T Cells Expressing Activated LFA-1 Are More Susceptible to Infection with Human Immunodeficiency Virus Type 1 Particles Bearing Host-Encoded ICAM-1 (original) (raw)
Abstract
The incorporation of host-derived proteins in nascent human immunodeficiency virus type 1 (HIV-1) particles is a well-established phenomenon. We recently demonstrated that the physical presence of host-encoded ICAM-1 glycoproteins on HIV-1 leads to a significant increase in virus infectivity in an ICAM-1/LFA-1-dependent fashion (J.-F. Fortin, R. Cantin, G. Lamontagne, and M. Tremblay, J. Virol. 71:3588–3596, 1997). We show here that conversion of LFA-1 to high affinity for ICAM-1 with the use of anti-LFA-1 antibodies (clones NKI-L16 and MEM83) markedly enhances the susceptibility of different target T-lymphoid cell lines, as well as of primary peripheral blood mononuclear cells, to infection by ICAM-1-bearing HIV-1 particles (6- to 95-fold). It is known that T-cell receptor (TCR) cross-linking induces a transient increase in LFA-1 affinity for ICAM-1. Treatment of peripheral blood mononuclear cells with anti-TCR antibodies (clone OKT3) resulted in a transient increase in susceptibility to infection by ICAM-1-positive virions that parallels the previously reported kinetics of the LFA-1/ICAM-1 adhesion mechanism. Our results led us to postulate that the strong interaction taking place between virally incorporated ICAM-1 and cell surface-activated LFA-1 markedly enhances the efficiency of virus binding and entry, thus favoring greater infection by ICAM-1-bearing HIV-1 particles. In view of the knowledge that primary HIV-1 isolates harbor host-derived ICAM-1 on their surfaces, these results provide new information about the role of host-derived ICAM-1 in the life cycle of HIV-1 and how it could positively modulate the dynamics of the viral infection, mainly in cellular compartments, such as the lymphoid tissues, where the level of cellular activation is high and where the probability of encountering a T cell expressing the activated LFA-1 form is also elevated.
In vivo, CD4+ T lymphocytes and monocytes-macrophages constitute the main reservoirs for the production and maintenance of human immunodeficiency virus type 1 (HIV-1) (48, 54). Infection of these cells occurs following the high-affinity interaction between the viral surface gp120 and the cell surface CD4 molecule (15). However, in order for the fusion to occur, the sole interaction between gp120 and CD4 is not sufficient (40), and the involvement of other molecules is required. These other cellular components, the so-called coreceptors, have been recently identified and characterized. Formerly called LESTR/HUMSTR/fusin, the chemokine receptor CXCR4 has been shown to act as the coreceptor for T-cell-tropic strains of HIV-1 (22). For macrophage-tropic HIV-1 isolates, the CCR5 molecule has been identified as the major coreceptor (16, 19), even though CCR3 and CCR2b are also used, but to a lesser extent (14, 18). Following ligation of gp120 with CD4, a high-affinity binding site for the chemokine receptor is created, thus leading to membrane fusion and virus entry (36, 58, 59). Besides these essential elements for viral entry, other cellular molecules could play important, although accessory, roles during the process of virus uptake.
It has been known for a while that HIV-1 can incorporate, besides its surface glycoproteins, a vast array of cell membrane molecules while budding out from the infected cell. For example, major histocompatibility complex class II (MHC-II) DR molecules were the first host constituents found embedded within HIV-1 particles and these were identified as a potential source of false-positive reactions in enzymatic screening tests (31). Many other cellular structures were found to be acquired by newly formed HIV-1, such as HLA-DP and -DQ, β2-microglobulin, CD44, CD55, and CD59, as well as LFA-1 and ICAM-1 adhesion molecules (6, 11, 12, 21, 29, 33, 52). It has also been suggested that the profile of virion-bound cellular constituents could be used as a marker to identify the virus-producing cell (1).
Recently, several studies investigated the functionality of host-derived molecules when present on the virion surface. The first, although indirect, evidence of the functionality of virally incorporated adhesion molecules came from the demonstration that anti-LFA-1 antibodies can act synergistically with antiserum to neutralize HIV-1 particles (28). More direct proof was provided by the demonstration that an increase in virion-incorporated HLA-DR and ICAM-1 resulted in enhanced infectivity toward CD4-negative cell lines (12). Saiffudin et al. demonstrated that CD55 and CD59, two glycosylphosphatidylinositol-linked complement control proteins, can protect HIV-1 from complement-mediated virolysis when incorporated into budding virions (52), while virion-incorporated host MHC-II molecules were shown to present bacterial superantigens (50). We have been able to demonstrate, by using mutagenized cell lines, that incorporation of MHC-II molecules within the viral envelope enhances the process of viral infection (9). Recently, we developed a transient-transfection-and-expression system that permits the production of virions differing only by the absence or the presence of a specific cell surface molecule on their surfaces. By using this new technical approach, we found that acquisition of cellular HLA-DR1 molecules by budding HIV-1 is associated with a 1.6- to 2.5-fold increase in virus infectivity (10). Moreover, we have shown that incorporation of host-derived ICAM-1 by progeny viruses leads to a 5- to 10-fold increase in HIV-1 infectivity, caused by an interaction between virally incorporated ICAM-1 and cell surface LFA-1 (23), an observation which has been corroborated by another group (49). This finding has great clinical relevance, considering that ICAM-1 is acquired by clinical HIV-1 isolates grown on primary mononuclear cells (4, 11, 24) and the in vivo HIV-1-producing cells are activated CD4+ T cells and macrophages, cells which are both known to express high levels of ICAM-1 (55). Therefore, it is likely that HIV-1 isolates found in vivo carry on their surfaces host-derived ICAM-1 glycoproteins.
The counterreceptor for ICAM-1 is LFA-1, a member of the integrin family that is expressed mainly on lymphocytes, granulocytes, monocytes, and macrophages, with elevated levels on memory T cells (53). The activation of leukocytes with various agents like phorbol esters and chemoattractant, or cross-linking of specific surface receptors such as the T-cell receptor (TCR)/CD3 complex, CD2, and MHC-II, induces LFA-1-mediated binding to ICAM-1 (17). This transient change in ICAM-1 binding is thought to involve both a variation in the affinity of LFA-1 for ICAM-1 caused by a conformational change and an increase in avidity mediated by clustering of the molecules (20). This dynamic regulation of integrins allows the cells that bear these molecules to convert rapidly from a nonadherent to an adherent phenotype and vice versa. Since LFA-1 can be expressed in two different conformational states, i.e., low versus high affinity for ICAM-1, we therefore examined whether the activation state of LFA-1 on the target cell surface could affect the overall susceptibility to infection by ICAM-1-bearing HIV-1 particles.
MATERIALS AND METHODS
Cells.
The 1G5 T-cell line, a Jurkat E6.1 derivative that harbors two stably integrated constructs consisting of the luciferase gene under the control of the HIV-1SF2 long terminal repeat (LTR) and Jurkat-tat, Sup-T1, and PM1 cells were obtained through the AIDS Research and Reference Reagent Program (Division of AIDS, National Institute of Allergy and Infectious Diseases), while 293T cells were kindly provided by W. C. Greene (The J. Gladstone Institutes, San Francisco, Calif.). Primary peripheral blood mononuclear cells (PBMCs) from healthy donors were isolated by Ficoll-Hypaque density gradient centrifugation and cultured in the presence of phytohemagglutinin P (PHA-P; Sigma, St. Louis, Mo.) at 3 μg/ml and recombinant human interleukin-2 at 30 U/ml for 3 days at 37°C in a 5% CO2 atmosphere prior to viral infection.
Plasmids.
pNL4-3 is a full-length infectious molecular clone of HIV-1 (2). Proviral plasmid pHXB-Luc was originally derived from pHXB-2D, in which a part of the nef gene was deleted and replaced with the luciferase reporter gene (13). This infectious molecular clone of HIV-1 was kindly provided by David Baltimore (Massachusetts Institute of Technology, Cambridge, Mass.). pCD1.8 is a eukaryotic expression vector containing the entire human ICAM-1 cDNA and was obtained from Timothy A. Springer (The Center for Blood Research, Boston, Mass.) (56).
Antibodies.
Anti-ICAM-1 (anti-CD54) antibody RR1/1.1.1 was kindly provided by Robert Rothlein (Boehringer Ingelheim, Ridgefield, Conn.) (51). NKI-L16 (anti-CD11a) was obtained from Carl C. Figdor (University Hospital Nijmegen, Nijmegen, The Netherlands) (34), while MEM30 and MEM83 (anti-CD11a) were kind gifts from Vaclav Horejsi (Institute of Molecular Genetics, Prague, Czech Republic) (5).
Production of virus stocks.
Viral particles differing only by the presence or the absence of the ICAM-1 molecule in their envelope were produced by CaPO4 transfection in 293T cells as described previously (23). Cotransfection of pNL4-3 or pHXB-Luc with pCD1.8 led to the production of virus stocks called ICAM-1/POS because such virions bear host-derived ICAM-1. Transfection of 293T cells with pNL4-3 or pHXB-Luc only resulted in the production of virus preparations named ICAM-1/NEG, since cellular ICAM-1 glycoproteins are not found embedded within such virions. Indeed, ultracentrifuged virus preparations harvested from 293T cells transiently transfected only with a molecular clone of HIV-1 are negative for ICAM-1 as monitored by an enzymatic assay (data not shown). Virus stocks were normalized for virion content by using a commercial assay for viral major core protein p24 (Organon Teknika, Durham, N.C.).
Virus infection.
Cells were first either left untreated or treated for 30 min at 37°C with anti-LFA-1 monoclonal antibody MEM30 (10 μg/ml), MEM83 (3 μg/ml), or NKI-L16 (1 μg/ml). These concentrations have been reported to either block (MEM30) (5) or activate (MEM83 and NKI-L16) LFA-1 (34, 35). Viral stocks were also left untreated or treated for 30 min at 37°C with anti-ICAM-1 monoclonal antibody RR1/1.1.1 (20 μg/ml). For the kinetic experiments, PBMCs were first incubated for 30 min on ice with OKT3 (2 μg/ml) before incubation at 37°C for the times indicated in Results with goat anti-mouse immunoglobulins (5 μg/ml). Infections were done with the amount of virus (standardized in term of p24 protein) necessary to infect 105 cells (1.5 ng of p24 for Jurkat-tat cells or 10 ng of p24 for other lymphoid cell lines and PBMCs). Infections were allowed to proceed for 90 min at 37°C. The cells were next washed twice with phosphate-buffered saline, resuspended in 200 μl of RPMI culture medium (supplemented with recombinant human interleukin-2 at 30 U/ml for PBMCs), and transferred to a 96-well flat-bottom tissue culture plate (Microtest III, Falcon, Becton Dickinson, Lincoln Park, N.J.). After a 24-h incubation period, a combination of 1 μM azidothymidine (3′-azido-2′,3′-dideoxythimidine) and 30 μM didanosine (2′,3′-dideoxyinosine) was added to abrogate any reinfection events. Finally, HIV-1-infected cells were incubated for an additional 24-h period (1G5 and Jurkat-tat cells) or 48 h (PBMCs and Sup-T1, PM1, and Jurkat E6.1 cells) at 37°C. After this final incubation, cells were lysed and luciferase activity was monitored as described previously (10, 23).
RESULTS
LFA-1 activation on 1G5 T-lymphoid cells increases susceptibility to infection by ICAM-1-positive HIV-1 particles.
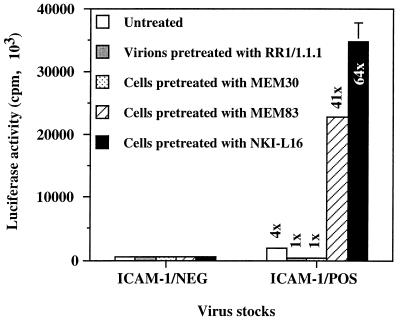
To test the hypothesis that LFA-1 activation on 1G5 T-lymphoid cells increases susceptibility to infection by ICAM-1-positive HIV-1 particles, we first inoculated 1G5 cells with virus stocks harvested from transiently transfected 293T cells as described previously (23). It should be noted that 1G5 is a clone of Jurkat E6.1 cells stably transfected with two copies of a construct consisting of the luciferase reporter gene placed under the control of the HIV-1 LTR (3). Upon virus infection, these cells express luciferase due to the viral Tat-mediated transactivation of the HIV-1 LTR. We then tested if antibody-mediated modification of the LFA-1 conformation could affect the susceptibility of 1G5 cells to infection by ICAM-1-free (ICAM-1/NEG) and ICAM-1-bearing (ICAM-1/POS) HIV-1 particles. This was achieved by pretreating or not pretreating 1G5 cells with MEM83 and NKI-L16, two anti-LFA-1 antibodies known to induce the switching of LFA-1 from low to high affinity for ICAM-1 (34, 35). Other experimental conditions consisted of virus preparations and target cells pretreated with RR1/1.1.1 (anti-ICAM-1) and MEM30 (anti-LFA-1), respectively, two antibodies known to abolish the ICAM-1/LFA-1 interaction (5, 41). For each condition, cells were infected with HIV-1NL4-3 ICAM-1/NEG and ICAM-1/POS preparations that were normalized according to p24 content. As measured by luciferase activity and in agreement with our previous study (23), ICAM-1-bearing viruses are more infectious (four times) than their ICAM-1-devoid counterparts (Fig. 1). The observation that addition of RR1/1.1.1 and MEM30 eliminates the enhancement of virus infectivity for ICAM-1-bearing particles is clear evidence of the role played by the physical interaction between virion-bound ICAM-1 and surface LFA-1 in enhancing the process of viral infection. Most strikingly, 1G5 cells are 41 to 64 times more susceptible to infection by ICAM-1/POS HIV-1NL4-3 particles than to infection by ICAM-1-negative virions when the cells are first pretreated with LFA-1-activating antibodies MEM83 and NKI-L16, respectively.
FIG. 1.
Effect of two LFA-1-activating antibodies on infection of 1G5 cells by ICAM-1/NEG and ICAM-1/POS virions. 1G5 cells were either left untreated or treated with various antibodies for 30 min at 37°C and then infected with ICAM-1/NEG or ICAM-1/POS HIV-1NL4-3 particles previously treated or not treated with ICAM-1-blocking antibody RR1/1.1.1. Cells were lysed 48 h later, and infection was evaluated by measuring the luciferase activity in the lysates. The results shown are means ± standard deviations of triplicate samples and are representative of three independent experiments. The fold increase over infection with ICAM-1-negative virus particles is indicated for each experimental condition. Luciferase activity in mock-infected cells was always less than 50 × 103 cpm.
Higher susceptibility to infection by ICAM-1-bearing HIV-1 conferred by the activated form of LFA-1 is seen in several T-lymphoid cell lines.
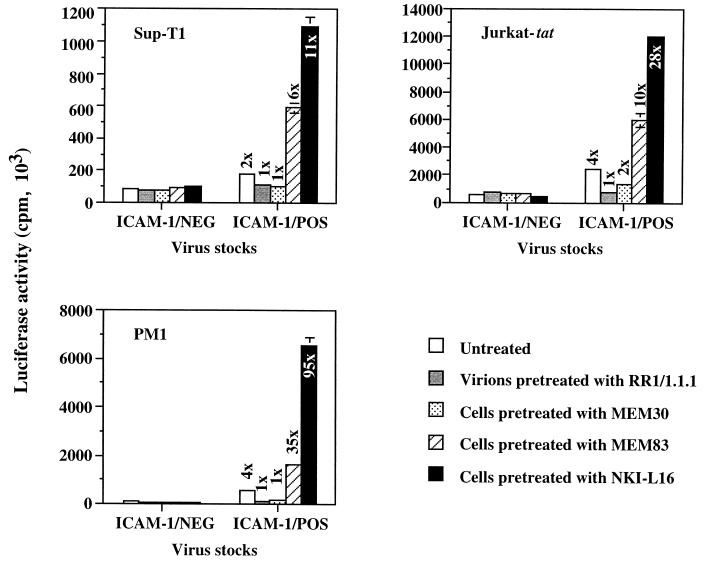
We next wanted to eliminate any cell type-specific phenomenon by using a more versatile system that is based on infection with recombinant luciferase-encoding HIV-1 particles. HXB-Luc virus particles bearing or not bearing host-encoded ICAM-1 on their surfaces were produced by transient transfection of 293T cells as described previously (10), and these virions were used to infect three different cell lines (Sup-T1, Jurkat-tat, and PM1). The authenticity of the phenomenon was proven by the fact that these cell lines were 6- to 95-fold more susceptible to infection by ICAM-1-bearing viruses than to infection by ICAM-1-free HXB-Luc particles when they were pretreated with LFA-1-activating antibodies (Fig. 2). The infection process with ICAM-1/NEG HXB-Luc particles was still unaffected by the treatment of target cells with LFA-1-activating antibodies. The LFA-1- and ICAM-1-blocking antibodies were again able to eliminate the infectivity advantage conferred by the presence on HIV-1 of host-encoded ICAM-1.
FIG. 2.
Effect of LFA-1-activating antibodies on infection of three different T-cell lines by ICAM-1/NEG and ICAM-1/POS HXB-Luc progeny viruses. Each cell line was either left untreated or treated with various antibodies for 30 min at 37°C prior to infection with ICAM-1/NEG or ICAM-1/POS HXB-Luc virions previously treated or not treated with ICAM-1-blocking antibody RR1/1.1.1. Cells were lysed 24 to 48 h later, and infection was evaluated by measuring the luciferase activity in the lysates. The results shown are means ± standard deviations of triplicate samples and are representative of three or four independent experiments. The fold increase over infection with ICAM-1-negative virus particles is indicated for each experimental condition. Luciferase activities in mock-infected Sup-T1, Jurkat-tat, and PM1 cells were always less than 35 × 103 cpm.
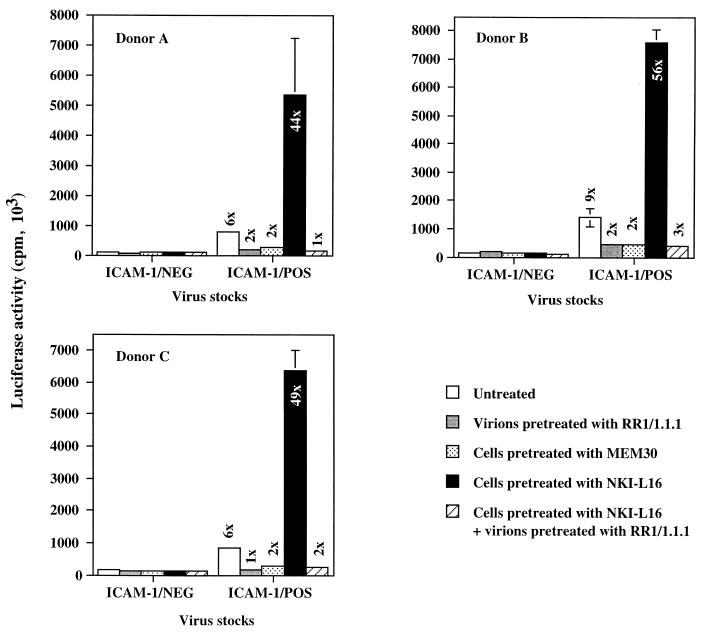
Enhanced susceptibility to infection by ICAM-1-bearing HIV-1 particles of primary target cells expressing the activated form of LFA-1.
To evaluate in a more physiological setting the effect mediated by the LFA-1 activation state on infection by ICAM-1-bearing virions, we performed similar experiments by using PHA-P-stimulated PBMCs isolated from different healthy donors as targets. A significant increase in cellular susceptibility to infection by ICAM-1/POS virions can still be seen upon treatment of the PBMCs with the LFA-1-activating NKI-L16 antibody, and this treatment had no measurable potentiating effect when infection was achieved with ICAM-1/NEG HXB-Luc virions (Fig. 3). For example, NKI-L16-mediated LFA-1 activation on the surface of PBMCs rendered these cells 44 to 56 times more susceptible to infection by ICAM-1/POS progeny viruses than to infection by ICAM-1-negative virions. Interestingly, the observed change in susceptibility of NKI-L16-treated PBMCs to viral infection was totally abrogated by pretreatment of ICAM-1-bearing HXB-Luc virions with RR1/1.1.1.
FIG. 3.
Effect of the NKI-L16 LFA-1-activating antibody on infection of PBMCs by ICAM-1/NEG and ICAM-1/POS HXB-Luc virions. PBMCs from three healthy donors were stimulated for 72 h with PHA. Thereafter, PBMCs were either left untreated or treated with various antibodies for 30 min at 37°C prior to infection with ICAM-1/NEG or ICAM-1/POS HXB-Luc progeny viruses previously treated or not treated with ICAM-1-blocking antibody RR1/1.1.1. Cells were lysed 48 h later, and infection was evaluated by measuring the luciferase activity in the lysates. The results shown are means ± standard deviations of triplicate samples and are representative of three independent experiments. The fold increase over infection with ICAM-1-negative virus particles is indicated for each experimental condition. Luciferase activities in mock-infected PBMCs ranged between 27 × 103 and 33 × 103 cpm.
It is known that PHA-stimulated PBMCs will grow for the first few days as cellular aggregates, primarily due to LFA-1/ICAM-1 interactions. The cells used in the experiment whose results are shown in Fig. 3 represent early blastogenic PBMCs that could putatively express activated LFA-1 on their surfaces. If this is the case, expression of LFA-1 molecules with a high affinity for ICAM-1 could, in turn, influence cellular susceptibility to infection with ICAM-1-bearing progeny viruses. However, the fact that the enhancement of virus infectivity (a 6- to 9-fold increase for untreated, PHA-stimulated PBMCs) conferred by the presence of host-encoded ICAM-1 is comparable to our previous findings (a 5- to 10-fold increase for untreated T-lymphoid and monocytoid cells) (23) indicates that expression of the activated form of LFA-1 on PBMCs treated for 3 days with PHA is negligible and does not influence susceptibility to infection with virions bearing cellular ICAM-1. This finding is in accord with previous observations indicating that PHA blasts (3 to 5 days of activation with PHA) do not show an ICAM-1 binding phenotype (39).
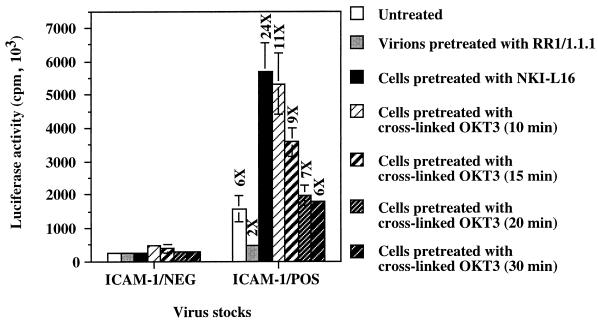
Anti-TCR-induced activation of LFA-1 renders T cells more susceptible to infection by ICAM-1-bearing HIV-1 particles.
The LFA-1 switch from low to high affinity for ICAM-1 has been reported to be transiently induced in T cells after stimulation through the TCR/CD3 complex (20). Therefore, our final goal was to induce LFA-1 high affinity for ICAM-1 by cross-linking the TCR to mimic antigen-specific T-lymphocyte cell-to-cell interactions. Results in Fig. 4 clearly demonstrate that antibody-mediated TCR stimulation of PHA-stimulated PBMCs increases their susceptibility to infection by ICAM-1/POS virions, as is the case for cells pretreated with LFA-1-activating antibody NKI-L16, while it has no detectable effect on cells inoculated with ICAM-1-free viruses. Interestingly, the kinetics of transient susceptibility to infection with ICAM-1-bearing progeny viruses, with a peak at 10 min, parallels the kinetics of the TCR-stimulated increase in LFA-1 affinity previously determined by Dustin and Springer when analyzing a cell-to-cell conjugate (20). These findings thus support the notion that the enhancement of cell susceptibility to infection with virions bearing host-derived ICAM-1 glycoproteins is due to the physical interaction between virion-bound host ICAM-1 glycoproteins and surface LFA-1 in the high-affinity state.
FIG. 4.
Kinetics of susceptibility of TCR-stimulated PBMCs to infection with ICAM-1/NEG and ICAM-1/POS HXB-Luc particles. PBMCs that had previously been treated for 72 h with PHA-P were incubated with cross-linked OKT3 antibodies for various times prior to infection with ICAM-1/NEG or ICAM-1/POS HXB-Luc viruses previously treated or not treated with ICAM-1-blocking antibody RR1/1.1.1. Cells were lysed 72 h later, and infection was evaluated by measuring the luciferase activity in the lysates. The results shown are means ± standard deviations of triplicate samples and are representative of two independent experiments. The fold increase over infection with ICAM-1-negative virus particles is indicated for each experimental condition. Luciferase activity in mock-infected PBMCs was less than 21 × 103 cpm.
DISCUSSION
The important role played by LFA-1 in cell-to-cell transmission of HIV-1 was recognized several years ago (32). However, its role in cell-free virus infection has been less well studied. In this study, our primary goal was to determine how the well-known conformational change in LFA-1 could affect the process of infection by ICAM-1-bearing HIV-1 particles.
In the context of the T-cell/antigen-presenting cell encounter, the increase in ICAM-1 binding strength following stimulation allows a stronger and longer interaction between the cells, thus permitting adequate signal exchange and optimal activation. Lymphoid organs are the anatomic sites where the generation of an immune response takes place. Propagation of antigen-specific immune responses occurs in these anatomic structures, since the lymphocytes migrate from the circulation and the lymphatics into secondary lymphoid organs, particularly the lymph nodes, during the antigen-specific immune response (47). In HIV-1-infected individuals, a heavy viral load is present in the lymphoid organs, at every stage of the disease, making them the major viral reservoirs (44). Moreover, in HIV-1-infected individuals, 25 to 50% of the CD4+ T cells found in lymph nodes are in an activated state and this strong activation is kept all through the evolution of the disease due to the constant stimulation of CD4+ T cells by the continuous presence of the immunogenic virions (45, 46).
With the experiments described in this paper, we have demonstrated that activation of LFA-1 on the surface of target T cells renders them much more susceptible to infection by ICAM-1-bearing HIV-1 particles than to infection by ICAM-1-negative virions. With the knowledge that clinical HIV-1 isolates grown on PBMCs do incorporate ICAM-1 on their surfaces (11) and taking into account the fact that T-cell activation leads to increased ICAM-1 binding, it is tempting to speculate that ICAM-1-bearing viruses should give rise to a more productive infection in the constantly activated environment prevailing in secondary lymphoid organs. This hypothesis is supported by the demonstration that 5 to 10 times more HIV-1-infected cells are detected in the lymphoid organs (lymph nodes, adenoids, and tonsils) than in peripheral blood (44).
It is also known that HIV-1 particles found trapped in the web of the dendritic cells are still infectious, even though they are covered with neutralizing antibodies and complement (30). Indeed, it has been demonstrated that the follicular dendritic cells covered by trapped virions can potently transmit cytopathic infection to CD4+ T cells, although the mechanism by which these neutralizing antibody-coated virions are still infectious is undefined (30). Berman and Nakamura demonstrated that in a cell-to-cell fusion system the presence of ICAM-1 on gp120-expressing cells is associated with a decrease in the ability of neutralizing antibodies to block syncytium formation (7). It has also been observed that ICAM-1-bearing virions are less susceptible to antibody-mediated neutralization than are their viral counterparts lacking host-encoded ICAM-1 on their surfaces (49). In view of the results shown in the present paper and in previously published work, it can be proposed that virion-bound host ICAM-1 could be partly responsible for the vigorous transmission of virus infection by dendritic cells despite a strong immune response. Activation of T cells by the dendritic cellular network coated with virions during antigen presentation could render these cells more susceptible to viral infection by at least two non-mutually exclusive processes: first, by creating in T cells an intracellular milieu favorable to HIV-1 reverse transcription and integration (57, 60) and, second, as shown in our study, by strengthening the LFA-1/ICAM-1 interaction.
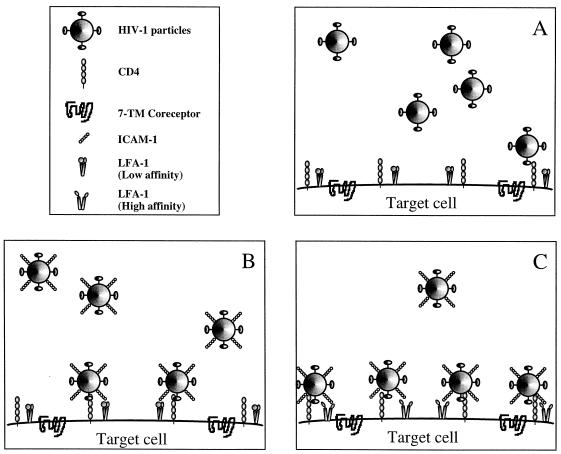
Our experimental design (i.e., a definite and standard incubation period to allow virus binding and entry) and the observation that pretreatment of ICAM-1-bearing virions with anti-ICAM-1 antibodies completely abrogates the higher susceptibility of cells expressing activated LFA-1 to infection with such HIV-1 particles (Fig. 3) have led us to propose the following mechanism to explain our findings. We postulate that the presence of activated LFA-1 strengthens the adhesion of ICAM-1-positive virions to target cells, ultimately resulting in greater susceptibility to virus infection due to a more efficient virus entry process (Fig. 5). However, due to the signaling nature of the LFA-1 molecule, we cannot rule out completely the possibility that some antibody-mediated outside-in signaling could alter the virus entry process by, for example, bringing CXCR4 into close proximity to LFA-1, thus favoring more efficiently entry of LFA-1-bound virions into the cell. It has been suggested that although a single gp120-CD4 interaction might be sufficient to lead to viral binding, numerous gp120-CD4 interactions are necessary to achieve virus adsorption and penetration (38). Therefore, after taking into account the reported weak association between gp120 and gp41 (25, 26), a phenomenon seen in vivo (26) that is linked with the loss of HIV-1 infectivity (42), the additional interactions between virions and target cells could compensate for a suboptimal number of gp120-CD4 associations. The initial binding event must then be rapid and strong to lead to efficient docking of the virus to its host cell. The interactions between virion-bound ICAM-1 and cell surface-activated LFA-1 will therefore result in increased efficiency or specificity of viral binding. It is possible that virus binding is analogous to adhesion of lymphocytes to endothelium, which involves initial binding to a first receptor to capture the lymphocyte, followed by binding to a second receptor (37). Secondary ICAM-1/LFA-1 interactions may allow the virus to browse the surface of the target cell until it finds the appropriate chemokine coreceptor.
FIG. 5.
Proposed model to explain the higher susceptibility of T cells expressing the activated form of LFA-1 to infection with ICAM-1-bearing virions. (A) Without any complementary interactions, the gp120/CD4 association enables the virus to attach to target cells with relatively high affinity. (B) The presence of additional interactions, such as that between virion-incorporated ICAM-1 and cell surface LFA-1, will increase the efficiency of viral binding and accelerate the process of virus entry. (C) In the context of cellular activation, the high-affinity LFA-1 on the surface of target cells will permit the ICAM-1-bearing viruses to attach more firmly to and enter the host cell more rapidly.
Previous studies have indicated that virus preparations from lymphoid cells are contaminated with microvesicles loaded with host proteins (8, 27). Data from our experiments permit us to rule out the possibility that higher susceptibility of T cells to infection by ICAM-1-bearing virions is not an indirect effect resulting from interactions between cellular contaminants and target cells. This postulate is based on the observation that incubation of studied T-cell lines with anti-LFA-1 antibodies (MEM83 and NKI-L16), a treatment which would induce cross-linking of surface LFA-1 molecules, as would be the case with ICAM-1-bearing microvesicles, neither activates HIV-1 LTR activity nor leads to increased susceptibility to infection by ICAM-1/NEG virions (Fig. 1 and 2). Moreover, the putative involvement of cellular contaminants in the observed phenomenon is unlikely considering that no host-derived ICAM-1 proteins could be detected in ultracentrifuged cell-free supernatants from 293T cells transiently expressing surface ICAM-1 (data not shown).
In summary, we have demonstrated that LFA-1 activation leads to a marked increase in the susceptibility of target T cells to infection by ICAM-1-bearing HIV-1 particles. Since clinical HIV-1 isolates do acquire host-encoded ICAM-1 glycoproteins, our observations have direct physiological relevance, as the incorporation process could help the virus to more efficiently target activated CD4+ T cells, which are known to contain an intracellular milieu favorable to productive viral infection and replication. LFA-1 has been previously shown to be important in HIV-1-mediated syncytium formation (32, 43). Therefore, it would be of interest to investigate whether the conformational state of LFA-1 can influence HIV-1-dependent cell-to-cell fusion events. Our study reinforces the idea that virally acquired host cell membrane molecules may play an important role in the pathogenesis of HIV-1 infection. Further studies are thus needed to better understand the role played by host molecules in the viral life cycle and how they could interfere with or be used in the design of new therapeutic strategies aimed at controlling this retroviral infection.
ACKNOWLEDGMENTS
We thank B. Barbeau for critical reading of the manuscript.
This study was supported by a grant to M.J.T. from the Medical Research Council of Canada (MT-14438). M.J.T. is the recipient of a scholarship from the Fonds de la Recherche en Santé du Québec (FRSQ). J.-F.F. is supported by a National Health Research and Development Program (NHRDP) Ph.D. fellowship. R.C. is the recipient of a Ph.D. fellowship from the FRSQ/Fonds pour la Formation de Chercheurs et l’Aide à la Recherche.
REFERENCES
- 1.Abbate I, Capobianchi M R, Fais S, Castilletti C, Mercuri F, Fei P C, Ameglio F, Dianzani F. Host cell antigenic profile acquired by HIV-1 is a marker of its cellular origin. Arch Virol. 1995;140:1849–1854. doi: 10.1007/BF01384347. [DOI] [PubMed] [Google Scholar]
- 2.Adachi A, Gendelman H E, Koenig S, Folks T, Willey R, Rabson A, Martin M A. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman transfected cells with an infectious molecular clone. J Virol. 1986;59:284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aguilar-Cordova E, Chinen J, Donehower L, Lewis D E, Belmont J W. A sensitive reporter cell line for HIV-1 tat activity, HIV-1 inhibitors, and T cell activation effects. AIDS Res Hum Retroviruses. 1994;10:295–301. doi: 10.1089/aid.1994.10.295. [DOI] [PubMed] [Google Scholar]
- 4.Bastiani L, Laal S, Kim M, Zolla-Pazner S. Host cell-dependent alterations in envelope components of human immunodeficiency virus type 1 virions. J Virol. 1997;71:3444–3450. doi: 10.1128/jvi.71.5.3444-3450.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bazil V, Stefanova I, Hilgert I, Kristofova H, Vanek S, Horejsi V. Monoclonal antibodies against leukocyte antigens. IV. Antibodies against the LFA-1 (CD11a/CD18) leukocyte-adhesion glycoprotein. Folia Biol (Praha) 1990;36:41–50. [PubMed] [Google Scholar]
- 6.Benkirane M, Blanc-Zouaoui D, Hirn M, Devaux C. Involvement of human leukocyte antigen class I molecules in human immunodeficiency virus infection of CD4-positive cells. J Virol. 1994;68:6332–6339. doi: 10.1128/jvi.68.10.6332-6339.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berman P W, Nakamura G R. Adhesion mediated by intercellular adhesion molecule 1 attenuates the potency of antibodies that block HIV-1 gp160-dependent syncytium formation. AIDS Res Hum Retroviruses. 1994;10:585–593. doi: 10.1089/aid.1994.10.585. [DOI] [PubMed] [Google Scholar]
- 8.Bess J W J, Gorelick R J, Bosche W J, Henderson L E, Arthur L O. Microvesicles are a source of contaminating cellular proteins found in purified HIV-1 preparations. Virology. 1997;230:134–144. doi: 10.1006/viro.1997.8499. [DOI] [PubMed] [Google Scholar]
- 9.Cantin R, Fortin J-F, Lamontagne G, Tremblay M. The acquisition of host major histocompatibility complex class II glycoproteins by human immunodeficiency virus type 1 accelerates the process of virus entry and infection in human T-lymphoid cells. Blood. 1997;90:1091–1100. [PubMed] [Google Scholar]
- 10.Cantin R, Fortin J-F, Lamontagne G, Tremblay M. The presence of host-derived HLA-DR1 on human immunodeficiency virus type 1 increases viral infectivity. J Virol. 1997;71:1922–1930. doi: 10.1128/jvi.71.3.1922-1930.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cantin R, Fortin J-F, Tremblay M. The amount of host HLA-DR proteins acquired by HIV-1 is virus strain- and cell type-specific. Virology. 1996;218:372–381. doi: 10.1006/viro.1996.0206. [DOI] [PubMed] [Google Scholar]
- 12.Castilletti C, Capobianchi M R, Fais S, Abbate I, Ficociello B, Ameglio F, Cordiali Fei P, Santini S M, Dianzani F. HIV type 1 grown on interferon γ-treated U937 cells shows selective increase in virion-associated intercellular adhesion molecule 1 and HLA-DR and enhanced infectivity for CD4-negative cells. AIDS Res Hum Retroviruses. 1995;11:547–553. doi: 10.1089/aid.1995.11.547. [DOI] [PubMed] [Google Scholar]
- 13.Chen B K, Saksela K, Andino R, Baltimore D. Distinct modes of human immunodeficiency virus type 1 proviral latency revealed by superinfection of nonproductively infected cell lines with recombinant luciferase-encoding viruses. J Virol. 1994;68:654–660. doi: 10.1128/jvi.68.2.654-660.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, Mackay C R, LaRosa G L, Newman W, Gerard N, Gerard C, Sodroski J. The β-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 15.Dalgleish A G, Beverly P C L, Clapham P R, Crawford D H, Greaves M F, Weiss R A. The CD4(T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984;312:763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- 16.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Mark Hill C, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 17.Diamond M S, Springer T A. The dynamic regulation of integrin adhesiveness. Curr Biol. 1994;4:506–517. doi: 10.1016/s0960-9822(00)00111-1. [DOI] [PubMed] [Google Scholar]
- 18.Doranz B J, Rucker J, Yi Y, Smyth R J, Samson M, Peiper S C, Parmentier M, Collman R G, Doms R W. A dual-tropic primary HIV-1 isolate that uses fusin and the β-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 19.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 20.Dustin M L, Springer T A. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature. 1989;341:619–624. doi: 10.1038/341619a0. [DOI] [PubMed] [Google Scholar]
- 21.Fais S, Capobianchi M R, Abbate I, Catilletti C, Gentile M, Cordiali Fei P, Ameglio F, Dianzani F. Unidirectional budding of HIV-1 at the site of cell-to-cell contact is associated with co-polarization of intercellular adhesion molecules (ICAM-1) and HIV-1 viral matrix protein. AIDS. 1995;9:329–335. [PubMed] [Google Scholar]
- 22.Feng Y, Broder C C, Kennedey P E, Berger E A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 23.Fortin J-F, Cantin R, Lamontagne G, Tremblay M. Host-derived ICAM-1 glycoproteins incorporated on human immunodeficiency virus type 1 are biologically active and enhance viral infectivity. J Virol. 1997;71:3588–3596. doi: 10.1128/jvi.71.5.3588-3596.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frank I, Stoiber H, Godar S, Stockinger H, Steindl F, Katinger H W D, Dierich M P. Acquisition of host cell-surface-derived molecules by HIV-1. AIDS. 1996;10:1611–1620. doi: 10.1097/00002030-199612000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Gelderblom H R, Hausmann E H S, Özel M, Pauli G, Koch M A. Fine structure of human immunodeficiency virus (HIV) and immunolocalization of structural proteins. Virology. 1987;156:171–176. doi: 10.1016/0042-6822(87)90449-1. [DOI] [PubMed] [Google Scholar]
- 26.Gelderblom H R, Reupke H, Pauli G. Loss of envelope antigens of HTLV-III/LAV, a factor in AIDS pathogenesis? Lancet. 1985;ii:1016–1017. doi: 10.1016/s0140-6736(85)90570-7. [DOI] [PubMed] [Google Scholar]
- 27.Gluschankof P, Mondor I, Gelderblom H S, Sattentau Q J. Cell membrane vesicles are a major contaminant of gradient-enriched human immunodeficiency virus type-1 preparations. Virology. 1997;230:125–133. doi: 10.1006/viro.1997.8453. [DOI] [PubMed] [Google Scholar]
- 28.Gomez M B, Hildreth J E K. Antibody to adhesion molecule LFA-1 enhances plasma neutralization of human immunodeficiency virus type 1. J Virol. 1995;69:4628–4632. doi: 10.1128/jvi.69.8.4628-4632.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo M M L, Hildreth J E K. HIV acquired functional adhesion receptors from host cells. AIDS Res Hum Retroviruses. 1995;11:1007–1013. doi: 10.1089/aid.1995.11.1007. [DOI] [PubMed] [Google Scholar]
- 30.Heath S L, Tew J G, Tew J G, Szakal A K, Burton G F. Follicular dendritic cells and human immunodeficiency virus infectivity. Nature. 1995;377:740–744. doi: 10.1038/377740a0. [DOI] [PubMed] [Google Scholar]
- 31.Henderson L E, Sowder R, Copeland T D, Oroszlan S, Arthur L O, Robey W G, Fischinger P J. Direct identification of class II histocompatibility DR proteins in preparations of human T-cell lymphotropic virus type III. J Virol. 1987;61:629–632. doi: 10.1128/jvi.61.2.629-632.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hildreth J E K, Orentas R J. Involvement of a leukocyte adhesion receptor (LFA-1) in HIV-induced syncytium formation. Science. 1989;244:1075–1078. doi: 10.1126/science.2543075. [DOI] [PubMed] [Google Scholar]
- 33.Hoxie J, Fitzharris T P, Yougbar P R, Matthews D M, Rackowski J L, Radka S F. Nonrandom association of cellular antigens with HTLV-III virions. Hum Immunol. 1987;18:39–52. doi: 10.1016/0198-8859(87)90111-x. [DOI] [PubMed] [Google Scholar]
- 34.Keizer G D, Visser W, Vliem M, Figdor C C. A monoclonal antibody (NKI-L16) directed against a unique epitope on the α-chain of human leukocyte function-associated antigen 1 induces homotypic cell-cell interactions. J Immunol. 1988;140:1393–1400. [PubMed] [Google Scholar]
- 35.Landis C R, Bennett R I, Hogg N. A novel LFA-1 activation epitope maps to the I domain. J Cell Biol. 1993;120:1519–1527. doi: 10.1083/jcb.120.6.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lapham C K, Ouyang J, Chandrasekhar B, Nguyen N Y, Dimitrov D S, Golding H. Evidence for cell-surface association between fusin and the CD4-gp120 complex in human cell lines. Science. 1996;274:602–605. doi: 10.1126/science.274.5287.602. [DOI] [PubMed] [Google Scholar]
- 37.Lawrence M B, Springer T A. Leukocyte roll on a selectin at physiological flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 1991;65:859–873. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- 38.Layne S P, Merges M J, Dembo M, Spouge J L, Nara P L. HIV requires multiple gp120 molecules for CD4-mediated infection. Nature. 1990;346:277–279. doi: 10.1038/346277a0. [DOI] [PubMed] [Google Scholar]
- 39.Lub M, van Kooyk Y, Figdor C G. Ins and out of LFA-1. Immunol Today. 1995;16:479–483. doi: 10.1016/0167-5699(95)80031-x. [DOI] [PubMed] [Google Scholar]
- 40.Maddon P J, Dalgleish A G, McDougal J S, Clapham P R, Weiss R A, Axel R. The CD4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986;47:333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- 41.Marlin S D, Springer T A. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1) Cell. 1987;51:813–819. doi: 10.1016/0092-8674(87)90104-8. [DOI] [PubMed] [Google Scholar]
- 42.McKeating J A, McKnight A, Moore J P. Differential loss of envelope glycoprotein gp120 from virions of human immunodeficiency virus type 1 isolates: effects on infectivity and neutralization. J Virol. 1991;65:852–860. doi: 10.1128/jvi.65.2.852-860.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pantaleo G, Butini L, Graziosi C, Poli G, Schnittman S M, Greenhouse J J, Gallin J I, Fauci A S. Human immunodeficiency virus (HIV) infection in CD4+ T lymphocytes genetically deficient in LFA-1: LFA-1 is required for HIV-mediated cell fusion but not for viral transmission. J Exp Med. 1991;173:511–514. doi: 10.1084/jem.173.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pantaleo G, Graziosi C, Butini L, Pizzo P A, Schnittman S M, Kotler D P, Fauci A S. Lymphoid organs function as major reservoirs for human immunodeficiency virus. Proc Natl Acad Sci USA. 1991;88:9838–9842. doi: 10.1073/pnas.88.21.9838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pantaleo G, Graziosi C, Fauci A S. The immunopathogenesis of human immunodeficiency virus infection. N Engl J Med. 1993;328:327–335. doi: 10.1056/NEJM199302043280508. [DOI] [PubMed] [Google Scholar]
- 46.Pantaleo, G., C. Graziosi, and A. S. Fauci. 1993. The role of lymphoid organs in the immunopathogenesis of HIV infection. AIDS 7(Suppl. 1)**:**s19–s23. [PubMed]
- 47.Parrott D M, Wilkinson P C. Lymphocyte locomotion and migration. Prog Allergy. 1981;28:193–284. [PubMed] [Google Scholar]
- 48.Popovic M, Gartner S. Isolation of HIV-1 from monocytes but not T lymphocytes. Lancet. 1987;ii:916. doi: 10.1016/s0140-6736(87)91403-6. [DOI] [PubMed] [Google Scholar]
- 49.Rizzuto C D, Sodroski J G. Contribution of virion ICAM-1 to human immunodeficiency virus infectivity and sensitivity to neutralization. J Virol. 1997;71:4847–4851. doi: 10.1128/jvi.71.6.4847-4851.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rossio J L, Bess J, Henderson L E, Cresswell P, Arthur L O. HLA class II on HIV particles is functional in superantigen presentation to human T cells: implications for HIV pathogenesis. AIDS Res Hum Retroviruses. 1995;11:1433–1439. doi: 10.1089/aid.1995.11.1433. [DOI] [PubMed] [Google Scholar]
- 51.Rothlein R, Dustin M L, Marlin S D, Springer T A. A human intercellular adhesion molecule (ICAM-1) distinct from LFA-1. J Immunol. 1986;137:1270–1274. [PubMed] [Google Scholar]
- 52.Saifuddin M, Parker C J, Peeples M E, Gorny M K, Zolla-Pazner S, Ghassemi M, Rooney I A, Atkinson J P, Spear G T. Role of virion-associated glycosylphosphatidylinositol-linked proteins CD55 and CD59 in complement resistance of cell line-derived and primary isolates of HIV-1. J Exp Med. 1995;182:501–509. doi: 10.1084/jem.182.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sanders M E, Makgoba M W, Sharrow S O, Stephany D, Springer T A, Young H A, Shaw S. Human memory T lymphocytes express increased levels of three cell adhesion molecules (LFA-3, CD2, and LFA-1) and three other molecules (UCHL1, CDw29, and Pgp-1) and have enhanced IFN-γ production. J Immunol. 1988;140:1401–1407. [PubMed] [Google Scholar]
- 54.Schnittman S M, Psallidopoulos M C, Lane H C, Thompson L, Baseler M, Massari F, Fox C H, Salzman N P, Fauci A S. The reservoir for HIV-1 in human peripheral blood is a T cell that maintains expression of CD4. Science. 1989;245:305–308. doi: 10.1126/science.2665081. [DOI] [PubMed] [Google Scholar]
- 55.Springer T A. Adhesion receptors of the immune system. Nature. 1990;346:425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 56.Staunton D E, Marlin S D, Stratowa C, Dustin M L, Springer T. Primary structure of ICAM-1 demonstrates interaction between members of the immunoglobulin and integrin families. Cell. 1988;52:925–933. doi: 10.1016/0092-8674(88)90434-5. [DOI] [PubMed] [Google Scholar]
- 57.Stevenson M, Stanwick T L, Dempsey M P, Lamonica C A. HIV-1 replication is controlled at the level of T cell activation and proviral integration. EMBO J. 1990;9:1551–1560. doi: 10.1002/j.1460-2075.1990.tb08274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Trkola A, Dragic T, Arthos J, Binley J M, Olson W C, Allaway G P, Cheng-Mayer C, Robinson J, Maddon P J, Moore J P. CD4-dependent, antibody-sensitive interactions between HIV-1 and its co-receptor CCR-5. Nature. 1996;384:184–187. doi: 10.1038/384184a0. [DOI] [PubMed] [Google Scholar]
- 59.Wu L, Gerard N P, Wyatt R, Choe H, Parolin C, Ruffing N, Borsetti A, Cardoso A A, Desjardin E, Newman W, Gerard C, Sodroski J. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature. 1996;384:179–183. doi: 10.1038/384179a0. [DOI] [PubMed] [Google Scholar]
- 60.Zack J A, Arrigo S J, Weitsman S R, Go A S, Haislip A, Chen I S Y. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990;61:213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]