Comparative Analyses of Intracellularly Expressed Antisense RNAs as Inhibitors of Human Immunodeficiency Virus Type 1 Replication (original) (raw)
Abstract
The antiviral activities of intracellularly expressed antisense RNAs complementary to the human immunodeficiency virus type 1 (HIV-1) pol, vif, and env genes and the 3′ long terminal repeat (LTR) sequence were evaluated in this comparative study. Retroviral vectors expressing the antisense RNAs as part of the Moloney murine leukemia virus LTR promoter-directed retroviral transcript were constructed. The CD4+ T-cell line CEM-SS was transduced with retroviral constructs, and Northern blot analyses showed high steady-state antisense RNA expression levels. The most efficient inhibition of HIV-1 replication was observed with the env antisense RNA, followed by the pol complementary sequence, leading to 2- to 3-log10 reductions in p24 antigen production even at high inoculation doses (4 × 104 50% tissue culture infective doses) of the HIV-1 strain HXB3. The strong antiviral effect correlated with a reduction of HIV-1 steady-state RNA levels, and with intracellular Tat protein production, suggesting that antisense transcripts act at an early step of HIV-1 replication. A lower steady-state antisense RNA level was detected in transduced primary CD4+ lymphocytes than in CEM-SS cells. Nevertheless, replication of the HIV-1 JR-CSF isolate was reduced with both the pol and env antisense RNA. Intracellularly expressed antisense sequences demonstrated more pronounced antiviral efficacy than the _trans_-dominant RevM10 protein, making these antisense RNAs a promising gene therapy strategy for HIV-1.
A number of gene therapy approaches have been explored to suppress human immunodeficiency virus type 1 (HIV-1) replication, including the use of _trans_-dominant proteins (3, 33), single-chain antibodies (15), antisense RNAs (4, 6, 10, 11, 18, 25, 26), RNA decoys (14, 32), and ribozymes (23, 39). The _trans_-dominant HIV-1 protein RevM10 was first evaluated in a clinical trial using genetically modified peripheral blood lymphocytes (PBLs) (38). Recently a ribozyme approach (13) and the use of a _trans_-dominant Rev combined with an antisense _trans_-acting responsive element (TAR)-based approach (21) have received NIH Recombinant Advisory Committee and Food and Drug Administration approval.
Intracellular expression of antisense RNAs is an attractive alternative gene therapy approach for HIV-1 disease. Antisense RNAs are highly specific and efficient inhibitors and have been described for both prokaryotic and eukaryotic systems (12, 20). Viral replication has been successfully inhibited by addition of in vitro-synthesized antisense oligonucleotides or by intracellular expression of antisense RNAs (9, 10). Inhibition of HIV-1 replication has been demonstrated for antisense RNAs targeted against several viral regulatory (4, 10, 11, 29, 30) and structural (6, 9, 18, 26) gene products. A few reports described long antisense sequences either expressed intracellularly by using retroviral vectors (6, 9, 26) or expressed by antibody-targeted liposomal delivery (25). The variable inhibition levels observed in those studies may reflect differences in antisense RNA expression levels or in secondary- and tertiary-RNA structures, which can affect the hybridization kinetics between two complementary RNAs (31), influencing the biological activity of these molecules.
In a previous study (36) we demonstrated that retrovirally expressed RNA complementary to the gag gene sequence (6) of HIV-1 is a very potent inhibitor of viral replication, even at high inoculation doses. In an extension of that initial study, the antiviral activities of sequences complementary to the pol, vif, and env genes as well as the 3′ long terminal repeat (LTR) were compared in HIV-1 infection experiments using a human CD4+ T-cell line (CEM-SS) and primary CD4+ T lymphocytes (PBLs). Retroviral vectors expressing chimeric RNAs containing 1,100- to 1,400-nucleotide (nt) complementary HIV-1 sequences were constructed. The most pronounced inhibition of HIV-1 replication was observed with an antisense sequence complementary to the HIV-1 env gene both in the CEM-SS cell line and in PBLs. This strong antiviral effect was further demonstrated in high-inoculation-dose infection experiments where reduction of the HIV-1 mRNAs correlated with low levels of Gag and Tat protein production, indicating that antisense RNA acts early during HIV-1 replication. Comparing the anti-HIV-1 efficacies of the antisense RNAs to that of the well-documented (3, 7, 17, 22) _trans_-dominant RevM10 protein demonstrated increased potency of antisense-RNA-mediated inhibition of HIV-1 replication.
MATERIALS AND METHODS
Retroviral vector construction.
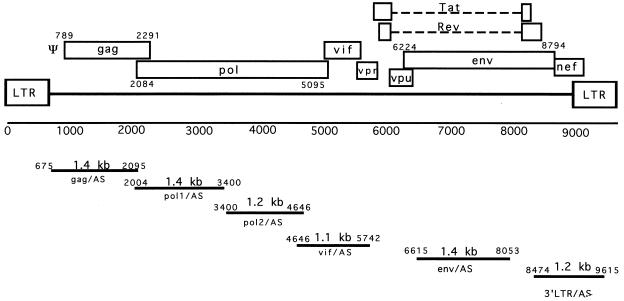
DNA restriction endonuclease fragments were derived from the HIV-1 HXB2 proviral genome as shown in Fig. 1. The 1,400-bp _Apa_I-_Pfl_MI fragment and the 1,246-bp _Pfl_MI-_Eco_RI fragment from the pol gene, the 1,100-bp _Eco_RI-_Eco_RI fragment from the vif gene, the 1,438-bp _Apa_LI-_Bsm_I fragment from the env gene, and the 1,260-bp _Bam_HI-_Hin_dIII fragment from the HIV 3′ LTR were cloned into the _Xho_I site of the pLN (19) retroviral vector in reverse orientation (Fig. 2A) to generate the pLN-pol1/AS, pLN-pol2/AS, pLN-vif/AS, pLN-env/AS, and pLN-3′LTR/AS vectors. The retroviral vector pLN-pol12/AS with the full-length pol sequence was constructed by inserting the 2,642-bp _Apa_I-_Eco_RI fragment into the pLN vector in reverse orientation. For the sense control vectors pLN-pol1/S and pLN-pol12/S, the 1,400-bp pol1 fragment and the 2,642-bp pol12 fragment were cloned in the sense orientation into the pLN vector. The pLN-790pol/AS vector was constructed by inserting the 790-bp _Bgl_II-_Nsi_I subfragment of the pol gene into the _Xho_I site of the pLN vector. Retroviral vectors (pLLyt2-pol1/AS, pLLyt2-pol1/S, pLLyt2-env/AS, and pLLyt2-env/S) were constructed by replacing the neo gene with the truncated mouse CD8 (Lyt2) cell surface marker (8) and used for the primary T-cell HIV infection experiments.
FIG. 1.
Schematic representation of the HIV-1 genome. The nucleotide positions, sizes, and positions of the restriction fragments used for antisense-vector construction are indicated.
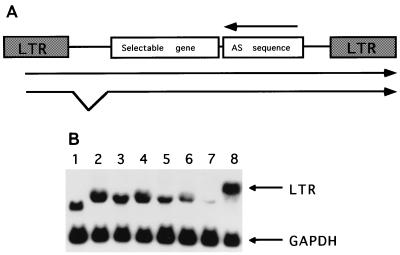
FIG. 2.
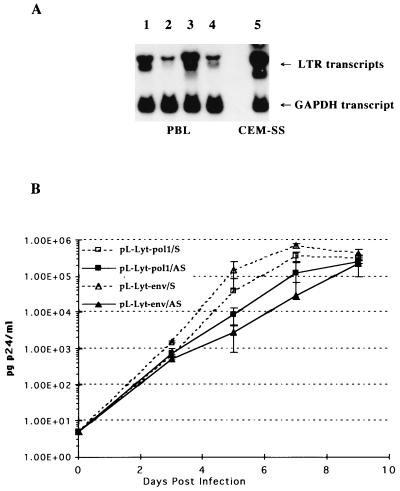
(A) Structure of the retroviral vectors containing the antisense sequences. The neomycin phosphotransferase gene and the Lyt2 gene were used as selectable marker genes. The antisense sequence together with the marker gene was expressed from the MoMLV LTR promoter. The arrow indicates the antisense orientation of the inserted HIV-1 sequences. (B) Northern blot analyses of antisense RNA expression in transduced CEM-SS cells. The recombinant transcripts carrying the antisense sequences were detected with a _neo_-specific probe. The lower panel shows the same blot hybridized with a GAPDH-specific probe as a internal standard. Lane 1, pLN vector; lane 2, pLN-gag/AS; lane 3, pLN-pol1/AS; lane 4, pLN-pol2/AS; lane 5, pLN-vif/AS; lane 6, pLN-env/AS; lane 7, pLN-3′LTR/AS; lane 8, pLN-pol12/AS.
Retroviral vector packaging.
Retroviral plasmids were cotransfected into ProGag cells (27) by the calcium phosphate transfection method. The supernatant from the transfected ProGag cells was used to transduce the amphotropic cell line ProPak-A (27) by centrifugation-enhanced retroviral gene transfer as described previously (8). Retroviral endpoint titers were determined on NIH 3T3 cells after drug selection (800 μg of G418 per ml), and the transduction efficacy of the Lyt2 vectors (8) was measured by fluorescence-activated cell sorter (FACS) analysis.
Transduction of the human T-cell line CEM-SS.
The human CD4+ T-cell line CEM-SS was transduced with ProPak-A-derived amphotropic supernatants by centrifugation-enhanced retroviral gene transfer and selected on G418 (800 μg/ml). The steady-state expression levels of the different antisense vectors were determined by analyzing total cellular RNA isolated from G418-resistant CEM-SS cells by Northern blotting.
Isolation and transduction of human PBLs.
Peripheral blood mononuclear cells were isolated from healthy donor buffy coats by density gradient centrifugation in Isoprep (Robbins Scientific Corporation, Sunnyvale, Calif.). CD4+ cells were enriched as described previously (36) by depletion with biotinylated anti-CD8 and anti-CD19 antibodies followed by streptavidin-conjugated magnetic beads (Dynabeads M-280; Dynal A.S., Oslo, Norway) and were cultured in Iscove’s modified Dulbecco minimal essential medium. Stimulated, CD4-enriched PBLs (2 × 106 cells) were transduced by centrifugation-enhanced retroviral gene transfer in the presence of Polybrene (8 μg/ml) (36). Lyt2 expression was analyzed 48 h after transduction by flow cytometry with anti-CD8 phycoerythrin-conjugated monoclonal antibodies. PBLs were further expanded and reactivated (36), and the CD4+ Lyt2+ cells were isolated by FACS (Vantage cell sorter; Becton Dickinson). After cell sorting, greater than 90% of the cell population was CD4 and Lyt2 positive.
HIV-1 infections.
A total of 106 transduced CEM-SS cells were inoculated in a 1-ml volume with various doses (4 × 102 to 4 × 105 50% tissue culture infective doses [TCID50]/ml) of HIV-1 (HXB3 or SF2) as described previously (36). The TCID50 of the HIV-1 isolates was defined by endpoint titration on CEM-SS cells. CD4 receptor molecule expression on CEM-SS cells was determined by FACS analyses with an anti-CD4 monoclonal antibody (Becton Dickinson). Transduced Lyt2+ CD4+ selected primary human T cells (5 × 104) were inoculated with 600 TCID50 of HIV-1 JR-CSF per ml in quadruplicate. The JR-CSF HIV-1 strain is a cloned patient isolate which has been amplified in human PBLs to generate our viral stock. In the PBL infection experiment, half of the culture supernatant was replaced daily for 9 days after inoculation and HIV-1 replication was measured by determination of the p24Gag antigen concentration in the culture supernatants by using an enzyme-linked immunosorbent assay (ELISA) kit (DuPont-NEN).
Detection of intracellular Tat and p24Gag antigens.
Transduced CEM-SS cells expressing RevM10 and antisense HIV-1 sequences were inoculated with 105 TCID50 of HIV-1 HXB3 per 106 cells per ml. At days 4, 6, and 8, cells were removed from the culture, washed and resuspended in cold phosphate-buffered saline, and fixed in ice-cold methanol for 30 min. The fixed cells were stained with a fluorescein isothiocyanate-conjugated anti-p24 monoclonal antibody (KC57; Coulter) for intracellular p24 detection or with a mouse anti-Tat immunoglobulin G1 antibody (Repligen) for intracellular Tat detection as described earlier (28). The samples were analyzed with a Becton-Dickinson FACScan.
Detection of antisense RNA in cells.
Total cellular RNA from CEM-SS cells and from activated PBLs was extracted with RNAzol (Cinna/Biotecx). RNA (10 μg per lane) was fractionated on 1.2% agarose–formaldehyde gels, transferred to Hybond N membranes (Amersham), and hybridized in Rapid-hyb buffer (Amersham). Oligonucleotides (100 ng) were radiolabeled with terminal transferase (Boehringer Mannheim), using [α-32P]dATP at a specific activity of 3 × 108 cpm/μg. DNA fragments were labeled with a random-priming kit (Boehringer Mannheim). The membranes were hybridized with the radiolabeled probe (5 × 106 cpm/ml) at 65°C for 1 h, washed with 1 × SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–0.1% sodium dodecyl sulfate at 65°C, and exposed to X-ray film or analyzed on a PhosphorImager (Molecular Dynamics).
RESULTS
Construction of antisense RNA-expressing retroviral vectors.
Antisense sequences longer than 1,000 nt were most effective in our previous Ψ-gag antisense RNA study (36). Therefore, we constructed a series of retroviral vectors expressing 1,100- to 1,400-nt sequences complementary to the pol, vif, and env genes and the 3′ LTR region of HIV-1 (Fig. 1) to evaluate their antiviral efficacies. To maintain similar fragment sizes, we divided the HIV-1 pol gene into two subfragments: the pol1 sequence, corresponding to the 5′ half of the gene, and the pol2 sequence, corresponding to the 3′ half of the gene. Figure 2A shows the general structure of antisense RNA-expressing retroviral vectors. We used the pLN parental vector (19) with the neomycin phosphotransferase gene as a selectable marker to generate the pLN-pol1/AS, pLN-pol2/AS, pLN-vif/AS, pLN-env/AS, and pLN-3′LTR/AS antisense vectors. Amphotropic retroviral vectors were generated in the ProPak-A packaging cell line (27). The Neor endpoints ranged from 2 × 105 to 4 × 106 CFU/ml, with the exception of the pLN-3′LTR vector, which had a titer of 1 × 104 CFU/ml. The CD4+ T-cell line CEM-SS was transduced with the amphotropic viral supernatants, and stable, drug-resistant cell populations were established. The steady-state RNA expression levels of the different antisense constructs were determined by Northern blot analyses. Comparable expression levels were observed, with the exception of the pLN-3′LTR/AS vector, which expressed a ∼20-fold-lower level of the recombinant transcript (Fig. 2B).
Inhibition of HIV-1 replication in CEM-SS cells.
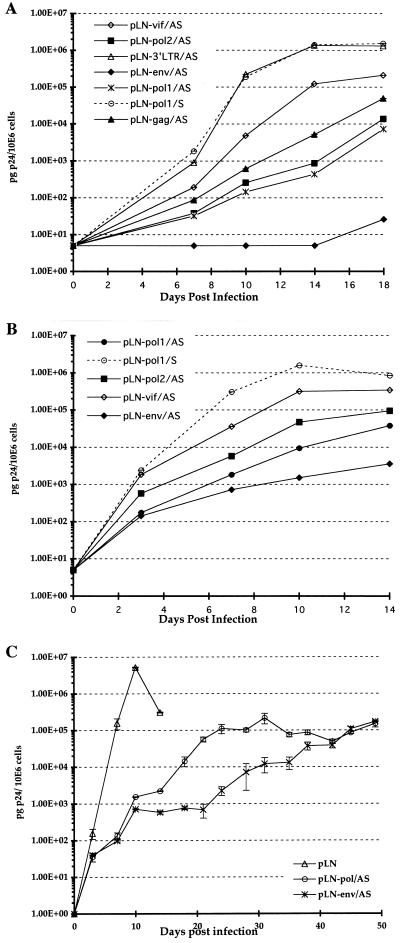
To compare the efficacies of the antisense sequences, transduced CEM-SS cells expressing complementary transcripts were inoculated with 4 × 102 TCID50 of HIV-1 HXB3 per ml. HIV-1 replication was monitored by measuring p24 antigen levels in the culture supernatant by ELISA. As negative controls, cells transduced with a vector containing the pol sequence in the sense orientation (pLN-pol/S) were used. The CD4 expression and the growth rate of the transduced cells expressing the different antisense or sense vector constructs were similar to those of the untransduced control CEM-SS cells (data not shown). Figure 3A shows the relative efficacies of the different antisense sequences, including the previously published Ψ-gag antisense sequence (36), at a low HIV-1 inoculation dose (4 × 102 TCID50/ml). CEM-SS cells expressing env antisense RNA showed almost complete suppression of HIV-1 replication, releasing 50 pg of p24/106 cells at day 18 postinoculation. We observed a 3-log10 reduction of p24 antigen production with the pol1 and pol2 antisense sequences, a 2-log10 reduction with the Ψ-gag antisense sequence (36), and a 1-log10 reduction with the vif antisense sequence (Fig. 3A). The 3′ LTR antisense construct was indistinguishable from the control vector, which might be explained by the low expression level of antisense transcript observed by Northern blotting (Fig. 2B). In the following experiment, we increased the HIV-1 inoculation dose 100-fold to 4 × 104 TCID50/ml and tested only the pol1, pol2, vif, and env antisense constructs (Fig. 3B). Overall, the onset of HIV-1 replication was much earlier and the replication kinetics were faster than in the experiment using the lower multiplicity of infection (MOI). At day 10, control CEM-SS cells (transduced with pLN-pol1/S) released high levels of p24 antigen into the culture supernatants (2 × 106 pg of p24/106 cells). However, at this time point HIV-1 replication was substantially inhibited in all CEM-SS cultures expressing antisense RNA relative to control cultures. Although HIV-1 replication was higher than in the previous experiment, the antisense env RNA was again the most potent inhibitor (3-log10 reduction), followed by pol1 and pol2 (2-log10 reduction), and the antisense vif sequence was the least potent antiviral inhibitor (1-log10 reduction). We have monitored HIV-1 infection in experiments for up to 50 days postinoculation in CEM-SS cells expressing the pol and env antisense RNAs (Fig. 3C). In control cells, viral replication peaked at day 14; the cells died and HIV-1 replication was not further analyzed in this population. We observed a gradual increase of p24Gag production in the cell populations expressing pol and env antisense RNA, and the cells remained viable. Even after 50 days, p24Gag levels in antisense-RNA-expressing cells were 2 log10 units lower than the peak p24 levels measured in the control cell population at day 14. This experiment indicates that although pol and env antisense RNA is not able to inhibit viral replication completely, it slows the spread of viral infectivity substantially. We obtained similar results when CEM-SS cells expressing antisense pol and env RNA were infected with the less cytopathic SF2 HIV-1 strain at 8 × 103 TCID50/ml and infection was monitored for 25 days (data not shown).
FIG. 3.
Inhibition of HIV-1 replication in transduced CEM-SS cells. (A) CEM-SS cell populations (106 cells/ml) were inoculated with 4 × 102 TCID50 of HIV-1 strain HXB3 per ml. B. Infection of transduced CEM-SS cell populations with a high HIV-1 inoculation dose, 4 × 104 TCID50/ml. The culture supernatants were tested for p24 antigen production by ELISA. Experiments were done in duplicate. (C) CEM-SS cell populations (106 cells/ml) were inoculated with 4 × 103 TCID50 of HIV-1 strain HXB3 per ml and HIV-1 replication was monitored for 50 days.
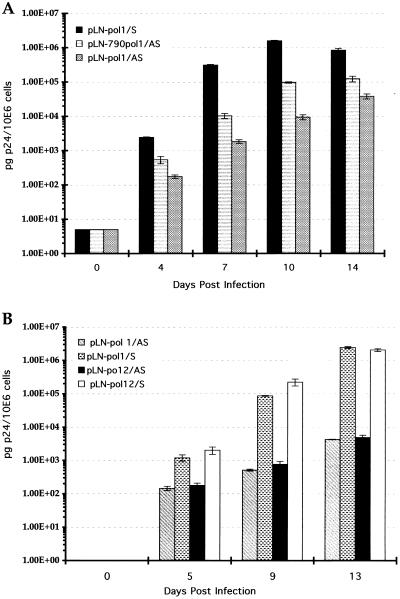
Effect of antisense RNA length on HIV-1 inhibition.
In our previous study (36), we demonstrated that the size of the retrovirally expressed Ψ-gag HIV-1 antisense transcript rather than a specific subfragment determined antiviral activity. To confirm that our observation was not specific to the Ψ-gag antisense RNA, we constructed a vector containing a shorter pol antisense fragment as described in Materials and Methods. The antiviral potencies of the 790-nt antisense pol fragment and the 1,400-nt pol1 fragment were compared at 4 × 103 TCID50 of HIV-1 HXB3 per ml. We observed approximately 50% lower anti-HIV-1 efficacy with the shorter pol1 sequence than with the 1,400-nt pol1 fragment (Fig. 4A). This experiment provided further evidence that the length of the retrovirally expressed antisense RNA is an important factor for antiviral efficacy. We also generated a vector containing an antisense transcript of the complete pol gene reading frame to determine whether increasing the antisense RNA length beyond 1,400 nt would result in increased antiviral efficacy. Figure 4B demonstrates that the 1,400-nt pol1 antisense sequence is as efficient in blocking HIV-1 replication as the 2,600-nt pol12 antisense RNA. Since pol1 and pol2 antisense RNAs yielded comparable levels of inhibition, this experiment suggests that other factors in addition to expression level and transcript length influence the efficacy of antisense RNA.
FIG. 4.
Evaluation of anti-HIV-1 efficacies of vectors containing different-length complementary pol sequences. (A) Anti-HIV-1 efficacies of pol1 deletion constructs. CEM-SS cells expressing the 1,400-nt pol1 antisense construct (pLN-pol1/AS), the 790-nt pol antisense construct (pLN-790pol/AS), and the sense pol1 construct (pLN-pol1/S) were inoculated with 4 × 103 TCID50 of HIV-1 strain HXB3 per ml. (B) CEM-SS cells expressing the 1,400-nt pol1 (pLN-pol1/AS) and the 2,600-nt pol12 (pLN-pol12/AS) antisense sequences were inoculated with 4 × 103 TCID50 of HIV-1 strain HXB3 per ml. The corresponding sense constructs (pLN-pol1/S and pLN-pol12/S) were used as controls.
Inhibition of HIV-1 replication in human PBLs.
While the evaluation of the anti-HIV-1 efficacies of antisense RNAs in T-cell lines is an adequate way to assess their relative efficacies, it is also important to demonstrate HIV-1 inhibition in primary CD4+ lymphocytes (PBLs). The steady-state expression levels of antisense transcripts were measured in stably transduced, CD4+ and Lyt2-selected and activated T lymphocytes. Quantitative RNA analyses of transduced PBLs showed that antisense-RNA expression was reduced about 40% relative to that in CEM-SS cells (Fig. 5A). Quadruplicate PBL cultures expressing either the pol1 or the env antisense transcript or the corresponding sense sequences were inoculated with the HIV-1 JR-CSF cloned primary isolate. Figure 5B shows that the CD4+ lymphocytes became readily infected with the JR-CSF isolate, as measured by the rapid increase of p24 antigen production. Virus replication in the antisense RNA-expressing cultures was decreased by 2 log10 units for the env antisense vector and by about 1 log10 unit for the pol1 antisense vector. The overall lower inhibition observed in PBLs might be the consequence of the reduced steady-state RNA levels in primary T cells. However, this result demonstrates a more efficient inhibition of replication of the HIV-1 JR-CSF isolate in primary CD4+ T cells with the env antisense sequence than with our previously published Ψ-gag sequence (36).
FIG. 5.
Antisense RNA expression and inhibition of HIV-1 replication in transduced PBLs. (A) Total cellular RNA was isolated from activated, CD4-enriched PBLs transduced with the pL-Lyt2-pol1/AS, pL-Lyt2/pol1/S, pL-Lyt2-env/AS, and pL-Lyt2/env/S vectors and selected for Lyt2 expression. The antisense transcripts were analyzed by Northern blotting with a radiolabeled Lyt2-specific probe. A GAPDH-specific probe was used to monitor the amount of RNA loaded. Lane 1, pL-Lyt2-pol1/AS; lane 2, pL-Lyt2-pol1/S; lane 3, pL-Lyt2-env/AS; lane 4, pL-Lyt2-env/S; lane 5, pL-Lyt2-pol1/AS. (B) Transduced CD4+, Lyt2-selected PBLs were activated (36) and infected with HIV-1 JR-CSF (600 TCID50/ml). Cultures were inoculated in quadruplicate, and p24 antigen production was measured.
Comparison of anti-HIV-1 efficacies of RevM10 and antisense RNAs.
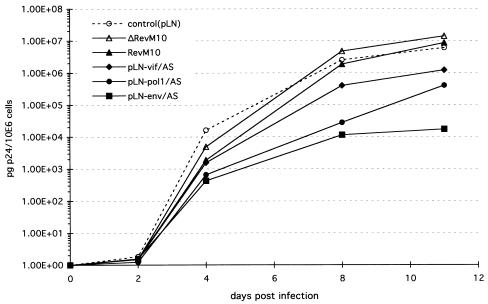
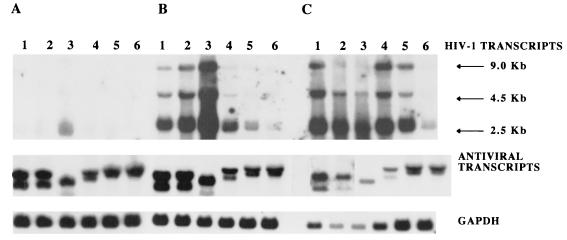
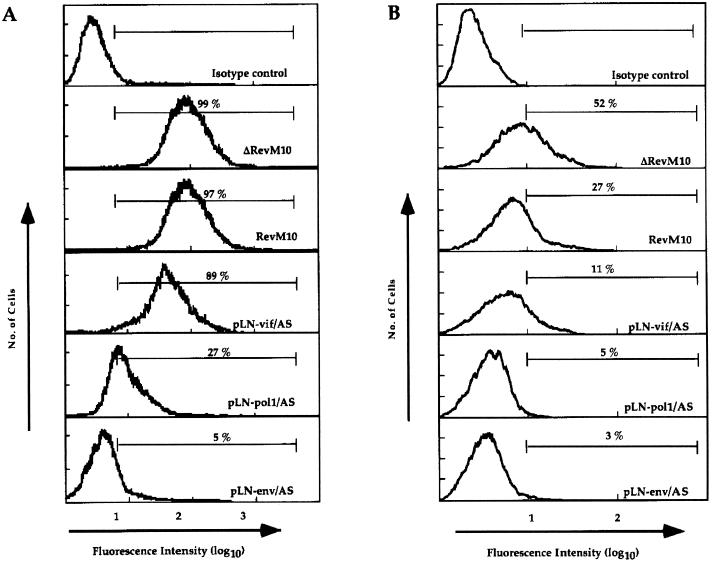
Next, we compared the antiviral potencies of vif, pol1, and env antisense sequences to that of RevM10, a _trans_-dominant form of the HIV-1 Rev protein, at a high HIV-1 inoculation dose. RevM10 acts posttranscriptionally (16), preventing the transport of full-length HIV-1 transcripts from the nucleus to the cytoplasm. To test at which step the antisense RNA interferes with the HIV-1 life cycle, we analyzed the effect of RevM10 or antisense sequences on HIV-1 RNA steady-state levels as well as on structural (p24Gag) and regulatory (Tat) protein expression. Polyclonal CEM-SS cell populations expressing RevM10, ΔRevM10 (24), or antisense vif, pol, or env sequences were inoculated with 105 TCID50 of HIV-1 HXB3 per ml (MOI, 0.1). The analyses of p24 antigen release into the cell supernatant indicated rapid progression of viral replication in the control cultures (pLN and ΔRevM10), as well as in the RevM10- and pLN-vif/AS-expressing cell populations (Fig. 6). In contrast, p24 production 2 orders of magnitude lower was observed with the cell lines expressing pLN-pol/AS and pLN-env/AS RNA. Total RNA samples isolated from HIV-1-infected cells at days 4, 6, and 8 postinoculation were used to determine HIV-1 mRNA and transgene expression levels. Northern blot analyses of day 4 samples showed low levels of HIV-1 transcripts in all cultures (Fig. 7A). At this time point, the steady-state expression levels of all recombinant transcripts were comparable. At day 6 postinfection (Fig. 7B), the cells transduced with the control vector (lane 3) and ΔRevM10 (lane 2) expressed high steady-state levels of HIV-1 transcripts. The cells transduced with RevM10 (lane 1) and the pLN-vif/AS vector (lane 4) expressed three- to fivefold less HIV-1 mRNA than the respective control cell populations, and the cells transduced with the pLN-pol/AS (lane 5) and pLN-env/AS (lane 6) vectors expressed very low levels of HIV-1 RNA (Fig. 7B). At this time point there were still comparable amounts of recombinant transcript present in all cultures (Fig. 7B, lower panel). Analyses of the day 8 RNA samples (Fig. 7C) demonstrated degradation and decreased amounts of all three RNA transcripts analyzed (HIV-1, vector transcripts, and the glyceraldehyde-3-phosphate dehydrogenase [GAPDH] transcript) in the control cell populations, probably due to HIV-1-induced cell death in these cultures. High levels of HIV-1 mRNA were detected in the RevM10- and pLN-vif/AS-expressing cells; the level increased about fivefold in the pLN-pol/AS-expressing cells but was still very low in the pLN-env/AS-expressing cells. At this time point, we also analyzed the intracellular p24Gag and Tat protein levels in the HIV-1-infected cell populations. FACS analysis of day 8 samples demonstrated that 27% of the pLN-pol/AS-expressing cells and only 5% of the pLN-env/AS-expressing cells produced detectable p24Gag protein (Fig. 8A), which correlates with the observed low HIV-1 transcript levels. At this time point, almost 100% of the CEM-SS cells expressing the RevM10 gene or pLN-vif/AS RNA were positive for intracellular p24Gag protein, although the pLN-vif/AS-expressing population produced lower p24 antigen levels (mean fluorescence intensity, 135).
FIG. 6.
Comparison of _trans_-dominant RevM10 and intracellularly expressed vif, pol1, and env antisense RNAs in high-inoculation-dose HIV-1 infection experiments. CEM-SS cells (106/ml) were inoculated with 105 TCID50 of HIV-1 strain HXB3 per ml, and viral replication was monitored by measuring p24Gag antigen production in the culture supernatant.
FIG. 7.
Detection of HIV-1, antisense, and RevM10 transcripts in CEM-SS cells inoculated with 105 TCID50 of HIV-1 strain HXB3 per ml. Total cellular RNA was isolated from 106 CEM-SS cells at days 4 (A), 6 (B), and 8 (C) postinfection and analyzed by Northern blotting. The HIV-specific transcripts were detected with a radiolabeled TAR-specific oligonucleotide probe, and expression of the ΔRevM10 and RevM10 transcripts was detected with a Rev-specific probe. After the filter was washed, a _neo_-specific probe which detected both RevM10 and ΔRevM10 and the antisense transcripts was used. A GAPDH-specific probe was used to monitor the amount of RNA loaded. Lane 1, RevM10; lane 2, ΔRevM10; lane 3, pLN (vector control); lane 4, pLN-vif/AS; lane 5, pLN-pol1/AS; lane 6, pLN-env/AS.
FIG. 8.
Analyses of intracellular p24Gag and Tat expression in HIV-1-infected CEM-SS cells. (A) Intracellular p24Gag expression was measured at day 8 postinoculation. The mean fluorescence intensities reflect the relative intracellular p24Gag expression levels. (B) Detection of Tat protein in transduced and HIV-1-infected CEM-SS cells. Aliquots of CEM-SS cells at day 8 postinfection were fixed in methanol, stained with a Tat-specific antibody, and analyzed with a FACScan.
Measuring the intracellular Tat protein levels gave similar results, although the sensitivity of this assay is lower and hence the assay is less accurate than for intracellular p24Gag protein detection. Figure 8B shows that antisense RNA-expressing cells produce substantially lower Tat protein levels (3, 5, and 11%) than the ΔRevM10- and RevM10-expressing cells (52 and 27%), which may explain the observed overall low HIV-1 transcript levels.
DISCUSSION
Highly efficient inhibition of HIV-1 replication with intracellularly expressed antiviral genes (2) would be an important step toward clinical gene therapy for HIV-1 disease. Several intracellularly expressed antisense RNA-based inhibition strategies using antisense transcripts of various lengths and targeted to different HIV-1 sequences have been described. Different vector systems, including Moloney murine leukemia virus (MoMLV)-based retroviral vectors (4, 9, 10, 34), HIV-based retroviral vectors (13), and adeno-associated virus vectors (5), have been reported as successful delivery vehicles.
In this study, we evaluated the efficacies of antisense sequences targeted against different HIV-1 genes and compared these efficacies to that of RevM10. MoMLV-based retroviral vectors expressed the antisense RNAs as part of the long transcript initiated from the RNA polymerase II-dependent viral LTR promoter. Constitutively expressed 1,100- to 1,400-nt sequences complementary to the pol, vif, and env genes and the 3′ LTR region were evaluated in CEM-SS cells against laboratory-adapted HIV-1 strains (HXB3 and SF2) at different inoculation doses. We observed the strongest antiviral efficacy with the pol and env antisense sequences, even at high inoculation doses. A 2.7-kb antisense sequence targeted against the tat, rev, and env genes of HIV-1 (9) and a 3.5-kb antisense sequence targeted against the SIVmac env gene region (34) have been previously described. Limited antiviral efficacy was observed with these antisense constructs even at low MOIs (9, 34). Contrary to these observations, we were able to show significant inhibition of HIV-1 replication with both pol and env antisense RNA even at the 105-TCID50/ml inoculation dose (MOI, 0.1). The potential differences in steady-state antisense RNA expression levels and the antisense RNA secondary structure may in part explain these experimental results.
Furthermore, we evaluated the anti-HIV-1 efficacies of pol and env antisense RNA in the clinically more relevant primary CD4+ T lymphocytes (PBLs). The steady-state expression level of antisense RNAs was lower in activated PBLs than in the CD4+ T-cell line CEM-SS, based on Northern blot analyses. Nevertheless, replication of the HIV-1 JR-CSF isolate was suppressed in PBLs transduced with retroviral vectors expressing pol or env antisense RNA. Comparing the anti-HIV efficacies of the different antisense RNAs to that of the well-established and widely used _trans_-dominant RevM10 gene further confirmed the potency of these antisense RNA sequences. With high viral inoculation doses (105 TCID50/ml), the anti-HIV-1 effect of the _trans_-dominant RevM10 protein was minimal, which is most likely due to the fact that RevM10 acts as a competitive inhibitor of the HIV-1 Rev protein (16). Previously, intracellularly expressed short antisense sequences targeted against the HIV-1 TAR sequence have been compared to RevM10 in PBL infection experiments (1, 35) using primary HIV-1 isolates. This short antisense RNA inhibited HIV-1 replication less efficiently than RevM10. Since we have demonstrated that the antisense-transcript length is important for antiviral efficacy (36), the limited anti-HIV-1 effect of the antisense TAR RNA observed in that study might be a reflection of the antisense-transcript size.
We also investigated the effect of antisense RNAs on HIV-1 transcript level and intracellular p24Gag and Tat protein expression. We observed a good correlation between HIV-1 transcript levels and intracellular p24Gag expression. Detection of the intracellular Tat protein was less sensitive and quantitative due to the lower efficiency of the staining reaction and the overall Tat expression level (28). However, the observed low Tat expression level indicated the same trend as the HIV-1 RNA and p24Gag expression data. The low steady-state HIV-1 transcript level, together with the suppression of p24Gag and Tat protein expression, suggests that intracellularly expressed antisense RNAs act early in the HIV-1 life cycle by reducing the HIV-1 mRNA level. Several reports suggested translational arrest as a mechanism of inhibition for antisense RNAs (26, 30). However, our results demonstrate that antisense RNA inhibits the accumulation of HIV-1 mRNA, supporting the hypothesis (5, 9, 11) that antisense-RNA-mediated effects are the consequence of hybrid duplex formation between the antisense RNA and the target mRNA, followed by the degradation of the RNA-RNA duplex. Degradation of the full-length HIV-1 transcript prior to splicing would lower the level of singly and multiply spliced transcripts present in antisense RNA-expressing cells. A similar result has been obtained with an antisense TAR sequence contained by an adeno-associated virus vector (5). It has been suggested that the antisense RNA effect depends on the ability of the complementary RNA to form hybrid duplexes with the target RNA and that hybrid duplex formation depends on the expression levels, length, and structure of antisense and target RNAs involved (37). A previously described (11) antisense Rev response element sequence has been shown to inhibit HIV-1 replication by using an HIV-1 based retroviral vector system in a transient-expression assay. The transient-inhibition data also indicated that decreased p24Gag antigen production correlated with the reduction of the level of full-length HIV-1 mRNA (11). In our experimental system, the relatively high, constant level of antisense RNA transcribed from the retroviral vector is sufficient to inactivate most of the RNA transcribed from the HIV-1 LTR. Other inhibition strategies, like that involving RevM10, are not able to facilitate HIV-1 RNA degradation, and as a consequence the level of regulatory gene expression is not inhibited, resulting in constant, steady-state Tat expression which in turn increases HIV-1 transcription levels.
In conclusion, we have demonstrated that antisense RNAs targeted against the HIV-1 pol and env genes are highly efficient in inhibiting HIV-1 replication. The high specificity and presumed nonimmunogenic nature of antisense RNAs are essential components of an effective HIV-1 gene therapy strategy.
ACKNOWLEDGMENTS
We thank Richard Rigg and Timothy Austin for constructive comments on the manuscript. The following reagents were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: CEM-SS cells (catalog no. 776) from P. Nara and HIV-1SF2 (ARV2) (catalog no. 275) from J. Levy.
REFERENCES
- 1.Bahner I, Zhou C, Yu X J, Guatelli J C, Kohn D B. Comparison of trans-dominant inhibitory mutant human immunodeficiency virus type 1 genes expressed by retroviral vectors in human T lymphocytes. J Virol. 1993;67:3199–3207. doi: 10.1128/jvi.67.6.3199-3207.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baltimore D. Intracellular immunization. Nature (London) 1988;335:395–396. doi: 10.1038/335395a0. [DOI] [PubMed] [Google Scholar]
- 3.Bevec D, Dobrovnik M, Hauber J, Böhnlein E. Inhibition of human immunodeficiency virus type 1 replication in human T cells by retroviral-mediated gene transfer of a dominant-negative rev trans-activator. Proc Natl Acad Sci USA. 1992;89:9870–9874. doi: 10.1073/pnas.89.20.9870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biasolo M A, Radaelli A, Del Pup L, Franchin E, De Giuli-Morghen C, Palù G. A new antisense tRNA construct for the genetic treatment of human immunodeficiency virus type 1 infection. J Virol. 1996;70:2154–2161. doi: 10.1128/jvi.70.4.2154-2161.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chatterjee S, Johnson P R, Wong K K. Dual-target inhibition of HIV-1 in vitro by means of adeno-associated virus antisense vector. Science. 1992;258:1485–1488. doi: 10.1126/science.1359646. [DOI] [PubMed] [Google Scholar]
- 6.Choli H, Fan B, Joshi R L, Ramezani A, Li X, Joshi S. Inhibition of HIV-1 multiplication in a human CD4+ lymphocytic cell line expressing antisense and sense RNA molecules containing HIV-1 packaging signal and rev response element(s) Antisense Res Dev. 1994;4:19–29. doi: 10.1089/ard.1994.4.19. [DOI] [PubMed] [Google Scholar]
- 7.Escaich S, Kalfoglou C, Plavec I, Kaushal S, Mosca J D, Böhnlein E. RevM10-mediated inhibition of HIV-1 replication in chronically infected T cells. Hum Gene Ther. 1995;6:625–634. doi: 10.1089/hum.1995.6.5-625. [DOI] [PubMed] [Google Scholar]
- 8.Forestell S P, Dando J S, Chen J, de Vries P, Böhnlein E, Rigg R J. Novel retroviral packaging cell lines: complementary tropism and improved vector production for efficient gene transfer. Gene Ther. 1997;4:19–28. doi: 10.1038/sj.gt.3300420. [DOI] [PubMed] [Google Scholar]
- 9.Gyotoky J I, El-Farrash M A, Fujimoto S, Germeraad W T, Watanabe Y, Teshigawara K, Harada S, Katsura Y. Inhibition of human immunodeficiency virus replication in human T cell line by antisense RNA expressed in the cell. Virus Genes. 1991;5:189–202. doi: 10.1007/BF00568969. [DOI] [PubMed] [Google Scholar]
- 10.Joshi S, Van Brunschot A, Asad S, Van Der Elst I, Read S E, Bernstein A. Inhibition of human immunodeficiency virus type 1 multiplication by antisense and sense RNA expression. J Virol. 1991;65:5524–5530. doi: 10.1128/jvi.65.10.5524-5530.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim J H, McLinden R J, Mosca J D, Vahey M T, Greene W C, Redfield R R. Inhibition of HIV replication by sense and antisense Rev response elements in HIV-based retroviral vectors. J Acquired Immune Defic Syndr. 1996;12:343–351. doi: 10.1097/00042560-199608010-00003. [DOI] [PubMed] [Google Scholar]
- 12.Knee R, Li A W, Murphy P R. Characterization and tissue-specific expression of rat basic fibroblast growth factor antisense mRNA and protein. Proc Natl Acad Sci USA. 1997;94:4943–4947. doi: 10.1073/pnas.94.10.4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leavitt M C, Yu M, Wong-Staal F, Looney D J. Ex vivo transduction and expansion of CD4+ lymphocytes from HIV+ donors: prelude to a ribozyme gene therapy trial. Gene Ther. 1996;3:599–606. [PubMed] [Google Scholar]
- 14.Lee S-W, Gallardo H F, Gilboa E, Smith C. Inhibition of human immunodeficiency virus type 1 in human T cells by a potent Rev response element decoy consisting of the 13-nucleotide minimal Rev-binding domain. J Virol. 1994;68:8254–8264. doi: 10.1128/jvi.68.12.8254-8264.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levy-Mintz P, Duan L, Zhang H, Hu B, Dornadula G, Zhu M, Kulkosky J, Bizub-Bender D, Skalka A M, Pomerantz R J. Intracellular expression of single-chain variable fragments to inhibit early stages of the viral life cycle by targeting human immunodeficiency virus type 1 integrase. J Virol. 1996;70:8821–8832. doi: 10.1128/jvi.70.12.8821-8832.1996. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 16.Malim M H, Böhnlein S, Hauber J, Cullen B R. Functional dissection of the HIV-1 Rev trans-activator—derivation of a trans-dominant repressor of Rev function. Cell. 1989;58:205–214. doi: 10.1016/0092-8674(89)90416-9. [DOI] [PubMed] [Google Scholar]
- 17.Malim M H, Freimuth W W, Liu J, Boyle T J, Lyerly H K, Cullen B R, Nabel G J. Stable expression of transdominant rev protein in human T cells inhibits human immunodeficiency virus replication. J Exp Med. 1992;176:1197–1201. doi: 10.1084/jem.176.4.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyer J, Nick S, Stamminger T, Grummt F, Jahn G, Lipps H J. Inhibition of HIV-1 replication by high-copy-number vector expressing antisense RNA for reverse transcriptase. Gene. 1993;129:263–268. doi: 10.1016/0378-1119(93)90277-a. [DOI] [PubMed] [Google Scholar]
- 19.Miller D A, Rosman G J. Improved retroviral vectors for gene transfer and expression. BioTechniques. 1989;7:980–988. [PMC free article] [PubMed] [Google Scholar]
- 20.Mizuno T, Chou M I, Inouye M. A unique mechanism regulating gene expression: translational inhibition by a complementary RNA transcript. Proc Natl Acad Sci USA. 1984;81:4123–4130. doi: 10.1073/pnas.81.7.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morgan R A, Walker R. Clinical protocol: gene therapy for AIDS using retroviral mediated gene transfer to deliver HIV-1 antisense TAR and transdominant Rev protein genes to syngeneic lymphocytes in HIV-1 infected identical twins. Hum Gene Ther. 1996;7:1281–1306. doi: 10.1089/hum.1996.7.10-1281. [DOI] [PubMed] [Google Scholar]
- 22.Nabel G J, Fox B A, Post L, Thompson C B, Woffendin C. A molecular genetic intervention for AIDS—effects of a transdominant negative form of Rev. Hum Gene Ther. 1995;5:79–92. doi: 10.1089/hum.1994.5.1-79. [DOI] [PubMed] [Google Scholar]
- 23.Ojwang J O, Hampel A, Looney D J, Wong-Staal F, Rappaport J. Inhibition of human immunodeficiency virus type 1 expression by a hairpin ribozyme. Proc Natl Acad Sci USA. 1992;89:10802–10806. doi: 10.1073/pnas.89.22.10802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plavec I, Agarwal M, Ho K E, Pineda M, Auten J, Baker J, Matsuzaki H, Escaich S, Bonyhadi M, Böhnlein E. High transdominant RevM10 protein levels are required to inhibit HIV-1 replication in cell lines and primary T cells: implication for gene therapy of AIDS. Gene Ther. 1997;4:128–139. doi: 10.1038/sj.gt.3300369. [DOI] [PubMed] [Google Scholar]
- 25.Renneisen K, Leserman L, Matthes E, Schröder H C, Müller W E G. Inhibition of expression of human immunodeficiency virus-1 in vitro by antibody-targeted liposomes containing antisense RNA to the env region. J Biol Chem. 1990;265:16337–16342. [PubMed] [Google Scholar]
- 26.Rhodes A, James W. Inhibition of human immunodeficiency virus replication in cell culture by endogenously synthesized antisense RNA. J Gen Virol. 1990;71:1965–1974. doi: 10.1099/0022-1317-71-9-1965. [DOI] [PubMed] [Google Scholar]
- 27.Rigg R J, Chen J, Dando J S, Forestell S P, Plavec I, Böhnlein E. A novel human amphotropic packaging cell line: high titer, complement resistance, and improved safety. Virology. 1996;218:290–295. doi: 10.1006/viro.1996.0194. [DOI] [PubMed] [Google Scholar]
- 28.Rigg R J, Dando J S, Escaich S, Plavec I, Böhnlein E. Detection of intracellular HIV-1 Rev protein by flow cytometry. J Immunol Methods. 1995;188:187–195. doi: 10.1016/0022-1759(95)00237-5. [DOI] [PubMed] [Google Scholar]
- 29.Sczakiel G, Pawlita M. Inhibition of human immunodeficiency virus type 1 replication in human T cells stably expressing antisense RNA. J Virol. 1991;65:468–472. doi: 10.1128/jvi.65.1.468-472.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sczakiel G, Oppenländer M, Rittner K, Pawlita M. Tat- and Rev-directed antisense RNA expression inhibits and abolishes replication of human immunodeficiency virus type 1: a temporal analysis. J Virol. 1992;66:5576–5581. doi: 10.1128/jvi.66.9.5576-5581.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sczakiel G, Homann M, Rittner K. Computer-aided search for effective antisense RNA target sequences of the human immunodeficiency virus type 1. Antisense Res Dev. 1993;3:45–52. doi: 10.1089/ard.1993.3.45. [DOI] [PubMed] [Google Scholar]
- 32.Sullenger B A, Gallardo H F, Ungers G E, Gilboa E. Overexpression of TAR sequences renders cells resistant to human immunodeficiency virus replication. Cell. 1990;63:601–608. doi: 10.1016/0092-8674(90)90455-n. [DOI] [PubMed] [Google Scholar]
- 33.Trono D, Feinberg M B, Baltimore D. HIV-1 gag mutants can dominantly interfere with the replication of the wild-type virus. Cell. 1989;59:113–120. doi: 10.1016/0092-8674(89)90874-x. [DOI] [PubMed] [Google Scholar]
- 34.Tung F Y T, Daniel M D. Targeted inhibition of immunodeficiency virus replication in lymphocytes through retroviral mediated gene transfer. Arch Virol. 1993;133:407–421. doi: 10.1007/BF01313779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vandendriessche T, Chuah M K L, Chiang L, Chang H-K, Ensoli B, Morgan R A. Inhibition of clinical human immunodeficiency virus (HIV) type 1 isolates in primary CD4+ T lymphocytes by retroviral vectors expressing anti-HIV genes. J Virol. 1995;69:4045–4052. doi: 10.1128/jvi.69.7.4045-4052.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Veres G, Escaich S, Baker J, Barske C, Kalfoglou C, Ilves H, Kaneshima H, Böhnlein E. Intracellular expression of RNA transcripts complementary to the human immunodeficiency virus type 1 gag gene inhibits viral replication in human CD4+ lymphocytes. J Virol. 1996;70:8792–8800. doi: 10.1128/jvi.70.12.8792-8800.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang S, Dolnick B J. Quantitative evaluation of intracellular sense:antisense RNA hybrid duplexes. Nucleic Acids Res. 1993;21:4383–4391. doi: 10.1093/nar/21.18.4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woffendin C, Ranga U, Yang Z-Y, Xu L, Nabel G J. Expression of a protective gene prolongs survival of T cells in human immunodeficiency virus infected patients. Proc Natl Acad Sci USA. 1996;93:2889–2894. doi: 10.1073/pnas.93.7.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou C, Bahner I, Larson G P, Zaia J A, Rossi J J, Kohn D B. Inhibition of HIV-1 in human T lymphocytes by retrovirally transduced anti-tat and rev hammerhead ribozymes. Gene. 1994;149:33–39. doi: 10.1016/0378-1119(94)90409-x. [DOI] [PubMed] [Google Scholar]