Cytosolic Gag p24 as an Index of Productive Entry of Human Immunodeficiency Virus Type 1 (original) (raw)
Abstract
We have investigated the cellular uptake of Gag p24 shortly after exposure of cells to human immunodeficiency virus (HIV) particles. In the absence of envelope glycoprotein on virions or of viral receptors or coreceptors at the cell surface, p24 was incorporated in intracellular vesicles but not detected in the cytosolic subcellular fraction. When appropriate envelope-receptor interactions could occur, the nonspecific vesicular uptake was still intense and cytosolic p24 represented 10 to 40% of total intracellular p24. The measurement of cytosolic p24 early after exposure to HIV type 1 is a reliable assay for investigating virus entry and early events leading to authentic cell infection.
The entry of human immunodeficiency virus type 1 (HIV-1) into target cells follows receptor-mediated attachment of viral particles to the cell surface. The cell surface receptor for HIV-1 is the CD4 molecule (7, 15), which promotes attachment of the particle to the cell surface. Fusion between the viral and plasma membranes leading to virus entry into the cytoplasm also requires interaction with a coreceptor. Various chemokine receptors ensure this function. The CXCR4 receptor is used by lymphotropic virus strains (10), whereas the entry of macrophage-tropic and of most primary isolates is processed through interaction with the CCR5 receptor (8, 9). Interactions with CD4 and with a coreceptor expose highly hydrophobic epitopes at the N terminus of the gp41 transmembrane component of envelope, leading to subsequent fusion between viral and cell membranes (6, 17, 34, 35).
Several observations have suggested that the fusion process takes place at the cell surface: (i) HIV infection is pH independent, whereas infection by most viruses entering through the endocytic pathway is inhibited by weak bases and ionophore agents (20, 32); (ii) HIV fusion images have been observed at the cell surface (11); (iii) endocytosis of CD4 is not required for entry (18, 20, 25, 28, 32); and (iv) mutant CXCR5 receptors which are not endocytosed in response to ligand binding still function as HIV coreceptors (2). However, other considerations led to the assumption that although HIV entry is clearly pH independent, it may not necessarily be endocytosis independent: (i) images of HIV particles internalized in endocytic vesicles and undergoing fusion with endosomal membranes have been observed (11, 27), (ii) pH-independent entry via endosomal vesicles has been reported for poliovirus (29), (iii) binding and cross-linking by multivalent virus particles may induce endocytic behavior of cell surface receptors different from that induced by their natural ligands, and (iv) endocytosis of CD4 and that of coreceptors have not been simultaneously examined after HIV exposure. Moreover, since studies of virus entry have been performed with cells where the endocytic pathway is active, it is difficult to determine whether particular fusion events at the cell surface or in endosomal vesicles give rise to productive infection.
With the aim of examining the role of endosomal HIV particle uptake, infection was synchronized by exposing cells to the virus at 4°C, cells were warmed at 37°C, and p24 was measured in the vesicular and cytosolic fractions of cell extracts. p24 was detected in intracellular vesicles regardless of whether exposure to virus particles could give rise to authentic infection or not. On the other hand, the detection of p24 in cytosolic fractions was strictly associated with authentic infectious events. However, it represented a minor fraction of intracellular p24. Thus, although vesicular uptake is quantitatively the main route of virus particle internalization, it is essentially a dead end with respect to cell infection.
MATERIALS AND METHODS
Cells, viruses, and reagents.
P4 cells are CD4-positive, HeLa cell-derived cells in which transactivation by Tat induces expression of the Escherichia coli lacZ gene from the HIV long terminal repeat (5). P4C5 cells are P4 cells constitutively expressing the CCR5 HIV coreceptor fused to the green fluorescent protein (2). HeLa, P4, and P4C5 cells were grown in Dulbecco modified Eagle medium (DMEM) supplemented with heat-inactivated fetal calf serum (FCS) and antibiotics at 37°C in 5% CO2. For P4 and P4C5 cells, G418 (Geneticin; 1 mg/ml; Gibco) and G418 plus hygromycin (300 μg/ml) were added to the culture media, respectively. NL43, JRCSF, and NL43Δenv were obtained by transfecting plasmids pNL43-2 (1), pJRCSF (24), and pNL43Δenv (3) into HeLa cells. HIV-1 pseudotypes with the vesicular stomatitis virus G glycoprotein (VSV-G) were produced by cotransfecting pNL43Δenv and pHCMV-G (36) into HeLa cells. pHCMV-G, a kind gift of A. Miyanohara, carries the VSV-G gene under the control of the human cytomegalovirus immediate-early promoter (36). Viral stocks were analyzed for their HIV-1 p24 content by enzyme-linked immunosorbent assay (kit from Dupont de Nemours) and frozen. The infectivity of viral supernatants was determined on P4 cells as described previously (23, 31). Bafilomycin A1 was from Sigma.
Cell fractionation assays. (i) HeLa and P4 cells.
A subconfluent 75-cm2 culture flask containing approximately 6 × 106 cells was exposed to an HIV suspension containing 200 to 1,000 ng of p24 in culture medium supplemented with 20 μg of DEAE-dextran per ml and 20 mM HEPES. After 30 to 60 min at 4°C (or, when stated, at 37°C), cells were washed three times in phosphate-buffered saline (PBS) at 4°C and either immediately treated with pronase or incubated at 37°C for 1 h before pronase treatment. Cells were treated with 1 ml of ice-cold DMEM with 20 mM HEPES and 7 mg of pronase per ml and were incubated for 10 min at 4°C. Cells were washed once in 1 ml of DMEM supplemented with 10% FCS and three times in ice-cold PBS to eliminate pronase and then were resuspended in 2 ml of swelling buffer (10 mM Tris-HCl [pH 8], 10 mM KCl, 1 mM EDTA) for 15 min at 4°C. Cells were then disrupted by Dounce homogenization (15 strokes, 7 ml B pestles); nuclei and cell debris were pelleted by centrifugation (3,000 rpm for 3 min in a Heraeus Varifuge centrifuge). The resulting postnuclear extracts were then ultracentrifuged at 60,000 rpm for 10 min at 4°C in a Beckman TL100 centrifuge. The supernatant representing the cytosolic fraction was adjusted to 0.5% Triton X-100, while the pellet was resuspended in lysis buffer (20 mM HEPES, 0.5% Triton X-100, 150 mM NaCl) to obtain the vesicular fraction. p24 concentrations were measured in both fractions by enzyme-linked immunosorbent assay.
(ii) CEM cells.
A total of 107 cells were washed in PBS and resuspended in an HIV suspension containing 300 ng of p24 in culture medium supplemented with 20 μg of DEAE-dextran per ml and 20 mM HEPES for 30 min at 4°C under gentle agitation. After successive washes in ice-cold PBS, cells were treated with pronase immediately or incubated at 37°C for 1 h before pronase treatment. For pronase treatment, cells were resuspended in a solution containing 1 ml of DMEM, 20 mM HEPES, and 0.1 mg of pronase per ml for 5 min at 4°C. Cells were washed once in 1 ml of DMEM supplemented with 10% FCS and three times in ice-cold PBS to eliminate pronase, and then they were resuspended in 2 ml of swelling buffer for 1 min at 4°C and lysed by Dounce homogenization (three strokes). The cytosolic and vesicular fractions were then prepared as for HeLa cells.
Immunofluorescence microscopy and confocal analysis.
HeLa and P4 cells were grown to 50% confluence on glass coverslips in 24-well plates, incubated for 2 h at 4°C with HIV-1NL43 (200 ng of p24 per well) in the presence of 20 μg of DEAE-dextran per ml, washed, and warmed at 37°C for 30 min. Cells were then fixed in 3.7% paraformaldehyde–PBS for 20 min and incubated for 10 min in 50 mM NH4Cl in order to quench free aldehydes. After a 15-min incubation in permeabilization buffer (0.5% saponin, 0.2% bovine serum albumin in PBS), cells were incubated for 1 h with a mixture of mouse anti-Gag monoclonal antibodies (MAbs) (a gift of F. Traincart, Institut Pasteur, Paris, France). Cells were then incubated with fluorescein isothiocyanate-conjugated goat anti-mouse Abs (Amersham), washed in permeabilization buffer and in PBS, and then mounted in a solution containing 133 mg of Mowiol (Hoechst) per ml, 33% glycerol, and 133 mM Tris HCl (pH 8.5). Cells were analyzed with a Leica TCS4D confocal microscope. Photographs were taken with Kodak Ektachrome 100 ASA film.
RESULTS AND DISCUSSION
Virus particle internalization in CD4-negative cells.
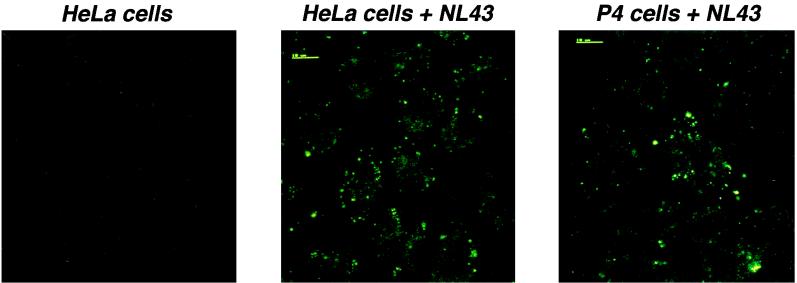
We have examined intracellular viral material after cell exposure to HIV-1. HeLa cells, which are not susceptible to HIV-1 infection, and P4 cells, which are CD4-positive HeLa cells that can be infected with lymphotropic HIV-1 strains, were incubated with equal amounts of the lymphotropic strain NL43 (200 ng of p24) for 2 h at 4°C, washed to remove unbound virus, and warmed to 37°C for 30 min. Cells were then analyzed by confocal fluorescence microscopy, using a mixture of anti-Gag MAbs. Multiple intracellular dots were observed inside cells of both types (Fig. 1). Staining was specific for p24, as it was absent in control uninfected cells. These data suggest that virus material is internalized into cells irrespectively of CD4 surface expression and with almost equal efficiencies in cells susceptible and not susceptible to HIV infection. It is therefore presumable that most of the internalized viral material does not participate in the infectious process. In agreement with a previous report showing HIV particles internalized in clathrin-coated vesicles (11), simultaneous detection of p24 and clathrin, p24 and the lysosomal Lamp1 protein, or p24 and endocytosed transferrin-rhodamine revealed partial colocalization (data not shown).
FIG. 1.
Confocal microscopy analysis of intracellular p24 after cell exposure to HIV-1. HeLa cells (middle panel) or P4 cells (right panel) were exposed to HIV-1NL43 for 2 h at 4°C, washed, warmed to 37°C for 30 min, fixed, and labeled with anti-Gag MAbs. Left panel, control HeLa cells not exposed to HIV-1.
Cytosolic p24 is associated with authentic infection.
We postulated that only viral material internalized into the cytosolic compartment would be relevant to infection. With the aim of testing this hypothesis, p24 was measured in subcellular cytosolic and vesicular fractions prepared 1 h after exposure to HIV-1 in various situations, some being compatible with infection and others not.
Accurate measurement of intracellular virus material requires that noninternalized virus particles attached to the cell surface be removed efficiently prior to cell lysis. To verify that this occurred, P4 cells were treated with various proteolytic enzymes and cell surface CD4 was analyzed by flow cytometry. Cell surface CD4 was totally removed by pronase treatment (7 mg/ml, 10 min at 4°C), thus potentially eliminating virus particles attached to the receptor (data not shown).
With the aim of verifying that p24 was not detectable in the subcellular fractions after exposure to HIV-1 under conditions not allowing virus entry, HeLa and P4 cells were exposed to NL43 (300 ng of p24) for 30 min at 4°C and immediately treated with pronase. The background p24 level was below 100 pg in postnuclear cell extracts, indicating that virus particles bound at the cell surface had been efficiently removed by pronase and had not been internalized into cells. Postnuclear extracts were separated into soluble cytosolic and sedimentable vesicular fractions by ultracentrifugation. Background p24 levels below 10 and 100 pg were measured in the cytosolic and vesicular fractions, respectively. The lysosomal Lamp1 and Lamp2 proteins were detected by Western blotting in the vesicular fraction only, indicating that the cytosolic fraction was free of detectable vesicular contaminants (data not shown).
Figure 2 shows results of experiments in which virus binding at 4°C for 30 min was followed by a 1-h incubation at 37°C in order to allow internalization. Total intracellular p24 represented 0.15% ± 0.04% of the viral input, whatever the target cell line. Vesicular fractions (pellet) contained p24 even in the absence of cell surface CD4 (HeLa cells) or when virus particles lacked envelope ligand (NL43Δenv). Western blot analysis indicated that the vesicular fraction also contained other components of viral particles (p17 and uncleaved or partially cleaved Gag polyprotein precursors [data not shown]). In contrast, cytosolic p24 (cytosol) was found only in CD4-positive cells (P4 cells) exposed to infectious NL43, the only combination relevant to virus infection. Whatever the virus input, cytosolic p24 represented roughly 10% of total intracellular p24, i.e., approximately 0.01% of the p24 amount used for infection (Fig. 2a). In these experiments, exposure of cells to virus was performed in the presence of DEAE-dextran in order to increase virus binding and infectivity (16). We examined whether DEAE-dextran affects the intracellular distribution of incoming viruses. When the polycationic reagent was omitted during virus exposure, total intracellular p24 values were significantly reduced. However, the distribution of p24 between the cytosolic and vesicular fractions was not modified (data not shown), indicating that DEAE-dextran does not favor nonspecific virus entry. Performing virus binding at 37°C instead of 4°C increased total intracellular p24 values but did not affect the distribution in the cytosolic and vesicular fractions (data not shown). Altogether, these observations suggest that the majority of intracellular p24, which was found in vesicles, does not participate in the infection process.
FIG. 2.
p24 levels in subcellular extracts of cells exposed to HIV-1. HeLa, P4, or P4C5 cells were exposed to strain NL43 (lymphotropic) or JRCSF (macrophage tropic) or to HIV particles devoid of envelope protein (NL43Δenv), respectively, for 30 min at 4°C. Cells were extensively washed and were incubated at 37°C for 1 h. Cells were then treated with pronase, and p24 amounts in subcellular fractions were measured. Total intracellular p24 levels (in picograms) are indicated over the bars. The percentages of cytosolic and vesicular p24 are shown inside the bars. (a) Dose-response analysis for NL43; (b) analysis of the entry of HIV-1 strains with different tropisms (input, 300 ng of p24); (c) analysis of the entry in CEM cells (input, 400 ng of p24). Data are representative of at least three experiments.
Experiments were performed with a macrophage-tropic HIV-1 strain, JRCSF (Fig. 2b). HeLa cells exposed to NL43, NL43Δenv, or JRCSF contained p24 in the vesicular fraction but not in the cytosol. P4 cells express the lymphotropic virus coreceptor CXCR4 but not the macrophage-tropic virus coreceptor CCR5. Cytosolic p24 represented 10% of total intracellular p24 after exposure to the lymphotropic virus NL43 but was at background levels after exposure to the macrophage-tropic virus JRCSF. P4 cell derivatives constitutively expressing CCR5 (P4C5 cells) were isolated (2). These cells are susceptible to infection with JRCSF (2). Cytosolic p24 represented 10% of total intracellular p24 after exposure of P4C5 cells to JRCSF. Therefore, significant cytosolic p24 levels were found only when envelope glycoproteins recognized relevant receptors on HeLa cell surfaces. These data indicate that the cytosolic release of p24 is specifically associated with the infection process.
Since lymphoid cells are natural targets of HIV infection, we repeated these experiments with the lymphoblastoid cell line CEM. Because these cells are more fragile than HeLa cells, pronase treatment and preparation of cell extracts were performed cautiously (see Materials and Methods). This impaired complete removal of virus particles attached at the cell surface, as observed by flow cytometry analysis (data not shown), and led to a contamination of both the vesicular and cytosolic fractions with extracellular p24. Contamination probably accounted for the detection of p24 in cytosolic fractions of CEM cells exposed to the negative control virus (NL43Δenv [Fig. 2c]). However, cytosolic p24 levels were much higher after exposure to NL43, which is infectious for CEM cells. CEM cells do not express the macrophage-tropic CCR5 coreceptor and are not susceptible to infection with JRCSF. Cytosolic p24 levels were significantly lower after exposure to JRCSF than after exposure to NL43 and represented, respectively, 23 and 44% of total p24 uptake. The presence of p24 in the cytosolic fractions of JRCSF-exposed CEM cells is probably the consequence of incomplete removal of virus particles attached at the cell surface. These data show that, in CEM cells, as well as in P4 cells, the detection of cytosolic p24 was associated with viral infection. Interestingly, after exposure to NL43, cytosolic p24 represented a much higher proportion of total intracellular p24 in CEM than in P4 cells (44% versus approximately 10%). This suggests that routing of viral material toward authentic entry pathways may be more efficient in natural HIV-1 lymphocytic targets than in epithelial cells.
Internalization of p24 in the cytosol is pH independent.
Bafilomycin A1 is an inhibitor of vacuolar proton-ATPases which impairs intracellular vesicle acidification (4). It is a potent inhibitor of the entry of viruses that require acidification for fusion, like VSV, and of endosomal and lysosomal enzymatic degradation systems (12, 19, 26). We tested whether these activities of bafilomycin A1 affect the entry of HIV particles coated with either the gp120 or gp41 envelope protein (NL43) or the VSV-G envelope protein (HIV/VSV).
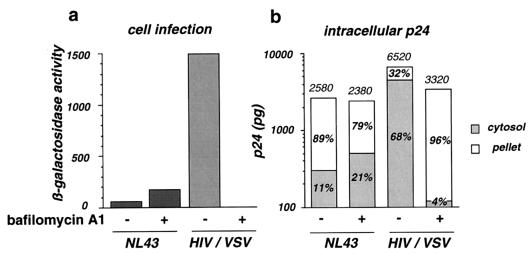
Infection of P4 cells with HIV-1 induces the transactivation of the integrated HIV long terminal repeat lacZ cassette by Tat. The β-galactosidase expression level reflects infection efficiency (5). In the presence of 0.5 μM bafilomycin A1, infection of P4 cells with NL43 increased twofold (Fig. 3a). Cytosolic p24 increased proportionally, whereas vesicular p24 slightly decreased (Fig. 3b). This observation suggests that part of the material internalized in vesicles can be subsequently released into the cytosol when the endosomal degradation pathway is interrupted. HIV-1 particles coated with the VSV-G envelope protein were 15-fold more infectious than NL43 for P4 cells. Cytosolic p24 was increased in the same proportion, representing 68% of total intracellular p24. As expected, infection with VSV-G-pseudotyped viruses was completely abolished in the presence of bafilomycin A1 (Fig. 3a). The loss of infectivity was associated with a 33-fold drop in cytosolic p24 amounts (Fig. 3b). In contrast, p24 accumulated in vesicles, where degradation was inhibited. These data confirmed that, independently of the cell surface receptors used for particle binding and of the entry pathway, cytosolic p24 is a valuable index of viral entry events leading to authentic infection.
FIG. 3.
Effect of bafilomycin A1 on HIV-1 infection efficiency and entry into P4 cells. Cells were either left untreated or treated with 0.5 μM bafilomycin A1 during virus exposure. (a) Infection was assessed by measuring β-galactosidase activity in cell extracts. Viral input corresponded to 1 ng of p24. (b) Intracellular p24 levels were measured in the cytosolic and vesicular fractions. Total intracellular p24 levels (in picograms) are indicated over the bars. The percentages of cytosolic and vesicular p24 are shown inside the bars. Viral input corresponded to 300 ng of p24 during 1 h at 37°C. HIV/VSV is a VSV-G pseudotype of NL43. Data are from one of three representative experiments.
The internalization of p24 in vesicles in the absence of appropriate envelope-receptor interaction probably results from nonspecific adhesion or aggregation of HIV-1 particles at the cell surface. Therefore, assays based on the measurement of total p24 uptake, irrespectively of its intracellular distribution, are not reliable indicators of productive HIV entry. Several incorrect conclusions have probably been drawn from artifactual observations in the past. In HeLa cells, HIV particles trapped in vesicles are unable to release virion cores into the cytosol. The vesicular pathway clearly appears to be a dead end that leads to degradation by lysosomal enzymes. Experiments with P4, P4C5, and, to a lesser extent, CEM cells indicate that, even when appropriate envelope-receptor interactions can take place, 50 to 90% of the intracellular viral material follows the endosomal degradation pathway. The very low proportion of the viral p24 input which reaches the cytosol (in the range of 0.01% of the input) is consistent with the low specific infectivity of HIV stocks, in which the ratio of infectious to total particles was estimated at 1:1,000 (14). Internalization into vesicles is not necessarily a dead end as long as fusion with vesicular membranes can occur before virions are degraded. This was illustrated by the high infectivity of VSV-G pseudotypes. It also probably accounts for increased entry of HIV-1NL43 when endosomal degradation was inhibited by bafilomycin A1. pH-independent entry through the vesicular pathway has been reported for poliovirus (30). This pathway may be important for differentiated macrophages, a key cell type in HIV replication in vivo (22), which exert continual and extensive endocytic activity as part of their natural function (27). It is also possible that CD4-independent endocytosis participates in the antibody-dependent enhancement of HIV-1 infectivity in macrophages (13, 21, 33).
This work reveals that, whatever the route leading to productive infection, a significant fraction of internalized particles are going to be destroyed in lysosomes. Measurements of cytosolic viral material is a reliable assay for authentic entry events and for investigating subsequent infectious processes.
ACKNOWLEDGMENTS
We thank N. Naffakh for suggestions and help in bafilomycin A1 experiments, E. Perret for confocal microscope analysis, and F. Traincart and A. Miyanohara for the kind gift of reagents.
This work was supported by grants from the Agence Nationale de Recherche contre le SIDA, Sidaction, and the Institut Pasteur.
REFERENCES
- 1.Adachi A, Gendelman H E, Koenig S, Folks T, Willey R, Rabson A, Martin M A. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986;59:284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amara A, Le Gall S, Schwartz O, Salamero J, Montes M, Loetscher P, Baggioloni M, Virelizier J L, Arenzana-Seisdedos F. HIV co-receptor down-regulation as anti-viral principle. SDF-1a dependent internalization of the chemokine receptor CXCR4 contributes to inhibition of HIV replication. J Exp Med. 1997;186:139–146. doi: 10.1084/jem.186.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borman A W, Quillent C, Charneau P, Dauguet C, Clavel F. Human immunodeficiency virus type 1 Vif− mutant particles from restrictive cells: role of Vif in correct particle assembly and infectivity. J Virol. 1995;69:2058–2067. doi: 10.1128/jvi.69.4.2058-2067.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowman E J, Siebers A, Altendorf K. Bafilomycins: a mass of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc Natl Acad Sci USA. 1988;85:7972–7976. doi: 10.1073/pnas.85.21.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charneau P, Mirabeau G, Roux P, Paulous S, Buc H, Clavel F. HIV-1 reverse transcription: a termination step at the center of the genome. J Mol Biol. 1994;241:651–662. doi: 10.1006/jmbi.1994.1542. [DOI] [PubMed] [Google Scholar]
- 6.Clapham P R. HIV and chemokines: ligands sharing cell-surface receptors. Trends Cell Biol. 1997;7:264–268. doi: 10.1016/S0962-8924(97)01075-1. [DOI] [PubMed] [Google Scholar]
- 7.Dalgleish A G, Beverley P C L, Clapham P R, Crawford D H, Greaves M F, Weiss R A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984;312:763–766. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- 8.Deng D, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, DiMarzio P, Mamron S, Sutton R E, Hill C M. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 9.Dragic T, Litvin V, Allsway S R, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 10.Feng Y, Broder C C, Kennedy P E, Berger E A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 11.Grewe C, Beck A, Gelderblom H R. HIV: early virus-cell interactions. J Acquired Immune Defic Syndr. 1990;3:965–974. [PubMed] [Google Scholar]
- 12.Guinea R, Carrasco L. Requirement for vacuolar protonATPase activity during entry of influenza virus into cells. J Virol. 1995;69:2306–2312. doi: 10.1128/jvi.69.4.2306-2312.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Homsy J, Meyer M, Tateno M, Clarkson S, Levy J A. The Fc and not CD4 receptor mediates antibody enhancement of HIV-1 infection in human cells. Science. 1989;244:1357–1360. doi: 10.1126/science.2786647. [DOI] [PubMed] [Google Scholar]
- 14.Kimpton J, Emerman M. Detection of replication-competent and pseudotyped human immunodeficiency virus with a sensitive cell line on the basis of activation of an integrated β-galactosidase gene. J Virol. 1992;66:2232–2239. doi: 10.1128/jvi.66.4.2232-2239.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klatzmann D, Champagne E, Chamaret S, Fruest J, Guetard D, Hercend T, Gluckman J, Montagnier L. T lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature. 1984;312:767–768. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- 16.Konopka K, Stamatatos L, Larsen C E, Davis B R, Duzgunes N. Enhancement of human immunodeficiency virus type 1 infection by cationic liposomes: the role of CD4, serum and liposome-cell interactions. J Gen Virol. 1991;72:2685–2696. doi: 10.1099/0022-1317-72-11-2685. [DOI] [PubMed] [Google Scholar]
- 17.Lapham C K, Ouyang J, Chandrasekhar B, Nguyen N Y, Dimitrov D S, Golding H. Evidence for cell-surface association between fusin and the CD4-gp120 complex in human cell lines. Science. 1996;274:602–605. doi: 10.1126/science.274.5287.602. [DOI] [PubMed] [Google Scholar]
- 18.Maddon P J, McDougal J S, Clapham P R, Dalgleish A G, Jamal S, Weiss R A, Axel R. HIV infection does not require endocytosis of its receptor, CD4. Cell. 1988;54:865–874. doi: 10.1016/s0092-8674(88)91241-x. [DOI] [PubMed] [Google Scholar]
- 19.Martínez C G, Guinea R, Benavente J, Carrasco L. The entry of reovirus into L cells is dependent on vacuolar proton-ATPase activity. J Virol. 1996;70:576–579. doi: 10.1128/jvi.70.1.576-579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McClure M O, Marsh M, Weiss R A. Human immunodeficiency virus infection of CD4-bearing cells occurs by a pH-independent mechanism. EMBO J. 1988;7:513–518. doi: 10.1002/j.1460-2075.1988.tb02839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKeating J A, Griffith P D, Weiss R A. HIV susceptibility conferred to human fibroblasts by cytomegalovirus-induced Fc receptor. Nature. 1990;343:659–661. doi: 10.1038/343659a0. [DOI] [PubMed] [Google Scholar]
- 22.Meltzer M S, Skillman D R, Hoover D L, Hanson B D, Turpin J A, Kalter D C, Gendelman H E. Macrophages and the immunodeficiency virus. Immunol Today. 1990;11:217–223. doi: 10.1016/0167-5699(90)90086-o. [DOI] [PubMed] [Google Scholar]
- 23.Oberlin E, Amara A, Bachelerie F, Bessia C, Virelizier J L, Arenzana-Seisdedos F, Schwartz O, Heard J M, Clark-Lewis I, Legler D F, Loetscher M, Baggiolini M, Moser B. The CXC chemokine, stromal cell derived factor 1 (SDF-1), is the ligand for LESTR/fusin and prevents infection by lymphocyte-tropic HIV-1 syncytium-inducing strains. Nature. 1996;382:833–835. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- 24.O’Brien W A, Koyanagi Y, Namazie A, Zhao J Q, Diagne A, Idler K, Zack J A, Chen I S. HIV-1 tropism for mononuclear phagocytes can be determined by regions of gp120 outside the CD4-binding domain. Nature. 1990;348:69–73. doi: 10.1038/348069a0. [DOI] [PubMed] [Google Scholar]
- 25.Orloff G M, Orloff S L, Kennedy M S, Maddon P J, McDougal J S. Penetration of CD4 T cells by HIV-1. J Immunol. 1991;146:2578–2587. [PubMed] [Google Scholar]
- 26.Palokangas H, Metsikko K, Vaananen K. Active vacuolar H+ ATPase is required for both endocytic and exocytic processes during viral infection of BHK-21 cells. J Biol Chem. 1994;269:17577–17585. [PubMed] [Google Scholar]
- 27.Pauza C D, Price T M. Human immunodeficiency virus infection of T cells and monocytes proceeds via receptor-mediated endocytosis. J Cell Biol. 1988;107:959–968. doi: 10.1083/jcb.107.3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pelchen-Matthews A, Clapham P, Marsh M. Role of CD4 endocytosis in human immunodeficiency virus infection. J Virol. 1995;69:8164–8168. doi: 10.1128/jvi.69.12.8164-8168.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pérez L, Carrasco L. Entry of poliovirus into cells does not require a low-pH step. J Virol. 1993;67:4543–4548. doi: 10.1128/jvi.67.8.4543-4548.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perez L, Carrasco L. Involvement of the vacuolar H(+)-ATPase in animal virus entry. J Gen Virol. 1994;75:2595–2606. doi: 10.1099/0022-1317-75-10-2595. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz O, Maréchal V, Danos O, Heard J M. Human immunodeficiency virus type 1 Nef increases the efficiency of reverse transcription in the infected cell. J Virol. 1995;69:4053–4059. doi: 10.1128/jvi.69.7.4053-4059.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stein B S, Gowda S D, Lifson J D, Penhallow R C, Bensch K G, Engleman E G. pH-independent HIV entry into CD4-positive cells via virus envelope fusion to the plasma membrane. Cell. 1987;49:659–668. doi: 10.1016/0092-8674(87)90542-3. [DOI] [PubMed] [Google Scholar]
- 33.Takeda A, Tuazon C U, Ennis F A. Antibody-enhanced infection by HIV-1 via Fc receptor-mediated entry. Science. 1988;242:580–583. doi: 10.1126/science.2972065. [DOI] [PubMed] [Google Scholar]
- 34.Trkola A, Dragic T, Arthos J, Binley J M, Olson W C, Alaway G P, Maddon P J, Moore J P. CD4-dependent, antibody-sensitive interactions between HIV-1 and its co-receptor CCR-5. Nature. 1996;384:184–187. doi: 10.1038/384184a0. [DOI] [PubMed] [Google Scholar]
- 35.Wu L, Gerard N P, Wyatt R, Choe H, Parolin C, Ruffing N, Borsetti A, Cardoso A A, Desjardin E, Newman W, Gerard C, Sodroski J. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature. 1996;384:179–183. doi: 10.1038/384179a0. [DOI] [PubMed] [Google Scholar]
- 36.Yee J K, Miyanohara A, LaPorte P, Bouic K, Burns J C, Friedmann T. A general method for the generation of high-titer, pantropic retroviral vectors: highly efficient infection of primary hepatocytes. Proc Natl Acad Sci USA. 1994;91:9564–9568. doi: 10.1073/pnas.91.20.9564. [DOI] [PMC free article] [PubMed] [Google Scholar]