3) or CsCl (AAV6) gradients. Virus stocks were incubated in 0.1% sodium dodecyl sulfate–0.2 mg of proteinase K per ml at 37°C for 3 h, extracted twice with phenol and chloroform and once with chloroform, and then precipitated with sodium acetate and ethanol. The DNA pellets were suspended in TE (10 mM Tris [pH 8], 1 mM EDTA), and the AAV strands were allowed to hybridize in 0.3 to 1.0 M NaCl for 2 h at 50 to 60°C. Double-stranded AAV DNA of approximately 5 kb was gel purified with a Qiaex II gel extraction kit (Qiagen, Chatsworth, Calif.) and end-filled with the Klenow fragment of DNA polymerase I, and _Xba_I linkers (dCTCTAGAG) were attached. The DNA was gel purified after linker ligation, cloned into the _Xba_I site of pACYC184, and propagated in recB recJ SURE cells (Stratagene, La Jolla, Calif.).
Plasmids that appeared to contain intact, full-length genomes of AAV3 and AAV6 were identified by restriction analysis. Plasmid clones of AAV3 were prescreened by colony hybridization and probed with the AAV2 genomic sequences. Infectious clones were identified by transfecting adenovirus-infected 293 cells with candidate plasmids (30 μg of each plasmid in a 15-cm-diameter dish), preparing crude lysates from each dish, and pelleting the lysate through sucrose, as described previously (48). Viral DNA was purified from each lysate as described above, and viral genome amplification was assayed by Southern analysis with a presumed viral genome fragment from one of the plasmid clones (for AAV6) or a fragment of plasmid pAAV/Ad (51) (for AAV3) as probes. The clone with the greatest signal from each serotype was designated pAAV3B or pAAV6 and selected for sequencing.
Sequence analysis.
Both strands of the proviral DNA (including the terminal repeats) contained in pAAV3B and pAAV6 were sequenced by adaptations of the dideoxy chain termination method (53) and read manually or by automated sequence analyzers. For pAAV6, the portion inside the terminal repeats was subcloned and the initial sequence was obtained with primers designed for the multiple cloning site. Additional internal sequence was obtained by using primers based on the sequences generated. The sequence of pAAV3B was obtained by subcloning sonicated fragments of the viral genome and using multiple-cloning-site primers in a shotgun strategy (35). Additional primers were designed to sequence any regions that were not completed by the shotgun strategy. To sequence through the terminal repeat regions, fragments of plasmid pAAV6 were first subcloned after digestion with _Ahd_I or _Bgl_I, which have single sites in the terminal repeat B and C regions, respectively. The sequences of the subclones from the _Ahd_I digest complemented those of the _Bgl_I digest. The terminal repeats in pAAV3B were directly sequenced after digestion with _Ahd_I or _Mwo_I. Portions of the sequencing were carried out by Seqwright DNA Sequencing (Houston, Tex.) and National Biosciences, Inc. (Plymouth, Minn.).
To sequence part of the AAV1 cap gene, 5 pg of purified AAV1 viral DNA was first amplified by two rounds of PCR, 30 cycles each, in a reaction mixture containing 6 mM MgSO4, 1.25 mM (each) deoxynucleoside triphosphate, 0.5 μM (each) primer, 1× ThermoPol buffer, and 10 mU of Vent DNA polymerase (New England Biolabs, Beverly, Mass.) per μl. The PCR primers used were 5′-CCTTTCCACAGCAGCTACGC-3′ and 5′-TGAAAGTGTCCATCCGTGTG-3′. The PCR product was purified by use of a QIAquick PCR purification kit (Qiagen), and 100 ng was subjected to sequencing with the AmpliTaqFS polymerase sequencing kit (Perkin-Elmer, Foster City, Calif.) and the primer 5′-CGGCTGATGAATCCTCT-3′ and analyzed on an Applied Biosystems Inc. (Foster City, Calif.) sequencer. All primers were designed based on the AAV6 sequence.
Sequence analysis was performed with the GAP program of the Wisconsin package of Genetics Computer Group (GCG) (Madison, Wis.), version 9.0, for both DNA and protein comparisons with default parameters. DNA and protein alignments were performed by using the PILEUP and PUBLISH programs of GCG.
Plasmids used.
pACYC184 (12), pALAPSN (47), pAV2 (30), pBluescript II KS+ (Stratagene), psub201 (52), and pVZ1 (26) have been described previously.
pAAV6Bgl was constructed by engineering two _Bgl_II sites in pAAV6. A 5′ _Bgl_II site was created in pAAV6 by changing two nucleotides (G to A at position 191 and C to T at position 194) with the oligonucleotide 5′-TCTAATACAAGATCTCCCTAAC-3′ by using published site-directed mutagenesis methods (29). A 3′ _Bgl_II site was created by linker (dCAGATCTG) insertion at the end-filled pAAV6 _Bst_EII site (position 4491). Helper plasmid pRepCap6 was constructed by isolating the _Bgl_II rep and cap fragment from pAAV6Bgl and ligating into the _Bam_HI site of cloning plasmid pVZ1. Vector plasmid pA6LAPSN was constructed by replacing the _Bgl_II rep and cap fragment of pAAV6Bgl with a _Cla_I fragment from pALAPSN containing the alkaline phosphatase and neomycin phosphotransferase reporter cassettes (end-filled and with _Bgl_II linkers [dCAGATCTG] attached).
pRepCap3 was constructed by inserting a _Bss_HII-_Apa_LI rep and cap fragment from pAAV3B (end-filled and with _Xba_I linkers [dCTCTAGAG] attached) containing nucleotides 163 to 4475 of the AAV3B genome into the _Xba_I site of pVZ1. The corresponding backbone fragment produced after removal of rep and cap (end-filled and with _Bgl_II linkers [dCAGATCTG] attached) was used to construct pA3LAPSN by ligation to the same _Cla_I LAPSN fragment used to create pA6LAPSN.
pRepCap2 consists of the _Xba_I fragment of psub201 containing the AAV2 rep and cap genes in the _Xba_I site of pBluescript II KS+ and was constructed by N. Inoue (University of Washington, Seattle). pA2LAPSN was constructed by inserting the _Bgl_II LAPSN fragment from pA3LAPSN into the psub201 _Xba_I backbone fragment (end-filled and with _Bgl_II linkers [dCAGATCTG] attached).
Transduction assays.
Except for K-562 cells, alkaline phosphatase transduction rates were measured by plating target cells at 5 × 104 cells per well in six-well dishes (Costar, Cambridge, Mass.) on day 1, adding 10 μl of 10-fold serial vector stock dilutions on day 2, and staining for alkaline phosphatase expression (18) on day 4. Each vector stock was adjusted to contain 5 × 107 viral genomes per μl, as determined by Southern analysis. For K-562 cells, 5 × 108 viral genomes (10 μl) were added to 104 cells in a 96-well plate (Nalge Nunc, Rochester, N.Y.). The infected cells were grown for 7 days with medium changes and then stained for alkaline phosphatase expression (18). Washes were carried out by centrifugation at 4,000 rpm in an Eppendorf 5415C centrifuge (Brinkmann Instruments, Inc., Westbury, N.Y.) for 5 min and resuspension in phosphate-buffered saline. After fixing, 0.01% bovine serum albumin in phosphate-buffered saline was added to the washes to minimize loss of cells. Total cell number was determined by using a hemocytometer, and alkaline phosphatase-positive cells were counted in wells of a 12-well dish (Nalge Nunc). The percentage of alkaline phosphatase-positive cells was calculated and used to determine the original number of transduced cells to measure the titer.
For serum inactivation experiments, 5 × 107 or 5 × 108 AAV vector particles were incubated with polyclonal rabbit anti-AAV2 serum dilutions (24) for 1 h at 37°C prior to addition to BHK21 cells. Serum dilutions of 1:20 and 1:100 were prepared in DMEM with 1% FBS after pooling of the serum from three rabbits previously exposed to AAV2 vectors (24).
DNA techniques.
Restriction enzymes, T4 DNA ligase, DNA polymerases, and phosphorylated linkers were from New England Biolabs. Proteinase K was from Boehringer Mannheim (Indianapolis, Ind.). Enzyme reactions were performed under the manufacturer’s recommended conditions. Plasmids were prepared by using Qiagen columns. DNA manipulations and Southern blot analysis were performed by standard procedures (50). Samples for alkaline gels were prepared by adding 2 μl of 10% sodium dodecyl sulfate to 10 μl of each CsCl fraction from an AAV stock preparation, boiling for 10 minutes, and then adding 2 μl of loading buffer (300 mM NaOH, 6 mM EDTA, 18% Ficoll Type 400, 0.15% bromocresol green, 0.25% xylene cyanol FF). Southern blots were quantitated with a PhosphorImager, model 400S (Molecular Dynamics, Sunnyvale, Calif.).
Nucleotide sequence accession numbers.
The nucleotide sequences of AAV3B and AAV6 determined in this study are available through GenBank under accession no. AF028705 and AF028704, respectively.
RESULTS
Infectious clones of AAV3 and AAV6.
Two strains of wild-type AAV were used to generate infectious clones. A sample of AAV3 was obtained from the ATCC, and a previously uncharacterized AAV strain that we have designated AAV6 was isolated from a laboratory stock of adenovirus type 5 that was found to be contaminated with AAV. Virion DNA was purified from stocks of AAV3 and AAV6 and cloned into the pACYC184 plasmid backbone after annealing complementary single strands, end-filling, and attaching linkers (see Materials and Methods). The pACYC184 backbone was chosen because it uses the low-copy-number p15A replication origin (15) and results in fewer rearrangements in the viral terminal repeats during propagation in Escherichia coli (data not shown). Plasmids that appeared to contain full-length inserts were identified by restriction digests and chosen for further analysis.
In order to identify infectious clones, crude viral stocks were prepared from cell lysates of adenovirus-infected 293 cells transfected with candidate plasmid clones. Southern analysis of purified DNA from these crude stocks identified plasmids that produced amplified viral DNAs. Five of 7 AAV3 clones and 10 of 11 AAV6 clones contained replication-competent proviruses, as determined by this assay. The plasmids that produced the greatest viral genome amplification for AAV3 and AAV6 were designated pAAV3B and pAAV6, respectively. The pAAV3B name was chosen to avoid confusion with the AAV3 clone produced by Muramatsu et al. (38), which was published while our study was being completed and is referred to here as AAV3A.
The infectious nature of the cloned proviruses was further demonstrated by infecting 293 cells with crude stocks generated by transfection with pAAV3B or pAAV6, adding adenovirus helper, and purifying AAV virions on CsCl gradients. Gradient fractions were analyzed by Southern blots of alkaline gels, and the full-length single-stranded virion signal was quantitated and plotted against the fraction density as shown in Fig. 1. For comparison, the same procedure was performed with the AAV2 infectious clone, pAV2 (30). The viral particles generated from pAAV3B and pAAV6 have densities of 1.40 to 1.41 g/cm3, as expected for wild-type AAVs, indicating that they produce infectious, packaged virions.
Sequence analysis.
Both strands of the entire cloned genomes of AAV3B and AAV6 were sequenced by a combination of subcloning and specific primer design. Sequencing of the terminal repeat regions was problematic due to polymerase stalling in the palindrome structure. To overcome this, the repeat regions were cut into two fragments by restriction enzyme digestion before being sequenced so that a complete palindrome could not form (see Materials and Methods). The aligned sequences of the AAV2, AAV3B, AAV4, and AAV6 genomes are shown in Fig. 2. The genetic structures of all four viruses were similar, including open reading frames, transcription units, and repeat structures. Sequence comparisons by the GCG GAP program showed that the AAV2, AAV3B, and AAV6 genomes were 82% identical. Identities with AAV4 were lower, ranging from 75 to 78%. The AAV3B sequence has 16 nucleotide differences compared to the sequence of AAV3A (38), and these are listed in Table 1.
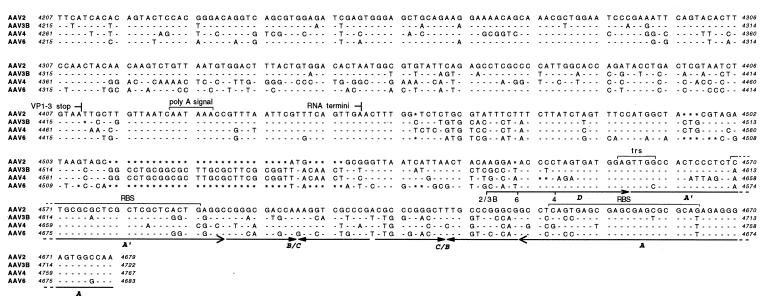
FIG. 2.
Sequences of infectious AAV clones. The DNA sequences of AAV2, AAV3B, AAV4 (14), and AAV6 are shown aligned, with nucleotides identical to those of AAV2 indicated by dashes and gaps indicated by asterisks. The positions of conserved genetic elements are shown, including the p5, p19, and p40 promoters, transcription start and stop sites, intron splice sites, polyadenylation signal (poly A signal), translation start and stop sites for the Rep (Rep 78, 68, 52, and 40) and capsid (VP1, VP2, and VP3) proteins, sp1 and sp1-like (GGT) binding sites (42, 43), Rep protein binding site (RBS) (13, 49), terminal resolution site (trs) (56), and the terminal repeat A, B, C, A′, and D domains. The AAV2 sequence (57) includes published corrections (11, 46).
TABLE 1.
Nucleotide differences between AAV3A and AAV3Ba
| AAV3A | AAV3B | ||
|---|---|---|---|
| Position(s) | Nucleotide(s) | Position(s) | Nucleotide(s) |
| 1–2 | TT | 1 | T |
| 370 | G | 369 | A |
| 425 | C | 424 | G |
| 426 | G | 425 | C |
| 1656 | G | 1655 | A |
| 1839 | G | 1838 | C |
| 2638 | G | 2637 | A |
| 2641 | G | 2640 | C |
| 2739 | A | 2738 | C |
| 3170 | G | 3169 | A |
| 3173 | G | 3172 | A |
| 3988 | G | 3987 | A |
| 4000 | C | 3999 | G |
| 4417–4419 | TGA | 4416–4417 | TA |
| 4483–4485 | TTA | 4481–4482 | TA |
| 4487–4489 | CTT | 4484–4485 | CT |
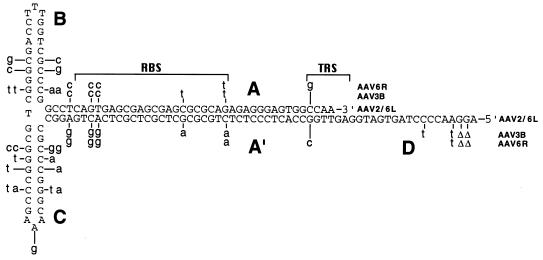
Although several nucleotide substitutions were noted in the terminal repeats, the base pairings that determine palindromic secondary structure were all conserved (Fig. 3). In the case of AAV6, the two repeats had different sequences, with the left repeat being identical to that of AAV2 and the right repeat having a unique sequence. An examination of the AAV4 sequence (14) also revealed different sequences of the left and right repeats, with the right A and A′ domains being unable to form a perfect palindrome. The first reported nucleotide in the AAV3A repeat is deleted in our isolate of AAV3B. A variable terminus has also been observed in AAV2 DNA, with 50% of the DNA molecules missing the first T residue and another 15% missing the first two nucleotides (TT) (19).
FIG. 3.
Terminal repeat secondary structure. The sequences of the terminal repeats of AAV2, AAV3B, and AAV6 are shown in the secondary structure predicted to exist in single-stranded vector genomes. The AAV2 and AAV6 left (AAV6L) sequences are shown in capital letters. The nucleotide differences in the AAV3B and AAV6 right (AAV6R) terminal repeats are shown in lowercase letters above or below the repeat structure. Nucleotide deletions are indicated by Δ. The A, B, C, A′, and D repeat domains, Rep binding site (RBS) (13, 49), and terminal resolution site (TRS) (56) are indicated.
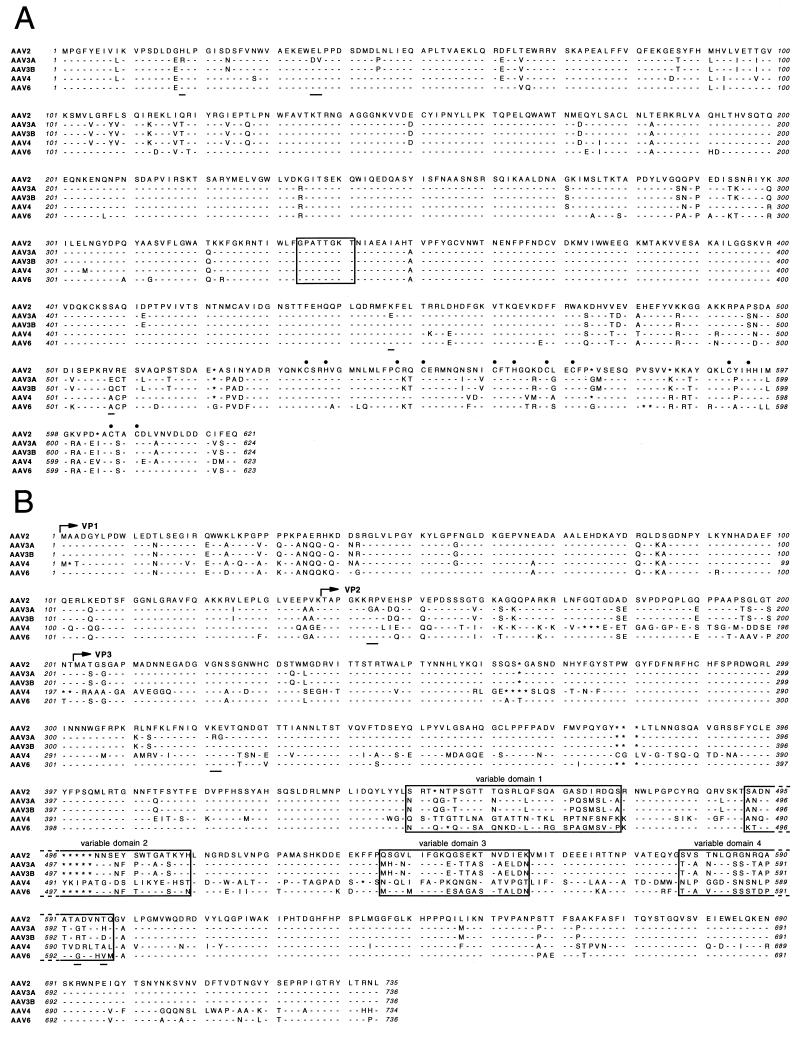
The predicted amino acid sequences of the Rep and Cap proteins from AAV2, AAV3A, AAV3B, AAV4, and AAV6 were aligned as shown in Fig. 4, and their homologies were determined by the GCG GAP program. The Rep78 proteins from these viruses were 89 to 93% identical to each other. The capsid VP1 proteins from AAV2, AAV3A or AAV3B, and AAV6 were 83 to 88% identical, while the AAV4 VP1 sequence was only 62 to 65% identical to those of the other serotypes. There were five amino acid differences between the Rep proteins of AAV3A and AAV3B and six amino acid differences between their Cap proteins. The AAV2 Rep78 ATP binding site (residues 334 to 341) (54, 58) and the zinc finger binding motif containing CXXH and CXXC sequences (residues 535 to 609) (10, 28) were completely conserved, as shown in Fig. 4A. Several variable domains were evident among the Cap proteins, four of which in particular contained unique sequences for each serotype (Fig. 4B).
FIG. 4.
Translated reading frames. The predicted amino acid sequences of the Rep78 (A) and capsid VP1, VP2, and VP3 proteins (B) are aligned for AAV2, AAV3A, AAV3B, AAV4, and AAV6. Amino acid identities with AAV2 sequences are indicated by dashes. Gaps are indicated by asterisks. Differences between AAV3B and AAV3A are also indicated by underlining. (A) The consensus ATP binding site (54, 58) is boxed, and the cysteine and histidine residues of the zinc finger binding motif (10, 28) are indicated by filled circles. (B) The start sites for each of the capsid proteins, VP1, VP2, and VP3, are indicated by arrows, and four variable domains (regions with significant sequence differences among all 4 serotypes, as determined by inspection) are boxed.
AAV6 is a variant of AAV1.
Although the sequence of AAV6 and serum neutralization studies (see below) suggest that AAV6 is a distinct serotype from AAV2 and AAV3, we wished to determine if our cloned isolate was related to the other known AAVs. Because it was a contaminant from a human adenovirus sample, one possibility was that AAV6 was related to AAV1, which frequently infects human populations (7, 40). We obtained a sample of AAV1 from the ATCC and isolated viral DNA from sucrose-pelleted virions. Southern analysis of the DNA digested with several restriction enzymes showed that many (but not all) restriction sites were conserved between AAV6 and AAV1 (data not shown). To further determine the relationship, a portion of AAV1 DNA containing a predicted variable region of the cap gene was amplified by PCR and sequenced with an internal primer. The sequence obtained was 96% identical to that of AAV6 (Fig. 5), resulting in one amino acid change out of the 139-amino-acid translated sequence (a lysine-to-glutamate substitution at position 531 of AAV6 VP1). The same DNA sequence of AAV6 was only 65% identical to those of AAV2 and AAV3B, resulting in 53 and 41 corresponding amino acid substitutions, respectively. These results suggest that AAV6 is closely related to AAV1, at least within this region of the genome. However, without additional sequence data from AAV1, we hesitate to conclude at this time that our AAV6 isolate is actually a member of the AAV1 serotype, especially since the first 507 nucleotides of AAV6 are identical in all but two positions to the AAV2 sequence (but not AAV3 or AAV4), suggesting that interserotype homologies can vary at different genomic positions. A comparison of AAV1 and AAV6 serologic cross-reactivity may be required to thoroughly resolve this issue.
FIG. 5.
Sequence comparison of AAV6 and AAV1. The variable region of the cap gene in AAV1 DNA was amplified by PCR and sequenced and is shown aligned with the corresponding sequence of AAV6. The vertical lines indicate nucleotide differences. Dots indicate an uncertainty. Numbers refer to the AAV6 genomic sequence in Fig. 2.
Vectors based on AAV3B and AAV6.
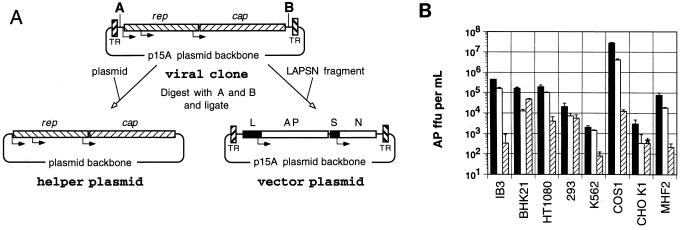
We constructed helper and vector plasmids for the production of vector stocks based on AAV3B and AAV6 (Fig. 6A). Vector plasmids pA3LAPSN and pA6LAPSN were derived from the original infectious clone plasmids pAAV3B and pAAV6, respectively, such that the rep and cap genes along with their promoters and polyadenylation signals were replaced by a fragment containing the human placental alkaline phosphatase gene under the control of the murine leukemia virus long terminal repeat promoter and the neomycin phosphotransferase gene under the control of the SV40 early promoter. Helper plasmids pRepCap3 and pRepCap6 were constructed by inserting the excised rep and cap gene fragments from pAAV3B and pAAV6, respectively, into a separate plasmid backbone. Analogous pA2LAPSN and pRepCap2 plasmids derived from AAV2 were also constructed.
FIG. 6.
Transduction by AAV vector serotypes. (A) Strategy for construction of helper and vector plasmids from the proviral clone. The viral clone was digested at the engineered restriction sites (shown as A and B) inside the terminal repeats (TR). The rep and cap genes were ligated into another plasmid backbone to create the helper plasmid, and the LAPSN fragment was inserted into the viral clone backbone inside the terminal repeats to create the vector plasmid. The positions of the murine leukemia virus long terminal repeat promoter (L), alkaline phosphatase gene (AP), SV40 early promoter (S), and neomycin phosphotransferase gene (N) are indicated. (B) AAV2-LAPSN (filled bars), AAV3-LAPSN (open bars), and AAV6-LAPSN (hatched bars) vector stocks were adjusted to contain 5 × 107 particles/μl and used to infect a panel of cell lines, and the numbers of cell foci expressing alkaline phosphatase (AP ffu) per ml of vector stock were determined (see Materials and Methods). Results are means ± standard deviations of three measurements. The asterisk indicates that the value was lower than the indicated amount (no stained cells were detected).
AAV-LAPSN vector stocks based on AAV2, AAV3B, and AAV6 were generated by cotransfection of adenovirus-infected 293 cells with the cognate helper and vector plasmids derived from each serotype. The titers of each of these stocks were approximately 5 × 1010 particles/ml, based on Southern analysis. A panel of cell lines was infected with each vector serotype at the same multiplicity of infection (particles/cell), and transduction was assayed by alkaline phosphatase expression 2 days later (Fig. 6B). A characteristic pattern of transduction efficiencies was observed for each vector serotype among the different cell lines tested. While the patterns for the AAV2 and AAV3B vectors were similar, with the AAV3B-LAPSN titers 1.4- to 13-fold lower than the AAV2-LAPSN titers, there was still significant variation between cell lines. The AAV6 vector transduction pattern was more distinct. For example, the AAV6-LAPSN titer on BHK21 cells was about 3-fold lower than that of AAV2-LAPSN and 4-fold higher than that of AAV3-LAPSN, while the AAV6-LAPSN titer was 300- to 2,300-fold lower than both of the other vector serotypes on COS1 cells. This variation in transduction efficiencies was not due to differences in reporter gene expression levels, since control experiments showed that each vector plasmid produced similar amounts of alkaline phosphatase after transfection into cells (data not shown). These results suggest that each vector serotype has a unique host range.
Because alkaline phosphatase assays could reflect transient transgene expression, neomycin phosphotransferase titers were determined for each vector serotype on HT-1080 cells, and the resulting titers were compared to those obtained by the alkaline phosphatase assay. Since the neo assay requires growth in G418 for 10 days, each colony should contain an integrated vector provirus (47, 48). The neo titers of each vector serotype were 15 to 30% of the alkaline phosphatase titers, indicating that vector genomes do integrate into host chromosomal DNA (data not shown).
Effects of anti-AAV2 serum on transduction.
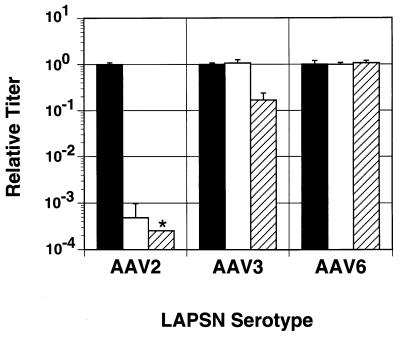
If the serotypes of AAV3B and AAV6 are different from that of AAV2, then they should not be neutralized by anti-AAV2 antibodies. We tested whether serum from rabbits exposed to AAV2 vectors could inactivate vectors based on each serotype by measuring the transduction efficiency after incubation with serum dilutions (Fig. 7) (24). At both 1:20 and 1:100 dilutions, pooled rabbit anti-AAV2 sera inactivated more than 99% of AAV2-LAPSN particles, as previously observed with other AAV2 vectors (24). Transduction by AAV6-LAPSN was not inhibited by this treatment, and transduction by AAV3-LAPSN was partially inhibited only at the 1:20 serum dilution. These results confirm that AAV3B and AAV6 are different serotypes from AAV2, with AAV6 being functionally more distinct.
FIG. 7.
Inactivation by anti-AAV2 serum. Vectors were left untreated (filled bars) or treated with 1:100 (open bars) or 1:20 (hatched bars) rabbit anti-AAV2 serum dilutions before infection of BHK21 cells. Two days after infection, the numbers of cell foci expressing alkaline phosphatase were determined by histochemical staining. The results (means ± standard deviations) are expressed as relative titers for each vector serotype after normalization of the untreated titers to 1.0 (mean ± standard deviation). The asterisk indicates a value lower than the indicated amount (no stained cells were detected).
DISCUSSION
We have sequenced two new infectious clones of AAV. As expected, our AAV3B sequence was very similar to that previously published for AAV3A (38). Both of these isolates were from the ATCC stock of AAV3, so the 16 nucleotide differences noted between the two clones may represent the genetic diversity present in the stock. AAV6 appears to be a variant of AAV1, based on limited sequence information from a variable region of the cap gene. Presumably the AAV6 isolate we cloned is a naturally occurring virus that at some point contaminated the laboratory adenovirus stock in which it was identified. This adenovirus sample was contaminated with AAV before being used in our laboratory, and we obtained the ATCC AAV1 isolate after characterizing AAV6, so we do not feel that the ATCC isolate was the original source. The differences between AAV1 and AAV6 would therefore reflect the genetic diversity of AAV1 isolates found in nature, assuming that AAV1 and AAV6 are ultimately shown to be the same serotype.
Difficulties have previously been encountered in attempts to isolate full-length infectious AAV clones in a single step, such that in some cases infectious viral genomes had to be reconstructed from cloned subgenomic fragments (14, 30, 38, 55). These cloning problems may have been due to genetic instability of the AAV terminal repeats, as parvoviral repeats often require propagation in recBC bacterial strains to avoid deletions (9). We have found that using lower-copy-number plasmids also decreases deletions in the AAV terminal repeats (data not shown), so our choice of the low-copy-number p15A plasmid replication origin (15) may have been important in allowing us to obtain full-length infectious clones.
The nucleotide sequences of AAV2, AAV3B, and AAV6 were 82% identical, with each sequence being equally divergent from the other two. The sequence of AAV4 was 75 to 78% identical to the other sequences. Regions where each isolate was the most divergent of the four DNA sequences were identified, and similar homology shifts were present in the protein sequences (Fig. 2 and 4), suggesting that the four AAVs evolved from a common ancestor. Important genetic elements were conserved among the viruses, many of which were previously noted in a comparison of AAV2 and AAV3A (38). These elements include the terminal repeat secondary structures, open reading frames, transcription start and stop sites, intron splice junctions, and the sp1 and sp1-like (GGT) binding sites previously identified upstream of the AAV2 p19 (43) and p40 (42) promoters.
The right terminal repeat of AAV6 was different from the left repeat, as were the AAV4 repeats. To determine if this was a consistent finding in AAV6 genomes, we sequenced the repeats of three other independently derived infectious AAV6 clones (data not shown). Two of the clones had the same terminal repeat sequences as the original AAV6 clone, but the third had left and right repeats that were identical to the right terminal repeat shown in Fig. 2. Recombination at genomic termini or replication from a circular intermediate with one repeat (60) could lead to exchange of left and right repeats, explaining this variability. Assuming that both repeats function in viral replication, it seems likely that all possible combinations would have been observed if enough independent clones were sequenced. Left and right repeat exchanges were previously observed when one AAV2 repeat was mutated and the progeny virions contained nearly all wild-type terminal repeats (8).
The Rep binding sites of AAV3B and the right terminal repeat of AAV6 differ from those of AAV2 (13, 49) at 4 and 3 positions, respectively. The AAV6 right terminal repeat also had one base change at the terminal resolution site and a CAGAG sequence in the loop of the B region (nucleotides 4608 to 4612) instead of the CAAAG sequence proposed to play a role in Rep binding (49). A nucleotide change in one of the loop regions appears in AAV4 as well. The significance of these differences is not clear, but they may affect replication rates or site-specific integration, the latter of which is dependent on the presence of a Rep binding site in the chromosomal integration locus (31).
The predicted amino acid sequences of the capsid proteins include several regions with significant variation between serotypes (Fig. 4B). Four domains in particular exhibit high variation, as indicated in Fig. 4B. These regions presumably play a role in serotype-specific functions such as determining antigen specificity and binding to host cell receptors. A separate domain at amino acids 21 to 42 of AAV2 is significantly different from those of the three other serotypes, which vary less in this region.
Vectors constructed from AAV2, AAV3B, and AAV6 had variable transduction rates on a panel of different cell lines. Transduction depends both on viral entry into the cell and conversion of single-stranded vector genomes into transcriptionally active forms. The events that create a transcriptionally active molecule may include integration into host chromosomal DNA (47) or conversion to double-stranded episomal vector genomes (17, 20). While it is possible that each AAV serotype interacts with specific host intracellular factors in ways that affect the conversion of vector genomes to transcriptionally active forms, it seems more likely that the transduction rates of different cell lines are due to distinct cell surface receptor interactions. Viral entry into the host cell is presumably mediated by capsid binding to a specific receptor protein, and the AAV2 receptor appears to be different from that of AAV1 and AAV3 (37). The unique transducing properties of each vector serotype can be explained by the use of distinct receptor molecules present at different levels in each cell line. A similar phenomenon occurs with retroviral vectors, where the host range of different vector pseudotypes is largely determined by the expression level of the particular receptor used (36).
Rabbit anti-AAV2 serum partially inhibited transduction by AAV3B vectors but had no effect on transduction by AAV6 vectors, suggesting that AAV2 is serologically more similar to AAV3B than to AAV6. Given the similar sequences of AAV6 and AAV1, these results are consistent with prior serological data showing that AAV2 and AAV3 are more closely related than AAV2 and AAV1 (40). The transduction patterns shown in Fig. 6 also suggest that the AAV2 host range is more like that of AAV3B than that of AAV6. These differences are likely due to specific amino acid changes on the exposed surface of the capsid protein and are not apparent as increased divergence at the nucleotide sequence level.
Our results suggest that vectors based on AAV3B and AAV6 will have potential advantages over AAV2 vectors. The different host ranges of these new vector serotypes may allow the transduction of cell types and tissues that are difficult or impossible to transduce with AAV2 vectors. Perhaps more importantly, these new vectors will make transduction possible in the presence of anti-AAV2 antibodies. This could be essential in treatments requiring multiple vector doses, as animal studies have shown that neutralizing antibodies can completely prevent transduction during vector readministration (24, 59). Similarly, new vector serotypes might also overcome the problem posed by the high prevalence of neutralizing antibodies against AAV2 in human populations (7, 40). As our understanding of the sequences responsible for receptor binding and antibody generation improves, desirable characteristics from different vector serotypes could be combined into hybrid vectors, allowing the transduction of specific cell types in the presence of diverse antibody profiles.
ACKNOWLEDGMENTS
We thank Roli Hirata and Jaclynn Mac for expert technical assistance and Naoki Inoue for plasmid constructs.
This work was supported by grants from the American Society of Hematology, the Cystic Fibrosis Foundation, the March of Dimes Birth Defects Foundation, and the Heart, Lung, and Blood Institute of the U.S. National Institutes of Health.
REFERENCES
- 1.Afione S A, Conrad C K, Kearns W G, Chunduru S, Adams R, Reynolds T C, Guggino W B, Cutting G R, Carter B J, Flotte T R. In vivo model of adeno-associated virus vector persistence and rescue. J Virol. 1996;70:3235–3241. doi: 10.1128/jvi.70.5.3235-3241.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander I E, Russell D W, Spence A M, Miller A D. Effects of gamma irradiation on the transduction of dividing and nondividing cells in brain and muscle of rats by adeno-associated virus vectors. Hum Gene Ther. 1996;7:841–850. doi: 10.1089/hum.1996.7.7-841. [DOI] [PubMed] [Google Scholar]
- 3.Atchison R W, Casto B C, Hammon W M. Adenovirus-associated defective virus particles. Science. 1965;149:754–756. doi: 10.1126/science.149.3685.754. [DOI] [PubMed] [Google Scholar]
- 4.Bantel Schaal U, zur Hausen H. Characterization of the DNA of a defective human parvovirus isolated from a genital site. Virology. 1984;134:52–63. doi: 10.1016/0042-6822(84)90271-x. [DOI] [PubMed] [Google Scholar]
- 5.Bertran J, Miller J L, Yang Y, Fenimore-Justman A, Rueda F, Vanin E F, Nienhuis A W. Recombinant adeno-associated virus-mediated high-efficiency, transient expression of the murine cationic amino acid transporter (ecotropic retroviral receptor) permits stable transduction of human HeLa cells by ecotropic retroviral vectors. J Virol. 1996;70:6759–6766. doi: 10.1128/jvi.70.10.6759-6766.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blacklow N R, Hoggan M D, Kapikian A Z, Austin J B, Rowe W P. Epidemiology of adenovirus-associated virus infection in a nursery population. Am J Epidemiol. 1968;88:368–378. doi: 10.1093/oxfordjournals.aje.a120897. [DOI] [PubMed] [Google Scholar]
- 7.Blacklow N R, Hoggan M D, Rowe W P. Serologic evidence for human infection with adenovirus-associated viruses. J Natl Cancer Inst. 1968;40:319–327. [PubMed] [Google Scholar]
- 8.Bohenzky R A, Berns K I. Interactions between the termini of adeno-associated virus DNA. J Mol Biol. 1989;206:91–100. doi: 10.1016/0022-2836(89)90526-3. [DOI] [PubMed] [Google Scholar]
- 9.Boissy R, Astell C R. An Escherichia coli recBC sbcB recF host permits the deletion-resistant propagation of plasmid clones containing the 5′-terminal palindrome of minute virus of mice. Gene. 1985;35:179–185. doi: 10.1016/0378-1119(85)90170-2. [DOI] [PubMed] [Google Scholar]
- 10.Carter B J, Trempe J P, Mendelson E. Adeno-associated virus gene expression and regulation. In: Tijssen P, editor. Handbook of parvoviruses. Vol. 1. Boca Raton, Fla: CRC Press; 1990. pp. 227–254. [Google Scholar]
- 11.Cassinotti P, Weitz M, Tratschin J D. Organization of the adeno-associated virus (AAV) capsid gene: mapping of a minor spliced mRNA coding for virus capsid protein 1. Virology. 1988;167:176–184. [PubMed] [Google Scholar]
- 12.Chang A C, Cohen S N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978;134:1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiorini J A, Wiener S M, Owens R A, Kyostio S R, Kotin R M, Safer B. Sequence requirements for stable binding and function of Rep68 on the adeno-associated virus type 2 inverted terminal repeats. J Virol. 1994;68:7448–7457. doi: 10.1128/jvi.68.11.7448-7457.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiorini J A, Yang L, Liu Y, Safer B, Kotin R M. Cloning of adeno-associated virus type 4 (AAV4) and generation of recombinant AAV4 particles. J Virol. 1997;71:6823–6833. doi: 10.1128/jvi.71.9.6823-6833.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cozzarelli N R, Kelly R B, Kornberg A. A minute circular DNA from Escherichia coli 15. Proc Natl Acad Sci USA. 1968;60:992–999. doi: 10.1073/pnas.60.3.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DuBridge R B, Tang P, Hsia H C, Leong P M, Miller J H, Calos M P. Analysis of mutation in human cells by using an Epstein-Barr virus shuttle system. Mol Cell Biol. 1987;7:379–387. doi: 10.1128/mcb.7.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrari F K, Samulski T, Shenk T, Samulski R J. Second-strand synthesis is a rate-limiting step for efficient transduction by recombinant adeno-associated virus vectors. J Virol. 1996;70:3227–3234. doi: 10.1128/jvi.70.5.3227-3234.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fields Berry S C, Halliday A L, Cepko C L. A recombinant retrovirus encoding alkaline phosphatase confirms clonal boundary assignment in lineage analysis of murine retina. Proc Natl Acad Sci USA. 1992;89:693–697. doi: 10.1073/pnas.89.2.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fife K H, Berns K I, Murray K. Structure and nucleotide sequence of the terminal regions of adeno-associated virus DNA. Virology. 1997;78:475–487. doi: 10.1016/0042-6822(77)90124-6. [DOI] [PubMed] [Google Scholar]
- 20.Fisher K J, Gao G P, Weitzman M D, DeMatteo R, Burda J F, Wilson J M. Transduction with recombinant adeno-associated virus for gene therapy is limited by leading-strand synthesis. J Virol. 1996;70:520–532. doi: 10.1128/jvi.70.1.520-532.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Georg Fries B, Biederlack S, Wolf J, zur Hausen H. Analysis of proteins, helper dependence, and seroepidemiology of a new human parvovirus. Virology. 1984;134:64–71. doi: 10.1016/0042-6822(84)90272-1. [DOI] [PubMed] [Google Scholar]
- 22.Graham F L, Smiley J, Russell W C, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977;36:59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- 23.Halbert C L, Alexander I E, Wolgamot G M, Miller A D. Adeno-associated virus vectors transduce primary cells much less efficiently than immortalized cells. J Virol. 1995;69:1473–1479. doi: 10.1128/jvi.69.3.1473-1479.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Halbert C L, Standaert T A, Aitken M L, Alexander I E, Russell D W, Miller A D. Transduction by adeno-associated virus vectors in the rabbit airway: efficiency, persistence, and readministration. J Virol. 1997;71:5932–5941. doi: 10.1128/jvi.71.8.5932-5941.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hargrove P W, Vanin E F, Kurtzman G J, Nienhuis A W. High-level globin gene expression mediated by a recombinant adeno-associated virus genome that contains the 3′ gamma globin gene regulatory element and integrates as tandem copies in erythroid cells. Blood. 1997;89:2167–2175. [PubMed] [Google Scholar]
- 26.Henikoff S, Eghtedarzadeh M K. Conserved arrangement of nested genes at the Drosophila Gart locus. Genetics. 1987;117:711–725. doi: 10.1093/genetics/117.4.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoggan M D, Blacklow N R, Rowe W P. Studies of small DNA viruses found in various adenovirus preparations: physical, biological, and immunological characteristics. Proc Natl Acad Sci USA. 1966;55:1467–1474. doi: 10.1073/pnas.55.6.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hörer M, Weger S, Butz K, Hoppe-Seyler F, Geisen C, Kleinschmidt J A. Mutational analysis of adeno-associated virus Rep protein-mediated inhibition of heterologous and homologous promoters. J Virol. 1995;69:5485–5496. doi: 10.1128/jvi.69.9.5485-5496.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kunkel T A, Roberts J D, Zakour R A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- 30.Laughlin C A, Tratschin J D, Coon H, Carter B J. Cloning of infectious adeno-associated virus genomes in bacterial plasmids. Gene. 1983;23:65–73. doi: 10.1016/0378-1119(83)90217-2. [DOI] [PubMed] [Google Scholar]
- 31.Linden R M, Winocour E, Berns K I. The recombination signals for adeno-associated virus site-specific integration. Proc Natl Acad Sci USA. 1996;93:7966–7972. doi: 10.1073/pnas.93.15.7966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lozzio C B, Lozzio B B. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood. 1975;45:321–334. [PubMed] [Google Scholar]
- 33.Macpherson I, Stoker M. Polyoma transformation of hamster cell clones—an investigation of genetic factors affecting cell competence. Virology. 1962;16:147–151. doi: 10.1016/0042-6822(62)90290-8. [DOI] [PubMed] [Google Scholar]
- 34.Malik P, McQuiston S A, Yu X J, Pepper K A, Krall W J, Podsakoff G M, Kurtzman G J, Kohn D B. Recombinant adeno-associated virus mediates a high level of gene transfer but less efficient integration in the K562 human hematopoietic cell line. J Virol. 1997;71:1776–1783. doi: 10.1128/jvi.71.3.1776-1783.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Messing J, Crea R, Seeburg P H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981;9:309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller A D, Wolgamot G. Murine retroviruses use at least six different receptors for entry into Mus dunni cells. J Virol. 1997;71:4531–4535. doi: 10.1128/jvi.71.6.4531-4535.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mizukami H, Young N S, Brown K E. Adeno-associated virus type 2 binds to a 150-kilodalton cell membrane glycoprotein. Virology. 1996;217:124–130. doi: 10.1006/viro.1996.0099. [DOI] [PubMed] [Google Scholar]
- 38.Muramatsu S, Mizukami H, Young N S, Brown K E. Nucleotide sequencing and generation of an infectious clone of adeno-associated virus 3. Virology. 1996;221:208–217. doi: 10.1006/viro.1996.0367. [DOI] [PubMed] [Google Scholar]
- 39.Muzyczka N. Use of adeno-associated virus as a general transduction vector for mammalian cells. Curr Top Microbiol Immunol. 1992;158:97–129. doi: 10.1007/978-3-642-75608-5_5. [DOI] [PubMed] [Google Scholar]
- 40.Parks W P, Boucher D W, Melnick J L, Taber L H, Yow M D. Seroepidemiological and ecological studies of the adenovirus-associated satellite viruses. Infect Immun. 1970;2:716–722. doi: 10.1128/iai.2.6.716-722.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parks W P, Melnick J L, Rongey R, Mayor H D. Physical assay and growth cycle studies of a defective adeno-satellite virus. J Virol. 1967;1:171–180. doi: 10.1128/jvi.1.1.171-180.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pereira D J, Muzyczka N. The adeno-associated virus type 2 p40 promoter requires a proximal Sp1 interaction and a p19 CArG-like element to facilitate Rep transactivation. J Virol. 1997;71:4300–4309. doi: 10.1128/jvi.71.6.4300-4309.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pereira D J, Muzyczka N. The cellular transcription factor SP1 and an unknown cellular protein are required to mediate Rep protein activation of the adeno-associated virus p19 promoter. J Virol. 1997;71:1747–1756. doi: 10.1128/jvi.71.3.1747-1756.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ponnazhagan S, Wang X S, Woody M J, Luo F, Kang L Y, Nallari M L, Munshi N C, Zhou S Z, Srivastava A. Differential expression in human cells from the p6 promoter of human parvovirus B19 following plasmid transfection and recombinant adeno-associated virus 2 (AAV) infection: human megakaryocytic leukaemia cells are non-permissive for AAV infection. J Gen Virol. 1996;77:1111–1122. doi: 10.1099/0022-1317-77-6-1111. [DOI] [PubMed] [Google Scholar]
- 45.Rasheed S, Nelson Rees W A, Toth E M, Arnstein P, Gardner M B. Characterization of a newly derived human sarcoma cell line (HT-1080) Cancer. 1974;33:1027–1033. doi: 10.1002/1097-0142(197404)33:4<1027::aid-cncr2820330419>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 46.Ruffing M, Heid H, Kleinschmidt J A. Mutations in the carboxy terminus of adeno-associated virus 2 capsid proteins affect viral infectivity: lack of an RGD integrin-binding motif. J Gen Virol. 1994;75:3385–3392. doi: 10.1099/0022-1317-75-12-3385. [DOI] [PubMed] [Google Scholar]
- 47.Russell D W, Miller A D, Alexander I E. Adeno-associated virus vectors preferentially transduce cells in S phase. Proc Natl Acad Sci USA. 1994;91:8915–8919. doi: 10.1073/pnas.91.19.8915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rutledge E A, Russell D W. Adeno-associated virus vector integration junctions. J Virol. 1997;71:8429–8436. doi: 10.1128/jvi.71.11.8429-8436.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ryan J H, Zolotukhin S, Muzyczka N. Sequence requirements for binding of Rep68 to the adeno-associated virus terminal repeats. J Virol. 1996;70:1542–1553. doi: 10.1128/jvi.70.3.1542-1553.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 51.Samulski R J, Chang L S, Shenk T. Helper-free stocks of recombinant adeno-associated viruses: normal integration does not require viral gene expression. J Virol. 1989;63:3822–3828. doi: 10.1128/jvi.63.9.3822-3828.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Samulski R J, Chang L S, Shenk T. A recombinant plasmid from which an infectious adeno-associated virus genome can be excised in vitro and its use to study viral replication. J Virol. 1987;61:3096–3101. doi: 10.1128/jvi.61.10.3096-3101.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saraste M, Sibbald P R, Wittinghofer A. The P-loop—a common motif in ATP- and GTP-binding proteins. Trends Biochem Sci. 1990;15:430–434. doi: 10.1016/0968-0004(90)90281-f. [DOI] [PubMed] [Google Scholar]
- 55.Senapathy P, Carter B J. Molecular cloning of adeno-associated virus variant genomes and generation of infectious virus by recombination in mammalian cells. J Biol Chem. 1984;259:4661–4666. [PubMed] [Google Scholar]
- 56.Snyder R O, Im D S, Ni T, Xiao X, Samulski R J, Muzyczka N. Features of the adeno-associated virus origin involved in substrate recognition by the viral Rep protein. J Virol. 1993;67:6096–6104. doi: 10.1128/jvi.67.10.6096-6104.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Srivastava A, Lusby E W, Berns K I. Nucleotide sequence and organization of the adeno-associated virus 2 genome. J Virol. 1983;45:555–564. doi: 10.1128/jvi.45.2.555-564.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wonderling R S, Kyöstiö S R M, Owens R A. A maltose-binding protein/adeno-associated virus Rep68 fusion protein has DNA-RNA helicase and ATPase activities. J Virol. 1995;69:3542–3548. doi: 10.1128/jvi.69.6.3542-3548.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xiao X, Li J, Samulski R J. Efficient long-term gene transfer into muscle tissue of immunocompetent mice by adeno-associated virus vector. J Virol. 1996;70:8098–8108. doi: 10.1128/jvi.70.11.8098-8108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xiao X, Xiao W, Li J, Samulski R J. A novel 165-base-pair terminal repeat sequence is the sole cis requirement for the adeno-associated virus life cycle. J Virol. 1997;71:941–948. doi: 10.1128/jvi.71.2.941-948.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zeitlin P L, Lu L, Rhim J, Cutting G, Stetten G, Kieffer K A, Craig R, Guggino W B. A cystic fibrosis bronchial epithelial cell line: immortalization by adeno-12-SV40 infection. Am J Respir Cell Mol Biol. 1991;4:313–319. doi: 10.1165/ajrcmb/4.4.313. [DOI] [PubMed] [Google Scholar]